Isoflurane Rescue Schizophrenia-Related Deficits through Parvalbumin-Positive Neurons in the Dentate Gyrus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and Drugs Administration
2.3. Stereotaxic Viral Injection
2.4. Sholl Analysis
2.5. BrdU Injection
2.6. Behavior Analysis
2.7. Open Field Test
2.8. Prepulse Inhibition of Acoustic Startle
2.9. T-Maze Test
2.10. Immunofluorescence
2.11. Western Blot
2.12. Slice Electrophysiology
2.13. Quantification and Statistical Analysis
3. Results
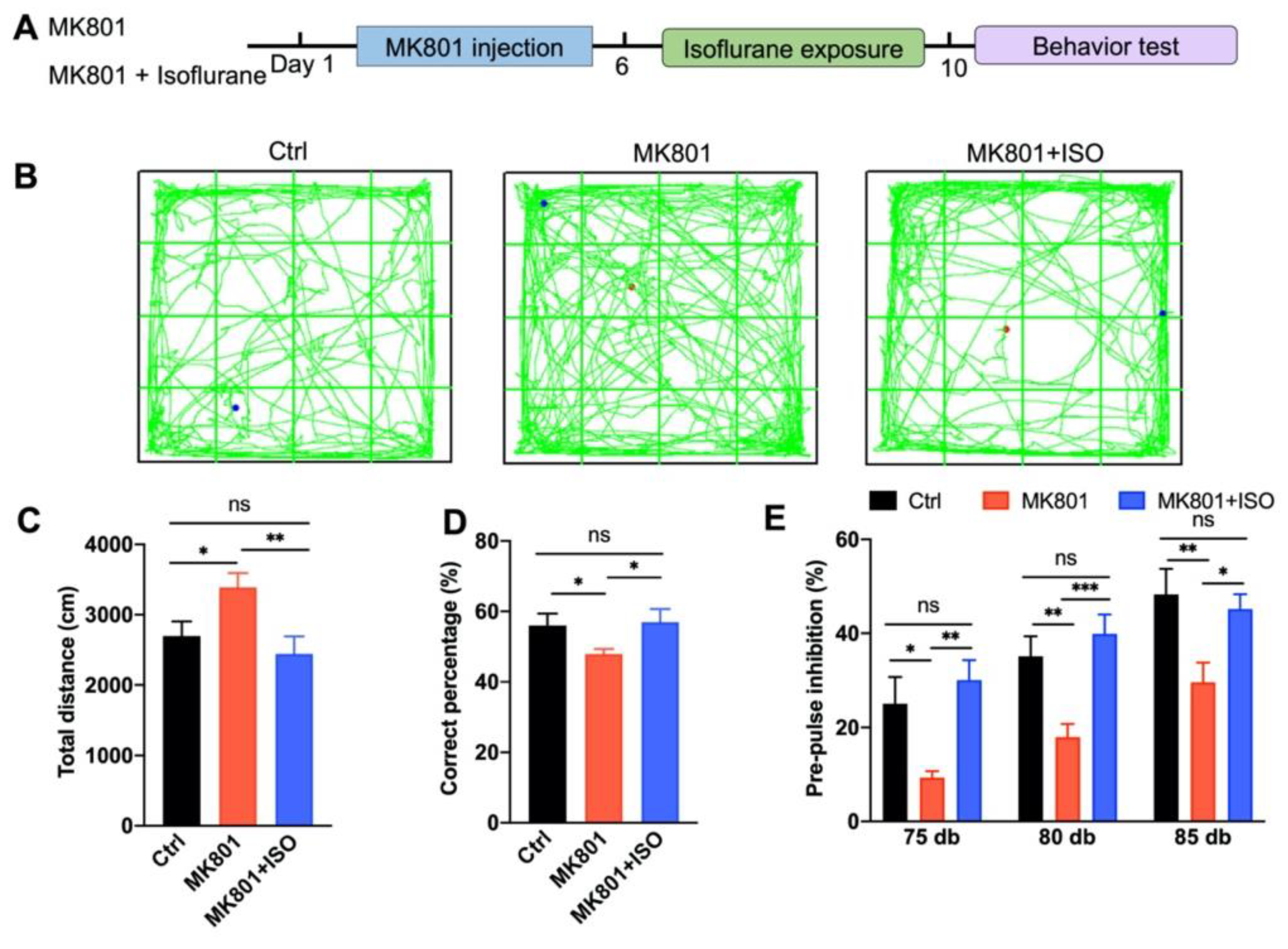
3.1. Isoflurane Treatment Ameliorates MK801-Induced Schizophrenia-Related Behavior Phenotypes
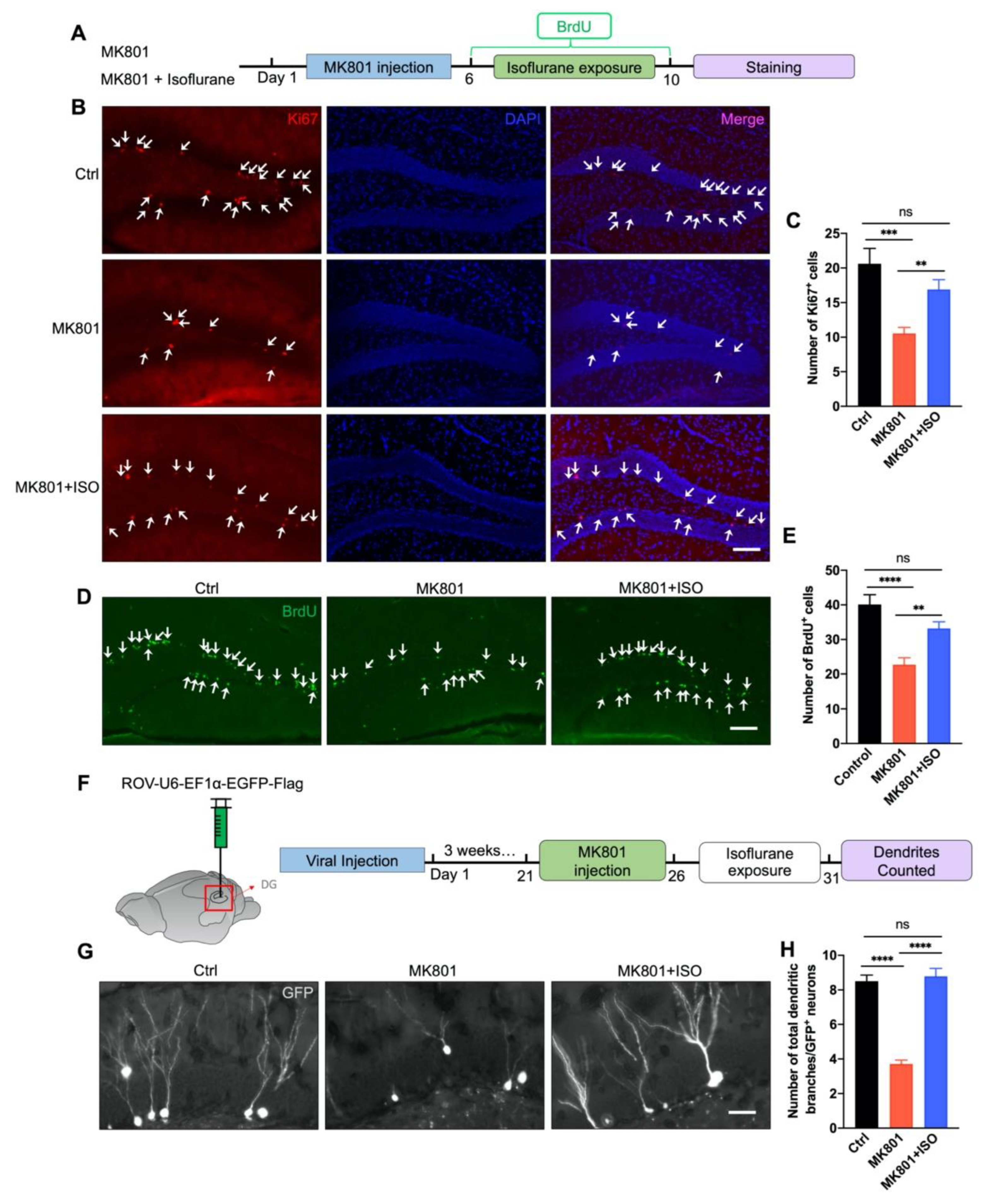
3.2. Isoflurane Reverses Adult Neurogenesis Deficits in the DG Induced by MK801
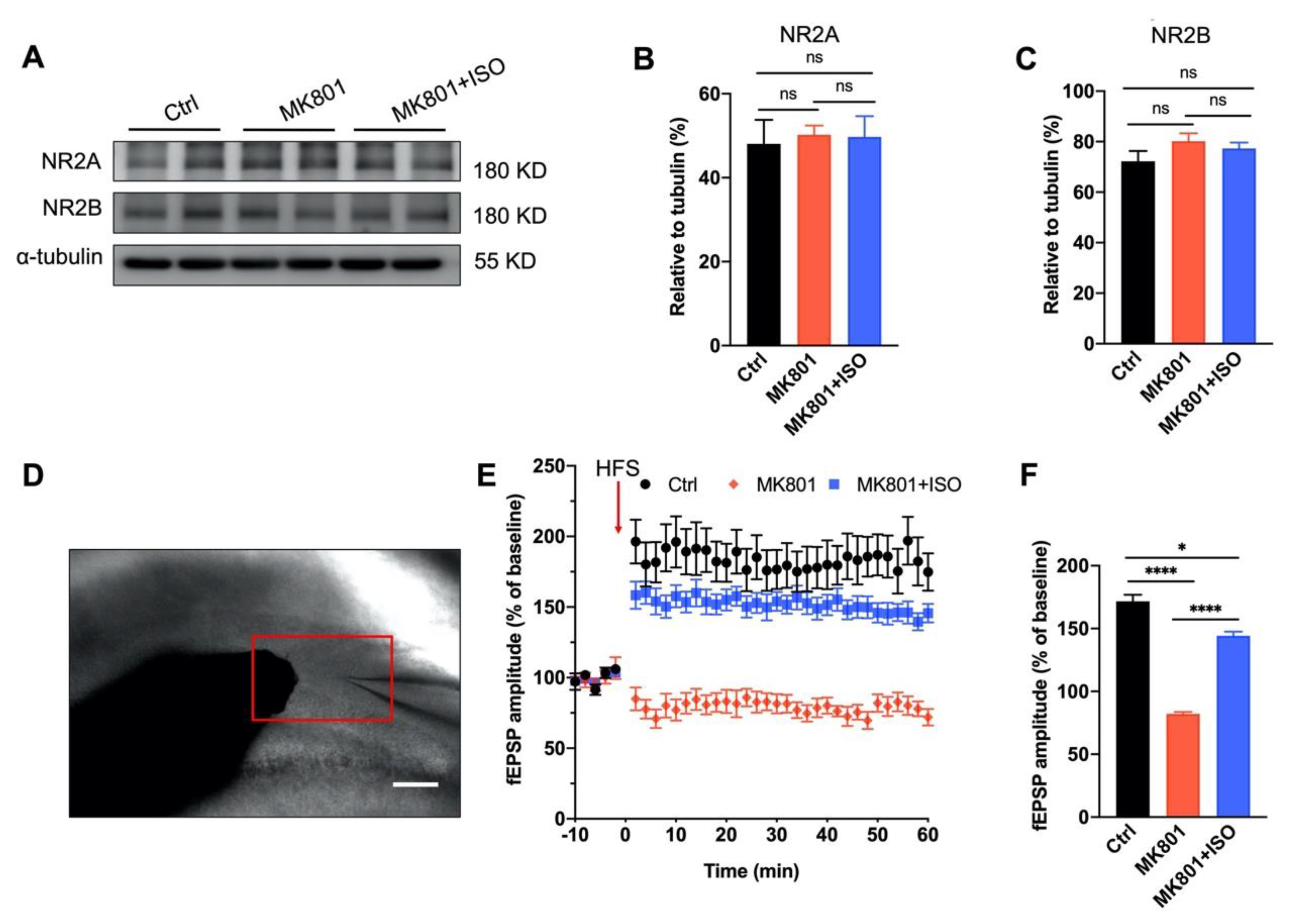
3.3. Synaptic Plasticity Deficit Induced by MK801 in the DG Can Be Reversed by Isoflurane Treatment
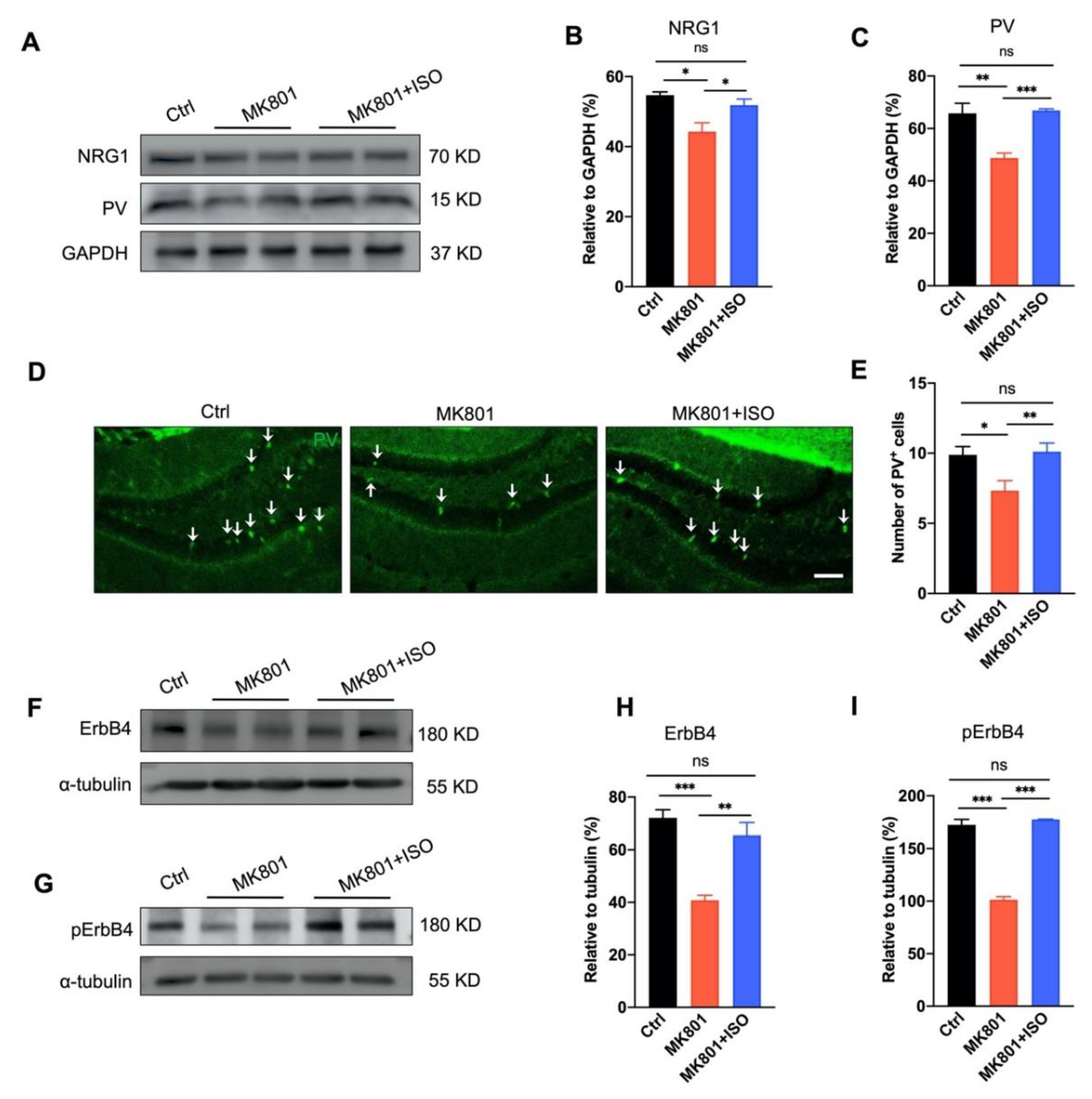
3.4. Isoflurane Inhalation Reverses PVIs Reduction and the Aberration of NRG1-ErbB4 Signaling in the DG Induced by MK801
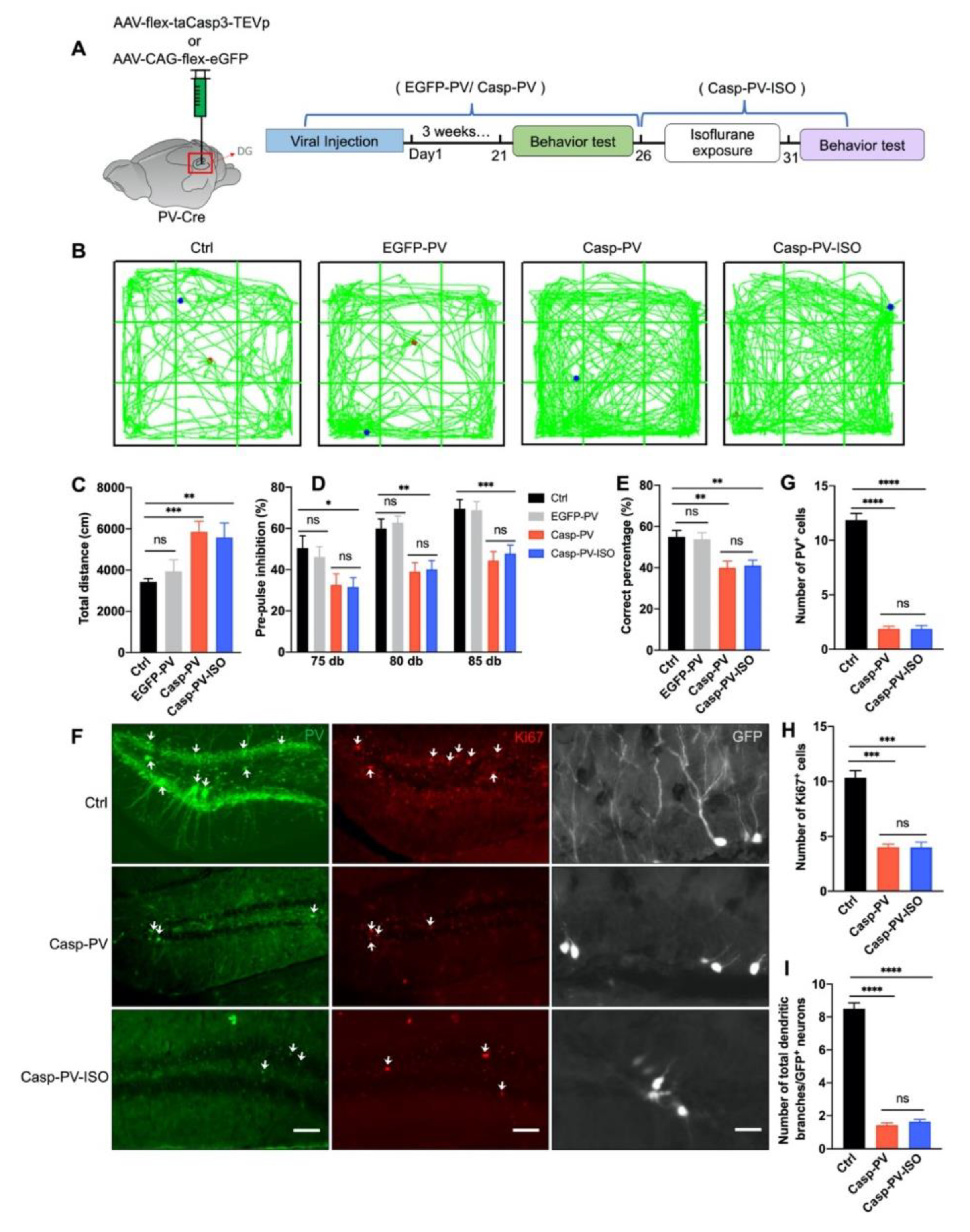
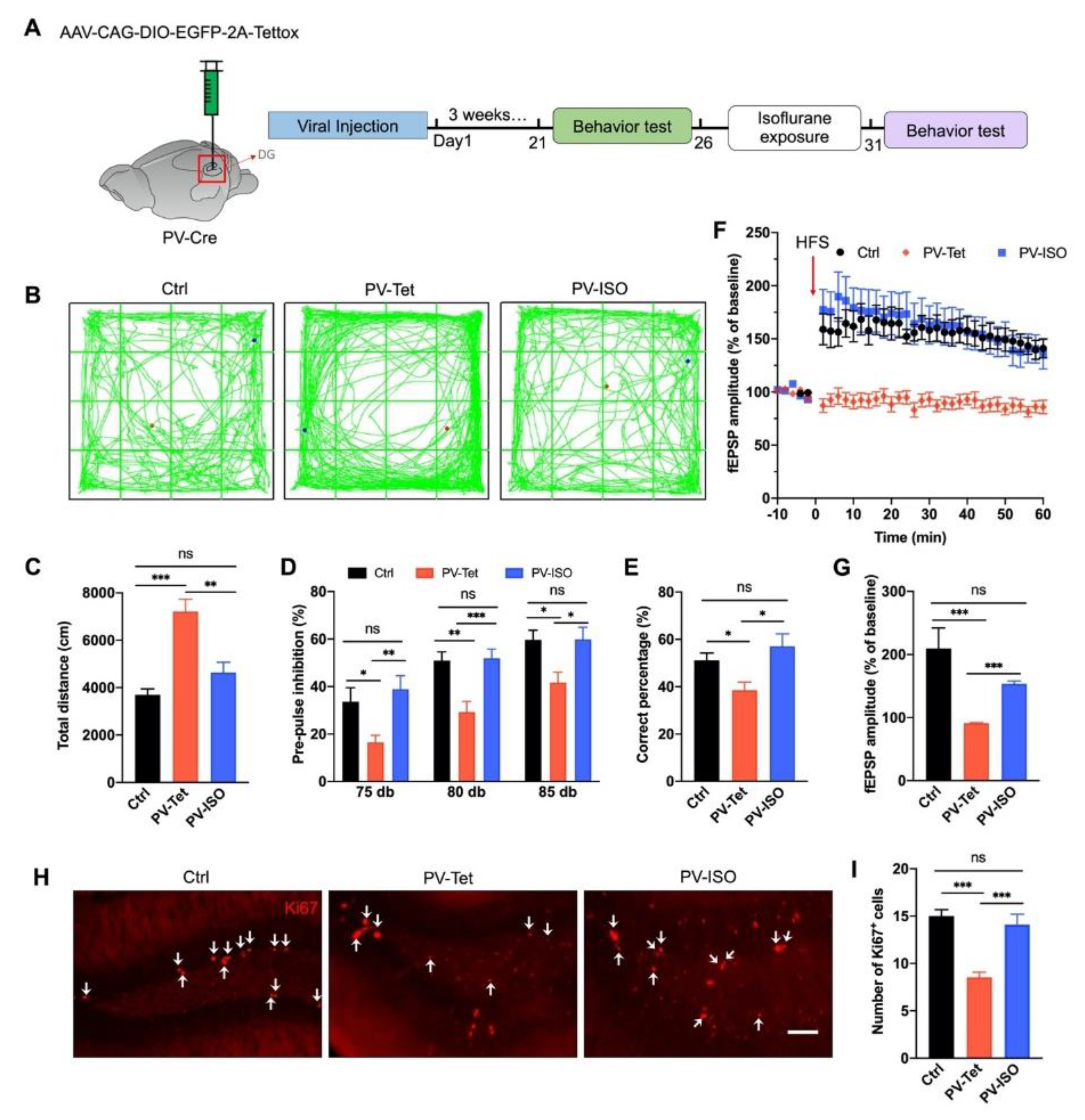
3.5. The Therapeutic Effect of Isoflurane Requires PVIs in the DG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, E.Q.; Shi, L.; Birnbaum, H.; Hudson, T.; Kessler, R. Annual Prevalence of Diagnosed Schizophrenia in the USA: A Claims Data Analysis Approach. Psychol. Med. 2006, 36, 1535–1540. [Google Scholar] [CrossRef]
- Chang, W.C.; Wong, C.S.M.; Chen, E.Y.H.; Lam, L.C.W.; Chan, W.C.; Ng, R.M.K.; Hung, S.F.; Cheung, E.F.C.; Sham, P.C.; Chiu, H.F.K.; et al. Lifetime Prevalence and Correlates of Schizophrenia-Spectrum, Affective, and Other Non-Affective Psychotic Disorders in the Chinese Adult Population. Schizophr. Bull. 2017, 43, 1280–1290. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Goldman, R.S.; Burdick, K.E.; Malhotra, A.K.; Lencz, T.; Patel, R.C.; Woerner, M.G.; Schooler, N.R.; Kane, J.M.; Robinson, D.G. Cognitive Improvement After Treatment With Second-Generation Antipsychotic Medications in First-Episode Schizophrenia: Is It a Practice Effect? Arch. Gen. Psychiatry 2007, 64, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Nagele, P.; Duma, A.; Kopec, M.; Gebara, M.A.; Parsoei, A.; Walker, M.; Janski, A.; Panagopoulos, V.N.; Cristancho, P.; Miller, J.P.; et al. Nitrous Oxide for Treatment-Resistant Major Depression: A Proof-of-Concept Trial. Biol. Psychiatry 2015, 78, 10–18. [Google Scholar] [CrossRef]
- Vargas, M.V.; Meyer, R.; Avanes, A.A.; Rus, M.; Olson, D.E. Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front. Psychiatry 2021, 12, 1691. [Google Scholar] [CrossRef] [PubMed]
- Theilmann, W.; Rosenholm, M.; Hampel, P.; Löscher, W.; Rantamäki, T. Lack of Antidepressant Effects of Burst-Suppressing Isoflurane Anesthesia in Adult Male Wistar Outbred Rats Subjected to Chronic Mild Stress. PLoS ONE 2020, 15, e0235046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Shi, Z.; Ling, N.; Qin, J.; Zhou, Q.; Wu, L.; Wang, Y.; Lin, C.; Ma, D.; Song, X. Sevoflurane Ameliorates Schizophrenia in a Mouse Model and Patients: Pre-Clinical and Clinical Feasibility Study. Curr. Neuropharmacol. 2022, 20, 1–12. [Google Scholar] [CrossRef]
- Lor, C.; Perouansky, M.; Pearce, R.A. Isoflurane Potentiation of GABAa Receptors Is Reduced but Not Eliminated by the Β3(N265m) Mutation. Int. J. Mol. Sci. 2020, 21, 9534. [Google Scholar] [CrossRef]
- Sloan, M.H.; Conard, P.F.; Karsunky, P.K.; Gross, J.B. Sevoflurane Versus Isoflurane: Induction and Recovery Characteristics with Single-Breath Inhaled Inductions of Anesthesia. Anesth. Analg. 1996, 82, 528–532. [Google Scholar]
- Shiraishi, Y.; Ikeda, K. Uptake and Biotransformation of Sevof Lurane in Humans: A Comparative Study of Sevof Lurane with Halothane, Enflurane, and Isoflurane. J. Clin. Anesth. 1990, 2, 381–386. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Cameron, H.A. Adult Neurogenesis and Mental Illness. Neuropsychopharmacology 2015, 40, 113–128. [Google Scholar] [CrossRef]
- Stratmann, G.; Sall, J.W.; May, L.D.V.; Bell, J.S.; Magnusson, K.R.; Rau, V.; Visrodia, K.H.; Alvi, R.S.; Ku, B.; Lee, M.T.; et al. Isoflurane Differentially Affects Neurogenesis and Long-Term Neurocognitive Function in 60-Day-Old and 7-Day-Old Rats. Anesthesiology 2009, 110, 834–848. [Google Scholar] [CrossRef]
- Song, J.; Sun, J.; Moss, J.; Wen, Z.; Sun, G.J.; Hsu, D.; Zhong, C.; Davoudi, H.; Christian, K.M.; Toni, N.; et al. Parvalbumin Interneurons Mediate Neuronal Circuitry-Neurogenesis Coupling in the Adult Hippocampus. Nat. Neurosci. 2013, 16, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Song, Y.; Lu, Y. Parvalbumin Interneuron Activation-Dependent Adult Hippocampal Neurogenesis Is Required for Treadmill Running to Reverse Schizophrenia-Like Phenotypes. Front. Cell Dev. Biol. 2020, 8, 86. [Google Scholar] [CrossRef]
- Antila, H.; Ryazantseva, M.; Popova, D.; Sipilä, P.; Guirado, R.; Kohtala, S.; Yalcin, I.; Lindholm, J.; Vesa, L.; Sato, V.; et al. Isoflurane Produces Antidepressant Effects and Induces TrkB Signaling in Rodents. Sci. Rep. 2017, 7, 7811. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Hawkins, L.M.; Stephenson, F.A. Appropriate NR1-NR1 Disulfide-Linked Homodimer Formation Is Requisite for Efficient Expression of Functional, Cell Surface N-Methyl-D-Aspartate NR1/NR2 Receptors. J. Biol. Chem. 2004, 279, 14703–14712. [Google Scholar] [CrossRef] [PubMed]
- Sepehrizadeh, Z.; Sahebgharani, M.; Ahmadi, S.; Shapourabadi, M.B.; Bozchlou, S.H.; Zarrindast, M.R. Morphine-Induced Behavioral Sensitization Increased the MRNA Expression of NMDA Receptor Subunits in the Rat Amygdala. Pharmacology 2008, 81, 333–343. [Google Scholar] [CrossRef]
- Babiec, W.E.; Guglietta, R.; Jami, S.A.; Morishita, W.; Malenka, R.C.; O’Dell, T.J. Ionotropic NMDA Receptor Signaling Is Required for the Induction of Long-Term Depression in the Mouse Hippocampal CA1 Region. J. Neurosci. 2014, 34, 5285–5290. [Google Scholar] [CrossRef]
- Rodríguez-Moreno, A.; Kohl, M.M.; Reeve, J.E.; Eaton, T.R.; Collins, H.A.; Anderson, H.L.; Paulsen, O. Presynaptic Induction and Expression of Timing-Dependent Long-Term Depression Demonstrated by Compartment-Specific Photorelease of a Use-Dependent NMDA Receptor Antagonist. J. Neurosci. 2011, 31, 8564–8569. [Google Scholar] [CrossRef]
- Packer, A.M.; Yuste, R. Dense, Unspecific Connectivity of Neocortical Parvalbumin-Positive Interneurons: A Canonical Microcircuit for Inhibition? J. Neurosci. 2011, 31, 13260–13271. [Google Scholar] [CrossRef]
- Beasley, C.L.; Zhang, Z.J.; Patten, I.; Reynolds, G.P. Selective Deficits in Prefrontal Cortical GABAergic Neurons in Schizophrenia Defined by the Presence of Calcium-Binding Proteins. Biol. Psychiatry 2002, 52, 708–715. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; Mei, Y.; Ling, Q. Ablation of ErbB4 in Parvalbumin-Positive Interneurons Inhibits Adult Hippocampal Neurogenesis through down-Regulating BDNF/TrkB Expression. J. Comp. Neurol. 2018, 526, 2482–2492. [Google Scholar] [CrossRef]
- Vohs, J.; Chambers, R.; O’Donnell, B.; Krishnan, G.; Morzorati, S. Auditory Steady State Responses in a Schizophrenia Rat Model. Int. J. Psychophysiol. 2013, 62, 1574–1583. [Google Scholar] [CrossRef]
- Bi, L.L.; Sun, X.D.; Zhang, J.; Lu, Y.S.; Chen, Y.H.; Wang, J.; Geng, F.; Liu, F.; Zhang, M.; Liu, J.H.; et al. Amygdala NRG1-ErbB4 Is Critical for the Modulation of Anxiety-like Behaviors. Neuropsychopharmacology 2015, 40, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, X.-D.; Hou, F.-Q.; Bi, L.-L.; Yin, D.-M.; Liu, F.; Chen, Y.-J.; Bean, J.C.; Jiao, H.-F.; Liu, X.; et al. Maintenance of GABAergic Activity by Neuregulin 1-ErbB4 in Amygdala for Fear Memory. Neuron 2014, 84, 835–846. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Z.; Melin, M.D.; Ng, Y.H.; Xu, W.; Südhof, T.C. A Central Amygdala to Zona Incerta Projection Is Required for Acquisition and Remote Recall of Conditioned Fear Memory. Nat. Neurosci. 2018, 21, 1515–1519. [Google Scholar] [CrossRef]
- Souery, D.; Oswald, P.; Massat, I.; Bailer, U.; Bollen, J.; Demyttenaere, K.; Kasper, S.; Lecrubier, Y.; Montgomery, S.; Serretti, A. Clinical Factors Associated with Treatment Resistance in Major Depressive Disorder: Results from a European Multicenter Study. J. Clin. Psychiatry 2007, 68, 1062–1070. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. The Physiological Approach: Functional Architecture of Working Memory and Disordered Cognition in Schizophrenia. Biol. Psychiatry 1999, 46, 650–661. [Google Scholar] [CrossRef]
- Tripathi, A.; Kar, S.K.; Shukla, R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin. Psychopharmacol. Neurosci. 2018, 16, 7–17. [Google Scholar] [CrossRef]
- Murray, A.J.; Woloszynowska-Fraser, M.U.; Ansel-Bollepalli, L.; Cole, K.L.H.; Foggetti, A.; Crouch, B.; Riedel, G.; Wulff, P. Parvalbumin-Positive Interneurons of the Prefrontal Cortex Support Working Memory and Cognitive Flexibility. Sci. Rep. 2015, 5, 16778. [Google Scholar] [CrossRef]
- Liu, Y.; Ouyang, P.; Zheng, Y.; Mi, L.; Zhao, J.; Ning, Y.; Guo, W. A Selective Review of the Excitatory-Inhibitory Imbalance in Schizophrenia: Underlying Biology, Genetics, Microcircuits, and Symptoms. Front. Cell Dev. Biol. 2021, 9, 664535. [Google Scholar] [CrossRef]
- Murueta-Goyena Larrañaga, A.; Lafuente Sánchez, J.V.; Bengoetxea Odriozola, H. The Role of Interneurons in Cognitive Impairment in Schizophrenia BT—Psychiatry and Neuroscience Update: From Translational Research to a Humanistic Approach—Volume III; Gargiulo, P.Á., Mesones Arroyo, H.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 201–212. ISBN 978-3-319-95360-1. [Google Scholar]
- Lisman, J.E.; Coyle, J.T.; Green, R.W.; Javitt, D.C.; Benes, F.M.; Heckers, S.; Grace, A.A. Circuit-Based Framework for Understanding Neurotransmitter and Risk Gene Interactions in Schizophrenia. Trends Neurosci. 2008, 31, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Bygrave, A.M.; Masiulis, S.; Nicholson, E.; Berkemann, M.; Barkus, C.; Sprengel, R.; Harrison, P.J.; Kullmann, D.M.; Bannerman, D.M.; Kätzel, D. Knockout of NMDA-Receptors from Parvalbumin Interneurons Sensitizes to Schizophrenia-Related Deficits Induced by MK-801. Transl. Psychiatry 2016, 6, e778. [Google Scholar] [CrossRef] [PubMed]
- Coan, E.J.; Saywood, W.; Collingridge, G.L. MK-801 Blocks NMDA Receptor-Mediated Synaptic Transmission and Long Term Potentiation in Rat Hippocampal Slices. Neurosci. Lett. 1987, 80, 111–114. [Google Scholar] [CrossRef]
- Wiescholleck, V.; Manahan-Vaughan, D. Persistent Deficits in Hippocampal Synaptic Plasticity Accompany Losses of Hippocampus-Dependent Memory in a Rodent Model of Psychosis. Front. Integr. Neurosci. 2013, 7, 12. [Google Scholar] [CrossRef][Green Version]
- Xi, D.; Li, Y.C.; Snyder, M.A.; Gao, R.Y.; Adelman, A.E.; Zhang, W.; Shumsky, J.S.; Gao, W.J. Erratum: Group II Metabotropic Glutamate Receptor Agonist Ameliorates Mk801-Induced Dysfunction of NMDA Receptors via the AKT/GSK-3B Pathway in Adult Rat Prefrontal Cortex. Neuropsychopharmacology 2013, 38, 1141. [Google Scholar] [CrossRef]
- Ruden, J.B.; Dugan, L.L.; Konradi, C. Parvalbumin Interneuron Vulnerability and Brain Disorders. Neuropsychopharmacology 2021, 46, 279–287. [Google Scholar] [CrossRef]
- Romón, T.; Mengod, G.; Adell, A. Expression of Parvalbumin and Glutamic Acid Decarboxylase-67 after Acute Administration of MK-801. Implications for the NMDA Hypofunction Model of Schizophrenia. Psychopharmacology 2011, 217, 231–238. [Google Scholar] [CrossRef]
- Cameron, H.A.; Tanapat, P.; Gould, E. Adrenal Steroids and N-Methyl-D-Aspartate Receptor Activation Regulate Neurogenesis in the Dentate Gyrus of Adult Rats through a Common Pathway. Neuroscience 1997, 82, 349–354. [Google Scholar] [CrossRef]
- Maeda, K.; Sugino, H.; Hirose, T.; Kitagawa, H.; Nagai, T.; Mizoguchi, H.; Takuma, K.; Yamada, K. Clozapine Prevents a Decrease in Neurogenesis in Mice Repeatedly Treated with Phencyclidine. J. Pharmacol. Sci. 2007, 103, 299–308. [Google Scholar] [CrossRef]
- Lucas, E.K.; Markwardt, S.J.; Gupta, S.; Meador-Woodruff, J.H.; Lin, J.D.; Overstreet-Wadiche, L.; Cowell, R.M. Parvalbumin Deficiency and GABAergic Dysfunction in Mice Lacking PGC-1α. J. Neurosci. 2010, 30, 7227–7235. [Google Scholar] [CrossRef]
- Mayer, A.R.; Hanlon, F.M.; Franco, A.R.; Teshiba, T.M.; Thoma, R.J.; Clark, V.P.; Canive, J.M. The Neural Networks Underlying Auditory Sensory Gating. Neuroimage 2009, 44, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Yoshizaki, K.; Kimura, R.; Suto, F.; Yanagawa, Y.; Osumi, N. A Sensitive Period for GABAergic Interneurons in the Dentate Gyrus in Modulating Sensorimotor Gating. J. Neurosci. 2013, 33, 6691–6704. [Google Scholar] [CrossRef] [PubMed]
- Lesh, T.A.; Niendam, T.A.; Minzenberg, M.J.; Carter, C.S. Cognitive Control Deficits in Schizophrenia: Mechanisms and Meaning. Neuropsychopharmacology 2011, 36, 316–338. [Google Scholar] [CrossRef]
- Korotkova, T.; Fuchs, E.C.; Ponomarenko, A.; von Engelhardt, J.; Monyer, H. NMDA Receptor Ablation on Parvalbumin-Positive Interneurons Impairs Hippocampal Synchrony, Spatial Representations, and Working Memory. Neuron 2010, 68, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gao, J.; Karlsson, N.; Li, Q.; Zhang, Y.; Huang, Z.; Li, H.; Kuhn, H.G.; Blomgren, K. Isoflurane Anesthesia Induced Persistent, Progressive Memory Impairment, Caused a Loss of Neural Stem Cells, and Reduced Neurogenesis in Young, but Not Adult, Rodents. J. Cereb. Blood Flow Metab. 2010, 30, 1017–1030. [Google Scholar] [CrossRef]
- Theilmann, W.; Alitalo, O.; Yorke, I.; Rantamäki, T. Dose-Dependent Effects of Isoflurane on TrkB and GSK3β Signaling: Importance of Burst Suppression Pattern. Neurosci. Lett. 2019, 694, 29–33. [Google Scholar] [CrossRef]
- Ren, X.; Rizavi, H.S.; Khan, M.A.; Bhaumik, R.; Dwivedi, Y.; Pandey, G.N. Alteration of Cyclic-AMP Response Element Binding Protein in the Postmortem Brain of Subjects with Bipolar Disorder and Schizophrenia. J. Affect. Disord. 2014, 152, 326–333. [Google Scholar] [CrossRef]
- Van Aken, H.; Fitch, W.; Graham, D.I.; Brüssel, T.; Themann, H. Cardiovascular and Cerebrovascular Effects of Isoflurane-Induced Hypotension in the Baboon. Anesth. Analg. 1986, 65, 565–574. [Google Scholar] [CrossRef]
- Li, C.-X.; Zhang, X. Evaluation of Prolonged Administration of Isoflurane on Cerebral Blood Flow and Default Mode Network in Macaque Monkeys Anesthetized with Different Maintenance Doses. Neurosci. Lett. 2018, 662, 402–408. [Google Scholar] [CrossRef]
- Stenroos, P.; Pirttimäki, T.; Paasonen, J.; Paasonen, E.; Salo, R.A.; Koivisto, H.; Natunen, T.; Mäkinen, P.; Kuulasmaa, T.; Hiltunen, M.; et al. Isoflurane Affects Brain Functional Connectivity in Rats 1 Month after Exposure. Neuroimage 2021, 234, 117987. [Google Scholar] [CrossRef]
- Brohan, J.; Goudra, B.G. The Role of GABA Receptor Agonists in Anesthesia and Sedation. CNS Drugs 2017, 31, 845–856. [Google Scholar] [CrossRef]
- McCartney, M.R.; Deeb, T.Z.; Henderson, T.N.; Hales, T.G. Tonically Active GABAA Receptors in Hippocampal Pyramidal Neurons Exhibit Constitutive GABA-Independent Gating. Mol. Pharmacol. 2007, 71, 539–548. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABA A Receptors: Their Function in the CNS and Implications for Disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Zhang, M.; Yin, D.-M.; Wen, L.; Ting, A.; Wang, P.; Lu, Y.-S.; Zhu, X.-H.; Li, S.-J.; Wu, C.-Y.; et al. ErbB4 in Parvalbumin-Positive Interneurons Is Critical for Neuregulin 1 Regulation of Long-Term Potentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 21818–21823. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lu, Y.-S.; Zhu, X.-H.; Li, X.-M.; Woo, R.-S.; Chen, Y.-J.; Yin, D.-M.; Lai, C.; Terry, A.V.; Vazdarjanova, A.; et al. Neuregulin 1 Regulates Pyramidal Neuron Activity via ErbB4 in Parvalbumin-Positive Interneurons. Proc. Natl. Acad. Sci. USA 2010, 107, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Su, F.; Ji, M.H.; Zhang, G.F.; Qiu, L.L.; Jia, M.; Gao, J.; Xie, Z.; Yang, J.J. Disruption of Hippocampal Neugegulin 1-ErbB4 Signaling Contributes to the Hippocampus-dependent Cognitive Impairment Induced by Isoflurane in Aged Mice. Anesthesiology 2015, 121, 79–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Jia, J.; Lu, Y.; Zheng, H. Isoflurane Rescue Schizophrenia-Related Deficits through Parvalbumin-Positive Neurons in the Dentate Gyrus. Biomedicines 2022, 10, 2759. https://doi.org/10.3390/biomedicines10112759
Peng H, Jia J, Lu Y, Zheng H. Isoflurane Rescue Schizophrenia-Related Deficits through Parvalbumin-Positive Neurons in the Dentate Gyrus. Biomedicines. 2022; 10(11):2759. https://doi.org/10.3390/biomedicines10112759
Chicago/Turabian StylePeng, Hualing, Jie Jia, Yisheng Lu, and Hua Zheng. 2022. "Isoflurane Rescue Schizophrenia-Related Deficits through Parvalbumin-Positive Neurons in the Dentate Gyrus" Biomedicines 10, no. 11: 2759. https://doi.org/10.3390/biomedicines10112759
APA StylePeng, H., Jia, J., Lu, Y., & Zheng, H. (2022). Isoflurane Rescue Schizophrenia-Related Deficits through Parvalbumin-Positive Neurons in the Dentate Gyrus. Biomedicines, 10(11), 2759. https://doi.org/10.3390/biomedicines10112759





