Quantitative Proteomics Indicate Radical Removal of Non-Small Cell Lung Cancer and Predict Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Olink-Proximity Extension Assay (PEA)
2.3. Confirmation of the Findings in Larger Cohorts
2.4. Statistical Analysis
3. Results
3.1. Proteomic Analysis
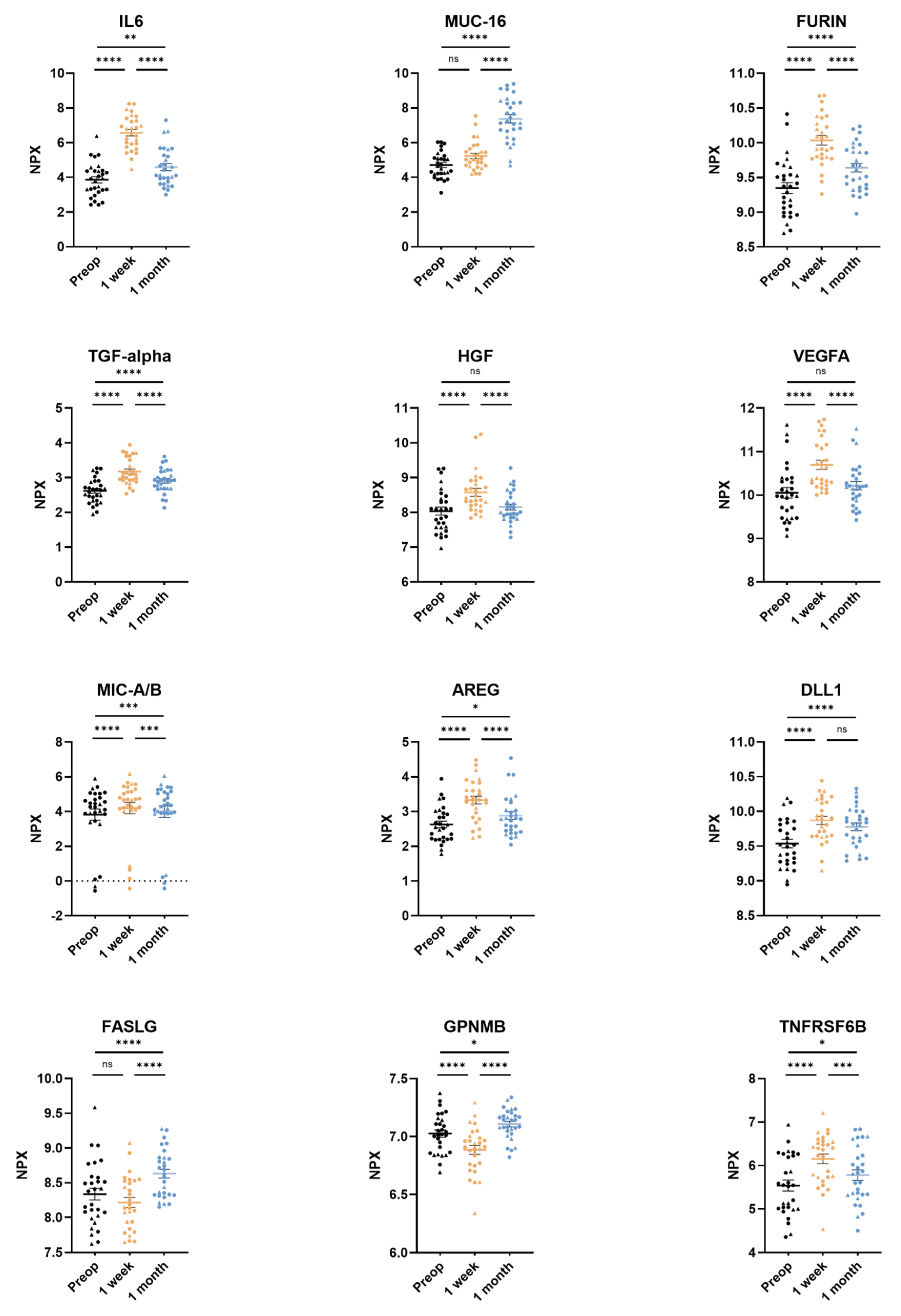
3.2. Comparing Three Timepoints: Pre-Op vs. 3–5 Days Post-Op vs. 1 Month Post-Op
3.3. Comparison of Dead or Relapsed NSCLC to Survivors without Relapse
3.4. Validation Using GEO DataSets Microarray Data
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 14 April 2022).
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 14 April 2022).
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef]
- Freedman, M.T.; Lo, S.C.; Seibel, J.C.; Bromley, C.M. Lung nodules: Improved detection with software that suppresses the rib and clavicle on chest radiographs. Radiology 2011, 260, 265–273. [Google Scholar] [CrossRef]
- Stiles, B.M.; Mirza, F.; Towe, C.W.; Ho, V.P.; Port, J.L.; Lee, P.C.; Paul, S.; Yankelevitz, D.F.; Altorki, N.K. Cumulative Radiation Dose From Medical Imaging Procedures in Patients Undergoing Resection for Lung Cancer. Ann. Thorac. Surg. 2011, 92, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Herout, V.; Heroutova, M.; Merta, Z.; Jr, I.C.; Brat, K. Transbronchial biopsy from the upper pulmonary lobes is associated with increased risk of pneumothorax—A retrospective study. BMC Pulm. Med. 2019, 19, 56. [Google Scholar] [CrossRef]
- Şahin, C.; Yılmaz, O.; Üçpınar, B.A.; Uçak, R.; Temel, U.; Başak, M.; Bayrak, A.H. Computed Tomography-guided Transthoracic Core Needle Biopsy of Lung Masses: Technique, Complications and Diagnostic Yield Rate. Sisli Etfal Hastan. Tip Bul. 2020, 54, 47–51. [Google Scholar] [CrossRef]
- Medford, A.R.L.; Agrawal, S.; Free, C.M.; Bennett, J.A. A Prospective Study of Conventional Transbronchial Needle Aspiration: Performance and Cost Utility. Respiration 2010, 79, 482–489. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Edu. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Zierhut, D.; Bettscheider, C.; Schubert, K.; Van Kampen, M.; Wannenmacher, M. Radiation therapy of stage I and II non-small cell lung cancer (NSCLC). Lung Cancer 2001, 34, 39–43. [Google Scholar] [CrossRef]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef] [PubMed]
- Galetta, D.; Solli, P.; Borri, A.; Petrella, F.; Gasparri, R.; Brambilla, D.; Spaggiari, L. Bilobectomy for Lung Cancer: Analysis of Indications, Postoperative Results, and Long-Term Outcomes. Ann. Thorac. Surg. 2012, 93, 251–258. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J.; Howington, J.; Feigenberg, S.; Movsas, B.; Pisters, K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cagle, P.T.; Allen, T.C.; Olsen, R.J. Lung Cancer Biomarkers: Present Status and Future Developments. Arch. Pathol. Lab. Med. 2013, 137, 1191–1198. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2015. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html (accessed on 17 September 2020).
- Al-Kattan, K. Disease recurrence after resection for stage I lung cancer. Eur. J. Cardio Thorac. Surg. 1997, 12, 380–384. [Google Scholar] [CrossRef]
- Taylor, M.D.; Nagji, A.S.; Bhamidipati, C.M.; Theodosakis, N.; Kozower, B.D.; Lau, C.L.; Jones, D.R. Tumor Recurrence After Complete Resection for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2012, 93, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Broggio, J.B.N.; Wong, K.; Poole, J.; Gildea, C.; Emmett, M.; Luchtenborg, M.; Kaur, J.; Butler, L.; Peet, M.; King, A. Cancer Survival in England: Adult, Stage at Diagnosis and Childhood—Patients Followed up to 2016. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalinengland/adultstageatdiagnosisandchildhoodpatientsfollowedupto2016#cancer-survival-by-stage-at-diagnosis-for-england-adults-diagnosed-in-2015-and-followed-up-to-2016-experimental-statistics (accessed on 14 April 2022).
- Sung, H.J.; Cho, J.Y. Biomarkers for the lung cancer diagnosis and their advances in proteomics. BMB Rep. 2008, 41, 615–625. [Google Scholar] [CrossRef]
- Shah, R.; Sabanathan, S.; Richardson, J.; Mearns, A.J.; Goulden, C. Results of surgical treatment of stage I and II lung cancer. J. Cardiovasc Surg. 1996, 37, 169–172. [Google Scholar]
- Van Meerbeeck, J.P.; Franck, C. Lung cancer screening in Europe: Where are we in 2021? Transl. Lung Cancer Res. 2021, 10, 2407–2417. [Google Scholar] [CrossRef]
- Kauczor, H.-U.; Baird, A.-M.; Blum, T.G.; Bonomo, L.; Bostantzoglou, C.; Burghuber, O.; Čepická, B.; Comanescu, A.; Couraud, S.; Devaraj, A.; et al. ESR/ERS statement paper on lung cancer screening. Eur. Radiol. 2020, 30, 3277–3294. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Black, W.C.; Chiles, C.; Church, T.R.; Gareen, I.F.; Gierada, D.S.; Mahon, I.; Miller, E.A.; Pinsky, P.F.; Sicks, J.D. Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. J. Thorac. Oncol. 2019, 14, 1732–1742. [Google Scholar] [CrossRef]
- NCI. Tumor Markers. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-fact-sheet (accessed on 14 April 2022).
- Siegfried, J.M.; Weissfeld, L.A.; Singh-Kaw, P.; Weyant, R.J.; Testa, J.R.; Landreneau, R.J. Association of Immunoreactive Hepatocyte Growth Factor with Poor Survival in Resectable Non-Small Cell Lung Cancer. Cancer Res. 1997, 57, 433–439. [Google Scholar] [PubMed]
- Scott, A.; Salgia, R. Biomarkers in lung cancer: From early detection to novel therapeutics and decision making. Biomark Med. 2008, 2, 577–586. [Google Scholar] [CrossRef]
- Paruk, F.; Chausse, J.M. Monitoring the post surgery inflammatory host response. J. Emerg. Crit. Care Med. 2019, 3, 47. [Google Scholar] [CrossRef]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health Eur. 2021, 10, 100179. [Google Scholar] [CrossRef] [PubMed]
- Ghadially, H.; Brown, L.; Lloyd, C.; Lewis, L.; Lewis, A.; Dillon, J.; Sainson, R.; Jovanovic, J.; Tigue, N.J.; Bannister, D.; et al. MHC class I chain-related protein A and B (MICA and MICB) are predominantly expressed intracellularly in tumour and normal tissue. Br. J. Cancer 2017, 116, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Blocking MICA/MICB Shedding Reactivates Antitumor Immunity. Cancer Discov. 2018, 8, OF20. [CrossRef][Green Version]
- Zhao, Y.; Chen, N.; Yu, Y.; Zhou, L.; Niu, C.; Liu, Y.; Tian, H.; Lv, Z.; Han, F.; Cui, J. Prognostic value of MICA/B in cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 96384–96395. [Google Scholar] [CrossRef] [PubMed]
- Okita, R.; Yukawa, T.; Nojima, Y.; Maeda, A.; Saisho, S.; Shimizu, K.; Nakata, M. MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2016, 65, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Djureinovic, D.; Pontén, V.; Landelius, P.; Al Sayegh, S.; Kappert, K.; Kamali-Moghaddam, M.; Micke, P.; Ståhle, E. Multiplex plasma protein profiling identifies novel markers to discriminate patients with adenocarcinoma of the lung. BMC Cancer 2019, 19, 741. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ramagopal, U.; Cheng, H.; Bonanno, J.B.; Toro, R.; Bhosle, R.; Zhan, C.; Almo, S.C. Crystal Structure of the Complex of Human FasL and Its Decoy Receptor DcR3. Structure 2016, 24, 2016–2023. [Google Scholar] [CrossRef]
- Bai, C.; Connolly, B.; Metzker, M.L.; Hilliard, C.A.; Liu, X.; Sandig, V.; Soderman, A.; Galloway, S.M.; Liu, Q.; Austin, C.P.; et al. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc. Natl. Acad. Sci. USA 2000, 97, 1230–1235. [Google Scholar] [CrossRef]
- Pitti, R.M.; Marsters, S.A.; Lawrence, D.A.; Roy, M.; Kischkel, F.C.; Dowd, P.; Huang, A.; Donahue, C.J.; Sherwood, S.W.; Baldwin, D.T.; et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 1998, 396, 699–703. [Google Scholar] [CrossRef]
- Ali, A.S.; Perren, A.; Lindskog, C.; Welin, S.; Sorbye, H.; Grönberg, M.; Janson, E.T. Candidate protein biomarkers in pancreatic neuroendocrine neoplasms grade 3. Sci. Rep. 2020, 10, 10639. [Google Scholar] [CrossRef]
- Tsao, M.-S.; Liu, N.; Chen, J.-R.; Pappas, J.; Ho, J.; To, C.; Viallet, J.; Park, M.; Zhu, H. Differential expression of Met/hepatocyte growth factor receptor in subtypes of non-small cell lung cancers. Lung Cancer 1998, 20, 1–16. [Google Scholar] [CrossRef]
- Masuya, D.; Huang, C.; Liu, D.; Nakashima, T.; Kameyama, K.; Haba, R.; Ueno, M.; Yokomise, H. The tumour–stromal interaction between intratumoral c-Met and stromal hepatocyte growth factor associated with tumour growth and prognosis in non-small-cell lung cancer patients. Br. J. Cancer 2004, 90, 1555–1562. [Google Scholar] [CrossRef]
- FranzeéN, B.; Viktorsson, K.; Kamali, C.; Darai-Ramqvist, E.; Grozman, V.; Arapi, V.; Hååg, P.; Kaminskyy, V.O.; Hydbring, P.; Kanter, L.; et al. Multiplex immune protein profiling of fine-needle aspirates from patients with non-small-cell lung cancer reveals signatures associated with PD-L1 expression and tumor stage. Mol. Oncol. 2021, 15, 2941–2957. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Pavlick, A.C.; Johnson, D.B.; Hart, L.L.; Infante, J.R.; Luke, J.J.; Lutzky, J.; Rothschild, N.E.; Spitler, L.E.; Cowey, C.L.; et al. A phase 2 study of glembatumumab vedotin, an antibody-drug conjugate targeting glycoprotein NMB, in patients with advanced melanoma. Cancer 2019, 125, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Zhang, L.; Li, X.L.; Cui, D.J.; Zheng, H.D.; Yang, S.Y.; Yang, W.L. Glycoprotein nonmetastatic B as a prognostic indicator in small cell lung cancer. APMIS 2014, 122, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Funk, T.; Fuchs, A.R.; Altdörfer, V.S.; Klein, R.; Autenrieth, S.E.; Müller, M.R.; Salih, H.R.; Henes, J.; Grünebach, F.; Dörfel, D. Monocyte-derived dendritic cells display a highly activated phenotype and altered function in patients with familial Mediterranean fever. Clin. Exper. Immunol. 2020, 201, 1–11. [Google Scholar] [CrossRef]
- Deacon, K.; Knox, A.J. Human airway smooth muscle cells secrete amphiregulin via bradykinin/COX-2/PGE2, inducing COX-2, CXCL8, and VEGF expression in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L237–L249. [Google Scholar] [CrossRef]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging Functions of Amphiregulin in Orchestrating Immunity, Inflammation, and Tissue Repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef]
- Sakamoto, K.; Arakawa, H.; Mita, S.; Ishiko, T.; Ikei, S.; Egami, H.; Hisano, S.; Ogawa, M. Elevation of circulating interleukin 6 after surgery: Factors influencing the serum level. Cytokine 1994, 6, 181–186. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nature Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Akira, S.; Hirano, T.; Taga, T.; Kishimoto, T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990, 4, 2860–2867. [Google Scholar] [CrossRef]
- Keewan, E.A.; Naser, S.A. The Role of Notch Signaling in Macrophages during Inflammation and Infection: Implication in Rheumatoid Arthritis? Cells 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.S.; Joshi, A.D.; Boniakowski, A.E.; Schaller, M.; Chung, J.; Allen, R.; Bermick, J.; Carson, W.F.; Henke, P.K.; Maillard, I.; et al. Notch Regulates Macrophage-Mediated Inflammation in Diabetic Wound Healing. Front. Immunol. 2017, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Belizon, A.; Balik, E.; Feingold, D.L.; Bessler, M.; Arnell, T.D.; Forde, K.A.; Horst, P.K.; Jain, S.; Cekic, V.; Kirman, I.; et al. Major abdominal surgery increases plasma levels of vascular endothelial growth factor: Open more so than minimally invasive methods. Ann. Surg. 2006, 244, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Weiss, S.J. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature 1995, 375, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Jiang, J.; Elliott, J.M.; Piacentini, L. Paradigmatic identification of MMP-2 and MT1-MMP activation systems in cardiac fibroblasts cultured as a monolayer. J. Cell Biochem. 2005, 94, 446–459. [Google Scholar] [CrossRef]
- Cordova, Z.M.; Grönholm, A.; Kytölä, V.; Taverniti, V.; Hämäläinen, S.; Aittomäki, S.; Niininen, W.; Junttila, I.; Ylipää, A.; Nykter, M.; et al. Myeloid cell expressed proprotein convertase FURIN attenuates inflammation. Oncotarget 2016, 7, 54392–54404. [Google Scholar] [CrossRef]
- Haridas, D.; Ponnusamy, M.P.; Chugh, S.; Lakshmanan, I.; Seshacharyulu, P.; Batra, S.K. MUC16: Molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014, 28, 4183–4199. [Google Scholar] [CrossRef]
- Kesimer, M.; Scull, M.; Brighton, B.; Demaria, G.; Burns, K.; O’Neal, W.; Pickles, R.J.; Sheehan, J.K. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009, 23, 1858–1868. [Google Scholar] [CrossRef]
- Singh, S.S.; Chauhan, S.B.; Kumar, A.; Kumar, S.; Engwerda, C.R.; Sundar, S.; Kumar, R. Amphiregulin in cellular physiology, health, and disease: Potential use as a biomarker and therapeutic target. J. Cell. Physiol. 2022, 237, 1143–1156. [Google Scholar] [CrossRef]
- Norum, H.M.; Michelsen, A.E.; Lekva, T.; Arora, S.; Otterdal, K.; Olsen, M.B.; Kong, X.Y.; Gude, E.; Andreassen, A.K.; Solbu, D.; et al. Circulating delta-like Notch ligand 1 is correlated with cardiac allograft vasculopathy and suppressed in heart transplant recipients on everolimus-based immunosuppression. Am. J. Transplant. 2019, 19, 1050–1060. [Google Scholar] [CrossRef]
- Jaaks, P.; Bernasconi, M. The proprotein convertase furin in tumour progression. Int. J. Cancer 2017, 141, 654–663. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef] [PubMed]
- Rappolee, D.A.; Mark, D.; Banda, M.J.; Werb, Z. Wound Macrophages Express TGF-α and Other Growth Factors in Vivo: Analysis by mRNA Phenotyping. Science 1988, 241, 708–712. [Google Scholar] [CrossRef] [PubMed]
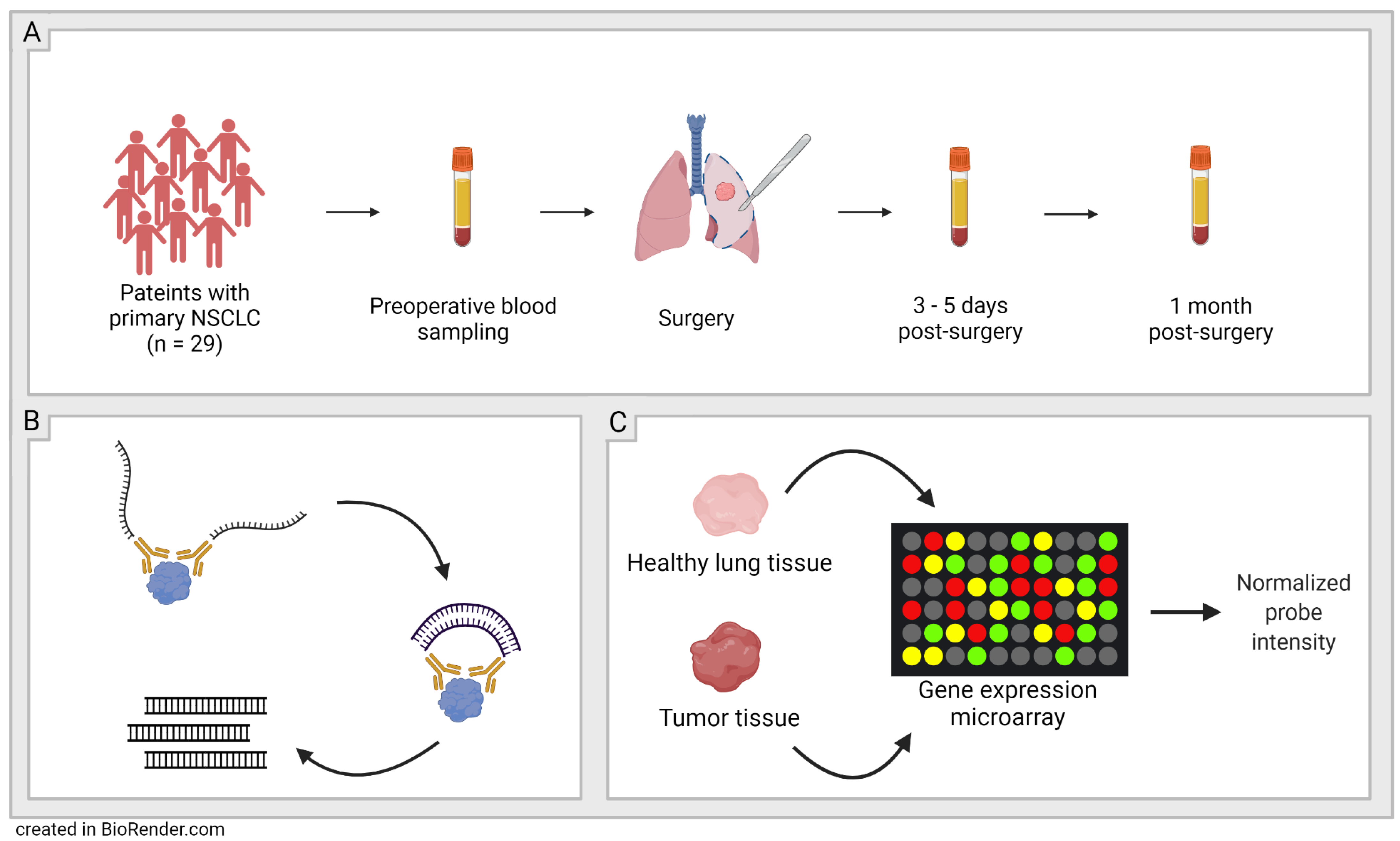

| n = 29 | |
|---|---|
| Sex, n (%) | |
| Male | 14 (48) |
| Female | 15 (52) |
| Age, years | |
| Mean (range) | 71 (46–84) |
| Mortality, n (%) | |
| Alive | 26 (90) |
| Deceased | 3 (10) |
| Time from diagnosis to death, days | |
| Mean (range) | 603 (264–786) |
| Time from surgery to death, days | |
| Mean (range) | 551 (211–736) |
| Comorbidities, n (%) | |
| None known | 17 (59) |
| Coronary artery disease | 2 (7) |
| Diabetes mellitus | 3 (10) |
| Hypertension | 10 (34) |
| Arrhythmias | 1 (3) |
| WHO performance status prior to surgery, n (%) | |
| 0 | 15 (52) |
| 1 | 14 (48) |
| Smoking history, n (%) | |
| Current | 4 (14) |
| Former (> 6 weeks) | 21 (72) |
| Never | 4 (14) |
| Histopathological classification, n (%) | |
| Adenocarcinoma | 21 (72) |
| Squamous cell carcinoma | 8 (28) |
| Tumor stage, n (%) | |
| IA | 13 (45) |
| IB | 8 (28) |
| IIA | 3 (10) |
| IIB | 2 (7) |
| IIIA | 3 (10) |
| Lung resection, n (%) | |
| Wedge resection | 2 (7) |
| Segmental resection | 2 (7) |
| Lobectomy | 23 (79) |
| Bilobectomy | 1 (3) |
| Pneumonectomy | 1 (3) |
| Radicality, n (%) | |
| R0 | 26 (90) |
| R1 | 3 (10) |
| Neoadjuvant therapy, n (%) | |
| Combined chemotherapy and radiotherapy | 2 (7) |
| Adjuvant therapy, n (%) | |
| Single therapy, chemotherapy | 6 (21) |
| Combined chemotherapy and radiotherapy | 1 (3) |
| Protein Abbreviation | Protein | NPX Preop (mean ± SD) | NPX 1 Week (mean ± SD) | NPX 1 Month (mean ± SD) | p-Value Preop vs. 1 Week | p-Value Preop vs. 1 Month | p-Value 1 Week vs. 1 Month |
|---|---|---|---|---|---|---|---|
| AREG | Amphiregulin | 2.63 ± 0.51 | 3.33 ± 0.59 | 2.88 ± 0.58 | p < 0.0001 | p = 0.0237 | p < 0.0001 |
| DLL1 | Delta-like protein 1 | 9.54 ± 0.33 | 9.87 ± 0.31 | 9.78 ± 0.29 | p < 0.0001 | p < 0.0001 | ns |
| Furin | Protein furin | 9.35 ± 0.41 | 10.03 ± 0.36 | 9.64 ± 0.33 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| IL-6 | Interleukin-6 | 3.86 ± 0.95 | 6.57 ± 0.97 | 4.59 ± 1.09 | p < 0.0001 | p = 0.0016 | p < 0.0001 |
| MIC-A/B | MHC class 1 polypeptide-related sequence A/B | 3.82 ± 0.70 | 4.20 ± 1.69 | 4.00 ± 1.70 | p < 0.0001 | p = 0.0009 | p = 0.0003 |
| MUC-16 | Mucin-16 | 4.71 ± 0.74 | 5.24 ± 0.81 | 7.37 ± 1.26 | ns | p < 0.0001 | p < 0.0001 |
| TGFα | Transforming growth factor alpha | 2.62 ± 0.35 | 3.18 ± 0.37 | 2.91 ± 0.34 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| TNFRSF6B | Tumor necrosis factor receptor superfamily member 6B | 5.54 ± 0.67 | 6.15 ± 0.57 | 5.78 ± 0.64 | p < 0.0001 | p = 0.0214 | p = 0.0003 |
| VEGFA | Vascular endothelial growth factor A | 10.05 ± 0.63 | 10.69 ± 0.55 | 10.22 ± 0.49 | p < 0.0001 | ns | p < 0.0001 |
| FASLG | Tumor necrosis factor ligand superfamily member 6 | 8.34 ± 0.45 | 8.22 ± 0.38 | 8.63 ± 0.34 | ns | p < 0.0001 | p < 0.0001 |
| GPNMB | Transmembrane glycoprotein NMB | 7.03 ± 0.17 | 6.89 ± 0.20 | 7.11 ± 0.13 | p < 0.0001 | p = 0.0207 | p < 0.0001 |
| HGF | Hepatocyte growth factor | 8.04 ± 0.60 | 8.58 ± 0.58 | 8.15 ± 0.45 | p < 0.0001 | ns | p < 0.0001 |
| Protein | GenBank | GEO | Gene Expression Cancer | Gene Expression Control | Significance |
|---|---|---|---|---|---|
| MIC-A | NM_000247 | GSE10072 | 8.00 ± 0.03 | 8.18 ± 0.04 | p = 0.0044 |
| MIC-A | NM_000247 | GSE19804 | 8.00 ± 0.06 | 8.19 ± 0.05 | p = 0.0166 |
| FASLG | AF288573 | GSE19804 | 4.42 ± 0.04 | 4.56 ± 0.05 | p = 0.0239 |
| Protein Abbreviation | Protein | Mode of Action |
|---|---|---|
| AREG | Amphiregulin | Cytokine in the epidermal growth factor family. Binds to epidermal growth factor receptors and activates signaling in inflammatory processes, cell metabolism, and the cell cycle. Produced by immune cells [63]. |
| DLL1 | Delta-like protein 1 | A NOTCH ligand. Regulates immune cells. Released from T-cells and eosinophilic cells. Secretion is enhanced by interleukin 1β. Has a positive correlation to systemic inflammation [64]. |
| Furin | Protein furin | Cleaves and activates matrix metalloproteases, integrins, and cadherins (cell adhesion molecules). Expression is upregulated by tissue hypoxia [65]. |
| IL-6 | Interleukin-6 | Involved in B-cell stimulation and induction of hepatic acute phase proteins. Increases thousand-fold in blood during inflammation. Signaling is dominated by signal transducer and activator of transcription 3 (STAT3) activation [66]. |
| MIC-A/B | MHC class 1 polypeptide-related sequence A/B | Cancer cell-surface molecules. Activates cytolytic properties in natural killer cells and cytotoxic T-cells. Shedding of MIC-A/B by cancer cells leads to their escape from cell-mediated antitumor immunity [37]. |
| MUC-16 | Mucin-16 | Glycoprotein is expressed by epithelial cells. Major component of mucus providing hydration and lubrication. Regulates mucosal defense of epithelial cells [61]. |
| TGFα | Transforming growth factor alpha | Expressed by wound macrophages. Mediates angiogenesis, epidermal regrowth, and formation of granulation tissue [67]. |
| TNFRSF6B | Tumor necrosis factor receptor superfamily member 6B | A soluble receptor also known as DcR3. Inhibits FASLG-induced cell death which potentially leads to the survival of malignant cells [42]. |
| VEGFA | Vascular endothelial growth factor A | Induces angiogenesis and is important in wound healing. Plasma levels have been proven to rise post-surgery corresponding to the extent of the operative intervention [57]. |
| FASLG | Tumor necrosis factor ligand superfamily member 6 | Produced by activated T-cells and natural killer cells. Induces cell death of damaged cells. Naturally higher expression in healthy tissue. The binding of DcR3 to FASLG inhibits its function [40]. |
| GPNMB | Transmembrane glycoprotein NMB | A transmembrane protein expressed by monocytic dendritic cells. Can inhibit T-cell activation [49]. Involved in metastasis of small-cell lung cancer [48]. |
| HGF | Hepatocyte growth factor | A proto-oncogene, the protein stimulates cell motility, invasion, and morphogenesis. Acts as a potent mitogen [44]. High expression in NSCLC correlates with poor overall survival [31]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodén, E.; Andreasson, J.; Hirdman, G.; Malmsjö, M.; Lindstedt, S. Quantitative Proteomics Indicate Radical Removal of Non-Small Cell Lung Cancer and Predict Outcome. Biomedicines 2022, 10, 2738. https://doi.org/10.3390/biomedicines10112738
Bodén E, Andreasson J, Hirdman G, Malmsjö M, Lindstedt S. Quantitative Proteomics Indicate Radical Removal of Non-Small Cell Lung Cancer and Predict Outcome. Biomedicines. 2022; 10(11):2738. https://doi.org/10.3390/biomedicines10112738
Chicago/Turabian StyleBodén, Embla, Jesper Andreasson, Gabriel Hirdman, Malin Malmsjö, and Sandra Lindstedt. 2022. "Quantitative Proteomics Indicate Radical Removal of Non-Small Cell Lung Cancer and Predict Outcome" Biomedicines 10, no. 11: 2738. https://doi.org/10.3390/biomedicines10112738
APA StyleBodén, E., Andreasson, J., Hirdman, G., Malmsjö, M., & Lindstedt, S. (2022). Quantitative Proteomics Indicate Radical Removal of Non-Small Cell Lung Cancer and Predict Outcome. Biomedicines, 10(11), 2738. https://doi.org/10.3390/biomedicines10112738








