Clinical and Research MRI Techniques for Assessing Spinal Cord Integrity in Degenerative Cervical Myelopathy—A Scoping Review
Abstract
1. Introduction
1.1. Epidemiology
1.2. Natural History
1.3. Current Diagnostic Options and Limitations
1.3.1. Clinical
1.3.2. Scoring Systems
1.3.3. Conventional MRI
1.3.4. Plain Radiographs and Computed Tomography (CT)
1.3.5. Electrophysiology
1.4. Novel qMRI Modalities and Parameters
1.5. Objective
‘What is known from the literature about existing clinical and novel research MRI techniques for assessing spinal cord integrity in patients with Degenerative Cervical Myelopathy (DCM)?’
2. Methodology
2.1. Data Sources
2.2. Selection Criteria
2.3. Synthesis of Results
3. Results
4. Discussion
4.1. Quantitative T1 and T2 Mapping
4.1.1. Principles
4.1.2. Application in DCM
4.2. Diffusion Tensor Imaging (DTI)
4.2.1. Principles
4.2.2. Application in DCM
4.3. Functional MRI (BOLD)
4.3.1. Principles
4.3.2. Application in DCM
4.4. Magnetic Resonance Spectroscopy (MRS)
4.4.1. Principles
4.4.2. Application in DCM
4.5. Magnetisation Transfer (MT)
4.5.1. Principles
4.5.2. Application in DCM
4.6. R2* or 1/T2*—A Promising Biomarker
4.6.1. Principles
4.6.2. Role of Iron in Neurodegenerative Disorders
4.6.3. Application in DCM
4.7. Quantitative Susceptibility Weighted Imaging (SWI)/Mapping—Another Promising Biomarker
4.7.1. Underlying Principle
4.7.2. Role of Calcium in Neurodegenerative Disorders
4.7.3. Application in DCM
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1.5TMRI | 1.5 Tesla magnetic resonance imaging |
| 3TMRI | 3 Tesla magnetic resonance imaging |
| AD | Alzheimer’s disease |
| ADC | Apparent diffusion coefficient |
| ALS | Amyotrophic lateral sclerosis |
| BOLD | Blood oxygen level dependent |
| Cho | Choline |
| CMS | Cervical myelopathy scale |
| CR | Compression Ratio |
| Cr | Creatine |
| CSM | Cervical spondylotic myelopathy |
| CT | Computed tomography |
| DBSI | Diffusion basis spectrum imaging |
| DCM | Degenerative cervical myelopathy |
| DNA | Deoxyribonucleic acid |
| DTI | Diffusion tensor imaging |
| DTT | Diffusion tensor tractography |
| DWI | Diffusion weighted imaging |
| EMG | Electromyography |
| EMS | European myelopathy scale |
| FA | Fractional anisotropy |
| FC | Functional connectivity |
| fFOV | Full field of view |
| fMRI | Functional MRI |
| Glx | Glutamate-glutamine |
| Ins | Myo-inositols |
| MCC | Maximum canal compromise |
| MEPs | Motor evoked potentials |
| mJOA | Modified Japanese Orthopaedic Association scale |
| MRI | Magnetic resonance imaging |
| MRS | Magnetic resonance spectroscopy |
| MS | Multiple sclerosis |
| MSCC | Maximum spinal cord compression |
| MT | Magnetization transfer |
| MTR | Magnetization transfer ratio |
| MWF | Myelin water fraction |
| NAA | N-acetylaspartate |
| NCS | Nerve conduction studies |
| NDI | Neck disability index |
| NPRS | Numeric pain rating scale |
| OPLL | Ossification of the posterior longitudinal ligaments |
| PD | Parkinson’s disease |
| qMRI | Quantitative magnetic resonance imaging |
| QSM | Quantitative susceptibility mapping |
| R2*MRI | R2* magnetic resonance imaging |
| rFOV | Reduced field of view |
| ROI | Region of interest |
| SMA | Supplementary motor area |
| SSEPs | Somatosensory evoked potentials |
| SWI | Susceptibility weighted imaging |
| T1WI | T1 weighted imaging |
| T2*WI | T2*-weighted imaging |
| T2WI | T2 weighted imaging |
| VOA | Volume of activation |
Appendix A. Classification Systems for DCM
| Modified Japanese Orthopaedic Association (mJOA) Score | ||
|---|---|---|
| Circle one | I. Motor dysfunction score of the upper extremities | |
| 0 | Inability to move hands | |
| 1 | Inability to eat with a spoon but able to move hands | |
| 2 | Inability to button shirt but able to eat with a spoon | |
| 3 | Able to button shirt with great difficulty | |
| 4 | Able to button shirt with slight difficulty | |
| 5 | No dysfunction | |
| Circle one | II. Motor dysfunction score of the lower extremities | |
| 0 | Complete loss of motor and sensory function | |
| 1 | Sensory preservation without ability to move legs | |
| 2 | Able to move legs but unable to walk | |
| 3 | Able to walk on flat floor with a walking aid (i.e., cane or crutch) | |
| 4 | Able to walk up and/or down stairs with hand rail | |
| 5 | Moderate to significant lack of stability but able to walk up and/or down stairs without hand rail | |
| 6 | Mild lack of stability but walk unaided with smooth reciprocation | |
| 7 | No dysfunction | |
| Circle one | III. Sensation | |
| 0 | Complete loss of hand sensation | |
| 1 | Severe sensory loss or pain | |
| 2 | Mild sensory loss | |
| 3 | No sensory loss | |
| Circle one | IV. Sphincter dysfunction | |
| 0 | Inability to urinate voluntarily | |
| 1 | Marked difficulty with micturition | |
| 2 | Mild to moderate difficulty with micturition | |
| 3 | Normal micturition | |
| Mild myelopathy | mJOA from 15 to 17 | |
| Moderate myelopathy | mJOA from 12 to 14 | |
| Severe myelopathy | mJOA from 0 to 11 | |
| Pain Numeric Rating Scale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. On a scale of 0 to 10, with 0 being no pain at all and 10 being the worst pain imaginable, how would you rate your pain RIGHT NOW. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| No Pain | Worst Pain Imaginable | |||||||||
| 2. On the same scale, how would you rate your USUAL level of pain during the last week. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| No Pain | Worst Pain Imaginable | |||||||||
| 3. On the same scale, how would you rate your BEST level of pain during the last week. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| No Pain | Worst Pain Imaginable | |||||||||
| 4. On the same scale, how would you rate your WORST level of pain during the last week. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| No Pain | Worst Pain Imaginable | |||||||||
| Neck Disability Index |
|---|
| Please answer every section and mark in each section only the one box that applies to you. |
| Section 1: Pain Intensity |
| I have no pain at the moment |
| The pain is very mild at the moment |
| The pain is moderate at the moment |
| The pain is fairly severe at the moment |
| The pain is very severe at the moment |
| The pain is the worst imaginable at the moment |
| Section 2: Personal Care (Washing, Dressing, etc.) |
| I can look after myself normally without causing extra pain |
| I can look after myself normally but it causes extra pain |
| It is painful to look after myself and I am slow and careful |
| I need some help but can manage most of my personal care |
| I need help every day in most aspects of self care |
| I do not get dressed. I wash with difficulty and stay in bed |
| Section 3: Lifting |
| I can lift heavy weights without extra pain |
| I can lift heavy weights but it gives extra pain |
| Pain prevents me lifting heavy weights off the floor, but I can manage if they are conveniently placed, for example on a table |
| Pain prevents me from lifting heavy weights but I can manage light to medium weights if they are conveniently positioned |
| I can only lift very light weights |
| I cannot lift or carry anything |
| Section 4: Reading |
| I can read as much as I want to with no pain in my neck |
| I can read as much as I want to with slight pain in my neck |
| I can read as much as I want with moderate pain in my neck |
| I can’t read as much as I want because of moderate pain in my neck |
| I can hardly read at all because of severe pain in my neck |
| I cannot read at all |
| Section 5: Headaches |
| I have no headaches at all |
| I have slight headaches, which come infrequently |
| I have moderate headaches, which come infrequently |
| I have moderate headaches, which come frequently |
| I have severe headaches, which come frequently |
| I have headaches almost all the time |
| Section 6: Concentration |
| I can concentrate fully when I want to with no difficulty |
| I can concentrate fully when I want to with slight difficulty |
| I have a fair degree of difficulty in concentrating when I want to |
| I have a lot of difficulty in concentrating when I want to |
| I have a great deal of difficulty in concentrating when I want to |
| I cannot concentrate at all |
| Section 7: Work |
| I can do as much work as I want to |
| I can only do my usual work, but no more |
| I can do most of my usual work, but no more |
| I cannot do my usual work |
| I can hardly do any work at all |
| I can’t do any work at all |
| Section 8: Driving |
| I can drive my car without any neck pain |
| I can drive my car as long as I want with slight pain in my neck |
| I can drive my car as long as I want with moderate pain in my neck |
| I can’t drive my car as long as I want because of moderate pain in my neck |
| I can hardly drive at all because of severe pain in my neck |
| I can’t drive my car at all |
| Section 9: Sleeping |
| I have no trouble sleeping |
| My sleep is slightly disturbed (less than 1 h sleepless) |
| My sleep is mildly disturbed (1–2 h sleepless) |
| My sleep is moderately disturbed (2–3 h sleepless) |
| My sleep is greatly disturbed (3–5 h sleepless) |
| My sleep is completely disturbed (5–7 h sleepless) |
| Section 10: Recreation |
| I am able to engage in all my recreation activities with no neck pain at all |
| I am able to engage in all my recreation activities, with some pain in my neck |
| I am able to engage in most, but not all of my usual recreation activities because of pain in my neck |
| I am able to engage in a few of my usual recreation activities because of pain in my neck |
| I can hardly do any recreation activities because of pain in my neck |
| I can’t do any recreation activities at all |
| Score:___/150 Transform to percentage score x 100 = %points |
| Scoring: For each section the total possible score is 5: if the first statement is marked the section score = 0, if the last statement is marked it = 5. If all ten sections are completed the score is calculated as follows: Example: 16 (total scored)50 (total possible score) x 100 = 32% |
| If one section is missed or not applicable the score is calculated: Example: 16 (total scored) 45 (total possible score) x 100 = 35.5% |
| Minimum Detectable Change (90% confidence): 5 points or 10 %points |
| EQ-5D |
|---|
| By placing a checkmark in one box in each group below, please indicate which statements best describe your own health state today. |
| Mobility |
| I have no problems in walking about |
| I have some problems in walking about |
| I am confined to bed |
| Self-Care |
| I have no problems with self-care |
| I have some problems washing or dressing myself |
| I am unable to wash or dress myself |
| Usual Activities (e.g., work, study, housework, family or leisure activities) |
| I have no problems with performing my usual activities |
| I have some problems with performing my usual activities |
| I am unable to perform my usual activities |
| Pain/Discomfort |
| I have no pain or discomfort |
| I have moderate pain or discomfort |
| I have extreme pain or discomfort |
| Anxiety/Depression |
| I am not anxious or depressed |
| I am moderately anxious or depressed |
| I am extremely anxious or depressed |
| Nurick Grading System | |
|---|---|
| Grade. | Definition |
| 0 | Signs or symptoms of root involvement, but without evidence of spinal cord disease. |
| I | Signs of spinal cord disease, but no walking difficulty. |
| II | Slight difficulty in walking that does not prevent full- time employment. |
| III | Walking difficulty that prevents full-time employment or the ability to do all housework but is not so severe as to require help from another person to ambulate. |
| IV | Able to walk only with help from another person or with the aid of a frame. |
| V | Bedridden or chairbound. |
| European Myelopathy Score | ||
|---|---|---|
| Upper motor neuron | ||
| 1 | Unable to walk, wheelchair | |
| Gait function | ||
| 2 | Walking on a flat ground only with cane or aid | |
| 3 | Climbing stairs only with aid | |
| 4 | Gait clumsy, but no aid necessary | |
| 5 | Normal walking and climbing stairs | |
| Upper motor neuron | ||
| 1 | Retention, no control over bladder and/or bowel function | |
| Bladder and bowel function | ||
| 2 | Inadequate micturition and urinary frequency | |
| 3 | Normal bladder and bowel function | |
| Lower motor neuron | ||
| 1 | Handwriting and eating with knife and fork impossible | |
| Hand function | ||
| 2 | Handwriting and eating with knife and fork impaired | |
| 3 | Handwriting, tying shoe laces or a tie clumsy | |
| 4 | Normal handwriting | |
| Posterior column | ||
| 1 | Getting dressed only with aid | |
| Proprioception and coordination | ||
| 2 | Getting dressed clumsily and slowly | |
| 3 | Getting dressed normally | |
| Paraesthesia/pain | ||
| 1 | Invalidity due to pain | |
| 2 | Endurable paraesthesia and pain | |
| 3 | No paraesthesia and pain | |
| Normal function | 17–18 | |
| Grade 1 | 13–16 | |
| Grade 2 | 9–12 | |
| Grade 3 | 5–8 | |
| Cooper Myelopathy Scale | |
|---|---|
| Upper extremity function | |
| Grade 0 | Intact |
| Grade 1 | Sensory symptoms only |
| Grade 2 | Mild motor deficit with some functional impairment |
| Grade 3 | Major functional impairment in at least one upper extremity but upper extremities useful for simple tasks |
| Grade 4 | No movement or flicker of movement in upper extremities; no useful function |
| Lower extremity function | |
| Grade 0 | Intact |
| Grade 1 | Walks independently but not normally |
| Grade 2 | Walks but needs cane or walker |
| Grade 3 | Stands but cannot walk |
| Grade 4 | Slight movement but cannot walk or stand |
| Grade 5 | Paralysis |
Appendix B. Database Search Strategy
- EBM Reviews—ACP Journal Club 1991 to November 2021
- Embase 1974 to 3 December 2021
- MEDLINE(R) All including Epub Ahead of Print, In-Process and Other Non-Indexed Citations, Daily and Versions(R) 1946-current

Appendix C. Article Study Characteristics
| No. | Author(s) | Year | Title | Study Design | Follow-Up Period (Months) | Subjects | qMRI Technique | qMRI Parameters Tested |
|---|---|---|---|---|---|---|---|---|
| 1 | Maki, Satoshi; Koda, Masao; Kitamura, Mitsuhiro; Inada, Taigo; Kamiya, Koshiro; Ota, Mitsutoshi; Iijima, Yasushi; Saito, Junya; Masuda, Yoshitada; Matsumoto, Koji; Kojima, Masatoshi; Obata, Takayuki; Takahashi, Kazuhisa; Yamazaki, Masashi; Furuya, Takeo | 2017 | Diffusion tensor imaging can predict surgical outcomes of patients with cervical compression myelopathy | Prospective Longitudinal | 6 | DCM = 26 | DTI | FA, MD |
| 2 | Bhosale, Sunil; Ingale, Pramod; Srivastava, Sudhir; Marathe, Nandan; Bhide, Prajakta | 2019 | Diffusion tensor imaging as an additional postoperative prognostic predictor factor in cervical myelopathy patients: An observational study | Prospective Longitudinal | 3 | DCM = 30 | DTI | FA, MD |
| 3 | Song, Ting; Chen, Wen-Jun; Huang, Jian-Wei; Cai, Ming-Jin; Dong, Tian-Fa; Li, Tang-Sheng; Yang, Bo; Zhao, Hong-Pu | 2011 | Diffusion tensor imaging in the cervical spinal cord | Prospective Longitudinal | 6 | DCM = 53 Healthy Controls = 20 | DTI | FA, ADC |
| 4 | Severino, Rocco; Nouri, Aria; Tessitore, Enrico | 2020 | Degenerative cervical myelopathy: How to identify the best responders to surgery? | Prospective Longitudinal | 12 | DCM = 36 | DTI | FA |
| 5 | Nukala, Monika; Abraham, Jini; Khandige, Ganesh; Shetty, Bharath K.; Rao, Arindam pol arjun | 2019 | Efficacy of diffusion tensor imaging in identification of degenerative cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 50 | DTI | FA, ADC |
| 6 | Ulubaba, Hilal Er; Saglik, Semih; Yildirim, Ismail Okan; Durak, Mehmet Akif | 2021 | Effectiveness of Diffusion Tensor Imaging in Determining Cervical Spondylotic Myelopathy | Prospective Cross-sectional | N/A | DCM = 54 | DTI | FA, ADC |
| 7 | Tian, Xiaonan; Zhang, Li; Zhang, Xuesong; Meng, Linghui; Li, Xiaona | 2021 | Correlations between preoperative diffusion tensor imaging and surgical outcome in patients with cervical spondylotic myelopathy | Retrospective Longitudinal | 12 | DCM = 95 | DTI | FA, ADC |
| 8 | Iwasaki, Motoyuki; Yokohama, Takumi; Oura, Daisuke; Furuya, Shou; Niiya, Yoshimasa; Okuaki, Tomoyuki | 2019 | Decreased Value of Highly Accurate Fractional Anisotropy Using 3-Tesla ZOOM Diffusion Tensor Imaging After Decompressive Surgery in Patients with Cervical Spondylotic Myelopathy: Aligned Fibers Effect | Prospective Longitudinal | 6 | DCM = 26Healthy Controls = 12 | DTI | FA |
| 9 | Toktas, Zafer Orkun; Kilic, Turker; Konya, Deniz; Tanrikulu, Bahattin; Koban, Orkun | 2016 | Diffusion tensor imaging of cervical spinal cord: A quantitative diagnostic tool in cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 21 | DTI | FA, ADC |
| 10 | Ellingson, Benjamin M.; Salamon, Noriko; Grinstead, John W.; Holly, Langston T. | 2014 | Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 48Healthy Controls = 9 | DTI | FA, ADC, MD |
| 11 | Han, X.; Ma, X.; Li, D.; Wang, J.; Jiang, W.; Cheng, X.; Li, G.; Guo, H.; Tian, W. | 2020 | The Evaluation and Prediction of Laminoplasty Surgery Outcome in Patients with Degenerative Cervical Myelopathy Using Diffusion Tensor MRI | Prospective Longitudinal | 6 | DCM = 55Healthy Controls = 20 | DTI | FA, MD |
| 12 | Guo, Xing; Yang, Xiaotian; Chen, Xukang; Zhao, Rui; Song, Yingchao; Liang, Meng; Sun, Haoran; Xue, Yuan | 2021 | Enhanced Information Flow From Cerebellum to Secondary Visual Cortices Leads to Better Surgery Outcome in Degenerative Cervical Myelopathy Patients: A Stochastic Dynamic Causal Modeling Study With Functional Magnetic Resonance Imaging | Prospective Longitudinal | 6 | DCM = 27Healthy Controls = 11 | fMRI (BOLD) | Effective connectivity (EC) |
| 13 | Rajasekaran, S.; Kanna, Rishi M.; Chittode, Vishnuprasath S.; Maheswaran, Anupama; Aiyer, Siddharth N.; Shetty, Ajoy P. | 2017 | Efficacy of Diffusion Tensor Imaging Indices in Assessing Postoperative Neural Recovery in Cervical Spondylotic Myelopathy | Prospective Longitudinal | 12 | DCM = 26 | DTI | ADC |
| 14 | Liu, Xiaojia; Qian, Wenshu; Jin, Richu; Li, Xiang; Luk, Keith Dk; Wu, Ed X.; Hu, Yong | 2016 | Amplitude of Low Frequency Fluctuation (ALFF) in the Cervical Spinal Cord with Stenosis: A Resting State fMRI Study | Prospective Cross-sectional | N/A | DCM = 18Healthy Controls = 25 | fMRI (BOLD) | Amplitude of low frequency fluctuation (ALFF) |
| 15 | Cui, Jiao-Long; Li, Xiang; Chan, Tin-Yan; Mak, Kin-Cheung; Luk, Keith Dip-Kei; Hu, Yong | 2015 | Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography | Prospective Cross-sectional | N/A | DCM = 23Healthy Controls = 20 | DTI | FA, MD |
| 16 | Nischal, Neha; Tripathi, Shalini; Singh, Jatinder Pal | 2020 | Quantitative Evaluation of the Diffusion Tensor Imaging Matrix Parameters and the Subsequent Correlation with the Clinical Assessment of Disease Severity in Cervical Spondylotic Myelopathy | Prospective Cross-sectional | N/A | DCM = 52 | DTI | FA, ADC |
| 17 | Peng, Xinji; Tan, Yongming; He, Laichang; Ou, Yangtao | 2020 | Alterations of functional connectivity between thalamus and cortex before and after decompression in cervical spondylotic myelopathy patients: A resting-state functional MRI study | Prospective Longitudinal | 3 | DCM = 43Healthy Controls = 43 | fMRI (BOLD) | BOLD signal |
| 18 | Tan, Yongming; Zhou, Fuqing; Liu, Zhili; Wu, Lin; Zeng, Xianjun; Gong, Honghan; He, Laichang | 2016 | Alteration of cerebral regional homogeneity within sensorimotor network in patients with cervical spondylotic myelopathy after spinal cord decompression: a resting-state functional MRI study | Prospective Longitudinal | 3 | DCM = 21Healthy Controls = 21 | fMRI (BOLD) | Regional homogeneity (ReHo) |
| 19 | Kowalczyk, Izabela; Bartha, Robert; Duggal, Neil | 2012 | Proton magnetic resonance spectroscopy of the motor cortex in cervical myelopathy | Prospective Cross-sectional | N/A | DCM = 24Healthy Controls = 11 | MRS | N-acetylaspartate/creatine |
| 20 | Lee, Seungbo; Chung, Tae-Sub; Kim, Sungjun; Yoo, Yeon Hwa; Yoon, Choon-Sik; Lee, Young Han; Suh, Jin-Suck; Jeong, Eun-Kee; Kim, In Seong; Park, Jung Hyun | 2015 | Accuracy of diffusion tensor imaging for diagnosing cervical spondylotic myelopathy in patients showing spinal cord compression | Prospective Cross-sectional | N/A | DCM = 33 | DTI | FA, MD |
| 21 | Wang, K.Y.; Idowu, O.; Orman, G.; Izbudak, I.; Thompson, C.B.; Myers, C.; Riley, L.H.; Carrino, J.A.; Flammang, A.; Gilson, W.; Sadowsky, C.L. | 2017 | Tract-Specific Diffusion Tensor Imaging in Cervical Spondylotic Myelopathy Before and After Decompressive Spinal Surgery: Preliminary Results | Prospective Longitudinal | 6 | DCM = 4Healthy Controls = 5 | DTI | FA, MD |
| 22 | Shabani, Saman; Kaushal, Mayank; Budde, Matthew; Schmit, Brian; Wang, Marjorie C.; Kurpad, Shekar | 2019 | Comparison between quantitative measurements of diffusion tensor imaging and T2 signal intensity in a large series of cervical spondylotic myelopathy patients for assessment of disease severity and prognostication of recovery | Prospective Longitudinal | 24 | DCM = 46 | DTI | FA |
| 23 | Duggal, N.; Rabin, D.; Bartha, R.; Barry, R.L.; Gati, J.S.; Kowalczyk, I.; Fink, M. | 2010 | Brain reorganization in patients with spinal cord compression evaluated using fMRI | Prospective Longitudinal | 6 | DCM = 12Healthy Controls = 10 | fMRI (BOLD) | Volume of Activation (VOA) |
| 24 | Jurova, Barbora; Mechl, Marek; Kerkovsky, Milos; Sprlakova-Pukova, Andrea; Kadanka, Zdenek; Nemec, Martin; Bednarik, Josef; Kovalova, Ivana; Dusek, Ladislav | 2017 | Spinal Cord MR Diffusion Properties in Patients with Degenerative Cervical Cord Compression | Prospective Cross-sectional | N/A | DCM = 130Healthy Controls = 71 | DTI | FA, ADC |
| 25 | Kara, Batuhan; Celik, Azim; Karadereler, Selhan; Ulusoy, Levent; Ganiyusufoglu, Kursat; Onat, Levent; Mutlu, Ayhan; Ornek, Ibrahim; Sirvanci, Mustafa; Hamzaoglu, Azmi | 2011 | The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI | Prospective Cross-sectional | N/A | DCM = 16 | DTI | FA, ADC |
| 26 | Maki, Satoshi; Koda, Masao; Ota, Mitsutoshi; Oikawa, Yoshihiro; Kamiya, Koshiro; Inada, Taigo; Furuya, Takeo; Takahashi, Kazuhisa; Masuda, Yoshitada; Matsumoto, Koji; Kojima, Masatoshi; Obata, Takayuki; Yamazaki, Masashi | 2018 | Reduced Field-of-View Diffusion Tensor Imaging of the Spinal Cord Shows Motor Dysfunction of the Lower Extremities in Patients with Cervical Compression Myelopathy | Prospective Cross-sectional | N/A | DCM = 20Healthy Controls = 10 | DTI | FA |
| 27 | Hassan, Talaat Ahmed Abd El Hameed; Assad, Ramy Edward; Belal, Shaimaa Atef | 2019 | MR diffusion tensor imaging of the spinal cord: can it help in early detection of cervical spondylotic myelopathy and assessment of its severity? | Prospective Cross-sectional | N/A | DCM = 30 | DTI | FA |
| 28 | Cloney, Michael Brendan; Smith, Zachary A.; Weber, Kenneth A.; Parrish, Todd B. | 2018 | Quantitative Magnetization Transfer MRI Measurements of the Anterior Spinal Cord Region are Associated with Clinical Outcomes in Cervical Spondylotic Myelopathy | Prospective Cross-sectional | N/A | DCM = 7Healthy Controls = 7 | MT | MTR |
| 29 | Salamon, Noriko; Woodworth, Davis C.; Holly, Langston T.; Ellingson, Benjamin M. | 2018 | Resting-State Functional Magnetic Resonance Imaging Connectivity of the Brain Is Associated with Altered Sensorimotor Function in Patients with Cervical Spondylosis | Prospective Cross-sectional | N/A | DCM = 24Healthy Controls = 17 | fMRI (BOLD) | Functional Connectivity (FC) |
| 30 | Wang, Chencai; Salamon, Noriko; Laiwalla, Azim; Holly, Langston T.; Ellingson, Benjamin M.; Islam, Sabah | 2021 | Supraspinal functional and structural plasticity in patients undergoing surgery for degenerative cervical myelopathy | Prospective Longitudinal | 3 | DCM = 19Healthy Controls = 16 | fMRI (BOLD) | Functional Connectivity (FC) |
| 31 | Baucher, G.; Rasoanandrianina, H.; Levy, S.; Pini, L.; Troude, L.; Roche, P. H.; Callot, V. | 2021 | T1 Mapping for Microstructural Assessment of the Cervical Spinal Cord in the Evaluation of Patients with Degenerative Cervical Myelopathy | Prospective Cross-sectional | N/A | DCM = 20Healthy Controls = 10 | Quantitative T1 | T1 |
| 32 | Banaszek, Anna; Bladowska, Joanna; Szewczyk, Pawel; Podgorski, Przemyslaw; Sasiadek, Marek | 2014 | Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease | Prospective Cross-sectional | N/A | DCM = 132Healthy Controls = 25 | DTI | FA, ADC |
| 33 | Ellingson, Benjamin M.; Salamon, Noriko; Hardy, Anthony J.; Holly, Langston T. | 2015 | Prediction of Neurological Impairment in Cervical Spondylotic Myelopathy using a Combination of Diffusion MRI and Proton MR Spectroscopy | Prospective Cross-sectional | N/A | DCM = 27Healthy Controls = 11 | DTI, MRS | FA, MD, Cho/NAA (Choline/N-acetylaspartate) |
| 34 | Salamon, N.; Ellingson, B.M.; Nagarajan, R.; Gebara, N.; Thomas, A.; Holly, L.T. | 2013 | Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T | Prospective Cross-sectional | N/A | DCM = 21Healthy Controls = 11 | MRS | NAA (N-acetylaspartate), Cho (choline), Myo-I (myo-inositol) ratio with Cr (creatine) |
| 35 | Chen, Zhao; Zhao, Rui; Wang, Qiu; Yu, Chunshui; Li, Fengtan; Liang, Meng; Zong, Yaqi; Zhao, Ying; Xiong, Wuyi; Su, Zhe; Xue, Yuan | 2020 | Functional Connectivity Changes of the Visual Cortex in the Cervical Spondylotic Myelopathy Patients: A Resting-State fMRI Study | Prospective Longitudinal | 3 | DCM = 30Healthy Controls = 20 | fMRI (BOLD) | Functional Connectivity (FC) |
| 36 | Bhagavatula, Indira Devi; Shukla, Dhaval; Sadashiva, Nishanth; Saligoudar, Praveen; Prasad, Chandrajit; Bhat, Dhananjaya I. | 2016 | Functional cortical reorganization in cases of cervical spondylotic myelopathy and changes associated with surgery | Prospective Longitudinal | 6 | DCM = 17Healthy Controls = 12 | fMRI (BOLD) | Volume of Activation (VOA) |
| 37 | Murphy, Rory K.; Sun, Peng; Han, Rowland H.; Griffin, Kim J.; Wagner, Joanne; Yarbrough, Chester K.; Wright, Neill M.; Dorward, Ian G.; Riew, K. Daniel; Kelly, Michael P.; Santiago, Paul; Zebala, Lukas P.; Trinkaus, Kathryn; Ray, Wilson Z.; Song, Sheng-Kwei | 2018 | Fractional anisotropy to quantify cervical spondylotic myelopathy severity | Prospective Cross-sectional | N/A | DCM = 14Healthy Controls = 7 | DTI | FA |
| 38 | Takenaka, Shota; Kan, Shigeyuki; Seymour, Ben; Makino, Takahiro; Sakai, Yusuke; Kushioka, Junichi; Tanaka, Hisashi; Watanabe, Yoshiyuki; Shibata, Masahiko; Yoshikawa, Hideki; Kaito, Takashi | 2020 | Resting-state Amplitude of Low-frequency Fluctuation is a Potentially Useful Prognostic Functional Biomarker in Cervical Myelopathy | Prospective Longitudinal | 6 | DCM = 28Healthy Controls = 28 | fMRI (BOLD) | Amplitude of low frequency fluctuation (ALFF) |
| 39 | Cui, Libin; Chen, Xueming; Liu, Yadong; Zhang, Yanjun; Kong, Chao; Guan, Yun | 2019 | Changes in diffusion tensor imaging indices of the lumbosacral enlargement correlate with cervical spinal cord changes and clinical assessment in patients with cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 40Healthy Controls = 42 | DTI | FA, ADC |
| 40 | Holly, Langston T.; Wang, Chencai; Salamon, Noriko; Woodworth, Davis C.; Ellingson, Benjamin M. | 2019 | Neck disability in patients with cervical spondylosis is associated with altered brain functional connectivity | Prospective Cross-sectional | N/A | DCM = 36Healthy Controls = 17 | fMRI (BOLD) | Functional Connectivity (FC) |
| 41 | Grabher, Patrick; David, Gergely; Mohammadi, Siawoosh; Freund, Patrick | 2017 | Neurodegeneration in the Spinal Ventral Horn Prior to Motor Impairment in Cervical Spondylotic Myelopathy | Prospective Cross-sectional | N/A | DCM = 20Healthy Controls = 18 | DTI | MD |
| 42 | Kerkovsky, M.; Jakubcova, B.; Mechl, M.; Kadanka, Z.; Kadanka Jr, Z.; Nemec, M.; Kovalova, I.; Bednarik, J. | 2015 | Multifactorial determination of the spinal cord diffusion properties in patients with cervical spondylotic spinal cord compression: A diffusion tensor imaging study | Prospective Cross-sectional | N/A | DCM = 130Healthy Controls = 71 | DTI | FA, ADC |
| 43 | Kowalczyk, I.; Bartha, R.; Duggal, N. | 2010 | Proton magnetic resonance spectroscopy of the motor cortex in cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 24Healthy Controls = 11 | MRS | NAA/Cr (N-acetylaspartate/creatine metabolite ratio) |
| 44 | Taha Ali, Tamer F.; Badawy, Ahmed E. | 2013 | Feasibility of 1H-MR Spectroscopy in evaluation of cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 34Healthy Controls = 11 | MRS | NAA/Cr (N-acetylaspartate/creatine metabolite ratio), Cho/Cr (Chloline/creatine ratio) |
| 45 | Aleksanderek, Izabela K.; Stevens, Todd; Goncalves, Sandy; Bartha, Robert; Duggal, Neil | 2017 | Metabolite and functional profile of patients with cervical spondylotic myelopathy | Prospective Longitudinal | 6 | DCM = 28Healthy Controls = 10 | fMRI (BOLD), MRS | Volume of Activation (VOA), NAA/Cr (N-acetylaspartate/creatine metabolite ratio) |
| 46 | Wen, Chun Yi; Cui, Jiao Long; Liu, Harris S.; Mak, Kin Cheung; Cheung, Wai Yuen; Luk, Keith D.K.; Hu, Yong | 2014 | Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy | Prospective Longitudinal | 6 to 24 | DCM = 45Healthy Controls = 20 | DTI | FA |
| 47 | Paliwal, Monica; Smith, Zachary A.; Weber, Kenneth A.; Mackey, Sean; Hopkins, Benjamin S.; Dahdaleh, Nader S.; Cantrell, Donald R.; Parrish, Todd D.; Hoggarth, Mark A.; Elliott, James M.; Dhaher, Yasin | 2020 | Magnetization Transfer Ratio and Morphometrics of the Spinal Cord Associates with Surgical Recovery in Patients with Degenerative Cervical Myelopathy | Prospective Longitudinal | 6 | DCM = 13Healthy Controls = 9 | MT | MTR |
| 48 | Martin, Allan R.; De Leener, Benjamin; Cohen-Adad, Julien; Kalsi-Ryan, Sukhvinder; Cadotte, David W.; Wilson, Jefferson R.; Tetreault, Lindsay; Nouri, Aria; Crawley, Adrian; Mikulis, David J.; Ginsberg, Howard; Massicotte, Eric M.; Fehlings, Michael G. | 2018 | Monitoring for myelopathic progression with multiparametric quantitative MRI | Prospective Longitudinal | 12 | DCM = 26 | DTI, MT | FA, MTR |
| 49 | Chen, Xueming; Kong, Chao; Feng, Shiqing; Guan, Hua; Yu, Zhenshan; Cui, Libin; Wang, Yanhui | 2016 | Magnetic resonance diffusion tensor imaging of cervical spinal cord and lumbosacral enlargement in patients with cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 10Healthy Controls = 10 | DTI | FA, ADC |
| 50 | Suleiman, Linda I.; Rosenthal, Brett D.; Bhatt, Surabhi A.; Hsu, Wellington K.; Patel, Alpesh A.; Parrish, Todd B.; Savage, Jason W.; Weber, Kenneth A. | 2018 | High-resolution magnetization transfer MRI in patients with cervical spondylotic myelopathy | Prospective Cross-sectional | N/A | DCM = 10Healthy Controls = 7 | MT | MTR |
| 51 | Nagashima, Hideki; Nanjo, Yoshiro; Teshima, Ryota; Morio, Yasuo; Meshitsuka, Shunsuke; Yamane, Koji | 2010 | High-resolution nuclear magnetic resonance spectroscopic study of metabolites in the cerebrospinal fluid of patients with cervical myelopathy and lumbar radiculopathy | Prospective Cross-sectional | N/A | DCM = 30Healthy Controls = 10 | MRS | Lactate, alanine, acetate, glutamate, pyruvate, citrate |
| 52 | Su, Qian; Zhao, Rui; Guo, Xing; Wang, ShuoWen; Tu, HaoYang; Yang, Fan | 2021 | Identification and Therapeutic Outcome Prediction of Cervical Spondylotic Myelopathy Based on the Functional Connectivity From Resting-State Functional MRI Data: A Preliminary Machine Learning Study | Retrospective Longitudinal | 6 | DCM = 53Healthy Controls = 47 | fMRI (BOLD) | Functional Connectivity (FC) |
| 53 | Yang, Young-Mi; Oh, Jae-Keun; Song, Ji-Sun; Yoo, Woo-Kyoung; Yoo, Je Hyun; Kwak, Yoon Hae; Kim, Seok Woo | 2017 | The functional relevance of diffusion tensor imaging in comparison to conventional MRI in patients with cervical compressive myelopathy | Prospective Cross-sectional | N/A | DCM = 20 | DTI | FA, ADC |
| 54 | Zhang, Meng-Ze; Liu, Jian-Fang; Jin, Dan; Wang, Chun-Jie; Zhao, Qiang; Lang, Ning; Yuan, Hui-Shu; Ou-Yang, Han-Qiang; Liu, Xiao-Guang; Liu, Zhong-Jun; Jiang, Liang; Zhang, Xian-Chang | 2021 | Utility of Advanced DWI in the Detection of Spinal Cord Microstructural Alterations and Assessment of Neurologic Function in Cervical Spondylotic Myelopathy Patients | Retrospective Longitudinal | 3 | DCM = 48Healthy Controls = 36 | DTI | FA |
| 55 | Xiangshui, M.; Xiangjun, C.; Xiaoming, Z.; Qingshi, Z.; Yi, C.; Chuanqiang, Q.; Xiangxing, M.; Chuanfu, L.; Jinwen, H. | 2010 | 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy | Prospective Cross-sectional | N/A | DCM = 84Healthy Controls = 21 | DTI | FA, ADC |
| 56 | He, Zhen; Wang, Nan; Kang, Liqing; Cui, Jiaolong; Wan, Yeda | 2020 | Analysis of pathological parameters of cervical spondylotic myelopathy using magnetic resonance imaging | Prospective Cross-sectional | N/A | DCM = 31Healthy Controls = 8 | DTI | FA |
| 57 | Mamata, Hatsuho; Jolesz, Ferenc A.; Maier, Stephan E. | 2005 | Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes | Prospective Cross-sectional | N/A | DCM = 79Healthy Controls = 11 | DTI | FA, ADC |
| 58 | Zheng, Weipeng; Chen, Haoyi; Wang, Ning; Jiang, Xin; Liang, YingJie; Xiao, Wende; Zhong, Bofu; Ju, Hongbin; Luo, Junnan; Wen, Shifeng; Xiong, Weifeng | 2018 | Application of Diffusion Tensor Imaging Cutoff Value to Evaluate the Severity and Postoperative Neurologic Recovery of Cervical Spondylotic Myelopathy | Retrospective Longitudinal | 12 to 24 | DCM = 61 | DTI | ADC, MD |
| 59 | Kanchiku, T.; Imajo, Y.; Suzuki, H.; Yoshida, Y.; Nishida, N.; Taguchi, T.; Suetomi, Y.; Nishijima, S. | 2016 | Application of diffusion tensor imaging for the diagnosis of segmental level of dysfunction in cervical spondylotic myelopathy | Retrospective Cross-sectional | N/A | DCM = 10Healthy Controls = 11 | DTI | FA, ADC |
| 60 | Uda, Takehiro; Takami, Toshihiro; Tsuyuguchi, Naohiro; Sakamoto, Shinichi; Yamagata, Toru; Ikeda, Hidetoshi; Nagata, Takashi; Ohata, Kenji | 2013 | Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla | Prospective Cross-sectional | N/A | DCM = 26Healthy Controls = 30 | DTI | FA, MD |
| 61 | Rajasekaran, S.; Kanna, Rishi M.; Balamurali, Gopalakrishnan; Shetty, Ajoy Prasad; Yerramshetty, Janardhan S.; Chittode, Vishnuprasath S. | 2014 | The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging | Prospective Cross-sectional | N/A | DCM = 35Healthy Controls = 40 | DTI | ADC |
| 62 | Maier, Ilko L; Hofer, Sabine; Eggert, Eva; Schregel, Katharina; Psychogios, Marios-Nikos; Frahm, Jens; Bähr, Mathias; Liman, Jan | 2020 | T1 Mapping Quantifies Spinal Cord Compression in Patients With Various Degrees of Cervical Spinal Canal Stenosis | Prospective Cross-sectional | N/A | DCM = 31Healthy Controls = 10 | Quantitative T1 | T1 |
| 63 | Albistegui-Dubois, Richard; Marehbian, Jonathan; Newton, Jennifer M.; Dong, Yun; Holly, Langston T.; Yan, Xiaohong; Dobkin, Bruce H. | 2008 | Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy: Laboratory investigation | Prospective Longitudinal | 6 | DCM = 8Healthy Controls = 6 | fMRI (BOLD) | Volume of Activation (VOA) |
| 64 | Hori, Masaaki; Fukunaga, Issei; Masutani, Yoshitaka; Nakanishi, Atsushi; Shimoji, Keigo; Kamagata, Koji; Asahi, Koichi; Hamasaki, Nozomi; Suzuki, Yuriko; Aoki, Shigeki | 2012 | New diffusion metrics for spondylotic myelopathy at an early clinical stage | Prospective Cross-sectional | N/A | DCM = 50 | DTI | FA, ADC |
| 65 | Vedantam, Aditya; Rao, Avinash; Kurpad, Shekar N.; Jirjis, Michael B.; Eckardt, Gerald; Schmit, Brian D.; Wang, Marjorie C. | 2017 | Diffusion Tensor Imaging Correlates with Short-Term Myelopathy Outcome in Patients with Cervical Spondylotic Myelopathy | Prospective Longitudinal | 3 | DCM = 27 | DTI | FA |
| 66 | Wang, Kun; Chen, Zhi; Shen, Hongxing; Zhang, Fan; Song, Qingxin; Hou, Canglong; Tang, Yixing; Wang, Jun; Chen, Shiyue; Bian, Yun; Hao, Qiang | 2017 | Evaluation of DTI Parameter Ratios and Diffusion Tensor Tractography Grading in the Diagnosis and Prognosis Prediction of Cervical Spondylotic Myelopathy | Prospective Longitudinal | 12 | DCM = 93Healthy Controls = 36 | DTI | FA, ADC |
| 67 | Sato, T.; Horikoshi, T.; Watanabe, A.; Uchida, M.; Ishigame, K.; Araki, T.; Kinouchi, H. | 2012 | Evaluation of cervical myelopathy using apparent diffusion coefficient measured by diffusion-weighted imaging | Prospective Longitudinal | 6 | DCM = 66 | DTI | ADC |
| 68 | Takenaka, Shota; Kan, Shigeyuki; Seymour, Ben; Makino, Takahiro; Sakai, Yusuke; Kushioka, Junichi; Tanaka, Hisashi; Watanabe, Yoshiyuki; Shibata, Masahiko; Yoshikawa, Hideki; Kaito, Takashi | 2019 | Towards prognostic functional brain biomarkers for cervical myelopathy: A resting-state fMRI study | Prospective Longitudinal | 6 | DCM = 28Healthy Controls = 28 | fMRI (BOLD) | Functional Connectivity (FC) |
References
- The Lancet Neurology. A focus on patient outcomes in cervical myelopathy. Lancet Neurol. 2019, 18, 615. [Google Scholar] [CrossRef]
- Tracy, J.A.; Bartleson, J. Cervical spondylotic myelopathy. Neurology 2010, 16, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Ogata, H.; Tokuhiro, A.; Takechi, H. Spinal cord injuries in Okayama Prefecture: An epidemiological study 88–89. J. UOEH 1993, 15, 209–215. [Google Scholar] [CrossRef] [PubMed]
- McKinley, W.O.; Seel, R.T.; Hardman, J.T. Nontraumatic spinal cord injury: Incidence, epidemiology, and functional outcome. Arch. Phys. Med. Rehabil. 1999, 80, 619–623. [Google Scholar] [CrossRef]
- New, P.W. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: Results from a retrospective study. Arch. Phys. Med. Rehabil. 2005, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- New, P.W.; Rawicki, H.B.; Bailey, M.J. Nontraumatic spinal cord injury: Demographic characteristics and complications. Arch. Phys. Med. Rehabil. 2002, 83, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- New, P.W.; Cripps, R.A.; Lee, B.B. Global maps of non-traumatic spinal cord injury epidemiology: Towards a living data repository. Spinal Cord 2014, 52, 97–109. [Google Scholar] [CrossRef]
- Biering-Sørensen, F.; Pedersen, V.; Clausen, S. Epidemiology of spinal cord lesions in Denmark. Spinal Cord 1990, 28, 105–118. [Google Scholar] [CrossRef]
- Ronen, J.; Goldin, D.; Bluvshtein, V.; Fishel, B.; Gelernter, I.; Catz, A. Survival after nontraumatic spinal cord lesions in Israel. Arch. Phys. Med. Rehabil. 2004, 85, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Catz, A.; Goldin, D.; Fishel, B.; Ronen, J.; Bluvshtein, V.; Gelernter, I. Recovery of neurologic function following nontraumatic spinal cord lesions in Israel. Spine 2004, 29, 2278–2282. [Google Scholar] [CrossRef][Green Version]
- Citterio, A.; Franceschini, M.; Spizzichino, L.; Reggio, A.; Rossi, B.; Stampacchia, G.; Mielolesioni, G.I.S.E. Nontraumatic spinal cord injury: An Italian survey. Arch. Phys. Med. Rehabil. 2004, 85, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Scivoletto, G.; Farchi, S.; Laurenza, L.; Molinari, M. Traumatic and non-traumatic spinal cord lesions: An Italian comparison of neurological and functional outcomes. Spinal Cord 2011, 49, 391–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schönherr, M.; Groothoff, J.; Mulder, G.; Eisma, W. Rehabilitation of patients with spinal cord lesions in The Netherlands: An epidemiological study. Spinal Cord 1996, 34, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; Fulford, G.; Harris, P.; Jellinek, E.; Kerr, W.; Kirkland, I.; Newsam, J.; Stark, G. A preliminary survey of the incidence and aetiology of spinal paralysis. Spinal Cord 1972, 10, 23–28. [Google Scholar] [CrossRef] [PubMed]
- New, P.W.; Farry, A.; Baxter, D.; Noonan, V. Prevalence of non-traumatic spinal cord injury in Victoria, Australia. Spinal Cord 2013, 51, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Tetreault, L.; Singh, A.; Karadimas, S.K.; Fehlings, M.G. Degenerative cervical myelopathy: Epidemiology, genetics, and pathogenesis. Spine 2015, 40, E675–E693. [Google Scholar] [CrossRef]
- Tu, J.; Vargas Castillo, J.; Das, A.; Diwan, A.D. Degenerative Cervical Myelopathy: Insights into Its Pathobiology and Molecular Mechanisms. J. Clin. Med. 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Badhiwala, J.H.; Witiw, C.D.; Nassiri, F.; Akbar, M.A.; Mansouri, A.; Wilson, J.R.; Fehlings, M.G. Efficacy and safety of surgery for mild degenerative cervical myelopathy: Results of the AOSpine North America and international prospective multicenter studies. Neurosurgery 2019, 84, 890–897. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Wilson, J.R. The natural history of degenerative cervical myelopathy. Neurosurg. Clin. 2018, 29, 21–32. [Google Scholar] [CrossRef]
- Nakamura, K.; Kurokawa, T.; Hoshino, Y.; Saita, K.; Takeshita, K.; Kawaguchi, H. Conservative treatment for cervical spondylotic myelopathy: Achievement and sustainability of a level of “no disability”. J. Spinal Disord. 1998, 11, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Robinson, P.K. Cervical myelopathy: A complication of cervical spondylosis. Brain 1956, 79, 483–510. [Google Scholar] [CrossRef] [PubMed]
- Bednarík, J.; Kadanka, Z.; Vohánka, S.; Stejskal, L.; Vlach, O.; Schröder, R. The value of somatosensory-and motor-evoked potentials in predicting and monitoring the effect of therapy in spondylotic cervical myelopathy: Prospective randomized study. Spine 1999, 24, 1593. [Google Scholar] [CrossRef] [PubMed]
- Kadanka, Z.; Mareš, M.; Bednarík, J.; Smrcka, V.; Krbec, M.; Stejskal, L.; Chaloupka, R.; Dagmar, S.; Novotný, O.; Urbánek, I. Approaches to spondylotic cervical myelopathy: Conservative versus surgical results in a 3-year follow-up study. Spine 2002, 27, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Kadaňka, Z.; Bednařík, J.; Novotný, O.; Urbánek, I.; Dušek, L. Cervical spondylotic myelopathy: Conservative versus surgical treatment after 10 years. Eur. Spine J. 2011, 20, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Kadaňka, Z.; Bednařík, J.; Voháňka, S.; Vlach, O.; Stejskal, L.; Chaloupka, R.; Filipovičová, D.; Šurelová, D.; Adamová, B.; Novotný, O. Conservative treatment versus surgery in spondylotic cervical myelopathy: A prospective randomised study. Eur. Spine J. 2000, 9, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Kadaňka, Z.; Mareš, M.; Bednařík, J.; Smrčka, V.; Krbec, M.; Chaloupka, R.; Dušek, L. Predictive factors for mild forms of spondylotic cervical myelopathy treated conservatively or surgically. Eur. J. Neurol. 2005, 12, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Toyama, Y.; Ishikawa, M.; Chiba, K.; Suzuki, N.; Fujimura, Y. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy: Does it predict the outcome of conservative treatment? Spine 2000, 25, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.; Bendebba, M.; Davis, J.D.; Ducker, T.B. Outcome of patients treated for cervical myelopathy: A prospective, multicenter study with independent clinical review. Spine 2000, 25, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Chiba, K.; Ishikawa, M.; Maruiwa, H.; Fujimura, Y.; Toyama, Y. Relationships between outcomes of conservative treatment and magnetic resonance imaging findings in patients with mild cervical myelopathy caused by soft disc herniations. Spine 2001, 26, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Nagata, K.; Goto, H.; Sonoda, K.; Ando, N.; Imoto, H.; Mashima, T.; Takamiya, Y. Conservative treatment for cervical spondylotic myelopathy: Prediction of treatment effects by multivariate analysis. Spine J. 2001, 1, 269–273. [Google Scholar] [CrossRef]
- Sumi, M.; Miyamoto, H.; Suzuki, T.; Kaneyama, S.; Kanatani, T.; Uno, K. Prospective cohort study of mild cervical spondylotic myelopathy without surgical treatment. J. Neurosurg. Spine 2012, 16, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Sumi, M.; Nishida, K.; Maeno, K.; Tadokoro, K.; Miyamoto, H.; Kurosaka, M.; Doita, M. Prognostic factors for deterioration of patients with cervical spondylotic myelopathy after nonsurgical treatment. Spine 2007, 32, 2474–2479. [Google Scholar] [CrossRef]
- Oshima, Y.; Seichi, A.; Takeshita, K.; Chikuda, H.; Ono, T.; Baba, S.; Morii, J.; Oka, H.; Kawaguchi, H.; Nakamura, K. Natural course and prognostic factors in patients with mild cervical spondylotic myelopathy with increased signal intensity on T2-weighted magnetic resonance imaging. Spine 2012, 37, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Tetreault, L.A.; Chapman, J.R.; Wilson, J.R.; Smith, J.S.; Martin, A.R.; Dettori, J.R.; Fehlings, M.G. Nonoperative versus operative management for the treatment degenerative cervical myelopathy: An updated systematic review. Glob. Spine J. 2017, 7, 35S–41S. [Google Scholar] [CrossRef]
- Karadimas, S.; Erwin, W.; Ely, C.; Dettori, J.; Fehlings, M. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine 2013, 38, S21–S36. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, L.A.; Karadimas, S.; Wilson, J.R.; Arnold, P.M.; Kurpad, S.; Dettori, J.R.; Fehlings, M.G. The natural history of degenerative cervical myelopathy and the rate of hospitalization following spinal cord injury: An updated systematic review. Glob. Spine J. 2017, 7, 28S–34S. [Google Scholar] [CrossRef] [PubMed]
- Kalsi-Ryan, S.; Karadimas, S.K.; Fehlings, M.G. Cervical spondylotic myelopathy: The clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 2013, 19, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, L.; Goldstein, C.L.; Arnold, P.; Harrop, J.; Hilibrand, A.; Nouri, A.; Fehlings, M.G. Degenerative cervical myelopathy: A spectrum of related disorders affecting the aging spine. Neurosurgery 2015, 77, S51–S67. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Munro, C.F.; Kotter, M.R. A novel insight into the challenges of diagnosing degenerative cervical myelopathy using web-based symptom Checkers. J. Med. Internet Res. 2019, 21, e10868. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; Mowforth, O.D.; Smith, E.K.; Kotter, M.R. Degenerative cervical myelopathy. BMJ 2018, 360, k186. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, L.; Kopjar, B.; Nouri, A.; Arnold, P.; Barbagallo, G.; Bartels, R.; Qiang, Z.; Singh, A.; Zileli, M.; Vaccaro, A. The modified Japanese Orthopaedic Association scale: Establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur. Spine J. 2017, 26, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Harrop, J.S.; Naroji, S.; Maltenfort, M.; Anderson, D.G.; Albert, T.; Ratliff, J.K.; Ponnappan, R.K.; Rihn, J.A.; Smith, H.E.; Hilibrand, A. Cervical myelopathy: A clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine 2010, 35, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Azad, T.D.; Tharin, S. Cervical spondylotic myelopathy. Clin. Spine Surg. 2016, 29, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Wilhelm, M.; Cook, A.E.; Petrosino, C.; Isaacs, R. Clinical tests for screening and diagnosis of cervical spine myelopathy: A systematic review. J. Manip. Physiol. Ther. 2011, 34, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.M.; McHugh, M.; Elgheriani, A.; Kolias, A.G.; Tetreault, L.A.; Hutchinson, P.J.; Fehlings, M.G.; Kotter, M.R. Reported outcome measures in degenerative cervical myelopathy: A systematic review. PLoS ONE 2016, 11, e0157263. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, B.; Tetreault, L.; Kalsi-Ryan, S.; Fehlings, M. Psychometric properties of the modified Japanese Orthopaedic Association scale in patients with cervical spondylotic myelopathy. Spine 2015, 40, E23–E28. [Google Scholar] [CrossRef]
- Revanappa, K.K.; Rajshekhar, V. Comparison of Nurick grading system and modified Japanese Orthopaedic Association scoring system in evaluation of patients with cervical spondylotic myelopathy. Eur. Spine J. 2011, 20, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Vitzthum, H.-E.; Dalitz, K. Analysis of five specific scores for cervical spondylogenic myelopathy. Eur. Spine J. 2007, 16, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Herdmann, J.; Linzbach, M.; Krzan, M.; Dvorak, J.; Bock, W. The European myelopathy score. In Cerebellar Infarct. Midline Tumors. Minimally Invasive Endoscopic Neurosurgery (MIEN); Springer: Berlin/Heidelberg, Germany, 1994; pp. 266–268. [Google Scholar]
- Lebl, D.R.; Hughes, A.; Cammisa, F.P., Jr.; O’leary, P.F. Cervical spondylotic myelopathy: Pathophysiology, clinical presentation, and treatment. HSS J. 2011, 7, 170–178. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Casey, A.; Laing, R.; Statham, P.; Fehlings, M.G. A summary of assessment tools for patients suffering from cervical spondylotic myelopathy: A systematic review on validity, reliability and responsiveness. Eur. Spine J. 2015, 24, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.C.; Craven, B.C. Psychometric analysis and critical appraisal of the original, revised, and modified versions of the Japanese Orthopaedic Association score in the assessment of patients with cervical spondylotic myelopathy. Neurosurg. Focus 2016, 40, E6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, Y.; Sun, Y.; Zhang, F.; Pan, S.; Liu, Z. Assessment of the minimum clinically important difference in neurological function and quality of life after surgery in cervical spondylotic myelopathy patients: A prospective cohort study. Eur. Spine J. 2015, 24, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Crockard, H. Comparison of seven different scales used to quantify severity of cervical spondylotic myelopathy and post-operative improvement. J. Outcome Meas. 2001, 5, 798–818. [Google Scholar] [PubMed]
- Iohom, G. Chapter 11—Clinical Assessment of Postoperative Pain. In Postoperative Pain Management; Shorten, G., Carr, D.B., Harmon, D., Puig, M.M., Browne, J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2006; pp. 102–108. [Google Scholar]
- Balestroni, G.; Bertolotti, G. EuroQol-5D (EQ–5D): An instrument for measuring quality of life. Monaldi Arch. Chest Dis. 2012, 78, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Martin, A.R.; Mikulis, D.; Fehlings, M.G. Magnetic resonance imaging assessment of degenerative cervical myelopathy: A review of structural changes and measurement techniques. Neurosurg. Focus 2016, 40, E5. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Kiyonaga, K.; Ohashi, T.; Sagara, M.; Miyazaki, S.; Inoue, A. Clinical value of magnetic resonance imaging for cervical myelopathy. Spine 1990, 15, 1088–1096. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, H.; Zhang, Y.; Li, Y.; Chen, L.; Chen, H.; Yuan, W. Do intramedullary spinal cord changes in signal intensity on MRI affect surgical opportunity and approach for cervical myelopathy due to ossification of the posterior longitudinal ligament? Eur. Spine J. 2011, 20, 1466–1473. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Yoo, W.-K.; Yoo, J.H.; Kwak, Y.H.; Oh, J.-K.; Song, J.-S.; Kim, S.W. The functional relevance of diffusion tensor imaging in comparison to conventional MRI in patients with cervical compressive myelopathy. Skelet. Radiol. 2017, 46, 1477–1486. [Google Scholar] [CrossRef]
- Houser, O.W.; Onofrio, B.M.; Miller, G.M.; Folger, W.N.; Smith, P.L. Cervical spondylotic stenosis and myelopathy: Evaluation with computed tomographic myelography. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 1994; Volume 69, pp. 557–563. [Google Scholar]
- Takahashi, M.; Yamashita, Y.; Sakamoto, Y.; Kojima, R. Chronic cervical cord compression: Clinical significance of increased signal intensity on MR images. Radiology 1989, 173, 219–224. [Google Scholar] [CrossRef]
- Suzuki, A.; Daubs, M.D.; Inoue, H.; Hayashi, T.; Aghdasi, B.; Montgomery, S.R.; Ruangchainikom, M.; Hu, X.; Lee, C.J.; Wang, C.J. Prevalence and motion characteristics of degenerative cervical spondylolisthesis in the symptomatic adult. Spine 2013, 38, E1115–E1120. [Google Scholar] [CrossRef]
- Muhle, C.; Metzner, J.; Weinert, D.; Falliner, A.; Brinkmann, G.; Mehdorn, M.H.; Heller, M.; Resnick, D. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. Am. J. Neuroradiol. 1998, 19, 1763–1771. [Google Scholar] [PubMed]
- Kang, Y.; Lee, J.; Koh, Y.; Hur, S.; Kim, S.; Chai, J. New MRI grading system for the cervical canal stenosis. AJR Am. J. Roentgenol. 2011, 197, W134–W140. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Yonenobu, K.; Hiroshima, K.; Ebara, S.; Yamashita, K.; Ono, K. Morphometry of the cervical spinal cord and its relation to pathology in cases with compression myelopathy. Spine 1988, 13, 1212–1216. [Google Scholar] [CrossRef]
- Okada, Y.; Ikata, T.; Yamada, H.; Sakamoto, R.; Katoh, S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine 1993, 18, 2024–2029. [Google Scholar] [CrossRef]
- Furlan, J.C.; Kailaya-Vasan, A.; Aarabi, B.; Fehlings, M.G. A novel approach to quantitatively assess posttraumatic cervical spinal canal compromise and spinal cord compression: A multicenter responsiveness study. Spine 2011, 36, 784–793. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Rao, S.C.; Tator, C.H.; Skaf, G.; Arnold, P.; Benzel, E.; Dickman, C.; Cuddy, B.; Green, B.; Hitchon, P. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury: Part II: Results of a multicenter study. Spine 1999, 24, 605–613. [Google Scholar] [CrossRef]
- Nouri, A.; Martin, A.R.; Kato, S.; Kermani, H.R.; Riehm, L.; Fehlings, M.G. The Relationship Between MRI Signal Intensity Changes, Clinical Presentation and Surgical Outcome in Degenerative Cervical Myelopathy: Analysis of a Global Cohort. Spine J. 2017, 17, S133–S134. [Google Scholar] [CrossRef]
- Uchida, K.; Nakajima, H.; Takeura, N.; Yayama, T.; Guerrero, A.R.; Yoshida, A.; Sakamoto, T.; Honjoh, K.; Baba, H. Prognostic value of changes in spinal cord signal intensity on magnetic resonance imaging in patients with cervical compressive myelopathy. Spine J. 2014, 14, 1601–1610. [Google Scholar] [CrossRef]
- Papadopoulos, C.A.; Karonis, P.; Papagelopoulos, P.J.; Karampekios, S.; Hadjipavlou, A.G. Surgical Decompression for Cervical Spondylotic Myelopathy: Correlation between Operative Outcomes and MRI of the Spinal Cord; SLACK Incorporated: Thorofare, NJ, USA, 2004. [Google Scholar]
- de Rota, J.J.F.; Meschian, S.; de Rota, A.F.; Urbano, V.; Baron, M. Cervical spondylotic myelopathy due to chronic compression: The role of signal intensity changes in magnetic resonance images. J. Neurosurg. Spine 2007, 6, 17–22. [Google Scholar]
- Mastronardi, L.; Elsawaf, A.; Roperto, R.; Bozzao, A.; Caroli, M.; Ferrante, M.; Ferrante, L. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J. Neurosurg. Spine 2007, 7, 615–622. [Google Scholar] [CrossRef]
- Yagi, M.; Ninomiya, K.; Kihara, M.; Horiuchi, Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on magnetic resonance imaging. J. Neurosurg. Spine 2010, 12, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, Y.; Kato, F.; Yoshihara, H.; Yanase, M.; Ito, K. MR T2 image classification in cervical compression myelopathy: Predictor of surgical outcomes. Spine 2007, 32, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Tetreault, L.; Côté, P.; Zamorano, J.J.; Dalzell, K.; Fehlings, M.G. Does magnetic resonance imaging improve the predictive performance of a validated clinical prediction rule developed to evaluate surgical outcome in patients with degenerative cervical myelopathy? Spine 2015, 40, 1092–1100. [Google Scholar] [CrossRef]
- Kato, F.; Yukawa, Y.; Suda, K.; Yamagata, M.; Ueta, T. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: Magnetic resonance imaging of over 1200 asymptomatic subjects. Eur. Spine J. 2012, 21, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Wessberg, P.; Danielson, B.I.; Willén, J. Comparison of Cobb angles in idiopathic scoliosis on standing radiographs and supine axially loaded MRI. Spine 2006, 31, 3039–3044. [Google Scholar] [CrossRef] [PubMed]
- Cowley, P. Neuroimaging of spinal canal stenosis. Magn. Reson. Imaging Clin. 2016, 24, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Karpova, A.; Arun, R.; Kalsi-Ryan, S.; Massicotte, E.M.; Kopjar, B.; Fehlings, M.G. Do quantitative magnetic resonance imaging parameters correlate with the clinical presentation and functional outcomes after surgery in cervical spondylotic myelopathy? A prospective multicenter study. Spine 2014, 39, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Z.; Zhang, F.; Shen, H.; Hou, T. A meta-analysis showing that high signal intensity on T2-weighted MRI is associated with poor prognosis for patients with cervical spondylotic myelopathy. J. Clin. Neurosci. 2011, 18, 1592–1595. [Google Scholar] [CrossRef]
- Tetreault, L.A.; Dettori, J.R.; Wilson, J.R.; Singh, A.; Nouri, A.; Fehlings, M.G.; Brodt, E.D.; Jacobs, W.B. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine 2013, 38, S89–S110. [Google Scholar] [CrossRef]
- Taylor, A. Mechanism and treatment of spinal-cord disorders associated with cervical spondylosis. Lancet 1953, 261, 717–720. [Google Scholar] [CrossRef]
- Xing, R.; Zhou, G.; Chen, Q.; Liang, Y.; Dong, J. MRI to measure cervical sagittal parameters: A comparison with plain radiographs. Arch. Orthop. Trauma Surg. 2017, 137, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Tang, J.A.; Smith, J.S.; Acosta, F.L.; Protopsaltis, T.S.; Blondel, B.; Bess, S.; Shaffrey, C.I.; Deviren, V.; Lafage, V. Cervical spine alignment, sagittal deformity, and clinical implications: A review. J. Neurosurg. Spine 2013, 19, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Waly, F.J.; Abduljabbar, F.H.; Fortin, M.; Nooh, A.; Weber, M. Preoperative computed tomography myelography parameters as predictors of outcome in patients with degenerative cervical myelopathy: Results of a systematic review. Glob. Spine J. 2017, 7, 521–528. [Google Scholar] [CrossRef]
- Naderi, S.; Özgen, S.; Pamir, M.N.; Özek, M.M.; Erzen, C. Cervical spondylotic myelopathy: Surgical results and factors affecting prognosis. Neurosurgery 1998, 43, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Höller, Y.; Brigo, F.; Frey, V.; Lochner, P.; Leis, S.; Golaszewski, S.; Trinka, E. The contribution of neurophysiology in the diagnosis and management of cervical spondylotic myelopathy: A review. Spinal Cord 2016, 54, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, C.; Meyer, B.U.; Machetanz, J.; Conrad, B. The value of magnetic stimulation in the diagnosis of radiculopathies. Muscle Nerve: Off. J. Am. Assoc. Electrodiagn. Med. 1993, 16, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bednařík, J.; Kadaňka, Z.; Voháňka, S.; Novotný, O.; Šurelová, D.; Filipovičová, D.; Prokeš, B. The value of somatosensory and motor evoked potentials in pre-clinical spondylotic cervical cord compression. Eur. Spine J. 1998, 7, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bednařík, J.; Sládková, D.; Kadaňka, Z.; Dušek, L.; Keřkovský, M.; Voháňka, S.; Novotný, O.; Urbánek, I.; Němec, M. Are subjects with spondylotic cervical cord encroachment at increased risk of cervical spinal cord injury after minor trauma? J. Neurol. Neurosurg. Psychiatry 2011, 82, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Bednarik, J.; Kadanka, Z.; Dusek, L.; Kerkovsky, M.; Vohanka, S.; Novotny, O.; Urbanek, I.; Kratochvilova, D. Presymptomatic spondylotic cervical myelopathy: An updated predictive model. Eur. Spine J. 2008, 17, 421–431. [Google Scholar] [CrossRef]
- Wilson, J.R.; Barry, S.; Fischer, D.J.; Skelly, A.C.; Arnold, P.M.; Riew, K.D.; Shaffrey, C.I.; Traynelis, V.C.; Fehlings, M.G. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine 2013, 38, S37–S54. [Google Scholar] [CrossRef]
- Feng, X.; Hu, Y.; Ma, X. Progression Prediction of Mild Cervical Spondylotic Myelopathy by Somatosensory-evoked Potentials. Spine 2020, 45, E560–E567. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Sutter, M.; Herdmann, J. Cervical myelopathy: Clinical and neurophysiological evaluation. Aging Spine 2005, 12, 99–105. [Google Scholar]
- Tsiptsios, I.; Fotiou, F.; Sitzoglou, K.; Fountoulakis, K. Neurophysiological investigation of cervical spondylosis. Electromyogr. Clin. Neurophysiol. 2001, 41, 305–313. [Google Scholar] [PubMed]
- Liu, H.; MacMillian, E.L.; Jutzeler, C.R.; Ljungberg, E.; MacKay, A.L.; Kolind, S.H.; Mädler, B.; Li, D.K.; Dvorak, M.F.; Curt, A. Assessing structure and function of myelin in cervical spondylotic myelopathy: Evidence of demyelination. Neurology 2017, 89, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Tamburelli, F.C.; Pilato, F.; Profice, P.; Ranieri, F.; Di Iorio, R.; Iodice, F.; Musumeci, G.; Di Lazzaro, V. The role of motor-evoked potentials in the management of cervical spondylotic myelopathy. Spine J. 2013, 13, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J. Chapter 11—Peripheral Nerves. In Essential Applications of Musculoskeletal Ultrasound in Rheumatology; Wakefield, R.J., D’Agostino, M.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2010; pp. 121–136. [Google Scholar]
- Mowforth, O.D.; Davies, B.M.; Goh, S.; O’Neill, C.P.; Kotter, M.R. Research inefficiency in degenerative cervical myelopathy: Findings of a systematic review on research activity over the past 20 years. Glob. Spine J. 2020, 10, 476–485. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Cohen-Adad, J. Chapter 3.1—Diffusion-Weighted Imaging of the Spinal Cord. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 123–145. [Google Scholar]
- Grabher, P.; Mohammadi, S.; Trachsler, A.; Friedl, S.; David, G.; Sutter, R.; Weiskopf, N.; Thompson, A.J.; Curt, A.; Freund, P. Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grabher, P.; Mohammadi, S.; David, G.; Freund, P. Neurodegeneration in the Spinal Ventral Horn Prior to Motor Impairment in Cervical Spondylotic Myelopathy. J. Neurotrauma 2017, 34, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Nouri, A.; Wilson, J.R.; Tetreault, L.; Crawley, A.P.; Mikulis, D.J.; Ginsberg, H. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open 2018, 8, e019809. [Google Scholar] [CrossRef]
- Yoo, W.-K.; Kim, T.H.; Hai, D.M.; Sundaram, S.; Yang, Y.M.; Park, M.S.; Kim, Y.C.; Kwak, Y.H.; Ohn, S.H.; Kim, S.W. Correlation of magnetic resonance diffusion tensor imaging and clinical findings of cervical myelopathy. Spine J. 2013, 13, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Kalsi-Ryan, S.; Cadotte, D.W.; Wilson, J.R.; Tetreault, L.; Nouri, A.; Crawley, A.; Mikulis, D.J. Monitoring for myelopathic progression with multiparametric quantitative MRI. PLoS ONE 2018, 13, e0195733. [Google Scholar]
- Wang, K.; Chen, Z.; Zhang, F.; Song, Q.; Hou, C.; Tang, Y.; Wang, J.; Chen, S.; Bian, Y.; Hao, Q. Evaluation of DTI parameter ratios and diffusion tensor tractography grading in the diagnosis and prognosis prediction of cervical spondylotic myelopathy. Spine 2017, 42, E202–E210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, L.; Hai, Y.; Liu, Y.; Ding, H.; Chen, X. Multi-shot echo-planar diffusion tensor imaging in cervical spondylotic myelopathy: A longitudinal study. Bone Jt. J. 2020, 102, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Aleksanderek, I.; Cohen-Adad, J.; Tarmohamed, Z.; Tetreault, L.; Smith, N.; Cadotte, D.W.; Crawley, A.; Ginsberg, H.; Mikulis, D.J. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. NeuroImage Clin. 2016, 10, 192–238. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.; Kalsi-Ryan, S.; Lange, S.; Tetreault, L.; Nouri, A.; Crawley, A.; Mikulis, D. Clinically feasible microstructural MRI to quantify cervical spinal cord tissue injury using DTI, MT, and T2*-weighted imaging: Assessment of normative data and reliability. Am. J. Neuroradiol. 2017, 38, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Summers, P.E.; Brooks, J.C.W.; Cohen-Adad, J. Chapter 4.1—Spinal Cord fMRI. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 221–239. [Google Scholar]
- Solanky, B.S.; De Vita, E. Chapter 5.1—Single Voxel MR Spectroscopy in the Spinal Cord: Technical Challenges and Clinical Applications. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–290. [Google Scholar]
- Laule, C.; MacKay, A. Chapter 3.5—T2 Relaxation. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 181–206. [Google Scholar]
- Kim, M.; Cercignani, M. Chapter 3.4—Magnetization Transfer. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 164–180. [Google Scholar]
- Brooks, J.C.W. Chapter 4.2—Physiological Noise Modeling and Analysis for Spinal Cord fMRI. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 240–257. [Google Scholar]
- Assaf, Y.; Alexander, D.C. Chapter 3.3—Advanced Methods to Study White Matter Microstructure. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 156–163. [Google Scholar]
- Abdel-Aziz, K.; Ciccarelli, O. Chapter 1.1—Rationale for Quantitative MRI of the Human Spinal Cord and Clinical Applications. In Quantitative MRI of the Spinal Cord; Cohen-Adad, J., Wheeler-Kingshott, C.A.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 3–21. [Google Scholar]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, techniques, and applications of T2 *-based MR imaging and its special applications. Radiographics 2009, 29, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Battiston, M.; Schneider, T.; Prados, F.; Grussu, F.; Yiannakas, M.C.; Ourselin, S.; Gandini Wheeler-Kingshott, C.A.; Samson, R.S. Fast and reproducible in vivo T1 mapping of the human cervical spinal cord. Magn. Reson. Med. 2018, 79, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Albistegui-Dubois, R.; Marehbian, J.; Newton, J.M.; Dong, Y.; Holly, L.T.; Yan, X.; Dobkin, B.H. Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy: Laboratory investigation. J. Neurosurg. Spine 2008, 9, 538–551. [Google Scholar]
- Aleksanderek, I.K.; Stevens, T.; Goncalves, S.; Bartha, R.; Duggal, N. Investigating metabolic and functional profiles of mild and moderate cervical spondylotic myelopathy: A MRS and fMRI study. Spine J. 2015, 15, S201. [Google Scholar] [CrossRef]
- Banaszek, A.; Bladowska, J.; Szewczyk, P.; Podgorski, P.; Sasiadek, M. Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease. Eur. Spine J. 2014, 23, 1523–1530. [Google Scholar] [CrossRef]
- Baucher, G.; Rasoanandrianina, H.; Levy, S.; Pini, L.; Troude, L.; Roche, P.H.; Callot, V. T1 Mapping for Microstructural Assessment of the Cervical Spinal Cord in the Evaluation of Patients with Degenerative Cervical Myelopathy. AJNR. Am. J. Neuroradiol. 2021, 42, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Bhagavatula, I.D.; Shukla, D.; Sadashiva, N.; Saligoudar, P.; Prasad, C.; Bhat, D.I. Functional cortical reorganization in cases of cervical spondylotic myelopathy and changes associated with surgery. Neurosurg. Focus 2016, 40, E2. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.; Ingale, P.; Srivastava, S.; Marathe, N.; Bhide, P. Diffusion tensor imaging as an additional postoperative prognostic predictor factor in cervical myelopathy patients: An observational study. J. Craniovertebral Junction Spine 2019, 10, 10–13. [Google Scholar]
- Chen, X.; Kong, C.; Feng, S.; Guan, H.; Yu, Z.; Cui, L.; Wang, Y. Magnetic resonance diffusion tensor imaging of cervical spinal cord and lumbosacral enlargement in patients with cervical spondylotic myelopathy. J. Magn. Reson. Imaging 2016, 43, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, R.; Wang, Q.; Yu, C.; Li, F.; Liang, M.; Zong, Y.; Zhao, Y.; Xiong, W.; Su, Z.; et al. Functional Connectivity Changes of the Visual Cortex in the Cervical Spondylotic Myelopathy Patients: A Resting-State fMRI Study. Spine 2020, 45, E272–E279. [Google Scholar] [CrossRef] [PubMed]
- Cloney, M.B.; Smith, Z.A.; Weber, K.A.; Parrish, T.B. Quantitative Magnetization Transfer MRI Measurements of the Anterior Spinal Cord Region are Associated with Clinical Outcomes in Cervical Spondylotic Myelopathy. Spine 2018, 43, 675–680. [Google Scholar] [CrossRef]
- Cui, J.-L.; Li, X.; Chan, T.-Y.; Mak, K.-C.; Luk, K.D.-K.; Hu, Y. Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur. Spine J. 2015, 24, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Chen, X.; Liu, Y.; Zhang, Y.; Kong, C.; Guan, Y. Changes in diffusion tensor imaging indices of the lumbosacral enlargement correlate with cervical spinal cord changes and clinical assessment in patients with cervical spondylotic myelopathy. Clin. Neurol. Neurosurg. 2019, 186, 105282. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.; Rabin, D.; Bartha, R.; Barry, R.L.; Gati, J.S.; Kowalczyk, I.; Fink, M. Brain reorganization in patients with spinal cord compression evaluated using fMRI. Neurology 2010, 74, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Salamon, N.; Grinstead, J.W.; Holly, L.T. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J. Off. J. North Am. Spine Soc. 2014, 14, 2589–2597. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Salamon, N.; Hardy, A.J.; Holly, L.T. Prediction of Neurological Impairment in Cervical Spondylotic Myelopathy using a Combination of Diffusion MRI and Proton MR Spectroscopy. PLoS ONE 2015, 10, e0139451. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, X.; Chen, X.; Zhao, R.; Song, Y.; Liang, M.; Sun, H.; Xue, Y. Enhanced Information Flow From Cerebellum to Secondary Visual Cortices Leads to Better Surgery Outcome in Degenerative Cervical Myelopathy Patients: A Stochastic Dynamic Causal Modeling Study With Functional Magnetic Resonance Imaging. Front. Hum. Neurosci. 2021, 15, 632829. [Google Scholar]
- Guo, X.; Yang, X.; Chen, X.; Zhao, R.; Song, Y.; Liang, M.; Sun, H.; Xue, Y. The Evaluation and Prediction of Laminoplasty Surgery Outcome in Patients with Degenerative Cervical Myelopathy Using Diffusion Tensor MRI. AJNR. Am. J. Neuroradiol. 2020, 41, 1745–1753. [Google Scholar]
- Hassan, T.A.A.E.H.; Assad, R.E.; Belal, S.A. MR diffusion tensor imaging of the spinal cord: Can it help in early detection of cervical spondylotic myelopathy and assessment of its severity? Egypt. J. Radiol. Nucl. Med. 2019, 50, 62. [Google Scholar] [CrossRef]
- He, Z.; Wang, N.; Kang, L.; Cui, J.; Wan, Y. Analysis of pathological parameters of cervical spondylotic myelopathy using magnetic resonance imaging. Clin. Neurol. Neurosurg. 2020, 189, 105631. [Google Scholar] [CrossRef] [PubMed]
- Holly, L.T.; Wang, C.; Salamon, N.; Woodworth, D.C.; Ellingson, B.M. Neck disability in patients with cervical spondylosis is associated with altered brain functional connectivity. J. Clin. Neurosci. 2019, 69, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Holly, L.T.; Wang, C.; Salamon, N.; Woodworth, D.C.; Ellingson, B.M. New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur. Radiol. 2012, 22, 1797–1802. [Google Scholar]
- Iwasaki, M.; Yokohama, T.; Oura, D.; Furuya, S.; Niiya, Y.; Okuaki, T. Decreased Value of Highly Accurate Fractional Anisotropy Using 3-Tesla ZOOM Diffusion Tensor Imaging After Decompressive Surgery in Patients with Cervical Spondylotic Myelopathy: Aligned Fibers Effect. World Neurosurg. X 2019, 4, 100056. [Google Scholar] [CrossRef]
- Jurova, B.; Mechl, M.; Kerkovsky, M.; Sprlakova-Pukova, A.; Kadanka, Z.; Nemec, M.; Bednarik, J.; Kovalova, I.; Dusek, L. Spinal Cord MR Diffusion Properties in Patients with Degenerative Cervical Cord Compression. J. Neuroimaging 2017, 27, 149–157. [Google Scholar]
- Kanchiku, T.; Imajo, Y.; Suzuki, H.; Yoshida, Y.; Nishida, N.; Taguchi, T.; Suetomi, Y.; Nishijima, S. Application of diffusion tensor imaging for the diagnosis of segmental level of dysfunction in cervical spondylotic myelopathy. Spinal Cord 2016, 54, 390–395. [Google Scholar]
- Kara, B.; Celik, A.; Karadereler, S.; Ulusoy, L.; Ganiyusufoglu, K.; Onat, L.; Mutlu, A.; Ornek, I.; Sirvanci, M.; Hamzaoglu, A. The role of DTI in early detection of cervical spondylotic myelopathy: A preliminary study with 3-T MRI. Neuroradiology 2011, 53, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kerkovsky, M.; Jakubcova, B.; Mechl, M.; Kadanka, Z.; Kadanka Jr, Z.; Nemec, M.; Kovalova, I.; Bednarik, J. Multifactorial determination of the spinal cord diffusion properties in patients with cervical spondylotic spinal cord compression: A diffusion tensor imaging study. Neuroradiology 2015, 57, S133. [Google Scholar]
- Kowalczyk, I.; Bartha, R.; Duggal, N. Proton magnetic resonance spectroscopy of the motor cortex in cervical myelopathy. Brain 2012, 135, 461–468. [Google Scholar] [CrossRef][Green Version]
- Kowalczyk, I.; Bartha, R.; Duggal, N. Proton magnetic resonance spectroscopy of the motor cortex in cervical spondylotic myelopathy. Can. J. Neurol. Sci. 2010, 37, S30. [Google Scholar]
- Lee, S.; Chung, T.-S.; Kim, S.; Yoo, Y.H.; Yoon, C.-S.; Lee, Y.H.; Suh, J.-S.; Jeong, E.-K.; Kim, I.S.; Park, J.H. Accuracy of diffusion tensor imaging for diagnosing cervical spondylotic myelopathy in patients showing spinal cord compression. Korean J. Radiol. 2015, 16, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, W.; Jin, R.; Li, X.; Luk, K.D.; Wu, E.X.; Hu, Y. Amplitude of Low Frequency Fluctuation (ALFF) in the Cervical Spinal Cord with Stenosis: A Resting State fMRI Study. PLoS ONE 2016, 11, e0167279. [Google Scholar] [CrossRef] [PubMed]
- Maier, I.L.; Hofer, S.; Eggert, E.; Schregel, K.; Psychogios, M.-N.; Frahm, J.; Bähr, M.; Liman, J. T1 Mapping Quantifies Spinal Cord Compression in Patients with Various Degrees of Cervical Spinal Canal Stenosis. Front. Neurol. 2020, 11, 574604. [Google Scholar] [CrossRef]
- Maki, S.; Koda, M.; Kitamura, M.; Inada, T.; Kamiya, K.; Ota, M.; Iijima, Y.; Saito, J.; Masuda, Y.; Matsumoto, K.; et al. Diffusion tensor imaging can predict surgical outcomes of patients with cervical compression myelopathy. Eur. Spine J. 2017, 26, 2459–2466. [Google Scholar] [CrossRef]
- Maki, S.; Koda, M.; Ota, M.; Oikawa, Y.; Kamiya, K.; Inada, T.; Furuya, T.; Takahashi, K.; Masuda, Y.; Matsumoto, K.; et al. Reduced Field-of-View Diffusion Tensor Imaging of the Spinal Cord Shows Motor Dysfunction of the Lower Extremities in Patients with Cervical Compression Myelopathy. Spine 2018, 43, 89–96. [Google Scholar] [CrossRef]
- Mamata, H.; Jolesz, F.A.; Maier, S.E. Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. J. Magn. Reson. Imaging 2005, 22, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.K.; Sun, P.; Han, R.H.; Griffin, K.J.; Wagner, J.; Yarbrough, C.K.; Wright, N.M.; Dorward, I.G.; Riew, K.D.; Kelly, M.P.; et al. Fractional anisotropy to quantify cervical spondylotic myelopathy severity. J. Neurosurg. Sci. 2018, 62, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Nanjo, Y.; Teshima, R.; Morio, Y.; Meshitsuka, S.; Yamane, K. High-resolution nuclear magnetic resonance spectroscopic study of metabolites in the cerebrospinal fluid of patients with cervical myelopathy and lumbar radiculopathy. Eur. Spine J. 2010, 19, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Nischal, N.; Tripathi, S.; Singh, J.P. Quantitative Evaluation of the Diffusion Tensor Imaging Matrix Parameters and the Subsequent Correlation with the Clinical Assessment of Disease Severity in Cervical Spondylotic Myelopathy. Asian Spine J. 2020, 15, 808–816. [Google Scholar] [CrossRef]
- Nukala, M.; Abraham, J.; Khandige, G.; Shetty, B.K.; Rao, A.p.a. Efficacy of diffusion tensor imaging in identification of degenerative cervical spondylotic myelopathy. Eur. J. Radiol. Open 2019, 6, 16–23. [Google Scholar] [CrossRef]
- Paliwal, M.; Smith, Z.A.; Weber, K.A.; Mackey, S.; Hopkins, B.S.; Dahdaleh, N.S.; Cantrell, D.R.; Parrish, T.D.; Hoggarth, M.A.; Elliott, J.M.; et al. Magnetization Transfer Ratio and Morphometrics of the Spinal Cord Associates with Surgical Recovery in Patients with Degenerative Cervical Myelopathy. World Neurosurg. 2020, 144, e939–e947. [Google Scholar] [CrossRef]
- Peng, X.; Tan, Y.; He, L.; Ou, Y. Alterations of functional connectivity between thalamus and cortex before and after decompression in cervical spondylotic myelopathy patients: A resting-state functional MRI study. NeuroReport 2020, 31, 365–371. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Kanna, R.M.; Balamurali, G.; Shetty, A.P.; Yerramshetty, J.S.; Chittode, V.S. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine 2014, 39, 1183–1189. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Kanna, R.M.; Chittode, V.S.; Maheswaran, A.; Aiyer, S.N.; Shetty, A.P. Efficacy of Diffusion Tensor Imaging Indices in Assessing Postoperative Neural Recovery in Cervical Spondylotic Myelopathy. Spine 2017, 42, 8–13. [Google Scholar] [CrossRef]
- Salamon, N.; Ellingson, B.M.; Nagarajan, R.; Gebara, N.; Thomas, A.; Holly, L.T. Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T. Spinal Cord 2013, 51, 558–563. [Google Scholar] [CrossRef]
- Salamon, N.; Woodworth, D.C.; Holly, L.T.; Ellingson, B.M. Resting-State Functional Magnetic Resonance Imaging Connectivity of the Brain Is Associated with Altered Sensorimotor Function in Patients with Cervical Spondylosis. World Neurosurg. 2018, 119, e740–e749. [Google Scholar]
- Sato, T.; Horikoshi, T.; Watanabe, A.; Uchida, M.; Ishigame, K.; Araki, T.; Kinouchi, H. Evaluation of cervical myelopathy using apparent diffusion coefficient measured by diffusion-weighted imaging. AJNR. Am. J. Neuroradiol. 2012, 33, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Severino, R.; Nouri, A.; Tessitore, E. Degenerative cervical myelopathy: How to identify the best responders to surgery? J. Clin. Med. 2020, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Kaushal, M.; Budde, M.; Schmit, B.; Wang, M.C.; Kurpad, S. Comparison between quantitative measurements of diffusion tensor imaging and T2 signal intensity in a large series of cervical spondylotic myelopathy patients for assessment of disease severity and prognostication of recovery. J. Neurosurg. Spine 2019, 31, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Chen, W.-J.; Huang, J.-W.; Cai, M.-J.; Dong, T.-F.; Li, T.-S.; Yang, B.; Zhao, H.-P. Diffusion tensor imaging in the cervical spinal cord. Eur. Spine J. 2011, 20, 422–428. [Google Scholar] [CrossRef]
- Su, Q.; Zhao, R.; Guo, X.; Wang, S.; Tu, H.; Yang, F. Identification and Therapeutic Outcome Prediction of Cervical Spondylotic Myelopathy Based on the Functional Connectivity From Resting-State Functional MRI Data: A Preliminary Machine Learning Study. Front. Neurol. 2021, 12, 711880. [Google Scholar] [CrossRef]
- Suleiman, L.I. High-resolution magnetization transfer MRI in patients with cervical spondylotic myelopathy. J. Clin. Neurosci. 2018, 51, 57–61. [Google Scholar] [CrossRef]
- Taha Ali, T.F.; Badawy, A.E. Feasibility of 1H-MR Spectroscopy in evaluation of cervical spondylotic myelopathy. Egypt. J. Radiol. Nucl. Med. 2013, 44, 93–99. [Google Scholar] [CrossRef]
- Takenaka, S.; Kan, S.; Seymour, B.; Makino, T.; Sakai, Y.; Kushioka, J.; Tanaka, H.; Watanabe, Y.; Shibata, M.; Yoshikawa, H.; et al. Resting-state Amplitude of Low-frequency Fluctuation is a Potentially Useful Prognostic Functional Biomarker in Cervical Myelopathy. Clin. Orthop. Relat. Res. 2020, 478, 1667–1680. [Google Scholar] [CrossRef]
- Takenaka, S.; Kan, S.; Seymour, B.; Makino, T.; Sakai, Y.; Kushioka, J.; Tanaka, H.; Watanabe, Y.; Shibata, M.; Yoshikawa, H.; et al. Towards prognostic functional brain biomarkers for cervical myelopathy: A resting-state fMRI study. Sci. Rep. 2019, 9, 10456. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, F.; Liu, Z.; Wu, L.; Zeng, X.; Gong, H.; He, L. Alteration of cerebral regional homogeneity within sensorimotor network in patients with cervical spondylotic myelopathy after spinal cord decompression: A resting-state functional MRI study. Chin. J. Radiol. 2016, 50, 495–499. [Google Scholar]
- Tian, X.; Zhang, L.; Zhang, X.; Meng, L.; Li, X. Correlations between preoperative diffusion tensor imaging and surgical outcome in patients with cervical spondylotic myelopathy. Am. J. Transl. Res. 2021, 13, 11461–11471. [Google Scholar] [PubMed]
- Toktas, Z.O.; Kilic, T.; Konya, D.; Tanrikulu, B.; Koban, O. Diffusion tensor imaging of cervical spinal cord: A quantitative diagnostic tool in cervical spondylotic myelopathy. J. Craniovertebral Junction Spine 2016, 7, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Uda, T.; Takami, T.; Tsuyuguchi, N.; Sakamoto, S.; Yamagata, T.; Ikeda, H.; Nagata, T.; Ohata, K. Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla. Spine 2013, 38, 407–414. [Google Scholar] [CrossRef]
- Ulubaba, H.E.; Saglik, S.; Yildirim, I.O.; Durak, M.A. Effectiveness of Diffusion Tensor Imaging in Determining Cervical Spondylotic Myelopathy. Turk. Neurosurg. 2021, 31, 67–72. [Google Scholar] [CrossRef]
- Vedantam, A.; Rao, A.; Kurpad, S.N.; Jirjis, M.B.; Eckardt, G.; Schmit, B.D.; Wang, M.C. Diffusion Tensor Imaging Correlates with Short-Term Myelopathy Outcome in Patients with Cervical Spondylotic Myelopathy. World Neurosurg. 2017, 97, 489–494. [Google Scholar] [CrossRef]
- Wang, C.; Salamon, N.; Laiwalla, A.; Holly, L.T.; Ellingson, B.M.; Islam, S. Supraspinal functional and structural plasticity in patients undergoing surgery for degenerative cervical myelopathy. J. Neurosurg. Spine 2021, 35, 185–193. [Google Scholar] [CrossRef]
- Wang, K.Y.; Idowu, O.; Orman, G.; Izbudak, I.; Thompson, C.B.; Myers, C.; Riley, L.H.; Carrino, J.A.; Flammang, A.; Gilson, W.; et al. Tract-Specific Diffusion Tensor Imaging in Cervical Spondylotic Myelopathy Before and After Decompressive Spinal Surgery: Preliminary Results. Clin. Neuroradiol. 2017, 27, 61–69. [Google Scholar] [CrossRef]
- Wen, C.Y.; Cui, J.L.; Liu, H.S.; Mak, K.C.; Cheung, W.Y.; Luk, K.D.K.; Hu, Y. Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy. Radiology 2014, 270, 197–204. [Google Scholar] [CrossRef]
- Xiangshui, M.; Xiangjun, C.; Xiaoming, Z.; Qingshi, Z.; Yi, C.; Chuanqiang, Q.; Xiangxing, M.; Chuanfu, L.; Jinwen, H. 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin. Radiol. 2010, 65, 465–473. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Liu, J.-F.; Jin, D.; Wang, C.-J.; Zhao, Q.; Lang, N.; Yuan, H.-S.; Ou-Yang, H.-Q.; Liu, X.-G.; Liu, Z.-J.; et al. Utility of Advanced DWI in the Detection of Spinal Cord Microstructural Alterations and Assessment of Neurologic Function in Cervical Spondylotic Myelopathy Patients. J. Magn. Reson. Imaging 2021, 55, 930–940. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, H.; Wang, N.; Jiang, X.; Liang, Y.; Xiao, W.; Zhong, B.; Ju, H.; Luo, J.; Wen, S.; et al. Application of Diffusion Tensor Imaging Cutoff Value to Evaluate the Severity and Postoperative Neurologic Recovery of Cervical Spondylotic Myelopathy. World Neurosurg. 2018, 118, e849–e855. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 mapping: Basic techniques and clinical applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Benneker, L.M.; Boesch, C.; Watanabe, T.; Obata, T.; Anderson, S.E. Classification of intervertebral disk degeneration with axial T2 mapping. Am. J. Roentgenol. 2007, 189, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; Rutt, B.K.; Peters, T.M. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn. Reson. Med. 2003, 49, 515–526. [Google Scholar] [CrossRef]
- Henderson, E.; McKinnon, G.; Lee, T.-Y.; Rutt, B.K. A fast 3D look-locker method for volumetric T1 mapping. Magn. Reson. Imaging 1999, 17, 1163–1171. [Google Scholar] [CrossRef]
- Wang, X.; Joseph, A.A.; Kalentev, O.; Merboldt, K.-D.; Voit, D.; Roeloffs, V.B.; van Zalk, M.; Frahm, J. High-resolution myocardial T 1 mapping using single-shot inversion recovery fast low-angle shot MRI with radial undersampling and iterative reconstruction. Br. J. Radiol. 2016, 89, 20160255. [Google Scholar] [CrossRef]
- Nöth, U.; Shrestha, M.; Schüre, J.-R.; Deichmann, R. Quantitative in vivo T2 mapping using fast spin echo techniques–A linear correction procedure. Neuroimage 2017, 157, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Lommers, E.; Simon, J.; Reuter, G.; Delrue, G.; Dive, D.; Degueldre, C.; Balteau, E.; Phillips, C.; Maquet, P. Multiparameter MRI quantification of microstructural tissue alterations in multiple sclerosis. NeuroImage Clin. 2019, 23, 101879. [Google Scholar] [CrossRef]
- Steenwijk, M.D.; Vrenken, H.; Jonkman, L.E.; Daams, M.; Geurts, J.J.; Barkhof, F.; Pouwels, P.J. High-resolution T1-relaxation time mapping displays subtle, clinically relevant, gray matter damage in long-standing multiple sclerosis. Mult. Scler. J. 2016, 22, 1279–1288. [Google Scholar] [CrossRef]
- Rasoanandrianina, H. Regional T1 mapping of the whole cervical spinal cord using an optimized MP2RAGE sequence. NMR Biomed. 2019, 32, e4142. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, N.; Haughton, V.M.; Anderson, P. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. Am. J. Neuroradiol. 2010, 31, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Pachowsky, M.L.; Kleyer, A.; Wegener, L.; Langenbach, A.; Simon, D.; Janka, R.; May, M.; Welsch, G.H. Quantitative T2 mapping shows increased degeneration in adjacent intervertebral discs following kyphoplasty. Cartilage 2020, 11, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Raudner, M.; Schreiner, M.M.; Hilbert, T.; Kober, T.; Weber, M.; Szelényi, A.; Windhager, R.; Juras, V.; Trattnig, S. Clinical implementation of accelerated T 2 mapping: Quantitative magnetic resonance imaging as a biomarker for annular tear and lumbar disc herniation. Eur. Radiol. 2021, 31, 3590–3599. [Google Scholar] [CrossRef] [PubMed]
- Chagawa, K.; Nishijima, S.; Kanchiku, T.; Imajo, Y.; Suzuki, H.; Yoshida, Y.; Taguchi, T. Normal values of diffusion tensor magnetic resonance imaging parameters in the cervical spinal cord. Asian Spine J. 2015, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wu, Y.; Song, P.; Qian, Y.; Wang, Y.; Xu, L.; Yin, M.; Zhang, R.; Tao, H.; Ge, P. A preliminary study of 3.0-T magnetic resonance diffusion tensor imaging in cervical spondylotic myelopathy. Eur. Spine J. 2018, 27, 1839–1845. [Google Scholar] [CrossRef]
- Guan, X.; Fan, G.; Wu, X.; Gu, G.; Gu, X.; Zhang, H.; He, S. Diffusion tensor imaging studies of cervical spondylotic myelopathy: A systemic review and meta-analysis. PLoS ONE 2015, 10, e0117707. [Google Scholar] [CrossRef] [PubMed]
- d’Avanzo, S.; Ciavarro, M.; Pavone, L.; Pasqua, G.; Ricciardi, F.; Bartolo, M.; Solari, D.; Somma, T.; de Divitiis, O.; Cappabianca, P.; et al. The Functional Relevance of Diffusion Tensor Imaging in Patients with Degenerative Cervical Myelopathy. J. Clin. Med. 2020, 9, 1828. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.I.A.; Saleh, A. Role of DTI in cases of cervical spondylosis presented with compression myelopathy: Could it explain the clinical radiological mismatch?! Egypt. J. Radiol. Nucl. Med. 2018, 49, 441–446. [Google Scholar] [CrossRef]
- Jones, J.G.A.; Cen, S.Y.; Lebel, R.M.; Hsieh, P.C.; Law, M. Diffusion Tensor Imaging Correlates with the Clinical Assessment of Disease Severity in Cervical Spondylotic Myelopathy and Predicts Outcome following Surgery. Am. J. Neuroradiol. 2013, 34, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K.; Pfeuffer, J. On the nature of the BOLD fMRI contrast mechanism. Magn. Reson. Imaging 2004, 22, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Anson, B.; MacManus, D.; Parker, G.; Davie, C.; Barker, G.; Moseley, I.; McDonald, W.; Miller, D. In vivo 1 H-magnetic resonance spectroscopy of the spinal cord in humans. Neuroradiology 2000, 42, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Saidha, S.; Chen, M.; Smith, S.A.; Prince, J.; Jones, C.; Diener-West, M.; Van Zijl, P.C.; Reich, D.S.; Calabresi, P.A. Spinal cord quantitative MRI discriminates between disability levels in multiple sclerosis. Neurology 2013, 80, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Petrella, J.R.; Grossman, R.I.; McGowan, J.C.; Campbell, G.; Cohen, J.A. Multiple sclerosis lesions: Relationship between MR enhancement pattern and magnetization transfer effect. Am. J. Neuroradiol. 1996, 17, 1041–1049. [Google Scholar] [PubMed]
- Serbruyns, L.; Leunissen, I.; van Ruitenbeek, P.; Pauwels, L.; Caeyenberghs, K.; Solesio-Jofre, E.; Geurts, M.; Cuypers, K.; Meesen, R.L.; Sunaert, S. Alterations in brain white matter contributing to age-related slowing of task switching performance: The role of radial diffusivity and magnetization transfer ratio. Hum. Brain Mapp. 2016, 37, 4084–4098. [Google Scholar] [CrossRef] [PubMed]
- Hankins, J.S.; McCarville, M.B.; Loeffler, R.B.; Smeltzer, M.P.; Onciu, M.; Hoffer, F.A.; Li, C.-S.; Wang, W.C.; Ware, R.E.; Hillenbrand, C.M. R2 * magnetic resonance imaging of the liver in patients with iron overload. Blood J. Am. Soc. Hematol. 2009, 113, 4853–4855. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rivera, L.A.; Schubert, T.; Johnson, K.M. Measurements of cerebral blood volume using quantitative susceptibility mapping, R2 * relaxometry, and ferumoxytol-enhanced MRI. NMR Biomed. 2019, 32, e4175. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.T.; Hidalgo, C. Noxious Iron–calcium connections in Neurodegeneration. Front. Neurosci. 2019, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Crichton, R.; Ward, R. Metal-Based Neurodegeneration: From Molecular Mechanisms to Therapeutic Strategies; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Crichton, R.; Crichton, R.R.; Boelaert, J.R. Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Craelius, W.; Migdal, M.; Luessenhop, C.; Sugar, A.; Mihalakis, I. Iron deposits surrounding multiple sclerosis plaques. Arch. Pathol. Lab. Med. 1982, 106, 397–399. [Google Scholar] [PubMed]
- Hametner, S.; Wimmer, I.; Haider, L.; Pfeifenbring, S.; Brück, W.; Lassmann, H. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 2013, 74, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kovacs, G.G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef]
- Schuh, C.; Wimmer, I.; Hametner, S.; Haider, L.; Van Dam, A.-M.; Liblau, R.S.; Smith, K.J.; Probert, L.; Binder, C.J.; Bauer, J. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol. 2014, 128, 247–266. [Google Scholar] [CrossRef]
- Bulk, M.; van der Weerd, L.; Breimer, W.; Lebedev, N.; Webb, A.; Goeman, J.J.; Ward, R.J.; Huber, M.; Oosterkamp, T.H.; Bossoni, L. Quantitative comparison of different iron forms in the temporal cortex of Alzheimer patients and control subjects. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Han, Y.-H.; Kang, B.-M.; Mun, C.-W.; Lee, S.-J.; Baik, S.-K. Quantitative assessment of subcortical atrophy and iron content in progressive supranuclear palsy and parkinsonian variant of multiple system atrophy. J. Neurol. 2013, 260, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Swaiman, K.F. Hallervorden-Spatz syndrome and brain iron metabolism. Arch. Neurol. 1991, 48, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, S.; Houlden, H. Neurodegeneration with brain iron accumulation. Handb. Clin. Neurol. 2018, 145, 157–166. [Google Scholar]
- Damulina, A.; Pirpamer, L.; Soellradl, M.; Sackl, M.; Tinauer, C.; Hofer, E.; Enzinger, C.; Gesierich, B.; Duering, M.; Ropele, S. Cross-sectional and Longitudinal Assessment of Brain Iron Level in Alzheimer Disease Using 3-T MRI. Radiology 2020, 296, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef]
- Ghadery, C.; Pirpamer, L.; Hofer, E.; Langkammer, C.; Petrovic, K.; Loitfelder, M.; Schwingenschuh, P.; Seiler, S.; Duering, M.; Jouvent, E. R2 * mapping for brain iron: Associations with cognition in normal aging. Neurobiol. Aging 2015, 36, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhu, W.; Zhan, C.; Zhao, L.; Wang, J.; Tian, Q.; Wang, W. Investigation on positive correlation of increased brain iron deposition with cognitive impairment in Alzheimer disease by using quantitative MR R2′ mapping. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 578. [Google Scholar] [CrossRef]
- Moon, Y.; Han, S.-H.; Moon, W.-J. Patterns of brain iron accumulation in vascular dementia and Alzheimer’s dementia using quantitative susceptibility mapping imaging. J. Alzheimer’s Dis. 2016, 51, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.H.O.; Santos, A.C.; Tumas, V.; Liu, M.; Zheng, W.; Haacke, E.M.; Salmon, C.E.G. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2. Magn. Reson. Imaging 2015, 33, 559–565. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, J.; Liang, J.; Ma, M.; Zhao, Q.; Lei, X.; Shi, C.; Luo, L. Evaluation of abnormal iron distribution in specific regions in the brains of patients with Parkinson’s disease using quantitative susceptibility mapping and R2* mapping. Exp. Ther. Med. 2020, 19, 3778–3786. [Google Scholar] [CrossRef] [PubMed]
- Wieler, M.; Gee, M.; Martin, W.W. Longitudinal midbrain changes in early Parkinson’s disease: Iron content estimated from R2 */MRI. Park. Relat. Disord. 2015, 21, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Lewis, M.M.; Styner, M.; Shaffer, M.L.; Sen, S.; Yang, Q.X.; Huang, X. Combined R2 * and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov. Disord. 2011, 26, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Langkammer, C.; Pichler, A.; Pinter, D.; Gattringer, T.; Bachmaier, G.; Ropele, S.; Fuchs, S.; Enzinger, C.; Fazekas, F. Dynamics of brain iron levels in multiple sclerosis: A longitudinal 3T MRI study. Neurology 2015, 84, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Langkammer, C.; Ropele, S.; Petrovic, K.; Wallner-Blazek, M.; Loitfelder, M.; Jehna, M.; Bachmaier, G.; Schmidt, R.; Enzinger, C. Determinants of brain iron in multiple sclerosis: A quantitative 3T MRI study. Neurology 2011, 77, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.J.; Blevins, G.; Lebel, R.M.; Seres, P.; Emery, D.J.; Wilman, A.H. Longitudinal MR imaging of iron in multiple sclerosis: An imaging marker of disease. Radiology 2014, 270, 186–196. [Google Scholar] [CrossRef]
- Paling, D.; Tozer, D.; Wheeler-Kingshott, C.; Kapoor, R.; Miller, D.H.; Golay, X. Reduced R2′ in multiple sclerosis normal appearing white matter and lesions may reflect decreased myelin and iron content. J. Neurol. Neurosurg. Psychiatry 2012, 83, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Curt, A.; Thompson, A.J.; Grabher, P.; Weiskopf, N.; Freund, P. Quantitative MRI of rostral spinal cord and brain regions is predictive of functional recovery in acute spinal cord injury. NeuroImage: Clin. 2018, 20, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Blomster, L.V.; Cowin, G.J.; Kurniawan, N.D.; Ruitenberg, M.J. Detection of endogenous iron deposits in the injured mouse spinal cord through high-resolution ex vivo and in vivo MRI. NMR Biomed. 2013, 26, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Mittal, S.; Wu, Z.; Neelavalli, J.; Cheng, Y.-C.N. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 1. Am. J. Neuroradiol. 2009, 30, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Wu, Z.; Neelavalli, J.; Haacke, E.M. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 2. Am. J. Neuroradiol. 2009, 30, 232–252. [Google Scholar] [CrossRef] [PubMed]
- Halefoglu, A.M.; Yousem, D.M. Susceptibility weighted imaging: Clinical applications and future directions. World J. Radiol. 2018, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Xu, Y.; Cheng, Y.C.N.; Reichenbach, J.R. Susceptibility weighted imaging (SWI). Magn. Reson. Med. 2004, 52, 612–618. [Google Scholar] [CrossRef]
- Yuste, R.; Majewska, A.; Holthoff, K. From form to function: Calcium compartmentalization in dendritic spines. Nat. Neurosci. 2000, 3, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Burnashev, N.; Rozov, A. Presynaptic Ca2+ dynamics, Ca2+ buffers and synaptic efficacy. Cell Calcium 2005, 37, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Konnerth, A. Determinants of postsynaptic Ca2+ signaling in Purkinje neurons. Cell Calcium 2005, 37, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, C.; Nunez, M.T. Calcium, iron and neuronal function. IUBMB Life 2007, 59, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, C.; Carrasco, M.A.; Muñoz, P.; Núñez, M.T. A role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antioxid. Redox Signal. 2007, 9, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Humeres, A.; Elgueta, C.; Kirkwood, A.; Hidalgo, C.; Núñez, M.T. Iron mediates N-methyl-D-aspartate receptor-dependent stimulation of calcium-induced pathways and hippocampal synaptic plasticity. J. Biol. Chem. 2011, 286, 13382–13392. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Hogan, E.; Gadsden Sr, R.; Spicer, K.; Shi, M.; Cox, R. Vascular permeability in experimental spinal cord injury. J. Neurol. Sci. 1985, 70, 275–282. [Google Scholar] [CrossRef]
- Happel, R.D.; Smith, K.P.; Banik, L.N.; Powers, M.J.; Hogan, E.L.; Balentine, J.D. Ca2+—Accumulation in experimental spinal cord trauma. Brain Res. 1981, 211, 476–479. [Google Scholar] [CrossRef]
- Young, W.; Koreh, I. Potassium and calcium changes in injured spinal cords. Brain Res. 1986, 365, 42–53. [Google Scholar] [CrossRef]
- Mohammed, W.; Xunning, H.; Haibin, S.; Jingzhi, M. Clinical applications of susceptibility-weighted imaging in detecting and grading intracranial gliomas: A review. Cancer Imaging 2013, 13, 186–195. [Google Scholar] [CrossRef]
- Wu, Z.; Mittal, S.; Kish, K.; Yu, Y.; Hu, J.; Haacke, E.M. Identification of calcification with MRI using susceptibility-weighted imaging: A case study. J. Magn. Reson. Imaging 2009, 29, 177–182. [Google Scholar] [CrossRef]
- Robinson, R.J.; Bhuta, S. Susceptibility-Weighted Imaging of the Brain: Current Utility and Potential Applications. J. Neuroimaging 2011, 21, e189–e204. [Google Scholar] [CrossRef]
- Thomas, B.; Somasundaram, S.; Thamburaj, K.; Kesavadas, C.; Kumar Gupta, A.; Bodhey, N.K.; Raman Kapilamoorthy, T. Clinical applications of susceptibility weighted MR imaging of the brain—A pictorial review. Neuroradiology 2008, 50, 105–116. [Google Scholar] [CrossRef]
- Nair, J.R.; Van Hecke, W.; De Belder, F.; Venstermans, C.; van den Hauwe, L.; Van Goethem, J.; Parizel, P.M. High-Resolution Susceptibility-Weighted Imaging at 3 T With a 32-Channel Head Coil: Technique and Clinical Applications. Am. J. Roentgenol. 2010, 195, 1007–1014. [Google Scholar] [CrossRef]
- Benzel, E.C.; Lancon, J.; Kesterson, L.; Hadden, T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J. Spinal Disord. 1991, 4, 286–295. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, M.; Beebe, A. The Numeric Pain Rating Scale Instructions Pain: Clinic Manual for Nursing Practice; 1989; Available online: http://nperesource.casn.ca/wp-content/uploads/2017/02/Numeric-Pain-Rating-Scale-Instructions.pdf (accessed on 24 August 2022).
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Devlin, N.; Parkin, D.; Janssen, B. An introduction to EQ-5D instruments and their applications. In Methods for Analysing and Reporting EQ-5D Data; Springer: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar]
- Nurjck, S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain 1972, 95, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Chiles, B.W., III; Leonard, M.A.; Choudhri, H.F.; Cooper, P.R. Cervical spondylotic myelopathy: Patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery 1999, 44, 762–769. [Google Scholar] [CrossRef] [PubMed]
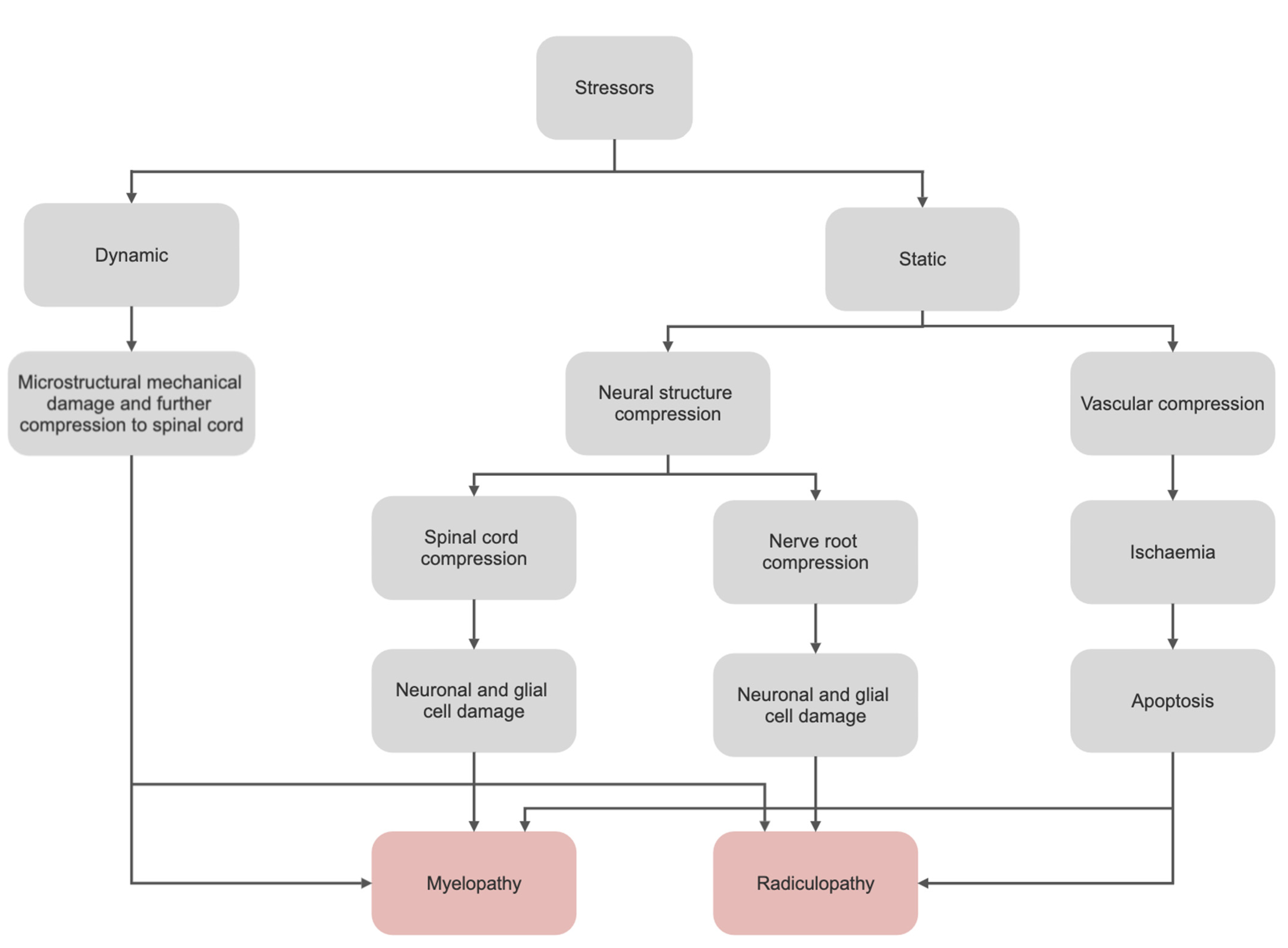
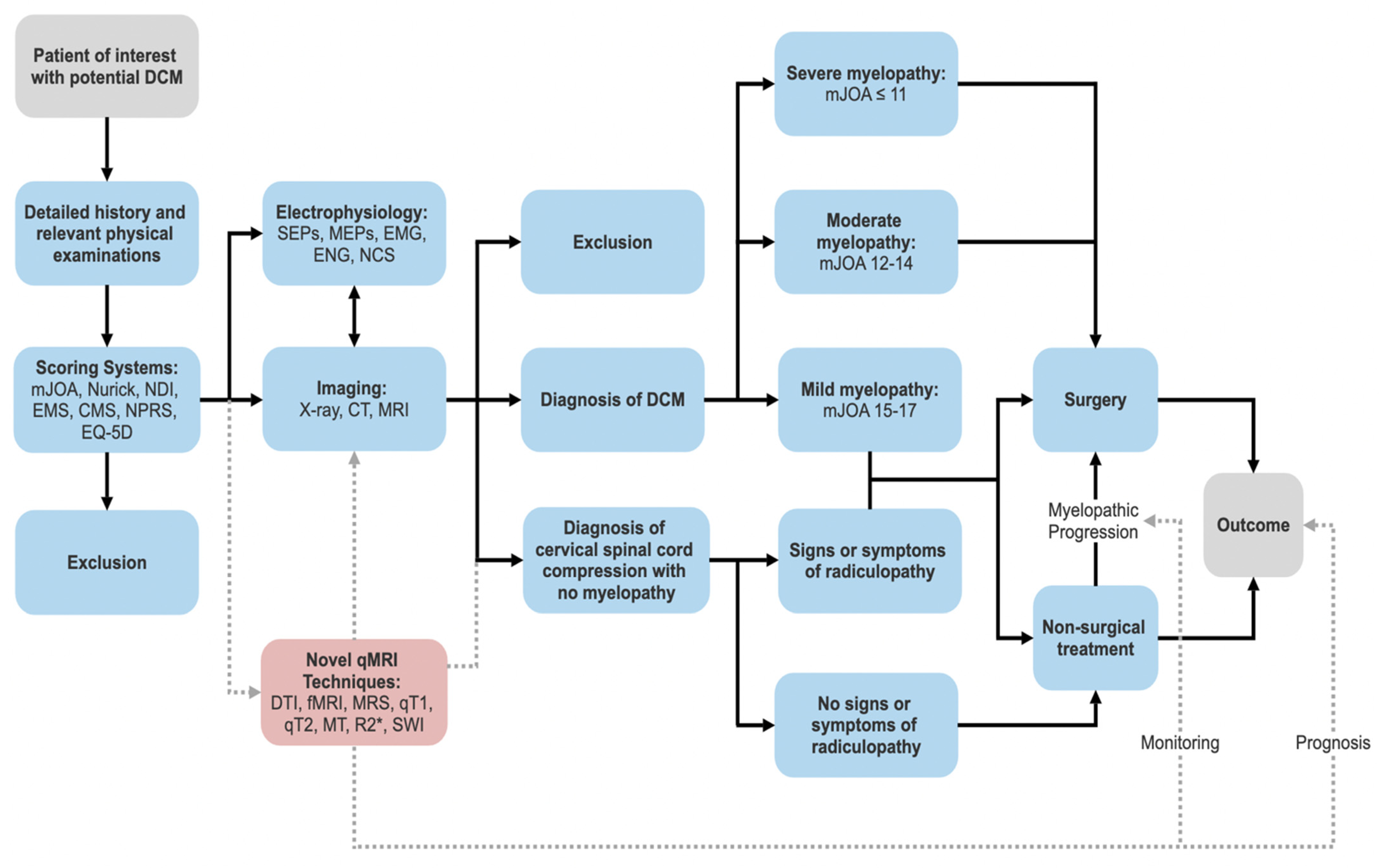
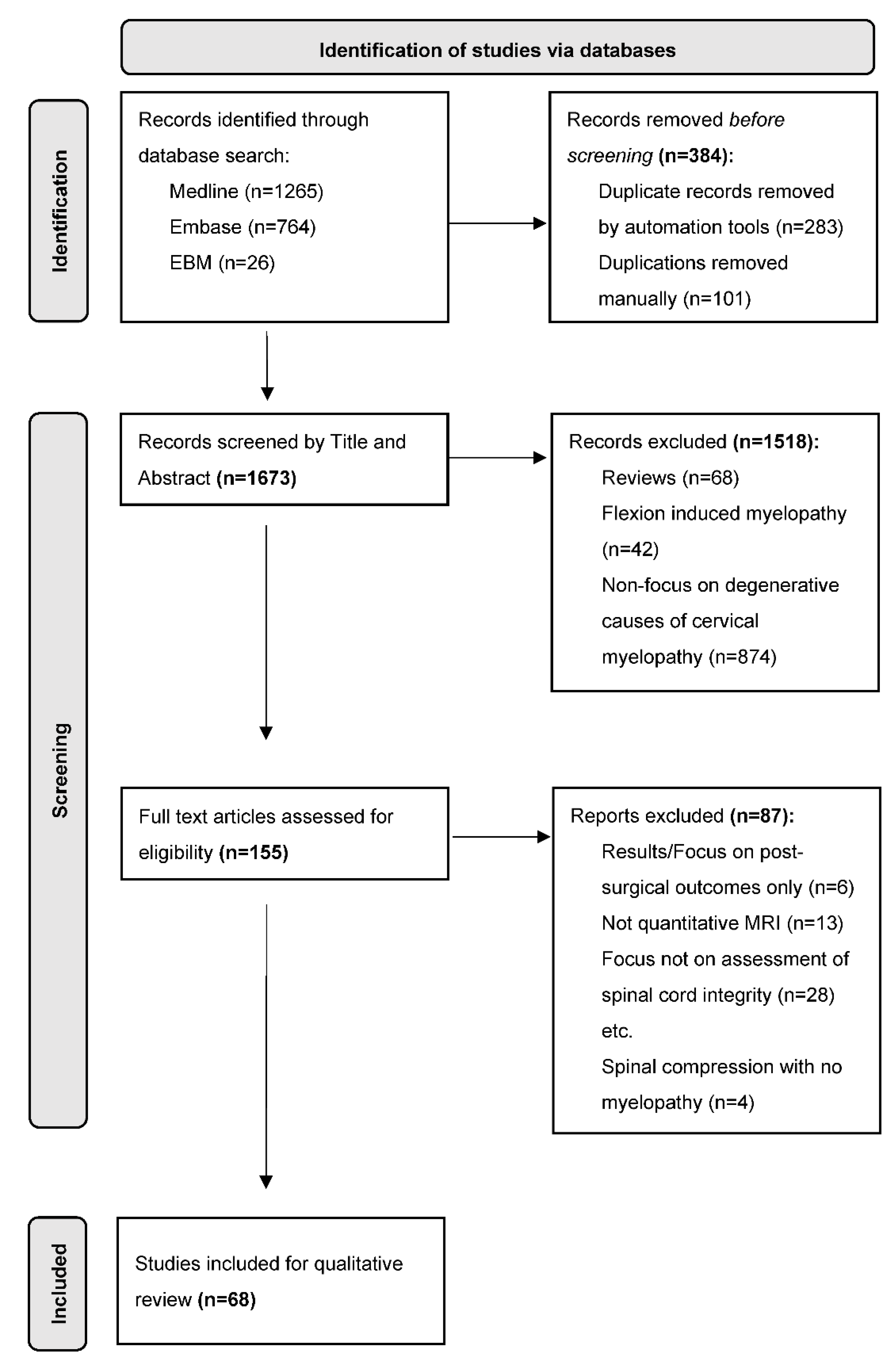
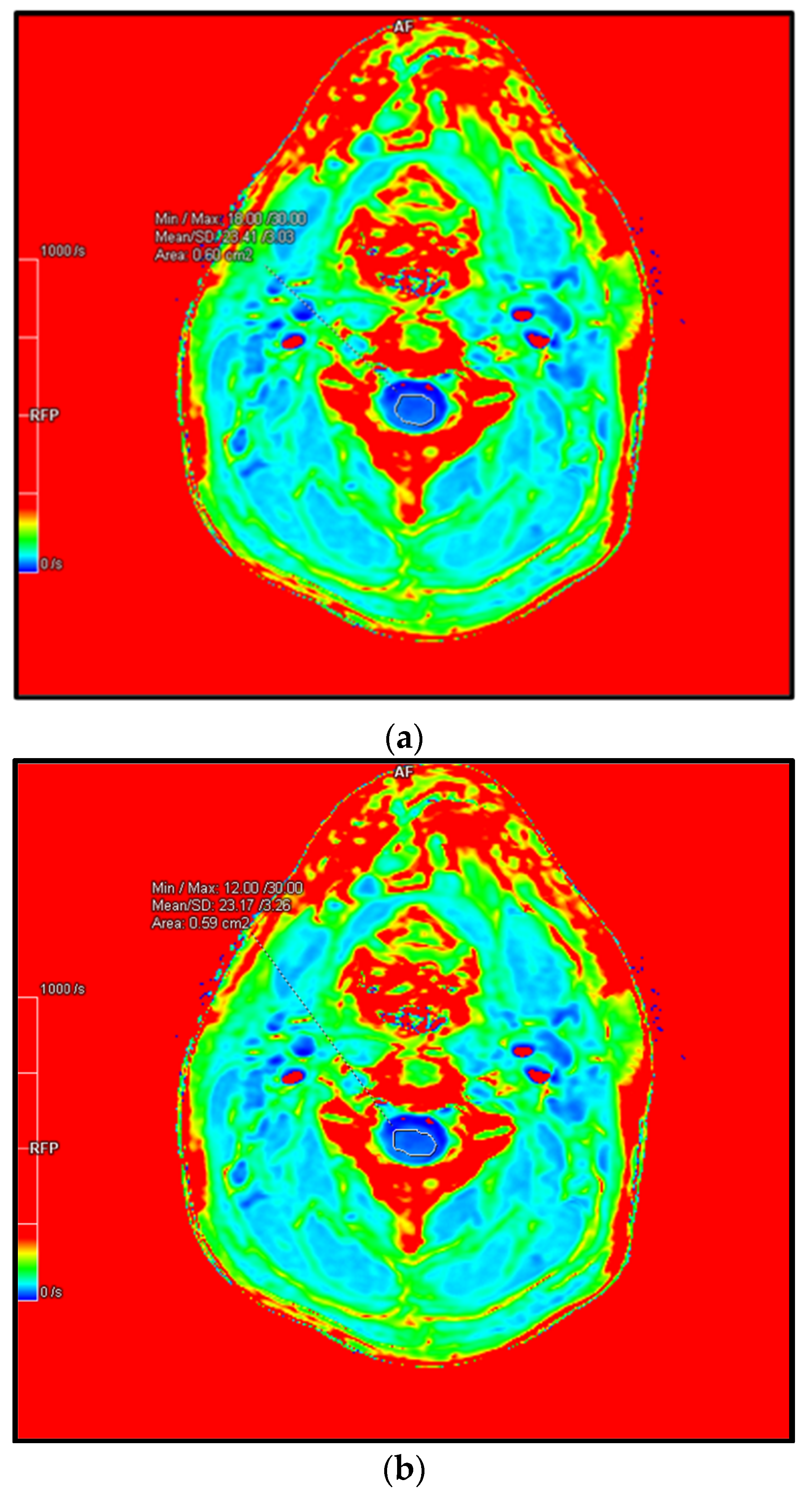
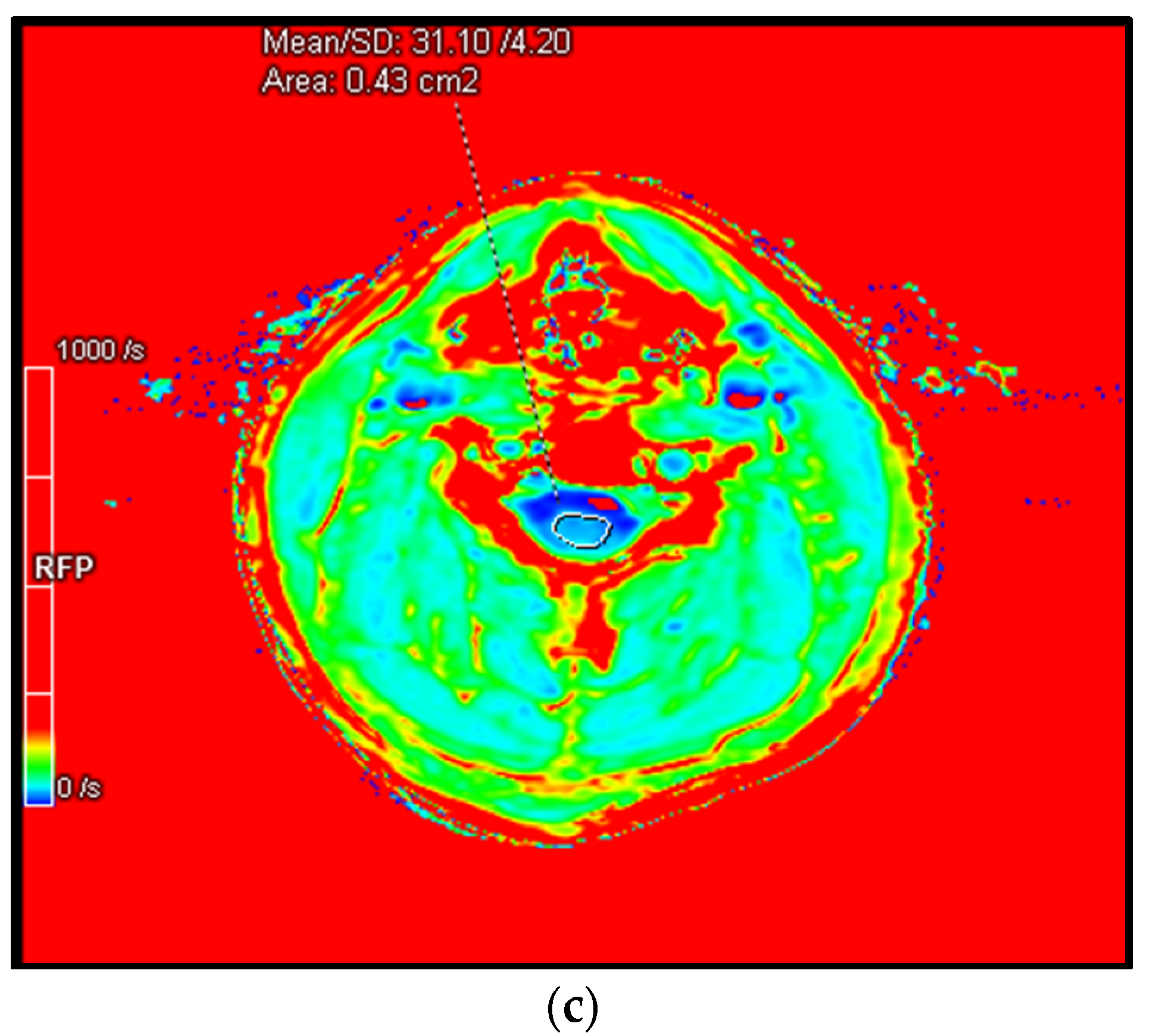
| Presenting Symptoms | Physical Signs | |
|---|---|---|
| Neck |
|
|
| Upper Limb |
|
|
| Lower Limb |
|
|
| Urinary/defecatory |
|
|
| System | Description | Benefits | Limitations |
|---|---|---|---|
| mJOA scale |
|
|
|
| Nurick scale |
|
|
|
| NDI |
|
|
|
| EMS |
|
|
|
| CMS |
|
|
|
| NPRS |
|
|
|
| EQ-5D |
|
|
|
| Additional scales that provide useful information in the context of DCM include the Myelopathy Disability Index, QuickDASH (assesses arm, shoulder and hand disability), the 30-Metre-Walk test, the Berg Balance Scale, GAITRite (a temporospatial gait analysis) and the Graded Redefined Assessment of Strength Sensibility and Prehension Myelopathy (GRASSP-M). | |||
| Sequence | Function | Quantitative Metrics | |
|---|---|---|---|
| Quantitative T1/T2 Mapping | Calculates the T1/T2 time of certain tissues and displays them on a parametric map. Reveals information about microstructural changes related to water, lipid, protein and iron content of tissues. | T1/T2 relaxation time | |
| DWI | DTI | Estimates the integrity of tissue microstructure through the modelling of water diffusion within the tissue. | FA [f], ADC, MD [g] |
| DTT | Tracks nerve fibres based on their FA values and can be elicited when fibres become interrupted, distorted or disorientated depending on the severity of spinal compression. | Volume and number of fibres | |
| DBSI | Quantifies axonal injury, inflammation and demyelination in DCM | Axonal injury, inflammation, demyelination. | |
| fMRI (BOLD) | Measures neuronal activity through associated changes detected in blood flow | FC, VOA | |
| MT | Provides information on the spinal cord structural integrity and derive information regarding myelination status | MTR | |
| MRS | Sensitive to metabolic changes that occur in pathology, reflecting important underlying biological mechanisms | Metabolite concentrations | |
| T2*-weighted imaging | Quantifies observable or effective T2 and is utilised to detect deoxyhaemoglobin, hemosiderin or methemoglobin in tissues and lesions. | R2* (=1/T2*) | |
| SWI/QSM | Sensitive to compounds that distort the magnetic field and alter phase of tissue and is therefore commonly used to detect blood products/haemorrhage and calcium | Tissue susceptibility | |
| qMRI Technique Utilised | Number of Studies | Overall Findings from the Included Literature |
|---|---|---|
| Quantitative T1 | 2 |
|
| Quantitative T2 | 0 | Nil |
| DTI | 43 |
|
| fMRI (BOLD) | 15 |
|
| MRS | 6 |
|
| MT | 4 |
|
| R2* or 1/T2* | 0 | Nil |
| SWI | 0 | Nil |
| Measurements | Min/Max (×10−3) | Mean (×10−3) | Standard Deviation (×10−3) | Area (cm2) |
|---|---|---|---|---|
| FA | 219/1000 | 629.16 | 201.72 | 0.35 |
| ADC | 186/1222 | 752.89 | 238.79 | 0.35 |
| Cervical Level | Min/Max (1/s) | Mean (1/s) | Standard Deviation | Area (cm2) |
|---|---|---|---|---|
| C2/3 | 18.00/30.00 | 23.41 | 3.03 | 0.60 |
| C3/4 | 12.00/30.00 | 23.17 | 3.26 | 0.59 |
| C4/5 | 18.00/44.00 | 31.40 | 4.20 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Sheldrick, K.; Das, A.; Diwan, A. Clinical and Research MRI Techniques for Assessing Spinal Cord Integrity in Degenerative Cervical Myelopathy—A Scoping Review. Biomedicines 2022, 10, 2621. https://doi.org/10.3390/biomedicines10102621
He B, Sheldrick K, Das A, Diwan A. Clinical and Research MRI Techniques for Assessing Spinal Cord Integrity in Degenerative Cervical Myelopathy—A Scoping Review. Biomedicines. 2022; 10(10):2621. https://doi.org/10.3390/biomedicines10102621
Chicago/Turabian StyleHe, Brandon, Kyle Sheldrick, Abhirup Das, and Ashish Diwan. 2022. "Clinical and Research MRI Techniques for Assessing Spinal Cord Integrity in Degenerative Cervical Myelopathy—A Scoping Review" Biomedicines 10, no. 10: 2621. https://doi.org/10.3390/biomedicines10102621
APA StyleHe, B., Sheldrick, K., Das, A., & Diwan, A. (2022). Clinical and Research MRI Techniques for Assessing Spinal Cord Integrity in Degenerative Cervical Myelopathy—A Scoping Review. Biomedicines, 10(10), 2621. https://doi.org/10.3390/biomedicines10102621







