Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis
Abstract
1. Introduction
2. Retrospective on Hemostasis and Thrombosis
3. The Identity of Thrombosis
3.1. Vascular Injury and Hemostasis
3.2. Intravascular Injury, Thrombosis and Thrombogenesis
3.3. Thrombosis as Generic Term
4. In Search of New Classification for Thrombotic Disorders
4.1. Issues to Be Considered in Classification of Thrombotic Disorders
4.2. Factors Caused by the Vascular Damage
4.3. Factors Caused by Associated Underlying Disease or Another Vascular Event
4.4. Factors Caused by Tropism, Endothelial Heterogeneity, Environmental and Gene Interaction
4.5. Thrombosis Due to Aberrant Hemostasis without Vascular Injury
5. ADAMTS13 Role in Thrombogenesis
6. Current Classification of Thrombotic Disorders without Molecular Mechanism
6.1. Currently Often Used Terms for the Phenotype
- Vessel milieu-designated thrombosis type
- Deep venous thrombosis (e.g., distal DVT, VTE, PTE)
- Superficial vein thrombosis
- Arterial thrombosis
- Microvascular thrombosis (e.g., TTP-like syndrome)
- Venous sinusoidal thrombosis (e.g., VOD)
- “DIC”
- Dysfunction expressive type
- Stroke (e.g., transient ischemic attack [TIA], AIS)
- Heart attack (e.g., Acute myocardial infarction)
- Coagulopathy
- Pelvic congestion syndrome
- Gangrene
- Thrombo-hemorrhagic syndrome
- Vascular localization type
- Cerebral artery thrombosis
- Aortic arch thrombosis
- Cerebral venous sinus thrombosis
- Coronary artery thrombosis
- Renal vein thrombosis
- PTE
- VTE
- Localized venous thrombosis (e.g., IVCT/SVCT, PVT, BCS, SVT)
- Symmetrical peripheral gangrene (SPG)
- Limb gangrene
- Organ localization type (but often the thrombotic nature is not recognized.)
- Encephalopathy
- PTE
- Acute respiratory distress syndrome (ARDS)
- Acute necrotizing pancreatitis
- Acute liver failure
- Hemolytic-uremic syndrome
- Rhabdomyolysis
- Multiorgan dysfunction syndrome (MODS)
- Pathologic expressive type
- TTP-like syndrome (e.g., arterial endotheliopathy)
- ITP-like syndrome (e.g., venous endotheliopathy)
- Acute necrotizing fasciitis
- Diabetic gangrene
- Undefined clinical syndrome type
- Heyde’s syndrome
- Susac syndrome
- Hemolytic-uremic syndrome
- Hereditary hemorrhagic telangiectasia
- Purpura fulminans
6.2. Proposed Novel Classification of Thrombotic Disorders Based on Thrombogenesis and Intrinsic Character
6.2.1. Microthrombosis
6.2.2. Macrothrombosis
6.2.3. Fibrin Clot Disease
6.2.4. Combined Micro-Macrothrombosis
6.2.5. Interpretation for Thrombotic Disorders as an Example in COVID-19 Infection
- Often vascular access, devices and mechanical ventilation would be needed in ICU for critically ill COVID-19 patients, which can contribute traumatic intravascular injury, culminating to combined micro-macrothrombosis [14].
- Traumatic injury penetrating to SET/EVT is suspected to release TF and activate TF path continuously in circulation and forms “fibrin meshes” via TF-FVIIa complex activating coagulation cascade, especially from the vascular device insertion area [11].
7. Diagnosis and Therapeutic Perspectives
- Different vascular milieux (arterial vs. venous)
- Depth, extent and site of vascular damage (ECs vs. ECs,/SET/EVT)
- Activated hemostasis and subsequent thrombogenesis in different-sized vasculatures (microvasculature vs. macrovasculature)
- Physiologic function of involved organ and tissue (e.g., brain vs. muscle)
- Interaction with environmental, genetic, and underlying pathologic factors
- In critically ill patients in hospital or ICU, early diagnosis of EA-VMTD is the first step preventing the complex forms of thrombotic disorders and then implementing proper therapy with antimicrothrombotic regimens.
- The effective prevention of macrothrombosis is to avoid vascular injury in the hospital and ICU with minimum vascular access and care. Its thromboprophylactic measure may provide only a limited value as long as the underlying endotheliopathy is persistent.
- Therapeutic regimens for EA-VMTD may include plasma exchange and clinical trials with antimicrothrombotic agents (e.g., rADAMTS13, N-acetyl cysteine) and complement inhibitors.
- The most effective approach preventing combined micro-macrothrombosis is diligence with absolute minimum of vascular access and intervention, and recognizing the potential of the deadly complications of VTE and gangrene syndromes.
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rossi, E.C. Comments on the early history of hemostasis. Med. Clin. N. Am. 1972, 56, 9–16. [Google Scholar] [CrossRef]
- Lillicrap, D.; Morrissey, J.H. Hemostasis and thrombosis 101-A challenge to energize. J. Thromb. Haemost. 2020, 18, 269. [Google Scholar] [CrossRef]
- Chang, J.C. Hemostasis based on a novel ‘two-path unifying theory’ and classification of hemostatic disorders. Blood. Coagul. Fibrinolysis 2018, 29, 573–584. [Google Scholar] [CrossRef]
- Chang, J.C. Thrombogenesis and thrombotic disorders based on ‘two-path unifying theory of hemostasis’: Philosophical, physiological, and phenotypical interpretation. Blood Coagul. Fibrinolysis 2018, 29, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Thrombocytopenia in critically ill patients due to vascular microthrombotic disease: Pathogenesis based on “two activation theory of the endothelium”. Vascul. Dis. Ther. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Harmening, D.M. Clinical Hematology and Fundamentals of Hemostasis, 3rd ed.; F.D. Davis: Philadelphia, PA, USA, 1997; pp. 481–508. [Google Scholar]
- Cohen, C.T.; Turner, N.A.; Moake, J.L. Production and control of coagulation proteins for factor X activation in human endothelial cells and fibroblasts. Sci. Rep. 2020, 10, 2005. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Hanlin, E.; Glurich, I.; Mazza, J.J.; Yale, S.H. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin. Med. Res. 2010, 8, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Maggio, E.; Pomero, F. Venous Thromboembolism in Sepsis: From Bench to Bedside. Biomedicines 2022, 10, 1651. [Google Scholar] [CrossRef]
- Chang, J.C.; Hawley, H.B. Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-macrothrombosis. Medicina 2021, 57, 1163. [Google Scholar] [CrossRef]
- Chang, J.C. Pathogenesis of Two Faces of DVT: New Identity of Venous Thromboembolism as Combined Micro-Macrothrombosis via Unifying Mechanism Based on Two-Path Unifying Theory of Hemostasis and Two-Activation Theory of the Endothelium. Life 2022, 12, 220. [Google Scholar] [CrossRef]
- Chang, J.C. Stroke Classification: Critical Role of Unusually Large von Willebrand Factor Multimers and Tissue Factor on Clinical Phenotypes Based on Novel Two-Path Unifying Theory of Hemostasis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620913634. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Acute Respiratory Distress Syndrome as an Organ Phenotype of Vascular Microthrombotic Disease: Based on Hemostatic Theory and Endothelial Molecular Pathogenesis. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619887437. [Google Scholar] [CrossRef]
- Chang, J.C. COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on "Two-Path Unifying Theory" of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy. Vasc. Health Risk Manag. 2021, 17, 273–298. [Google Scholar] [CrossRef]
- Dakay, K.; Cooper, J.; Bloomfield, J.; Overby, P.; Mayer, S.A.; Nuoman, R.; Sahni, R.; Gulko, E.; Kaur, G.; Santarelli, J.; et al. Cerebral Venous Sinus Thrombosis in COVID-19 Infection: A Case Series and Review of The Literature. J. Stroke Cerebrovasc. Dis. 2021, 30, 105434. [Google Scholar] [CrossRef]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, P.; Maroules, M. Splanchnic vein thrombosis in COVID-19: A review of literature. Dig. Liver Dis. 2020, 52, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Novara, E.; Molinaro, E.; Benedetti, I.; Bonometti, R.; Lauritano, E.C.; Boverio, R. Severe acute dried gangrene in COVID-19 infection: A case report. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5769–5771. [Google Scholar]
- Pourdowlat, G.; Naderi, Z.; Seif, F.; Mansouri, D.; Raji, H. Acrocyanosis and digital necrosis are associated with poor prognosis in COVID-19. Clin. Case Rep. 2020, 8, 2769–2772. [Google Scholar] [CrossRef] [PubMed]
- Rose-Sauld, S.; Dua, A. COVID toes and other cutaneous manifestations of COVID-19. J. Wound Care 2020, 29, 486–487. [Google Scholar] [CrossRef]
- Chang, J.C. Sepsis and septic shock: Endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb. J. 2019, 17, 10. [Google Scholar] [CrossRef]
- Chang, J.C. Disseminated intravascular coagulation: New identity as endotheliopathy-associated vascular microthrombotic disease based on in vivo hemostasis and endothelial molecular pathogenesis. Thromb. J. 2020, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. TTP-like syndrome: Novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb. J. 2018, 16, 20. [Google Scholar] [CrossRef]
- Chang, J.C. Pathogenesis of Ebola Viral Haemorrhagic Fever: TTP-like Syndrome Associated with Hepatic Coagulopathy. J. Prev. Infect. Control 2017, 3, 1–4. [Google Scholar] [CrossRef]
- Chang, J.C. Molecular pathogenesis of endotheliopathy and endotheliopathic syndromes, leading to inflammation and microthrombosis, and various hemostatic clinical phenotypes based on “two activation theory of the endothelium” and “two-path unifying theory of hemostasis. Medicina 2022, 58, 1311. [Google Scholar]
- Bray, M.A.; Sartain, S.E.; Gollamudi, J.; Rumbaut, R.E. Microvascular thrombosis: Experimental and clinical implications. Transl. Res. 2020, 225, 105–130. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Viral hemorrhagic fevers due to endotheliopathy-associated disseminated intravascular microthrombosis and hepatic coagulopathy: Pathogenesis based on “two activation theory of the endothelium”. Clin. Microbiol. Infect. Dis. 2017, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Cugno, M.; Meroni, P.L.; Gualtierotti, R.; Griffini, S.; Grovetti, E.; Torri, A.; Lonati, P.; Grossi, C.; Borghi, M.O.; Novembrino, C.; et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J. Autoimmun. 2021, 116, 102560. [Google Scholar] [CrossRef]
- Kerr, H.; Richards, A. Complement-mediated injury and protection of endothelium: Lessons from atypical haemolytic uraemic syndrome. Immunobiology 2012, 217, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Koch, A.E. Vascular endothelium and immune responses: Implications for inflammation and angiogenesis. Rheum. Dis. Clin. N. Am. 2004, 30, 97–114. [Google Scholar] [CrossRef]
- Murdaca, G.; Colombo, B.M.; Cagnati, P.; Gulli, R.; Spanò, F.; Puppo, F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 2012, 224, 309–317. [Google Scholar] [CrossRef]
- Ai, J.; Hong, W.; Wu, M.; Wei, X. Pulmonary vascular system: A vulnerable target for COVID-19. MedComm 2021, 2, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Naresh, K.N.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Susi, P. Special Issue: Virus Receptors and Viral Tropism. Viruses 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, H.C.; Samama, M.M. The significance of endothelial heterogeneity in thrombosis and hemostasis. Semin. Thromb. Hemost. 2010, 36, 286–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aird, W.C.; Kwaan, H.C. Under-recognized significance of endothelial heterogeneity: Hemostasis, thrombosis, and beyond. Semin. Thromb. Hemost. 2010, 36, 225–226. [Google Scholar] [CrossRef]
- Luft, T.; Dreger, P.; Radujkovic, A. Endothelial cell dysfunction: A key determinant for the outcome of allogeneic stem cell transplantation. Bone Marrow Transplant. 2021, 56, 2326–2335. [Google Scholar] [CrossRef]
- van Deuren, M.; Brandtzaeg, P.; van der Meer, J.W. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 2000, 13, 144–166. [Google Scholar] [CrossRef]
- Ortolan, L.S.; Avril, M.; Xue, J.; Seydel, K.B.; Zheng, Y.; Smith, J.D. Plasmodium falciparum Parasite Lines Expressing DC8 and Group A PfEMP1 Bind to Brain, Intestinal, and Kidney Endothelial Cells. Front. Cell. Infect. Microbiol. 2022, 12, 813011. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 2007, 100, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Zöller, B.; García de Frutos, P.; Hillarp, A.; Dahlbäck, B. Thrombophilia as a multigenic disease. Haematologica 1999, 84, 59–70. [Google Scholar]
- Zhu, J.; Guo, W.-M.; Yao, Y.-Y.; Zhao, W.-L.; Pan, L.; Cai, X.; Ju, B.; Sun, G.-L.; Wang, H.-L.; Chen, S.-J.; et al. Tissue factors on acute promyelocytic leukemia and endothelial cells are differently regulated by retinoic acid, arsenic trioxide and chemotherapeutic agents. Leukemia 1999, 13, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, A.C.; Gruel, Y.; Barsotti, J.; Castellani, L.; Marchand, M.; Guerois, C.; Leclerc, M.H.; Delahousse, B.; Griguer, P.; Leroy, J. The white clot syndrome or heparin associated thrombocytopenia and thrombosis (WCS or HATT) (26 cases). Int. Angiol. 1985, 4, 303–310. [Google Scholar] [PubMed]
- Warkentin, T.E. Heparin-induced thrombocytopenia: Pathogenesis and management. Br. J. Haematol. 2003, 121, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M. Heparin-induced thrombocytopenia: An update. Thromb. J. 2005, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Reardon, B.; Pasalic, L.; Favaloro, E.J. The Intriguing Relationships of von Willebrand Factor, ADAMTS13 and Cardiac Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 115. [Google Scholar] [CrossRef]
- Ye, Z.; Zheng, J. Verification of the Role of ADAMTS13 in the Cardiovascular Disease Using Two-Sample Mendelian Randomization. Front. Genet. 2021, 12, 660989. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kisucka, J.; Brill, A.; Walsh, M.T.; Scheiflinger, F.; Wagner, D.D. ADAMTS13: A new link between thrombosis and inflammation. J. Exp. Med. 2008, 205, 2065–2074. [Google Scholar] [CrossRef]
- Uemura, M.; Tatsumi, K.; Matsumoto, M.; Fujimoto, M.; Matsuyama, T.; Ishikawa, M.; Iwamoto, T.-A.; Mori, T.; Wanaka, A.; Fukui, H.; et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood 2005, 106, 922–924. [Google Scholar] [CrossRef]
- Delrue, M.; Siguret, V.; Neuwirth, M.; Joly, B.; Beranger, N.; Sène, D.; Chousterman, B.G.; Voicu, S.; Bonnin, P.; Mégarbane, B.; et al. von Willebrand factor/ADAMTS13 axis and venous thromboembolism in moderate-to-severe COVID-19 patients. Br. J. Haematol. 2021, 192, 1097–1100. [Google Scholar] [CrossRef]
- Bittar, L.F.; de Paula, E.V.; Mello, T.B.; Siqueira, L.H.; Orsi, F.L.; Annichino-Bizzacchi, J.M. Polymorphisms and mutations in vWF and ADAMTS13 genes and their correlation with plasma levels of FVIII and vWF in patients with deep venous thrombosis. Clin. Appl. Thromb. Hemost. 2011, 17, 514–518. [Google Scholar] [CrossRef]
- Newnham, M.; South, K.; Bleda, M.; Auger, W.R.; Barberà, J.A.; Bogaard, H.; Bunclark, K.; Cannon, J.E.; Delcroix, M.; Hadinnapola, C.; et al. The ADAMTS13-VWF axis is dysregulated in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801805. [Google Scholar] [CrossRef] [PubMed]
- Llobet, D.; Tirado, I.; Vilalta, N.; Vallvé, C.; Oliver, A.; Vázquez-Santiago, M.; Mateo, J.; Millón, J.; Fontcuberta, J.; Souto, J. Low ADAMTS13 levels are associated with venous thrombosis risk in women. Thromb. Res. 2017, 157, 38–40. [Google Scholar] [CrossRef]
- Obermeier, H.L.; Riedl, J.; Ay, C.; Koder, S.; Quehenberger, P.; Bartsch, R.; Kaider, A.; Zielinski, C.C.; Pabinger, I. The role of ADAMTS-13 and von Willebrand factor in cancer patients: Results from the Vienna Cancer and Thrombosis Study. Res. Pract. Thromb. Haemost. 2019, 3, 503–514. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.G. Progress in disseminated intravascular coagulation. Calif. Med. 1969, 111, 186–198. [Google Scholar] [PubMed]
- Bick, R.L. Disseminated intravascular coagulation: A review of etiology, pathophysiology, diagnosis, and management: Guidelines for care. Clin. Appl. Thromb. Hemost. 2002, 8, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Disseminated intravascular coagulation (DIC): Is it fact or fancy? Blood Coagul. Fibrinolysis 2018, 29, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Consonni, R.; Marchetti, M.; Locatelli, G.; Garattini, E.; Passerini, C.G.; Gordon, S.G.; Barbui, T. Cancer procoagulant and tissue factor are differently modulated by all-trans-retinoic acid in acute promyelocytic leukemia cells. Blood 1998, 92, 143–151. [Google Scholar] [CrossRef]
- Hambley, B.C.; Tomuleasa, C.; Ghiaur, G. Coagulopathy in Acute Promyelocytic Leukemia: Can We Go Beyond Supportive Care? Front. Med. 2021, 8, 722614. [Google Scholar] [CrossRef]
- David, S.; Mathews, V. Mechanisms and management of coagulopathy in acute promyelocytic leukemia. Thromb. Res. 2018, 164 (Suppl. 1), S82–S88. [Google Scholar] [CrossRef]
- Mackman, N. The role of tissue factor and factor VIIa in hemostasis. Anesth. Analg. 2009, 108, 1447–1452. [Google Scholar] [CrossRef]
- Schreuder, M.; Reitsma, P.H.; Bos, M.H.A. Blood coagulation factor Va’s key interactive residues and regions for prothrombinase assembly and prothrombin binding. J. Thromb. Haemost. 2019, 17, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, A.A. Acute promyelocytic leukemia. Can. Med. Assoc. J. 1967, 97, 57–63. [Google Scholar] [PubMed]
- Ghosh, S.K.; Bandyopadhyay, D.; Ghosh, A. Symmetrical peripheral gangrene: A prospective study of 14 consecutive cases in a tertiary-care hospital in eastern India. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 214–218. [Google Scholar] [CrossRef]
- Sil, A.; Chakraborty, U.; Chandra, A.; Biswas, S.K. COVID-19 associated symmetrical peripheral gangrene: A case series. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102356. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, S.Z.; MacRae, E.A.; Harding, K.G.; Patel, G.K. Symmetrical peripheral digital gangrene following severe Plasmodium falciparum malaria-induced disseminated intravascular coagulopathy. Int. Wound J. 2010, 7, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, N.; Vasa, C.V.; Breakstone, S.; Axman, W. Symmetrical peripheral gangrene of bilateral feet and unilateral hand after administration of vasopressors during septic shock. BMJ Case Rep. 2018, 2018, bcr2017223602. [Google Scholar] [CrossRef]
- Ramachandran, R.; Pillai, A.V.; Raja, S.; Sailesh, S. Axillary artery thrombosis resulting in upper limb amputation as a COVID-19 sequela. BMJ Case Rep. 2021, 14, e240981. [Google Scholar] [CrossRef]
- Biswal, J.K.; Mohanty, S.K.; Behera, S.N.; Swain, S.K.; Sahoo, A.K. Acute Limb Ischemia: A Catastrophic COVID-19 Sequel Leading to Amputation. Cureus 2021, 13, e16456. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Hafizi, S.; Rezaei, N. Inflammation in venous thromboembolism: Cause or consequence? Int. Immunopharmacol. 2015, 28, 655–665. [Google Scholar] [CrossRef]
- Khan, I.A.; Karmakar, S.; Chakraborty, U.; Sil, A.; Chandra, A. Purpura fulminans as the presenting manifestation of COVID-19. Postgrad. Med. J. 2021, 97, 473. [Google Scholar] [CrossRef]
- Zhitny, V.P.; Lyons, M.; Perloff, A.; Menezes, J.; Pistorio, A.; Baynosa, R. COVID-19 association with purpura fulminans: Report of a life threatening complication in a fully vaccinated patient. J. Surg. Case Rep. 2022, 2022, rjac095. [Google Scholar] [CrossRef] [PubMed]
- Acherjee, T.; Bastien, B.; Rodriguez-Guerra, M.A.; Salman, S.; Ali, N. Digital Ischemia as an Initial Presentation in a COVID-19-Positive Patient Without Any Significant Respiratory Symptoms. Cureus 2021, 13, e14054. [Google Scholar] [CrossRef] [PubMed]
- Galanis, N.; Stavraka, C.; Agathangelidis, F.; Petsatodis, E.; Giankoulof, C.; Givissis, P. Coagulopathy in COVID-19 infection: A case of acute upper limb ischemia. J. Surg. Case Rep. 2020, 2020, rjaa204. [Google Scholar] [CrossRef] [PubMed]
- Galyfos, G.; Sianou, A.; Frountzas, M.; Vasilios, K.; Vouros, D.; Theodoropoulos, C.; Michalopoulou, V.; Sigala, F.; Filis, K. Acute limb ischemia among patients with COVID-19 infection. J. Vasc. Surg. 2022, 75, 326–342. [Google Scholar] [CrossRef]
- Hanif, M.; Ali, M.J.; Haider, M.A.; Naz, S.; Ahmad, Z. Acute Upper Limb Ischemia Due To Arterial Thrombosis in a Mild COVID-19 Patient: A Case Report. Cureus 2020, 12, e10349. [Google Scholar] [CrossRef]
- Chaudhary, H.; Mohan, M.; Jain, A.; Kumar, V.; Takia, L.; Sudhakar, M.; Angurana, S.A.; Jindal, A.K. Acral Gangrene: Ugly Cousin of COVID Toes in Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2? Pediatr. Infect. Dis. J. 2021, 40, e312–e313. [Google Scholar] [CrossRef]
- Singh, B.; Mechineni, A.; Kaur, P.; Ajdir, N.; Maroules, M.; Shamoon, F.; Bikkina, F.S.A.M. Acute Intestinal Ischemia in a Patient with COVID-19 Infection. Korean J. Gastroenterol. 2020, 76, 164–166. [Google Scholar] [CrossRef]
- Alhomoud, M.; Alhobayb, T.; Armitage, K. COVID-19 infection triggering Thrombotic Thrombocytopenic Purpura. IDCases. 2021, 26, e01256. [Google Scholar] [CrossRef]
- Dhingra, G.; Maji, M.; Mandal, S.; Vaniyath, S.; Negi, G.; Nath, U.K. COVID 19 infection associated with thrombotic thrombocytopenic purpura. J. Thromb. Thrombolysis 2021, 52, 504–507. [Google Scholar] [CrossRef]
- Tehrani, H.A.; Darnahal, M.; Vaezi, M.; Haghighi, S. COVID-19 associated thrombotic thrombocytopenic purpura (TTP); A case series and mini-review. Int. Immunopharmacol. 2021, 93, 107397. [Google Scholar] [CrossRef]
- Capecchi, M.; Mocellin, C.; Abbruzzese, C.; Mancini, I.; Prati, D.; Peyvandi, F. Dramatic presentation of acquired thombotic thrombocytopenic purpura associated with COVID-19. Haematologica 2020, 105, e540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Zuo, Y.; Yalavarthi, S.; Hunker, K.L.; Knight, J.S.; Kanthi, Y.; Obi, A.T.; Ganesh, S.K. SARS-CoV-2 Spike Protein S1-Mediated Endothelial Injury and Pro-Inflammatory State Is Amplified by Dihydrotestosterone and Prevented by Mineralocorticoid Antagonism. Viruses 2021, 13, 2209. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Moake, J.L. Factor VIII Is Synthesized in Human Endothelial Cells, Packaged in Weibel-Palade Bodies and Secreted Bound to ULVWF Strings. PLoS ONE 2015, 10, e0140740. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.; Moake, J.L.; Bernardo, A.; Ball, C.; Wang, Y.; Arya, M.; Nolasco, L.; Turner, N.; Berndt, M.C.; Anvari, B.; et al. P-selectin anchors newly released ultralarge von Willebrand factor multimers to the endothelial cell surface. Blood 2004, 103, 2150–2156. [Google Scholar] [CrossRef]
- Reeve, S.R.; Abbitt, K.B.; Cruise, T.D.; Hose, D.R.; Lawford, P.V. A method for the automated processing and analysis of images of ULVWF-platelet strings. Platelets 2013, 24, 226–234. [Google Scholar] [CrossRef] [PubMed]
- De Ceunynck, K.; De Meyer, S.F.; Vanhoorelbeke, K. Unwinding the von Willebrand factor strings puzzle. Blood 2013, 121, 270–277. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Goerge, T.; Schneider, S.W.; Wagner, D.D. Formation of platelet strings and microthrombi in the presence of ADAMTS-13 inhibitor does not require P-selectin or beta3 integrin. J. Thromb. Haemost. 2007, 5, 583–589. [Google Scholar] [CrossRef]




| Hemostasis | Coagulation | Thrombosis + | |
|---|---|---|---|
| Term concept | Philosophical | Physiological | Structural |
| Implied meaning | Natural process in vivo | Artificial process in vitro | Pathological process in vivo |
| Physiologic process at bleeding site | |||
| Involved location | Blood vessel wall | Laboratory test tube | Intravascular lumen |
| Vessel wall in extravascular trauma | |||
| End products | Hemostatic plug | Fibrin mesh/fibrin clot * | Microthrombi/macrothrombus |
| Critical role in | Vascular wall injury | Coagulation test | Thrombogenesis |
| Hemorrhage | |||
| Involved components | Endothelium | TF/thromboplastin | ULVWF/FVIII from ECs |
| SET | Coagulation factors | Platelets from circulation | |
| EVT | Fibrinogen | Coagulation proteins/serine proteases | |
| Blood from circulation | Fibrinogen | ||
| TF from SET/EVT | |||
| End results | Determined by the depth | Determined by participating | Determined by ULVWF/FVIII, platelets, TF, |
| of vascular damage | coagulation factors | hemostatic factors and unifying mechanism | |
| Inciting events | Endotheliopathy | Coagulation tests for PT and aPTT | Microthrombosis (i.e., EA-VMTD) |
| Vascular injury | Macrothrombosis (e.g., DVT) | ||
| Combined micro-macrothrombosis (e.g., VTE) |
| (1) Hemostatic principles | ||
| (1) Hemostasis can be activated only by vascular injury. | ||
| (2) Hemostasis must be activated through ULVWF path and/or TF path. | ||
| (3) Hemostasis is the same process in both hemorrhage and thrombosis. | ||
| (4) Hemostasis is the same process in both arterial thrombosis and venous thrombosis. | ||
| (5) Level of vascular damage (endothelium/SET/EVT) determines different clinical phenotypes of hemorrhage and thrombosis. | ||
| (2) Major participating components | ||
| Components | Origin | Mechanism |
| (1) ECs/SET/EVT | Blood vessel wall/EVT | Protective barrier |
| (2) ULVWF/FVIII | ECs | Endothelial exocytosis/anchoring and microthrombogenesis |
| (3) Platelets | Circulation | Adhesion to ULVWF to form microthrombi/assembling and microthrombogenesis |
| (4) TF | SET and EVT | Release from tissue due to vascular injury/leading to fibrinogenesis |
| (5) Coagulation factors | Circulation | Activation to fibrin mesh and fibrin clot/participating in fibrinogenesis |
| (3) Vascular injury and hemostatic phenotypes | ||
| Injury-induced damage | Involved hemostatic path | Level of Vascular Injury and examples |
| (1) Endothelium | ULVWF | Level 1 damage—microthrombosis (e.g., TIA [focal]; Heyde’s syndrome [local]; EA-VMTD [disseminated]) |
| (2) Endothelium/SET | ULVWF + sTF | Level 2 damage—macrothrombosis (e.g., AIS; DVT; PTE; AA) |
| (3) Endothelium/SET/EVT | ULVWF + eTF | Level 3 damage—macrothrombosis with hemorrhage (e.g., THS; THMI) |
| (4) EVT alone | eTF | Level e damage—fibrin clot disease (e.g., AHS [e.g., SDH; EDH]; ICH) |
| Hemostatic phenotypes | Causes | Genesis |
| (1) Hemorrhage | External bodily injury | Trauma-induced external bleeding (e.g., accident; assault; self-inflicted) |
| (2) Hematoma | Internal EVT injury | Obtuse trauma-induced bleeding (e.g., tissue and cavitary hematoma) |
| (3) Thrombosis | Intravascular injury | Intravascular injury (e.g., atherosclerosis; sepsis; indwelling catheter; surgery) |
| The depth of intravascular wall injury |
| ECs injury: ULVWF and FVIII release (e.g., sepsis causing EA-VMTD) |
| ECs and SET injury: ULVWF and FVIII and TF release (e.g., vascular trauma causing DVT) |
| ECs and SET/EVT injury: ULVWF and FVIII and TF (e.g., vascular causing THS) |
| The extent of injury affecting vascular tree system |
| Focal/local/multifocal (e.g., TIA, DVT, Susac syndrome) |
| Regional (e.g., vascular trauma, Kasabach-Merritt syndrome) |
| Disseminated (e.g., endothelial damage/dysfunction due to sepsis, vaccination, envenomation and others) |
| The vascular milieux system |
| Venous (e.g., ITP-like syndrome, VOD, DVT, IVCT/SVCT, VTE, PTE, CVST) |
| Arterial (e.g., TTP-like syndrome, arterial thrombosis, AMI, AIS, SPG, diabetic gangrene) |
| Microvasculature (e.g., capillaries, arterioles, venules, and hepatic sinusoids, causing microthrombosis such as ARDS, encephalopathy, ALF, HUS, WFS) |
| Macrovasculature (e.g., aorta, artery and “minute” arteries, and vena cava, and vein, causing macrothrombosis such as aortic aneurysm, AIS, AMI, SPG, DVT, IVC/SVC, CVST) |
| The locality of vascular injury |
| Tropism (e.g., bacteria, virus, fungus, parasite causing organotropism such as WFS syndrome due to N. menningococcus) |
| Endothelial heterogeneity (e.g., gene expression of host in specific vascular system such as ITP-like syndrome in venous system) |
| Trauma site |
| Interaction with non-hemostatic factors |
| Environmental factor (e.g., pathogen, vaccine, venom, toxin, drug, chemical, trauma) |
| Hemostasis altering genetic factor (e.g., thrombophilic genes such as PC, PS, FV-Leiden, antithrombin, ADAMTS13, and others) |
| Hereditary disease (e.g., Fabry’s disease, HERNS syndrome, Degos disease, hereditary hemorrhagic telangiectasia) |
| Clinical Phenotype | Arterial Endotheliopathy | Venous Endothelipathy |
|---|---|---|
| Underlying pathology | aEA-VMTD | vEA-VMTD |
| Physiological/hemodynamic difference in vascular milieu | Efferent circulation from the heart (O2 delivery) | Afferent circulation into the heart (CO2 disposal) |
| Tissue hypoxia (e.g., microthromboangiitis obliterans) | Pulmonary circulatory congestion (e.g., ARDS) | |
| High pressure flow | Low pressure flow | |
| High shear stress | Low shear stress | |
| Capillary and arteriolar microvascular event | Venous and pulmonary microvascular event | |
| Insignificant role of NETosis | Significant role of NETosis | |
| Primary cause | ||
| Vascular injury (ECs) | Sepsis-induced arterial microvascular endotheliopathy | Sepsis-induced venous endotheliopathy |
| Vaccine-induced venous endotheliopathy | ||
| Vascular pathology site | Disseminated aEA-VMTD at microvasculature | Transient or “silent” vEA-VMTD at venous system |
| Activated hemostatic path | ULVWF path | ULVWF path |
| Thrombosis component | Microthrombi strings in the microvasculature | Microthrombi strings in venous system |
| Microthrombotic event | Disseminated VMTD | Silent microthrombosis with microthrombolysis |
| Clinical phenotypes | TTP-like syndrome | ITP-like syndrome |
| consumptive thrombocytopenia | consumptive thrombocytopenia | |
| MAHA | MAHA (mild and rare: e.g., ARDS) | |
| MODS/MOIS | MOIS |
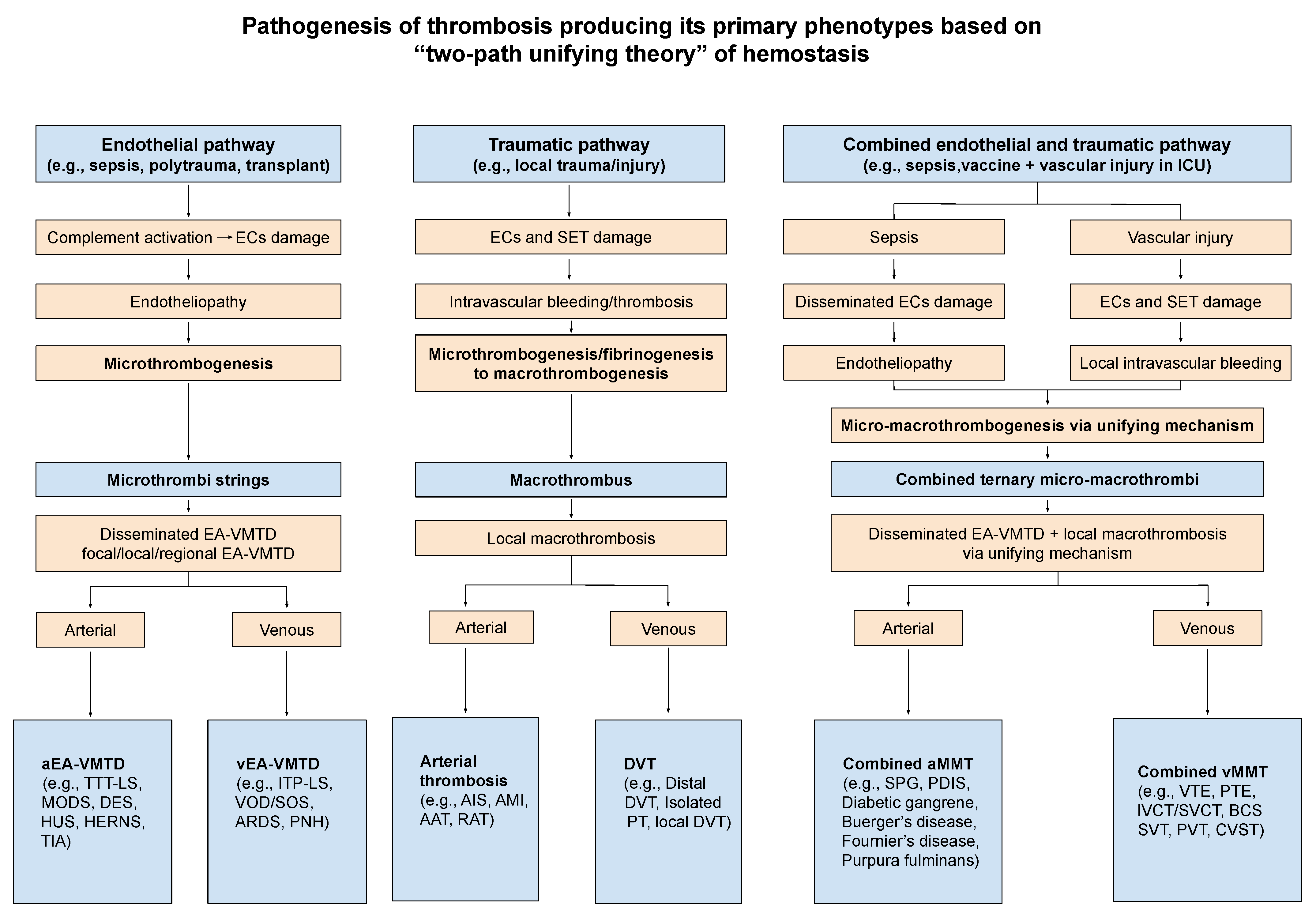
| Microthrombosis |
| ● aEA-VMTD |
| Mechanism: via microthrombogenesis from activated ULVWF path due to ECs injury of capillaries and arterioles |
| Disseminated aEA-VMTD (e.g., TTP-like syndrome, MODS, MVMI, microthrombotic encephalopathy, HUS) |
| Regional aEA-VMTD (e.g., Kasabach-Merritt syndrome, Heyde’s syndrome) |
| Multifocal aEA-VMTD (e.g., HERNS syndrome, Susac syndrome, diabetic retinal microaneurysm) |
| Focal aEA-VMTD (e.g., TIA) |
| ● vEA-VMTD |
| Mechanism: via microthrombogenesis from activated ULVWF path due to venous ECs injury |
| Disseminated vEA-VMTD (e.g., “silent” ITP-like syndrome, ARDS) |
| Regional vEA-VMTD (e.g., VOD) |
| Macrothrombosis |
| ● Arterial macrothrombosis |
| Mechanism: via macrothrombogenesis from activated ULVWF path and TF path due to arterial ECs and SET/EVT injury |
| Localized arterial thrombosis (e.g., AIS, THS, AMI, aortic aneurysm-associated thrombosis) |
| Multifocal partially obstructive macrothrombosis (e.g., chronic atherosclerosis?) |
| ● Venous macrothrombosis |
| Mechanism: via macrothrombogenesis from activated ULVWF path and TF path due to venous ECs and SET/EVT injury |
| Localized venous thrombosis (e.g., distal DVT, superficial venous thrombosis) |
| Fibrin clot disease |
| ● Arterial/venous fibrin clot disease |
| Mechanism: via pathologic fibrinogenesis from activated aberrant TF path due to overexpressed TF in APL |
| Disseminated fibrin clot disease and hemorrhagic syndrome (e.g., true DIC in APL) |
| Localized hematoma (?) (e.g., internal bleeding including SDH, EDH, AHS) |
| Combined micro-macrothrombosis (due to two different vascular injuries: e.g., sepsis and vascular injury in ICU ) |
| ● Arterial combined micro-macrothrombosis |
| Mechanism: via combined micro-macrothrombogenesis from activated ULVWF path and TF path in arterial system |
| Peripheral gangrene syndrome (e.g., SPG, PDIS, Buerger’s disease, Fournier’s disease, purpura fulminans, acrocyanosis) |
| ● Venous combined micro-macrothrombosis |
| Mechanism: via combined micro-macrothrombogenesis from activated ULVWF path and TF path in venous system |
| Venous circulatory congestion syndrome (e.g., VTE, PTE, IVCT/SVCT, SVT, BCS, PVT, CVST) |
| Non-hemostatic thrombotic syndromes |
| ● Disseminated VMTD |
| Mechanism: excess of ULVWF due to ADAMTS13 deficiency leading to intravascular microthrombogenesis |
| ADAMTS13 gene mutation-associated vascular microthrombotic disease (GA-VMTD: Hereditary TTP) |
| ADAMTS13 gene antibody-associated vascular microthrombotic disease (AA-VMTD: Acquired TTP) |
| ● HIT-WCS |
| Mechanism: platelet thrombi due to heparin-PF4 complex reacting with heparin-PF4 complex antibody |
| White clot syndrome |
| ● Fibrin clot disease in APL * |
| Mechanism: activated aberrant TF path from overexpressed TF of APL without vascular injury |
| APL coagulopathy with hemorrhagic syndrome (i.e., fibrin clot disease; true DIC) |
| aEA-VMTD | vEA-VMTD | aMT | vMT | FCD | Combined aMMT | Combined vMMT | |
|---|---|---|---|---|---|---|---|
| Typical example | TTP-like syndrome | ITP-like syndrome | AIS | Distal DVT | APL | SPG | VTE |
| Thrombocytopenia | + | + | - | - | + (due to leukemia) | + | + |
| Fibrinogen | Increased/decreased | Increased/decreased | Normal | Normal | Decreased | Markedly increased | May be increased |
| ULVWF/VWF antigen | Markedly increased | May be increased | Normal | Normal | Normal expected | Markedly increased | Maybe increased |
| FVIII | Markedly increased | Increased | Normal | Normal | Markedly decreased | Increased | May be increased |
| FV | Normal | Normal | Normal | Normal | Decreased | Decreased (?) | Decreased (?) |
| ADAMTS13 | Low | Low | Normal | Normal | Normal expected | Likely low | Likely low |
| FSPs | +/- | +/- | Normal | Normal | Positive | Positive | Positive |
| D-dimer | Normal | Normal | +/- | +/- | Increased | Markedly increased | Markedly increased |
| MAHA | + | +/- | - | - | - | + | - |
| MODS | + | +/- | - | - | - | + | - |
| Inflammation | + | + | - | - | ? | + | + |
| C5b-9 involvement | Expected to be + | Expected to be + | - | - | ? | Expected to be + | Expected to be + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.C. Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis. Biomedicines 2022, 10, 2706. https://doi.org/10.3390/biomedicines10112706
Chang JC. Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis. Biomedicines. 2022; 10(11):2706. https://doi.org/10.3390/biomedicines10112706
Chicago/Turabian StyleChang, Jae Chan. 2022. "Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis" Biomedicines 10, no. 11: 2706. https://doi.org/10.3390/biomedicines10112706
APA StyleChang, J. C. (2022). Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis. Biomedicines, 10(11), 2706. https://doi.org/10.3390/biomedicines10112706






