Identification of COPD Inflammatory Endotypes Using Repeated Sputum Eosinophil Counts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Sputum Measurements
2.4. qPCR Detection of Common Respiratory Pathogens
2.5. Blood Measurements
2.6. Statistical Analysis
3. Results
3.1. Repeated Sputum Eosinophils Counts
3.2. Sputum Eosinophil Counts and BEC
3.3. Bacterial Colonisation and Sputum Eosinophil Counts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodruff, P.G.; Agusti, A.; Roche, N.; Singh, D.; Martinez, F.J. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: Making progress towards personalised management. Lancet 2015, 385, 1789–1798. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Roche, N.; Halpin, D.; Agusti, A.; Wedzicha, J.A.; Martinez, F.J. Current Controversies in the Pharmacological Treatment of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 194, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Monteiro, W.; Ward, R.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, I.D. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2000, 356, 1480–1485. [Google Scholar] [CrossRef]
- Brightling, C.E.; McKenna, S.; Hargadon, B.; Birring, S.; Green, R.; Siva, R.; Berry, M.; Parker, D.; Monteiro, W.; Pavord, I.D.; et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005, 60, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Bafadhel, M.; Brightling, C.E.; Sciurba, F.C.; Curtis, J.L.; Martinez, F.J.; Pasquale, C.B.; Merrill, D.D.; Metzdorf, N.; Petruzzelli, S.; et al. Blood Eosinophil Counts in Clinical Trials for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 660–671. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Higham, A.; Beech, A.; Wolosianka, S.; Jackson, N.; Long, G.; Kolsum, U.; Southworth, T.; Pham, T.H.; Sridhar, S.; McCrae, C.; et al. Type 2 inflammation in eosinophilic chronic obstructive pulmonary disease. Allergy 2021, 76, 1861–1864. [Google Scholar] [CrossRef]
- Kolsum, U.; Damera, G.; Pham, T.H.; Southworth, T.; Mason, S.; Karur, P.; Newbold, P.; Singh, D. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J. Allergy Clin. Immunol. 2017, 140, 1181–1184.e7. [Google Scholar] [CrossRef] [Green Version]
- Beech, A.; Lea, S.; Li, J.; Jackson, N.; Mulvanny, A.; Singh, D. Airway Bacteria Quantification Using Polymerase Chain Reaction Combined with Neutrophil and Eosinophil Counts Identifies Distinct COPD Endotypes. Biomedicines 2021, 9, 1337. [Google Scholar] [CrossRef]
- Farrier, J.N.; Farrier, S.; Haworth, S.; Beech, A.N. Can we justify the continued use of botulinum toxin A in the management of myofascial pain? Br. J. Oral Maxillofac. Surg. 2020, 58, 1133–1138. [Google Scholar] [CrossRef]
- Wang, Z.; Locantore, N.; Haldar, K.; Ramsheh, M.Y.; Beech, A.S.; Ma, W.; Brown, J.R.; Tal-Singer, R.; Barer, M.R.; Bafadhel, M.; et al. Inflammatory Endotype-associated Airway Microbiome in Chronic Obstructive Pulmonary Disease Clinical Stability and Exacerbations: A Multicohort Longitudinal Analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 1488–1502. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Faner, R.; Oscullo, G.; de la Rosa, D.; Soler-Cataluna, J.J.; Ballester, M.; Agusti, A. Inhaled Steroids, Circulating Eosinophils, Chronic Airway Infection, and Pneumonia Risk in Chronic Obstructive Pulmonary Disease. A Network Analysis. Am. J. Respir. Crit. Care Med. 2020, 201, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Martinez, F.J.; Papi, A.; Pavord, I.D.; Wedzicha, J.A.; Vogelmeier, C.F.; Halpin, D.M.G. Blood Eosinophils and Chronic Obstructive Pulmonary Disease: A GOLD Science Committee 2022 Review. Am. J. Respir. Crit. Care Med. 2022, 206, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Oshagbemi, O.A.; Burden, A.M.; Braeken, D.C.W.; Henskens, Y.; Wouters, E.F.M.; Driessen, J.H.M.; Maitland-van der Zee, A.H.; de Vries, F.; Franssen, F.M.E. Stability of Blood Eosinophils in Patients with Chronic Obstructive Pulmonary Disease and in Control Subjects, and the Impact of Sex, Age, Smoking, and Baseline Counts. Am. J. Respir. Crit. Care Med. 2017, 195, 1402–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.K.; Lee, J.K.; Lee, C.H.; Hwang, Y.I.; Kim, H.; Park, D.; Hwang, K.E.; Kim, S.H.; Jung, K.S.; Yoo, K.H.; et al. The Association Between Eosinophil Variability Patterns and the Efficacy of Inhaled Corticosteroids in Stable COPD Patients. Int. J. Chronic. Obstr. Pulm. Dis. 2020, 15, 2061–2070. [Google Scholar] [CrossRef]
- Long, G.H.; Southworth, T.; Kolsum, U.; Donaldson, G.C.; Wedzicha, J.A.; Brightling, C.E.; Singh, D. The stability of blood Eosinophils in chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 15. [Google Scholar] [CrossRef]
- Southworth, T.; Beech, G.; Foden, P.; Kolsum, U.; Singh, D. The reproducibility of COPD blood eosinophil counts. Eur. Respir. J. 2018, 52, 1800427. [Google Scholar] [CrossRef]
- Higham, A.; Leow-Dyke, S.; Jackson, N.; Singh, D. Stability of Eosinophilic Inflammation in COPD Bronchial Biopsies. Eur. Respir. J. 2020, 56, 2000622. [Google Scholar] [CrossRef]
- Ahmed, A.U. An Overview of Inflammation: Mechanism and Consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Mulvanny, A.; Pattwell, C.; Beech, A.; Southworth, T.; Singh, D. Validation of Sputum Biomarker Immunoassays and Cytokine Expression Profiles in COPD. Biomedicines 2022, 10, 1949. [Google Scholar] [CrossRef] [PubMed]
- Hajiro, T.; Nishimura, K.; Tsukino, M.; Ikeda, A.; Koyama, H.; Izumi, T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, 1185–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M.; Littlejohns, P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992, 145, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Bafadhel, M.; McCormick, M.; Saha, S.; McKenna, S.; Shelley, M.; Hargadon, B.; Mistry, V.; Reid, C.; Parker, D.; Dodson, P.; et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration 2012, 83, 36–44. [Google Scholar] [CrossRef]
- Pizzichini, E.; Pizzichini, M.M.; Gibson, P.; Parameswaran, K.; Gleich, G.J.; Berman, L.; Dolovich, J.; Hargreave, F.E. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am. J. Respir. Crit. Care Med. 1998, 158, 1511–1517. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Boorsma, M.; Lutter, R.; van de Pol, M.A.; Out, T.A.; Jansen, H.M.; Jonkers, R.E. Repeatability of inflammatory parameters in induced sputum of COPD patients. COPD J. Chronic Obstr. Pulm. Dis. 2007, 4, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Monterio, W.; Green, R.H.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, D. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: Safety and repeatability. Respir. Med. 2001, 95, 999–1002. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bafadhel, M.; Haldar, K.; Spivak, A.; Mayhew, D.; Miller, B.E.; Tal-Singer, R.; Johnston, S.L.; Ramsheh, M.Y.; Barer, M.R.; et al. Lung microbiome dynamics in COPD exacerbations. Eur. Respir. J. 2016, 47, 1082–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southworth, T.; Higham, A.; Kolsum, U.; Li, J.; Scott, T.; Dungwa, J.; Sridhar, S.; Pham, T.H.; Newbold, P.; Singh, D. The relationship between airway immunoglobulin activity and eosinophils in COPD. J. Cell Mol. Med. 2021, 25, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Beech, A.S.; Lea, S.; Kolsum, U.; Wang, Z.; Miller, B.E.; Donaldson, G.C.; Wedzicha, J.A.; Brightling, C.E.; Singh, D. Bacteria and sputum inflammatory cell counts; a COPD cohort analysis. Respir. Res. 2020, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Van Rossem, I.; Hanon, S.; Verbanck, S.; Vanderhelst, E. Blood Eosinophil Counts in Chronic Obstructive Pulmonary Disease: Adding Within-Day Variability to the Equation. Am. J. Respir. Crit. Care Med. 2022, 205, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Barker, B.L.; Mistry, V.; Pancholi, M.; Brightling, C.; Bafadhel, M. Are sputum and blood biomarkers of inflammation repeatable in stable COPD? Thorax 2012, 67, A155–A156. [Google Scholar] [CrossRef] [Green Version]
- Landis, S.H.; Suruki, R.; Hilton, E.; Compton, C.; Galwey, N.W. Stability of Blood Eosinophil Count in Patients with COPD in the UK Clinical Practice Research Datalink. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 382–388. [Google Scholar] [CrossRef]
- Bafadhel, M.; Pavord, I.D.; Russell, R.E.K. Eosinophils in COPD: Just another biomarker? Lancet Respir. Med. 2017, 5, 747–759. [Google Scholar] [CrossRef]
- McCulloch, E.; Lucas, C.; Ramage, G.; Williams, C. Improved early diagnosis of Pseudomonas aeruginosa by real-time PCR to prevent chronic colonisation in a paediatric cystic fibrosis population. J. Cyst. Fibros. 2011, 10, 21–24. [Google Scholar] [CrossRef]
- Garcha, D.S.; Thurston, S.J.; Patel, A.R.; Mackay, A.J.; Goldring, J.J.; Donaldson, G.C.; McHugh, T.D.; Wedzicha, J.A. Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 2012, 67, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
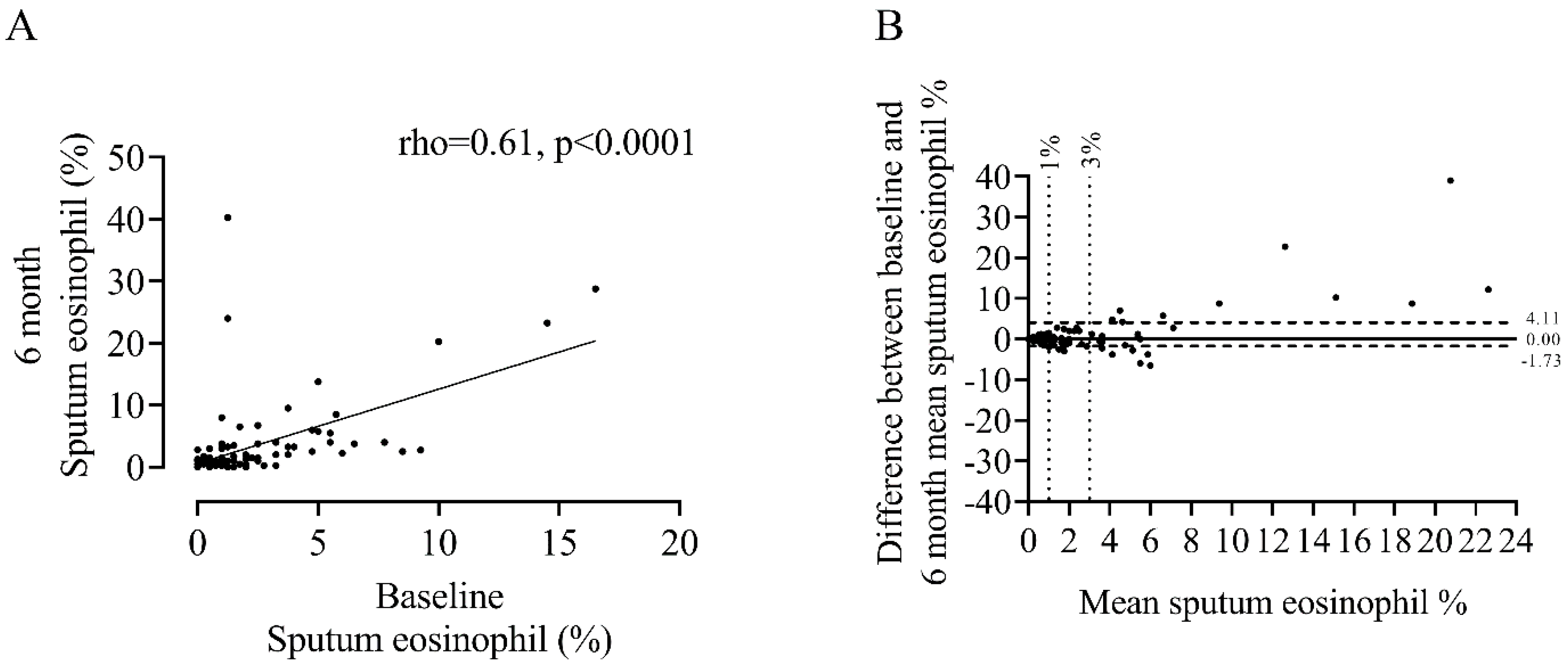
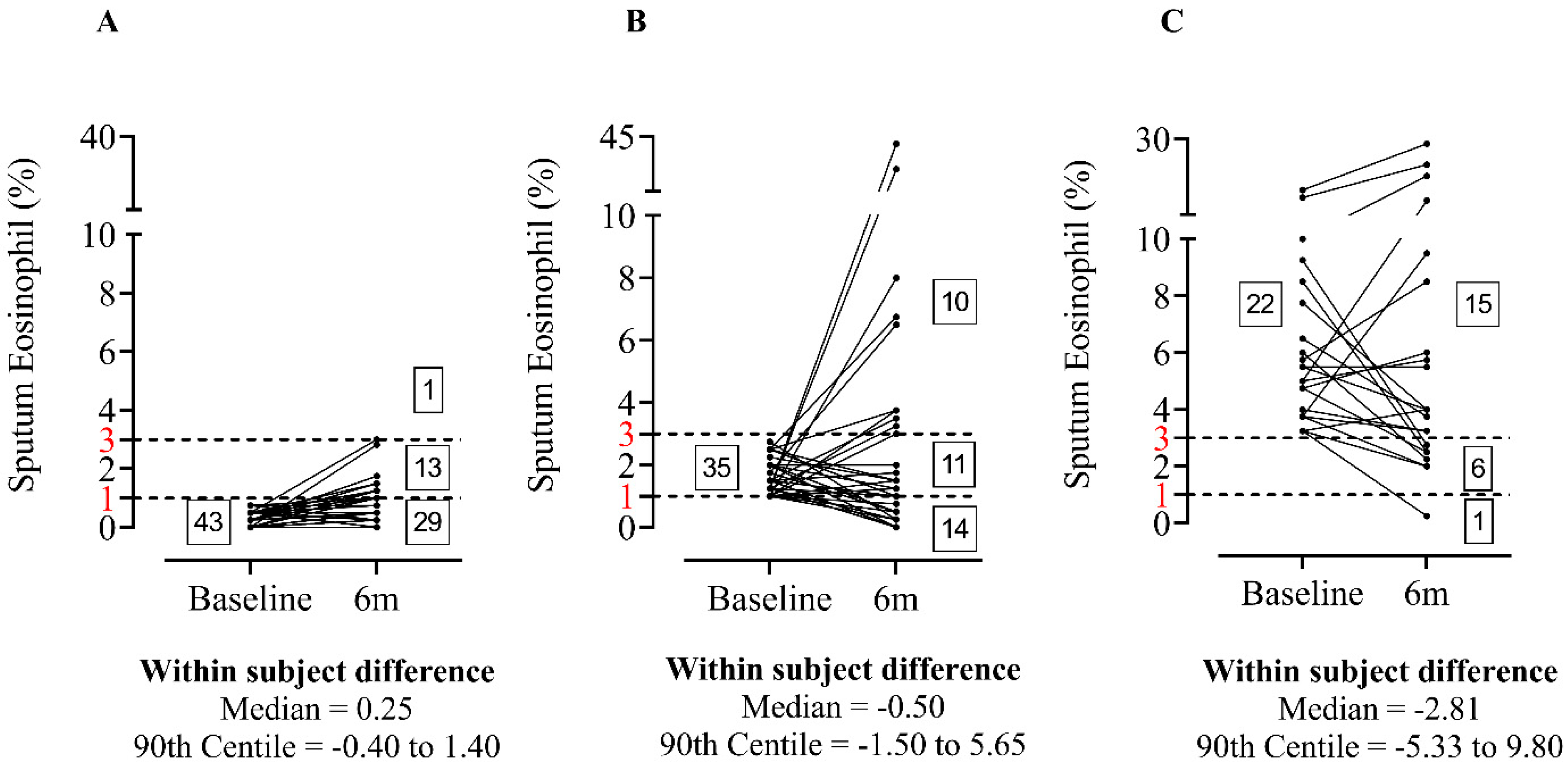


| Characteristic | n = 100 |
|---|---|
| Gender n (Female/Male) | 34/66 |
| Age | 65.2 (7.5) |
| Smoking status (Current %) | 42.0 |
| Pack years | 42.3 (18.9) |
| BMI (kg/m2) | 27.8 (5.1) |
| Exacerbations (1 year period) | 0.89 (1.2) |
| 0 (%) | 52.4 |
| 1 (%) | 23.8 |
| ≥2 (%) | 23.8 |
| Post FEV1 (L) | 1.8 (0.5) |
| Post FEV1 (%) | 63.4 (17.4) |
| GOLD Category (%) | |
| 1 | 16.0 |
| 2 | 65.0 |
| 3 | 19.0 |
| 4 | 0.0 |
| mMRC | 3.0 [0.0–4.0] |
| CAT | 19.3 (7.4) |
| SGRQ-C (Total) | 47.7 (18.3) |
| Atopy (%) | 10.0 |
| Chronic bronchitis (%) | 74.6 |
| ICS Use (%) | 66.0 |
| LABA + LAMA + ICS (%) | 53.0 |
| LABA + LAMA (%) | 7.0 |
| ICS only (%) | 2.0 |
| LABA only (%) | 1.0 |
| LAMA only (%) | 14.0 |
| No inhaled medication (%) | 9.0 |
| Sputum characteristics | |
| Sputum total cell count × 106/g | 7.49 [0.62–100.9] |
| Sputum Neutrophil (%) | 73.63 [15.25–99.50] |
| Sputum Eosinophil (%) | 1.00 [0.00–16.50] |
| Sputum Lymphocyte (%) | 0.25 [0.00–4.75] |
| Sputum Macrophage (%) | 18.00 [0.50–79.50] |
| Sputum Epithelial Cells (%) | 2.13 [0.00–5.25] |
| Sputum Neutrophil cell count × 106/g | 4.65 [0.03–98.08] |
| Sputum Eosinophil cell count × 106/g | 0.08 [0.00–2.45] |
| Sputum Lymphocyte cell count × 106/g | 0.01 [0.00–0.64] |
| Sputum Macrophage cell count × 106/g | 1.22 [0.04–10.13] |
| Sputum Epithelial cell count × 106/g | 0.13 [0.00–2.45] |
| Baseline Sputum Characteristic | EosinophilLOW n = 43 | EosinophilINT n = 35 | EosinophilHIGH n = 22 | p-Value |
|---|---|---|---|---|
| Sputum total cell count × 106/g | 8.53 [0.96–100.9] | 9.53 [0.62–58.78] | 6.30 [1.43–49.40] | 0.27 |
| Sputum Neutrophil (%) | 74.50 [15.25–99.50] | 77.75 [29.75–97.00] | 67.25 [24.25–87.50] | 0.13 |
| Sputum Eosinophil (%) | 0.25 [0.00–0.75] | 1.50 [1.00–2.75] *** | 5.25 [3.25–16.50] ***,+++ | <0.0001 |
| Sputum Lymphocyte (%) | 0.00 [0.00–4.75] | 0.25 [0.00–2.00] | 0.00 [0.00–3.50] | 0.79 |
| Sputum Macrophage (%) | 18.00 [0.50–79.50] | 15.25 [1.25–60.00] | 19.50 [1.50–54.00] | 0.62 |
| Sputum Epithelial Cells (%) | 1.25 [0.00–60.50] | 2.13 [0.00–40.50] | 2.75 [0.00–16.25] | 0.31 |
| Sputum Neutrophil cell count × 106/g | 5.15 [0.03–98.08] | 6.74 [0.32–57.01] | 2.90 [0.35–36.11] | 0.11 |
| Sputum Eosinophil cell count × 106/g | 0.03 [0.00–0.34] | 0.13 [0.00–0.59] *** | 0.29 [0.00–2.45] *** | <0.0001 |
| Sputum Lymphocyte cell count × 106/g | 0.02 [0.00–0.64] | 0.01 [0.00–0.26] | 0.00 [0.00–0.21] | 0.09 |
| Sputum Macrophage cell count × 106/g | 1.25 [0.04–10.13] | 1.24 [0.08–3.51] | 1.05 [0.10–3.41] | 0.54 |
| Sputum Epithelial cell count × 106/g | 0.12 [0.00–2.45] | 0.16 [0.00–1.95] | 0.13 [0.00–0.73] | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beech, A.; Jackson, N.; Singh, D. Identification of COPD Inflammatory Endotypes Using Repeated Sputum Eosinophil Counts. Biomedicines 2022, 10, 2611. https://doi.org/10.3390/biomedicines10102611
Beech A, Jackson N, Singh D. Identification of COPD Inflammatory Endotypes Using Repeated Sputum Eosinophil Counts. Biomedicines. 2022; 10(10):2611. https://doi.org/10.3390/biomedicines10102611
Chicago/Turabian StyleBeech, Augusta, Natalie Jackson, and Dave Singh. 2022. "Identification of COPD Inflammatory Endotypes Using Repeated Sputum Eosinophil Counts" Biomedicines 10, no. 10: 2611. https://doi.org/10.3390/biomedicines10102611
APA StyleBeech, A., Jackson, N., & Singh, D. (2022). Identification of COPD Inflammatory Endotypes Using Repeated Sputum Eosinophil Counts. Biomedicines, 10(10), 2611. https://doi.org/10.3390/biomedicines10102611





