Transcranial Magnetic Stimulation Following a Paired Associative Stimulation Protocol Based on a Video Game Neuromodulates Cortical Excitability and Motor Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Transcranial Magnetic Stimulation
2.3. Electromyography (EMG) Recordings
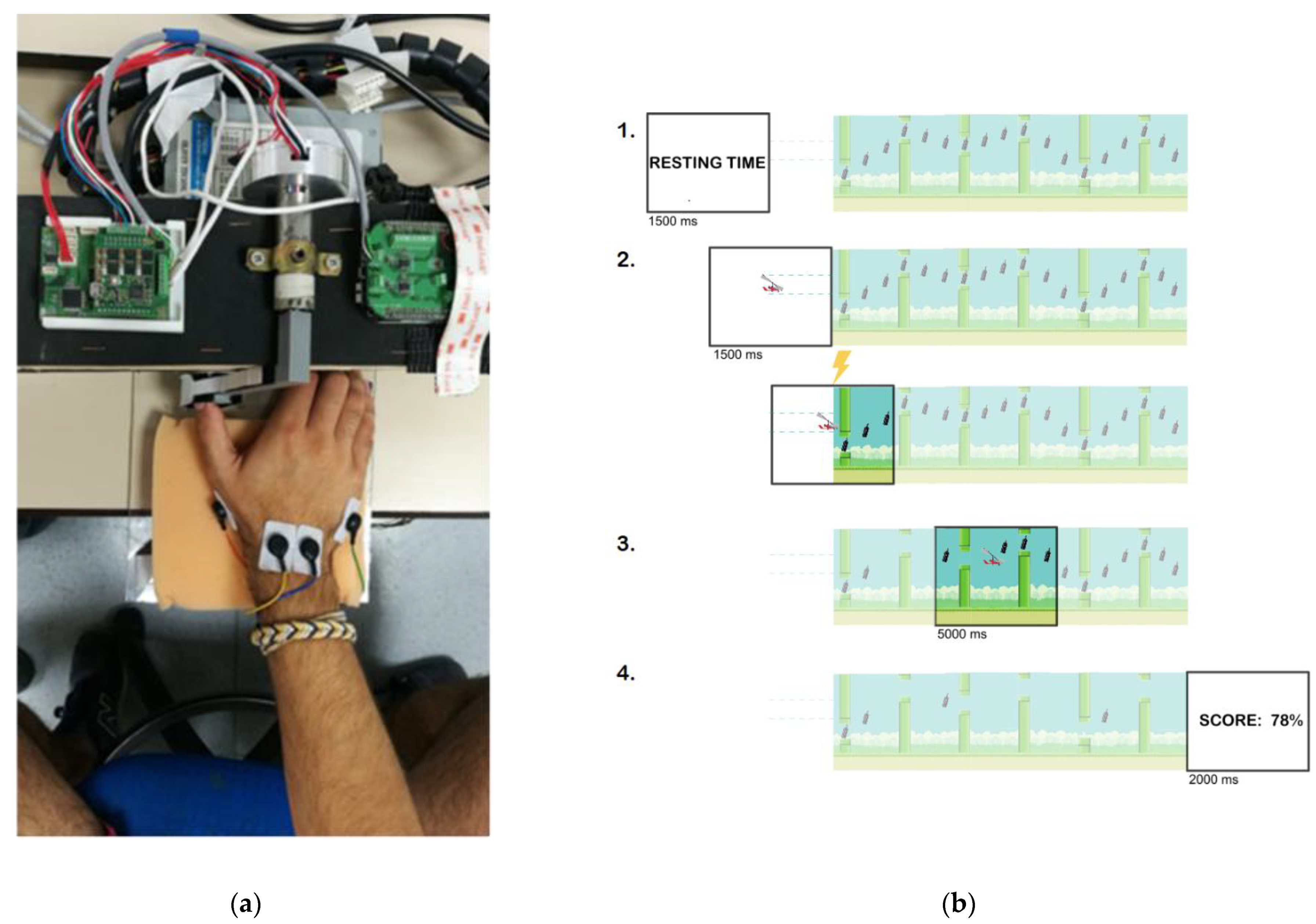
2.4. Movement-Related Dynamic Task (MRDT)
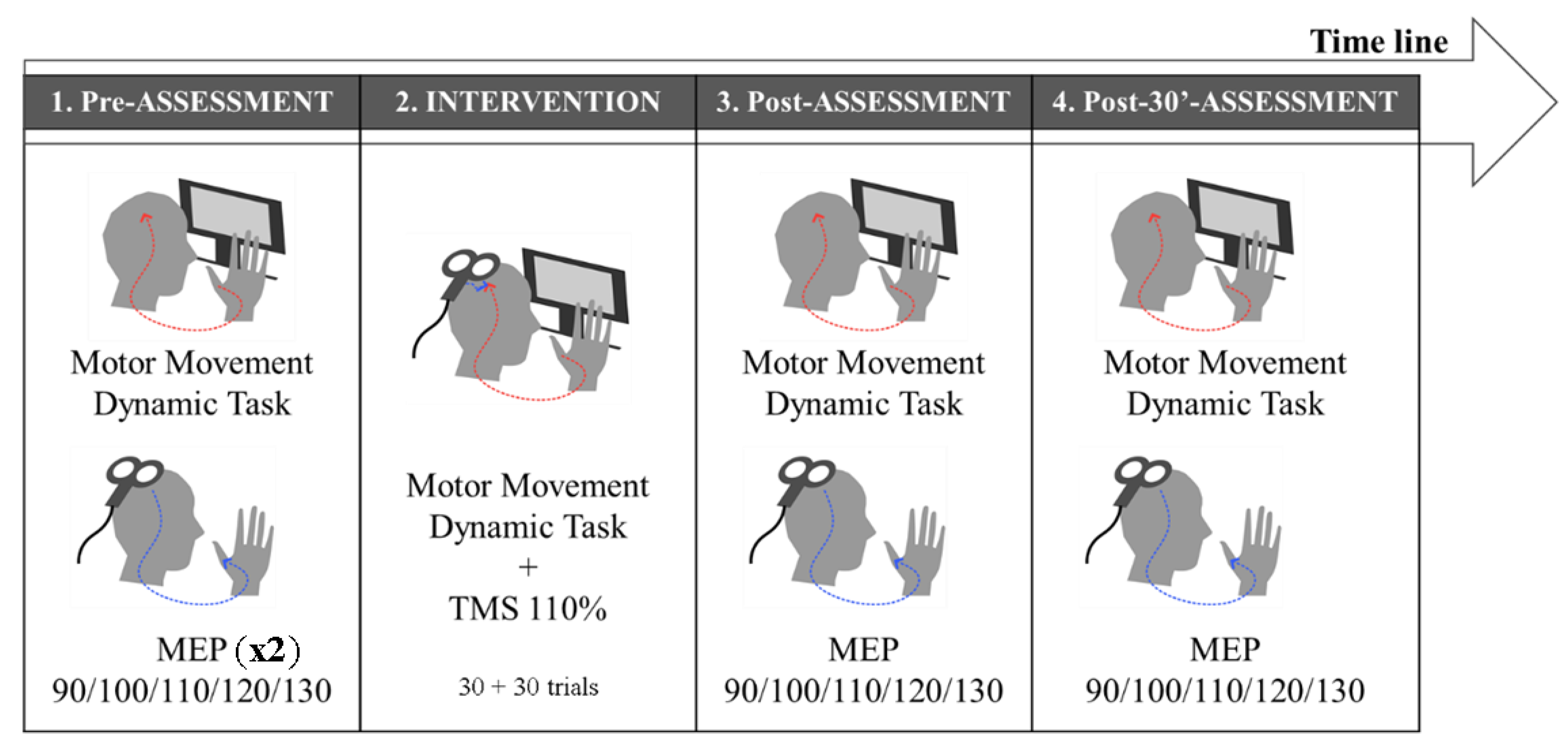
2.5. Experimental Design
2.6. Statistical Analysis
3. Results
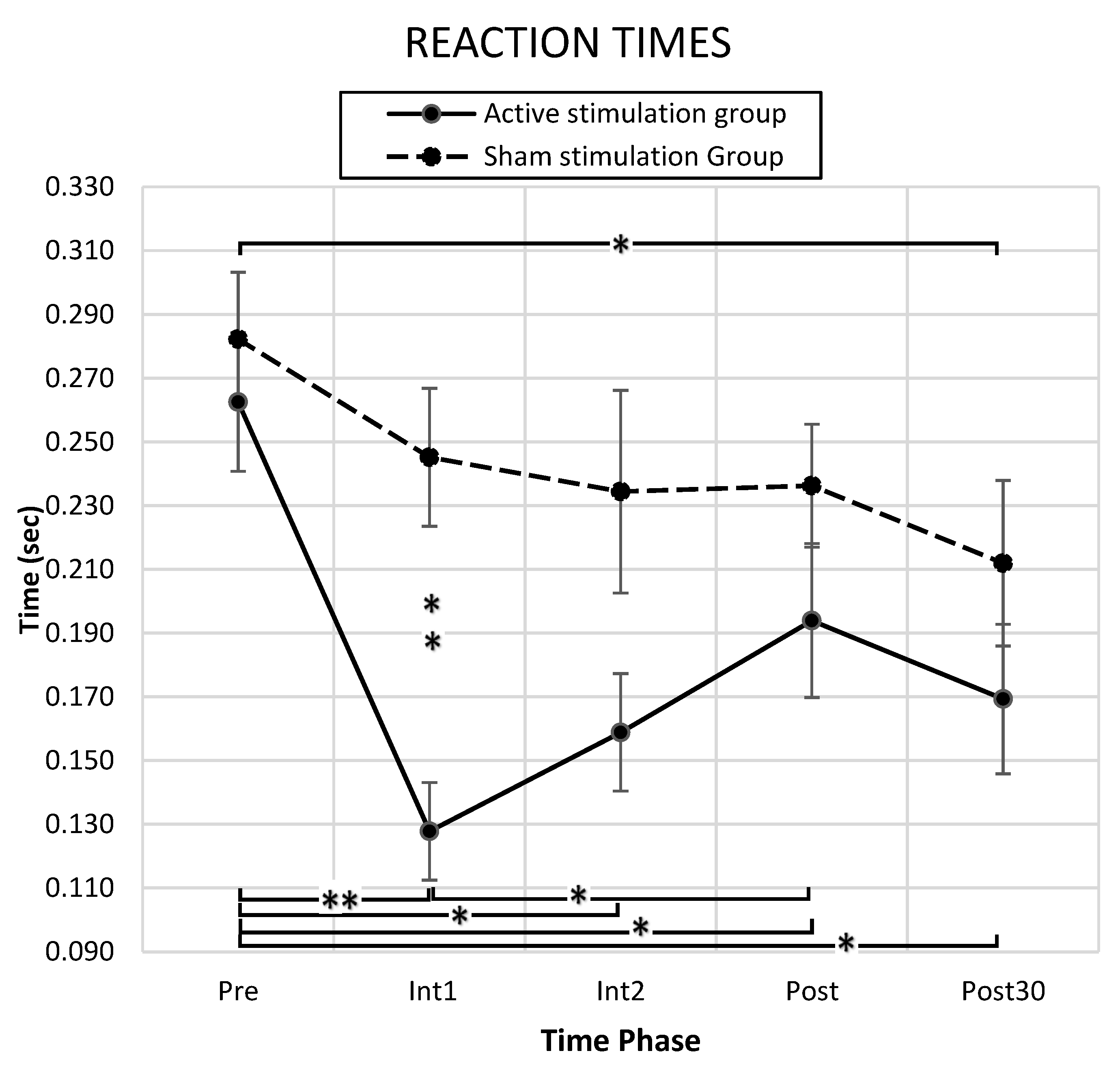
3.1. Behavioral Results
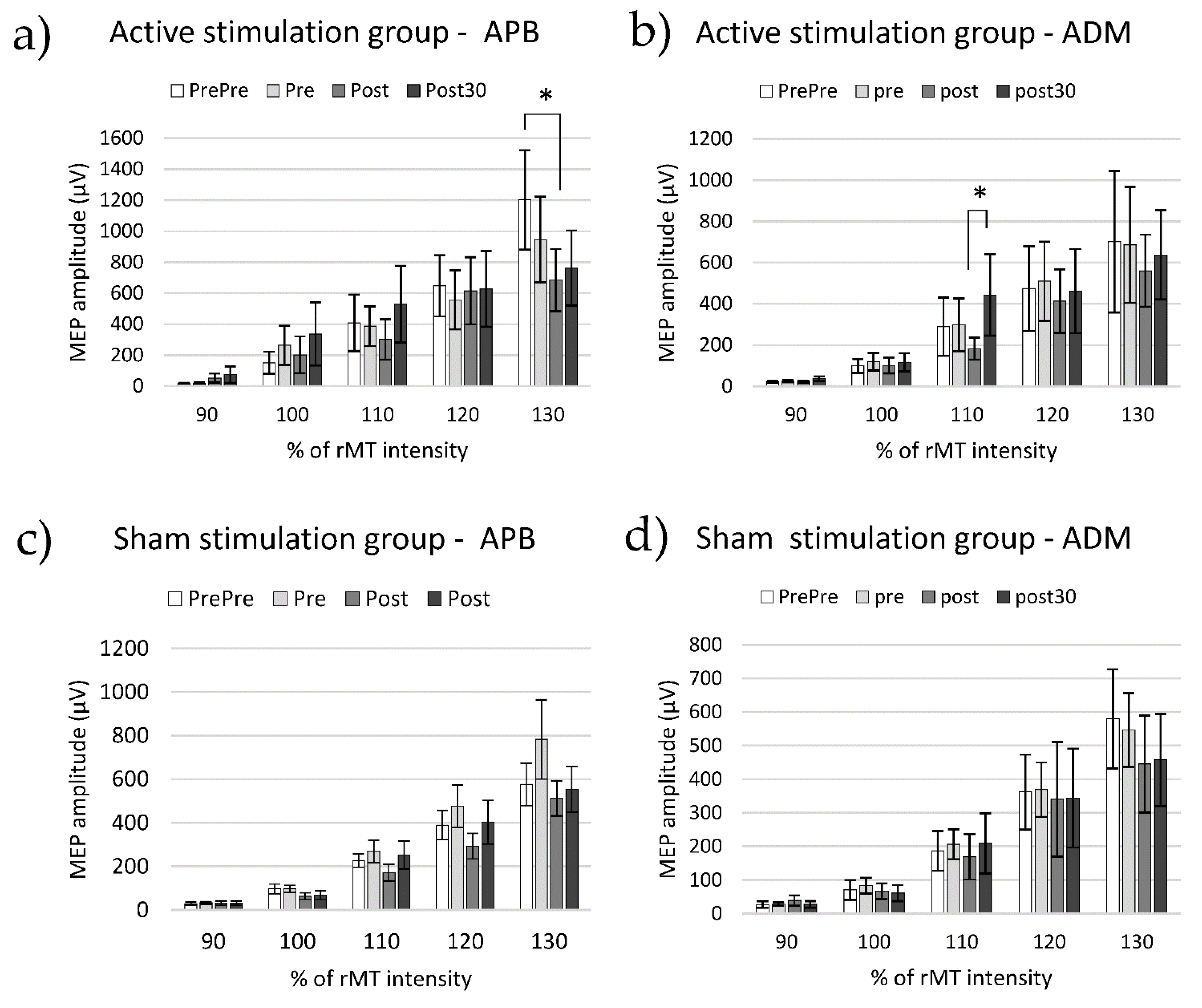
3.2. Cortico-Spinal Excitability
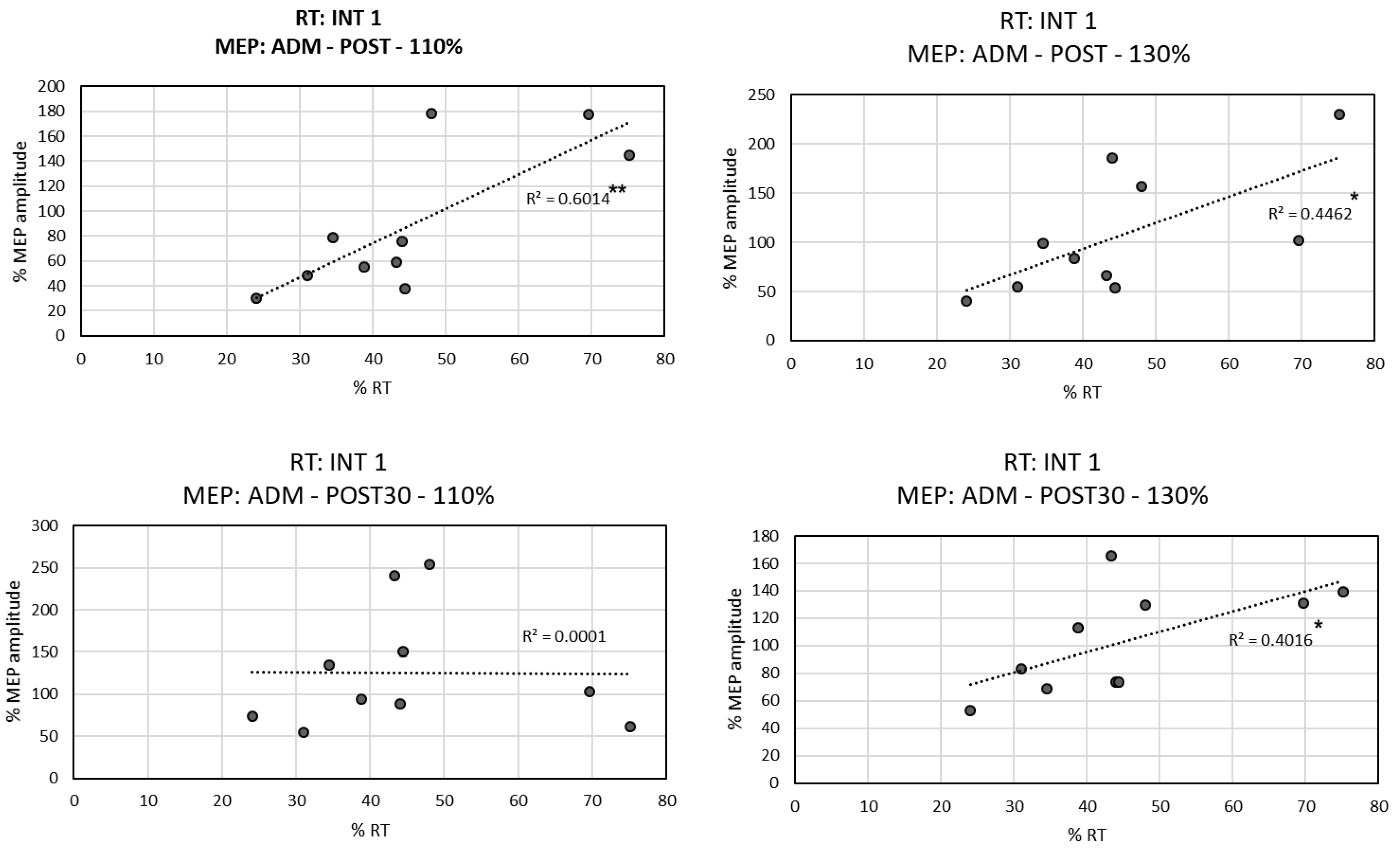
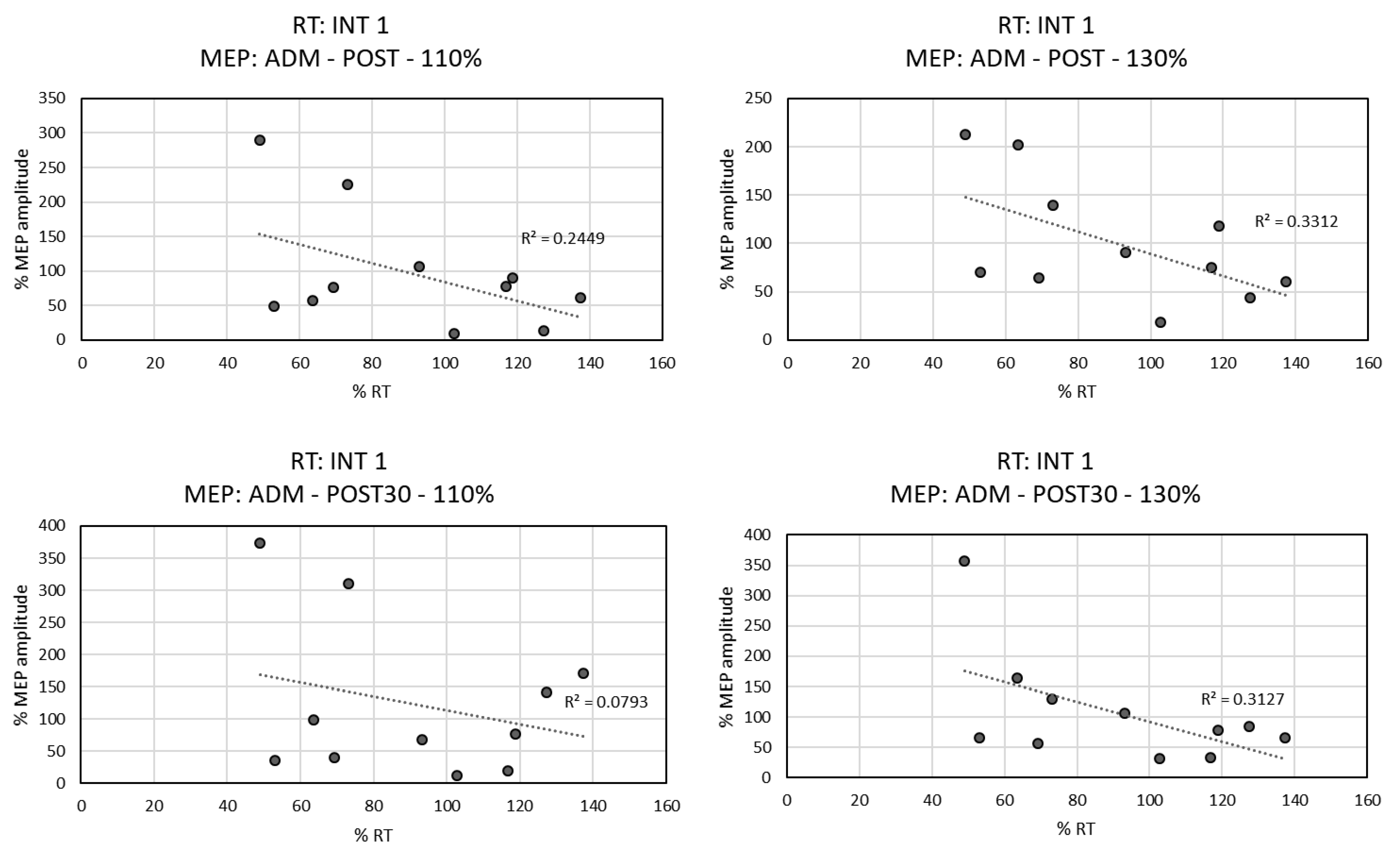
3.3. Correlation between RTs and MEPs
4. Discussion
4.1. PAS Effects on Behavioral Indices
4.1.1. RT Index
4.1.2. TE and the Number of Collected and Avoided Items
4.2. PAS Effects on MEPs
4.2.1. APB Muscle
4.2.2. ADM Muscle
4.3. Why Are the MEP Effects Different Depending on the TMS Pulse Intensity?
4.4. Methodological Considerations and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayford, M.; Siegelbaum, S.A.; Kandel, E.R. Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 2012, 4, a005751. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Poo, M.M. Spike timing-dependent plasticity: From synapse to perception. Physiol. Rev. 2006, 86, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.P.; Lomo, T. Long-lasting potentiation of synapic transmission in the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Egger, V.; Feldmeyer, D.; Sakmann, B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat. Neurosci. 1999, 2, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Miller, K.D.; Abbott, L.F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 2000, 3, 919–926. [Google Scholar] [CrossRef]
- Sjöström, P.J.; Turrigiano, G.G.; Nelson, S.B. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 2001, 32, 1149–1164. [Google Scholar] [CrossRef] [Green Version]
- Tzounopoulos, T.; Kim, Y.; Oertel, D.; Trussell, L.O. Cell-specific, spike timing–dependent plasticities in the dorsal cochlear nucleus. Nat. Neurosci. 2004, 7, 719–725. [Google Scholar] [CrossRef]
- D’amour, J.A.; Froemke, R.C. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 2015, 86, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Froemke, R.C. Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci. 2015, 38, 195–219. [Google Scholar] [CrossRef] [Green Version]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 325, 1106–1107. [Google Scholar] [CrossRef]
- San Agustín, A.; Moreno, J.C. Ethical Aspects of Transcranial Magnetic Stimulation for Neuroenhancement. Dilemata 2021, 34, 121–132. [Google Scholar]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000, 123, 572–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classen, J.; Wolters, A.; Stefan, K.; Wycislo, M.; Sandbrink, F.; Schmidt, A.; Kunesch, E. Paired associative stimulation. Suppl. Clin. Neurophysiol. 2004, 57, 9. [Google Scholar] [CrossRef]
- Ziemann, U.; Iliać, T.V.; Pauli, C.; Meintzschel, F.; Ruge, D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004, 24, 1666–1672. [Google Scholar] [CrossRef] [Green Version]
- Frantseva, M.V.; Fitzgerald, P.B.; Chen, R.; Möller, B.; Daigle, M.; Daskalakis, Z.J. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill leaning. Cereb. Cortex 2008, 18, 990–996. [Google Scholar] [CrossRef] [Green Version]
- Tolmacheva, A.; Savolainen, S.; Kirveskari, E.; Lioumis, P.; Kuusela, L.; Brandstack, N.; Shulga, A. Long-term paired associative stimulation enhances motor output of the tetraplegic hand. J. Neurotrauma 2017, 34, 2668–2674. [Google Scholar] [CrossRef]
- Wessel, M.J.; Zimerman, M.; Hummel, F.C. Non-invasive brain stimulation: An interventional tool for enhancing behavioral training after stroke. Front. Hum. Neurosci. 2015, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Brihmat, N.; Tarri, M.; De Boissezon, X.; Gasq, D.; Loubinoux, I.; Marque, P.; Castel-Lacanal, E. Effect of the association of motor imagery exercises and paired associative stimulation in stroke patients (MIPAS). Ann. Phys. Rehabil. Med. 2018, 61, e28. [Google Scholar] [CrossRef]
- San Agustín, A.; Pons, J.L. Paired associative stimulation protocols with transcranial magnetic stimulation for motor cortex potentiation. In Proceedings of the School and Symposium on Advanced Neurorehabilitation (SSNR2018), Baiona, Spain, 16–21 September2018; p. 44. [Google Scholar]
- Thabit, M.N.; Ueki, Y.; Koganemaru, S.; Fawi, G.; Fukuyama, H.; Mima, T. Movement-related cortical stimulation can induce human motor plasticity. J. Neurosci. 2010, 30, 11529–11536. [Google Scholar] [CrossRef] [Green Version]
- San Agustín, A.; Pons Rovira, J.L. Paired Associative Stimulation for Memory Facilitation. In Proceedings of the School and Symposium On Advanced Neurorehabilitation (SSNR2019), Baiona, Spain, 15–20 September 2019; pp. 37–39. [Google Scholar]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Rossini, P.M. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef] [Green Version]
- The Math Works, Inc. MATLAB, Version 2013b; The Math Works: Natik, MA, USA, 2013; Available online: https://www.mathworks.com (accessed on 11 September 2022).
- IBM Corp. IBM SPSS Statistics for Windows, Version 2019; IBM: Atlanta, GA, USA, 2019; Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 11 September 2022).
- Stinear, C.M.; Coxon, J.P.; Byblow, W.D. Primary motor cortex and movement prevention: Where Stop meets Go. Neurosci. Biobehav. Rev. 2009, 33, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Muir, R.B.; Lemon, R.N. Corticospinal neurons with a special role in precision grip. Brain Res. 1983, 261, 312–316. [Google Scholar] [CrossRef]
- Raos, V.; Umiltá, M.A.; Murata, A.; Fogassi, L.; Gallese, V. Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J. Neurophysiol. 2006, 95, 709–729. [Google Scholar] [CrossRef] [PubMed]
- Brasil-Neto, J.P.; Pascual-Leone, A.; Valls-Solé, J.; Cammarota, A.; Cohen, L.G.; Hallett, M. Postexercise depression of motor evoked potentials: A measure of central nervous system fatigue. Exp. Brain Res. 1993, 93, 181–184. [Google Scholar] [CrossRef]
- Samii, A.; Wassermann, E.M.; Hallett, M. Post-exercise depression of motor evoked potentials as a function of exercise duration. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control. 1997, 105, 352–356. [Google Scholar] [CrossRef]
- Sacco, P.; Newberry, R.; McFadden, L.; Brown, T.; McComas, A.J. Depression of human electromyographic activity by fatigue of a synergistic muscle. Muscle Nerve 1997, 20, 710–717. [Google Scholar] [CrossRef]
- Zijdewind, I.; Zwarts, M.J.; Kernell, D. Potentiating and fatiguing cortical reactions in a voluntary fatigue test of a human hand muscle. Exp. Brain Res. 2000, 130, 529–532. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Tonali, P.A.; Mazzone, P.; Insola, A.; Pilato, F.; Rothwell, J.C. Direct demonstration of reduction of the output of the human motor cortex induced by a fatiguing muscle contraction. Exp. Brain Res. 2003, 149, 535–538. [Google Scholar] [CrossRef]
- Perretti, A.; Balbi, P.; Orefice, G.; Trojano, L.; Marcantonio, L.; Brescia-Morra, V.; Santoro, L. Post-exercise facilitation and depression of motor evoked potentials to transcranial magnetic stimulation: A study in multiple sclerosis. Clin. Neurophysiol. 2004, 115, 2128–2133. [Google Scholar] [CrossRef]
- Milanović, S.; Filipović, S.R.; Blesić, S.; Ilić, T.V.; Dhanasekaran, S.; Ljubisavljević, M. Paired-associative stimulation can modulate muscle fatigue induced motor cortex excitability changes. Behav. Brain Res. 2011, 223, 30–35. [Google Scholar] [CrossRef]
- Patton, H.D.; Vahé, E.A. Single-and multiple-unit analysis of cortical stage of pyramidal tract activation. J. Neurophysiol. 1954, 17, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Day, B.L.; Thompson, P.D.; Dick, J.P.; Nakashima, K.; Marsden, C.D. Different sites of action of electrical and magnetic stimulation of the human brain. Neurosci. Lett. 1987, 75, 101–106. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Restuccia, D.; Oliviero, A.; Profice, P.; Ferrara, L.; Insola, A.; Rothwell, J.C. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp. Brain Res. 1998, 119, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Mazzone, P.; Insola, A.; Ranieri, F.; Tonali, P.A. Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurol. Res. 2003, 25, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Mazzone, P.; Rothwell, J.C. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin. Neurophysiol. 2004, 115, 255–266. [Google Scholar] [CrossRef]
- Berardelli, A.; Inghilleri, M.; Cruccu, G.; Manfredi, M. Descending volley after electrical and magnetic transcranial stimulation in man. Neurosci. Lett. 1990, 112, 54–58. [Google Scholar] [CrossRef]
- Thompson, P.D.; Day, B.L.; Crockard, H.A.; Calder, I.; Murray, N.M.; Rothwell, J.C.; Marsden, C.D. Intra-operative recording of motor tract potentials at the cervico-medullary junction following scalp electrical and magnetic stimulation of the motor cortex. J. Neurol. Neurosurg. Psychiatry 1991, 54, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Burke, D.J.; Hicks, R.; Stephen, J.; Woodforth, I.; Crawford, M. The site of activation of the corticospinal system by transcranial magnetic and electrical stimulation of the human motor cortex. In Spasticity; Springer: Berlin/Heidelberg, Germany, 1993; pp. 57–66. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Saturno, E.; Pilato, F.; Insola, A.; Rothwell, J.C. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control. 1998, 109, 397–401. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Agustín, A.; Asín-Prieto, G.; Moreno, J.C.; Oliviero, A.; Pons, J.L. Transcranial Magnetic Stimulation Following a Paired Associative Stimulation Protocol Based on a Video Game Neuromodulates Cortical Excitability and Motor Behavior. Biomedicines 2022, 10, 2632. https://doi.org/10.3390/biomedicines10102632
San Agustín A, Asín-Prieto G, Moreno JC, Oliviero A, Pons JL. Transcranial Magnetic Stimulation Following a Paired Associative Stimulation Protocol Based on a Video Game Neuromodulates Cortical Excitability and Motor Behavior. Biomedicines. 2022; 10(10):2632. https://doi.org/10.3390/biomedicines10102632
Chicago/Turabian StyleSan Agustín, Arantzazu, Guillermo Asín-Prieto, Juan C. Moreno, Antonio Oliviero, and José L. Pons. 2022. "Transcranial Magnetic Stimulation Following a Paired Associative Stimulation Protocol Based on a Video Game Neuromodulates Cortical Excitability and Motor Behavior" Biomedicines 10, no. 10: 2632. https://doi.org/10.3390/biomedicines10102632
APA StyleSan Agustín, A., Asín-Prieto, G., Moreno, J. C., Oliviero, A., & Pons, J. L. (2022). Transcranial Magnetic Stimulation Following a Paired Associative Stimulation Protocol Based on a Video Game Neuromodulates Cortical Excitability and Motor Behavior. Biomedicines, 10(10), 2632. https://doi.org/10.3390/biomedicines10102632





