Implantable Peripheral Nerve Stimulation for Peripheral Neuropathic Pain: A Systematic Review of Prospective Studies
Abstract
:1. Introduction
2. Materials and Methods
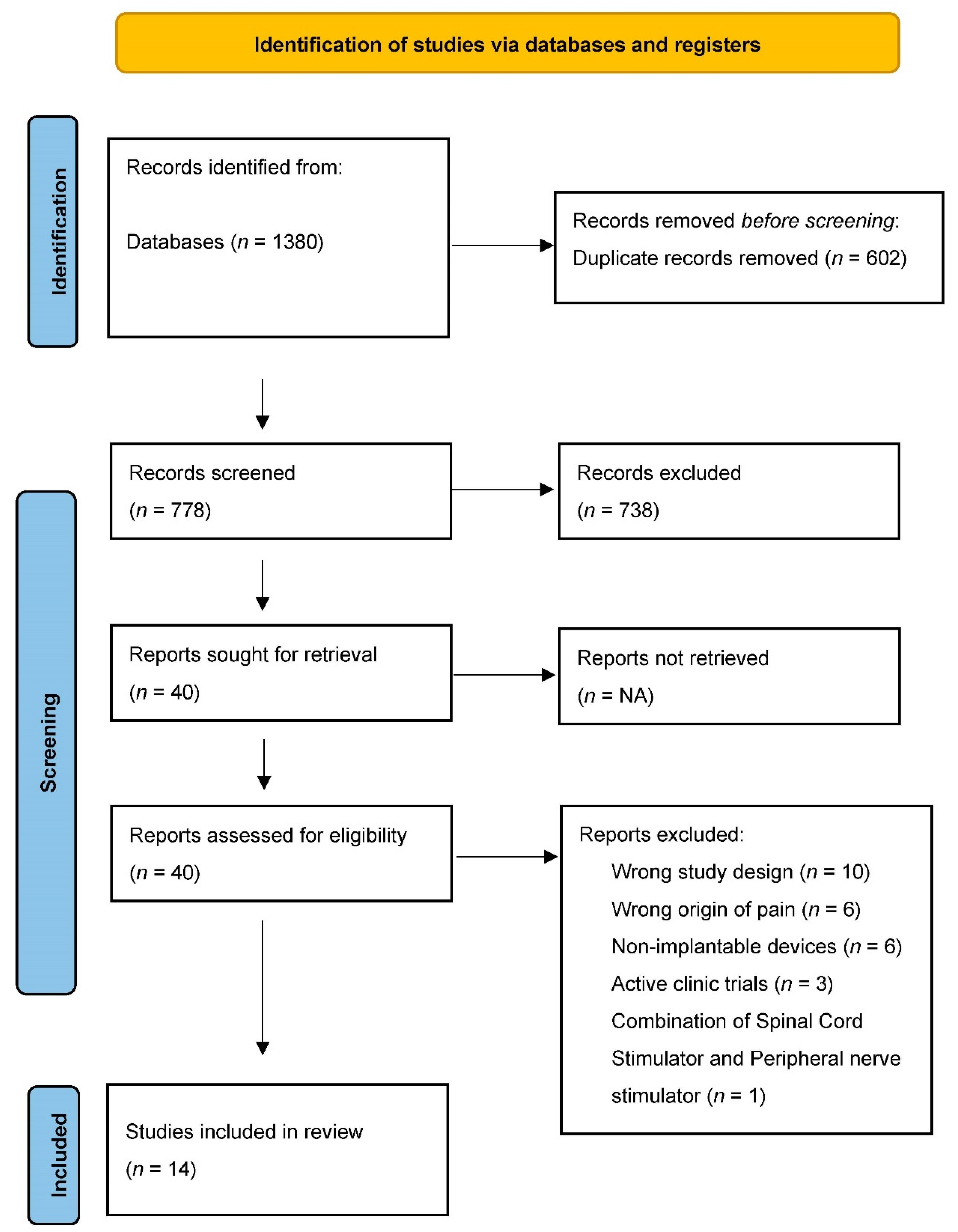
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
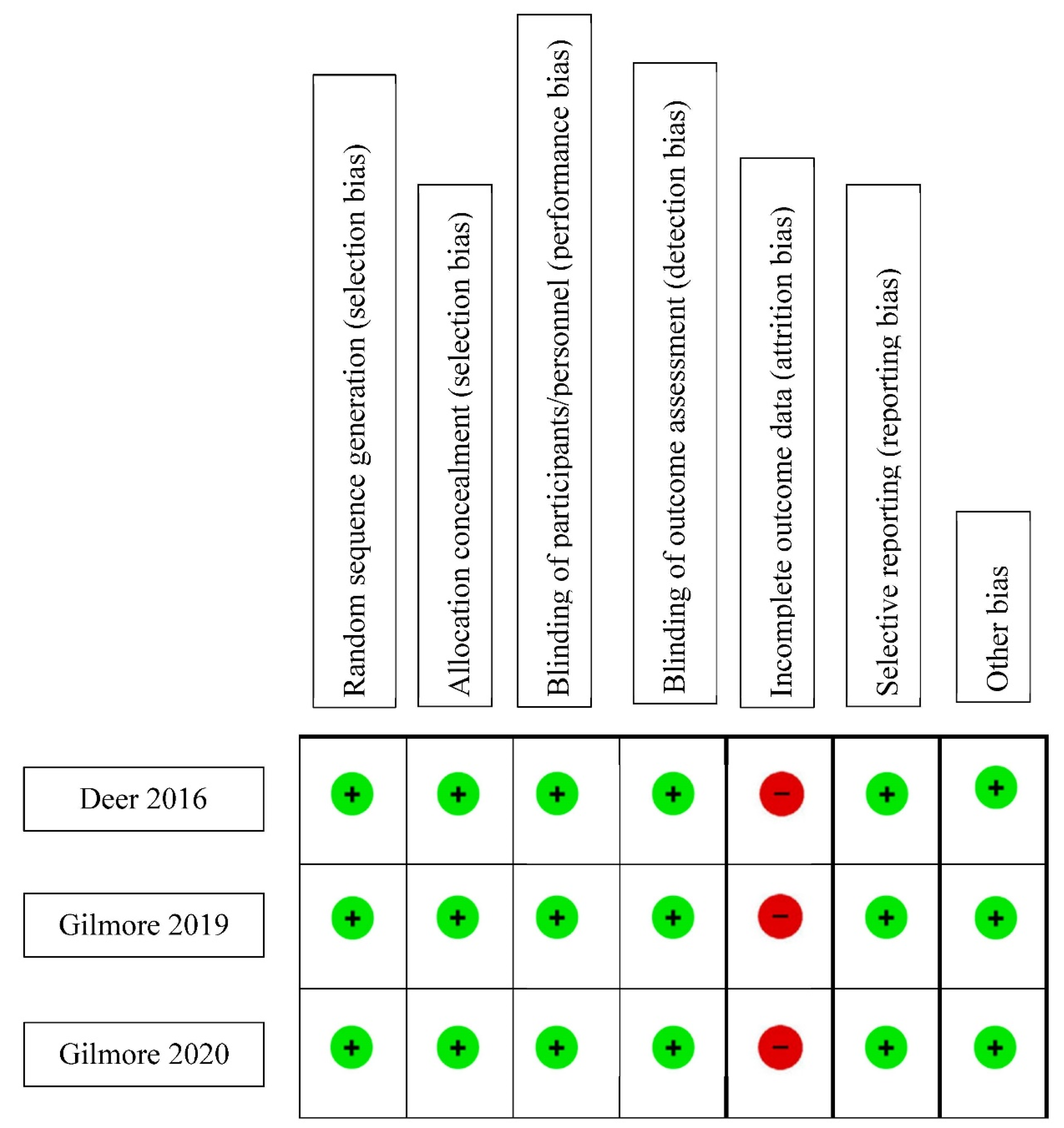
2.4. Assessment of Risk of Bias
2.5. Quality Assessment
3. Results
3.1. Type of Neuropathic Pain
3.1.1. Complex Regional Pain Syndrome (CRPS)
3.1.2. Shoulder Pain
3.1.3. Phantom Limb Pain (PLP)
3.1.4. Post-Surgical Pain
3.1.5. Mononeuropathy
3.1.6. Bias Assessment
3.1.7. Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shelden, C. Depolarization in the treatment of trigeminal neuralgia. Evaluation of compression and electrical methods; clinical concept of neurophysiological mechanism. In Pain; Little, Brown: Boston, MA, USA, 1966; pp. 373–386. [Google Scholar]
- Wall, P.D.; Sweet, W.H. Temporary Abolition of Pain in Man. Science 1967, 155, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Long, D.M. Peripheral nerve stimulation in the treatment of intractable pain. J. Neurosurg. 1976, 45, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Long, D.M. Electrical Stimulation for the Control of Pain. Arch. Surg. 1977, 112, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Sweet, W.H. Control of Pain by Direct Electrical Stimulation of Peripheral Nerves. Neurosurgery 1976, 23, 103–111. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; D’Souza, R.S. Peripheral Nerve Stimulation: The Evolution in Pain Medicine. Biomedicines 2021, 10, 18. [Google Scholar] [CrossRef]
- Weiner, R.L.; Reed, K.L.; Weiner, F.R.L. Peripheral Neurostimulation for Control of Intractable Occipital Neuralgia. Neuromodulation Technol. Neural Interface 1999, 2, 217–221. [Google Scholar] [CrossRef]
- Fontaine, D. Spinal cord stimulation for neuropathic pain. Rev. Neurol. 2021, 177, 838–842. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Kubrova, E.; Her, Y.F.; Barman, R.A.; Smith, B.J.; Alvarez, G.M.; West, T.E.; Abd-Elsayed, A. Dorsal Root Ganglion Stimulation for Lower Extremity Neuropathic Pain Syndromes: An Evidence-Based Literature Review. Adv. Ther. 2022, 39, 4440–4473. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.H.; D’Souza, R.; Wie, C.; Covington, S.; Maita, M.; Freeman, J.; Maloney, J. Mechanism of Action of Peripheral Nerve Stimulation. Curr. Pain Headache Rep. 2021, 25, 47. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Eldabe, S.; Falowski, S.M.; A Huntoon, M.; Staats, P.S.; Cassar, I.R.; Crosby, N.D.; Boggs, J.W. Peripherally Induced Reconditioning of the Central Nervous System: A Proposed Mechanistic Theory for Sustained Relief of Chronic Pain with Percutaneous Peripheral Nerve Stimulation. J. Pain Res. 2021, 14, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, C.A.; Kapural, L.; McGee, M.J.; Boggs, J. Percutaneous Peripheral Nerve Stimulation for Chronic Low Back Pain: Prospective Case Series With 1 Year of Sustained Relief Following Short-Term Implant. Pain Pract. 2019, 20, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Malinowski, M.N.; Chopra, P.R.; Francio, V.T.; Budwany, R.; Deer, T.R. A narrative review and future considerations of spinal cord stimulation, dorsal root ganglion stimulation and peripheral nerve stimulation. Curr. Opin. Anaesthesiol. 2021, 34, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Frederico, T.N.; Freitas, T.D.S. Peripheral Nerve Stimulation of the Brachial Plexus for Chronic Refractory CRPS Pain of the Upper Limb: Description of a New Technique and Case Series. Pain Med. 2020, 21 (Suppl. 1), S18–S26. [Google Scholar] [CrossRef]
- Oswald, J.; Shahi, V.; Chakravarthy, K.V. Prospective case series on the use of peripheral nerve stimulation for focal mononeuropathy treatment. Pain Manag. 2019, 9, 551–558. [Google Scholar] [CrossRef]
- Wilson, R.D.; Bennett, M.E.; Nguyen, V.Q.; Bock, W.C.; O’Dell, M.W.; Watanabe, T.K.; Amundson, R.H.; Hoyen, H.A.; Chae, J. Fully Implantable Peripheral Nerve Stimulation for Hemiplegic Shoulder Pain: A Multi-Site Case Series With Two-Year Follow-Up. Neuromodulation Technol. Neural Interface 2017, 21, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.D.S.; Fonoff, E.T.; Neto, O.R.M.; Kessler, I.M.; Barros, L.M.; Guimaraes, R.W.; Azevedo, M.F. Peripheral Nerve Stimulation for Painful Mononeuropathy Secondary to Leprosy: A 12-Month Follow-Up Study. Neuromodulation Technol. Neural Interface 2017, 21, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sokal, P.; Harat, M.; Zieliński, P.; Kieronska, S. Tibial nerve stimulation with a miniature, wireless stimulator in chronic peripheral neuropathic pain. J. Pain Res. 2017, 10, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Voorbrood, C.E.H.; Burgmans, J.P.J.; Van Dalen, T.; Breel, J.; Clevers, G.J.; Wille, F.; Simmermacher, R.K.J. An algorithm for assessment and treatment of postherniorrhaphy pain. Hernia 2015, 19, 571–577. [Google Scholar] [CrossRef]
- Wilson, R.D.; Harris, M.A.; Gunzler, D.D.; Bennett, M.E.; Chae, J. Percutaneous Peripheral Nerve Stimulation for Chronic Pain in Subacromial Impingement Syndrome: A Case Series. Neuromodulation Technol. Neural Interface 2014, 17, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevanato, G.; Devigili, G.; Eleopra, R.; Fontana, P.; Lettieri, C.; Baracco, C.; Guida, F.; Rinaldo, S.; Bevilacqua, M. Chronic post-traumatic neuropathic pain of brachial plexus and upper limb: A new technique of peripheral nerve stimulation. Neurosurg. Rev. 2014, 37, 473–480. [Google Scholar] [CrossRef]
- Rauck, R.L.; Cohen, S.P.; Gilmore, C.A.; North, J.M.; Kapural, L.; Zang, R.H.; Grill, J.H.; Boggs, J.W. Treatment of Post-Amputation Pain With Peripheral Nerve Stimulation. Neuromodulation Technol. Neural Interface 2013, 17, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Levy, R.M.; Rosenfeld, E.L. Prospective Clinical Study of a New Implantable Peripheral Nerve Stimulation Device to Treat Chronic Pain. Clin. J. Pain 2010, 26, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Hassenbusch, S.J.; Stanton-Hicks, M.; Schoppa, D.; Walsh, J.G.; Covington, E.C. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J. Neurosurg. 1996, 84, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, C.A.; Ilfeld, B.M.; Rosenow, J.M.; Li, S.; Desai, M.J.; Hunter, C.W.; Rauck, R.L.; Nader, A.; Mak, J.; Cohen, S.P.; et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg. Anesth. Pain Med. 2019, 45, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, C.; Ilfeld, B.; Rosenow, J.; Li, S.; Desai, M.; Hunter, C.; Rauck, R.; Kapural, L.; Nader, A.; Mak, J.; et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial. Reg. Anesth. Pain Med. 2019, 44, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.; Pope, J.; Benyamin, R.; Vallejo, R.; Friedman, A.; Caraway, D.; Staats, P.; Grigsby, E.; McRoberts, W.P.; McJunkin, T.; et al. Prospective, Multicenter, Randomized, Double-Blinded, Partial Crossover Study to Assess the Safety and Efficacy of the Novel Neuromodulation System in the Treatment of Patients With Chronic Pain of Peripheral Nerve Origin. Neuromodulation Technol. Neural Interface 2016, 19, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Regnier, S.M.; Chen, J.; Gabriel, R.A.; Chakravarthy, K.V. A review of the StimRouter® peripheral neuromodulation system for chronic pain management. Pain Manag. 2021, 11, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.; D’Souza, R.S.; Hagedorn, J.M.; Pritzlaff, S.; Sayed, D.; Azeem, N.; Abd-Elsayed, A.; Escobar, A.; A Huntoon, M.; Lam, C.M.; et al. Evidence-Based Clinical Guidelines from the American Society of Pain and Neuroscience for the Use of Implantable Peripheral Nerve Stimulation in the Treatment of Chronic Pain. J. Pain Res. 2022, 15, 2483–2504. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.S.; Barman, R.; Joseph, A.; Abd-Elsayed, A. Evidence-Based Treatment of Painful Diabetic Neuropathy: A Systematic Review. Curr. Pain Headache Rep. 2022, 26, 583–594. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.S.; Langford, B.; Dombovy-Johnson, M.; Abd-Elsayed, A. Neuromodulation Interventions for the Treatment of Painful Diabetic Neuropathy: A Systematic Review. Curr. Pain Headache Rep. 2022, 26, 365–377. [Google Scholar] [CrossRef]
- Dones, I.; Levi, V. Spinal Cord Stimulation for Neuropathic Pain: Current Trends and Future Applications. Brain Sci. 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Gargya, A.; Singh, H.; Sivanesan, E.; Gulati, A. Mechanism of Peripheral Nerve Stimulation in Chronic Pain. Pain Med. 2020, 21 (Suppl. 1), S6–S12. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Torebjörk, H.E.; Hallin, R.G. Responses in human A and C fibres to repeated electrical intradermal stimulation. J. Neurol. Neurosurg. Psychiatry 1974, 37, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, P.D.; Gutnick, M. Properties of afferent nerve impulses originating from a neuroma. Nature 1974, 248, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.; Jain, S.; Hunter, C.; Chakravarthy, K. Neurostimulation for Intractable Chronic Pain. Brain Sci. 2019, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Frießem, C.; Wiegand, T.; Eitner, L.; Maier, C.; Mainka, T.; Vollert, J.; Enax-Krumova, E.K. Effects of Spinal Cord and Peripheral Nerve Stimulation Reflected in Sensory Profiles and Endogenous Pain Modulation. Clin. J. Pain 2019, 35, 111–120. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Q.; Cheong, Y.-K.; Shechter, R.; Sdrulla, A.; He, S.-Q.; Tiwari, V.; Dong, X.; Wacnik, P.; Meyer, R.; et al. Comparison of intensity-dependent inhibition of spinal wide-dynamic range neurons by dorsal column and peripheral nerve stimulation in a rat model of neuropathic pain. Eur. J. Pain 2014, 18, 978–988. [Google Scholar] [CrossRef]
- Papuć, E.; Rejdak, K. The role of neurostimulation in the treatment of neuropathic pain. Ann. Agric. Environ. Med. 2013, 1, 14–17. [Google Scholar]
- Zuo, K.J.; Gordon, T.; Chan, K.M.; Borschel, G.H. Electrical stimulation to enhance peripheral nerve regeneration: Update in molecular investigations and clinical translation. Exp. Neurol. 2020, 332, 113397. [Google Scholar] [CrossRef]
- A Power, H.; Morhart, M.J.; Olson, J.L.; Chan, K.M. Postsurgical Electrical Stimulation Enhances Recovery Following Surgery for Severe Cubital Tunnel Syndrome. Neurosurgery 2020, 86, 769–777. [Google Scholar] [CrossRef]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2009, 223, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.N.; Olson, J.L.; Morhart, M.J.; Chan, K.M. Electrical stimulation enhances sensory recovery: A randomized controlled trial. Ann. Neurol. 2015, 77, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.; Seikaly, H.; Chan, K.M.; Beaudry, R.; Rychlik, S.; Olson, J.; Curran, M.; Dziegielewski, P.; Biron, V.; Harris, J.; et al. Intraoperative Brief Electrical Stimulation of the Spinal Accessory Nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection: A double-blinded, randomized controlled trial. J. Otolaryngol. Head Neck Surg. 2018, 47, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics 2016, 13, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Euser, A.M.; Zoccali, C.; Jager, K.J.; Dekker, F.W. Cohort Studies: Prospective versus Retrospective. Nephron Clin. Pract. 2009, 113, c214–c217. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.-Y.; In, J. Considerations for crossover design in clinical study. Korean J. Anesthesiol. 2021, 74, 293–299. [Google Scholar] [CrossRef]
- Origoni, M.; Leone Roberti Maggiore, U.; Salvatore, S.; Candiani, M. Neurobiological Mechanisms of Pelvic Pain. BioMed Res. Int. 2014, 2014, 903848. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.R.; Borkowski, G.P.; Albers, J.W.; Levin, K.H.; Barohn, R.J.; Mitsumoto, H. Obturator mononeuropathy caused by pelvic cancer: Six cases. Neurology 1993, 43, 1489. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Her, Y.F. Stimulation holiday rescues analgesia after habituation and loss of efficacy from 10-kilohertz dorsal column spinal cord stimulation. Reg. Anesth. Pain Med. 2022. [Google Scholar] [CrossRef]
| Author/Year | Study Design | Study Funding Source | Mean Age of Subjects | Type of Interventions | Waveform Settings | Sample Size | Follow-Up Period | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Frederico 2020 [14] | Prospective longitudinal case series | No funding | 42.2 | 7 days PNS trial. Patients with >50% improvement in pain had permanent PNS implants. | Pulse width = 210 μs, freq = 40–60 Hz, and amplitude from 0.6 mA to 1.7 mA. | 14 (10 were included) | 12 months | 8/10 patients (80%) have >50 pain reduction and 2/10 have 30% pain reduction on VAS scale. 60.2% improvement in neuropathic pain. | 21.9% improvement in quality of life. |
| Glimore 2020 (12 Month Follow up for Gilmore 2019) [25] | Multicenter, randomized, double-blind, placebo-controlled, partial-crossover study | Industry and non-industry funding | 46.5 | Temporary PNS | Asymmetric charge-balanced biphasic pulse train. Pulse width = 10–200 μs, freq = 100 Hz, and amplitude 1–30 mA | 47 (26 were included ana analyzed for efficacy) | 12 months | 67% of participants in group 1 (i.e., treatment group) had >50% reductions in pain in all qualifying regions of RLP and PLP. 0% of group 2 (i.e., placebo group) reported ≥50% reductions in average weekly pain at the end of the placebo period. | 56% of participants in group 1 (i.e., treatment group) reported ≥50% reductions in pain interference in all qualifying regions of RLP and PLP at the end of the 12-month follow-up, compared with 18% in group 2 at the end of the placebo period. Reduction in pain interference with general activity, walking, sleeping, enjoyment of life by 55%, 39%, 63% and 65%, respectively. For treatment group, average BDI-II score was 55% lower than baseline at the end of 8 weeks of PNS and remained 33% lower than baseline at 12 months. |
| Glimore 2019 [26] | Multicenter, randomized, double-blind, placebo-controlled, partial-crossover study | Industry and non-industry funding | 46.5 | Temporary PNS | Asymmetric charge-balanced biphasic pulse train. Pulse width = 10–200 μs, freq = 100 Hz, and amplitude 1–30 mA | 47 (26 were included ana analyzed for efficacy) | 12 months | 7/12 (58%) of patients receiving PNS reported >50% pain relief compared to only 2/14 (14%) in placebo group during weeks 1–4 of therapy. Among these 7 patients’ average reductions in RLP and PLP were 73% and 69%, respectively. 8/12 (687%) of patients receiving PNS reported >50% pain relief compared to only 2/14 (14%) in placebo group during weeks 5–8 of therapy. Among these 7 patients’ average reductions in RLP and PLP were 56% and 72%, respectively. After crossing over at week 4, patients in the placebo group reported only significant improvement in PLP but not RLP, subjects reporting >50% pain improvement remained 2/14 (14%). | 8/10 (80%) of patients receiving PNS reported >50% reductions in average pain interference in all qualifying regions of RLP and PLP at the end of the treatment period compared to 2/13 (15%) in placebo group. PGIC score was 2.2 in PNS group compared to 0.6 in placebo group. After crossing over at week 4, PGIC increased to 1.3 in placebo group. |
| Oswold 2019 [15] | Prospective case series | Industry funding | N/A | Permanent PNS | Phase duration: 70–500 ms, freq = 0–200 Hz and amplitude of 1–30 mA | 39 patients (42 PNS implants | 6 months | 78% of patients had improvement in their pain, with an average of 71% reduction. Average VAS pain score decreased from 8 cm pre procedural to 2 cm post-implants. Greatest reduction in pain scores with lateral femoral cutaneous nerve (100% reduction). Smallest pain score improvement (29%) with the intercostal nerve stimulation. | 100% of patients reported improvement in their physical activity with an average improvement of 72%. Greatest noted with the brachial plexus (80%) and suprascapular nerve (80%) and smallest in the intercostal nerve (40%). |
| Wilson 2018 [16] | Case series | Industry and non-industry funding | 62.7 | Temporary PNS | Pulse width ranges from 40–200 ms, freq = 12 Hz, and amplitude of 20 mA. | 28 (5 underwent permanent implantation and were analyzed for efficacy) | 24 months | 100% of patients have pain reduction > 50% at 6 and 12 month follow up and about 80% had pain improvement at 24 months follow up. | Improvement in pain interference with ADL measured by BPI-SF9 by 93.5%, 95.9% and 91.1% at 6, 12 and 24 months, respectively, compared to end of sham period. Improvement in pain during shoulder external ROM by 46.2%, 56.7% at 6, 12 months, respectively, compared to end of sham period. Global impression of change by the patients were more towards much improvement. |
| Freitas 2017 [24] | Prospective longitudinal case series | No funding | 32 | 7 days trial. Patients with >50% improvement in pain had permanent implants. | Low frequency tonic stimulation (Pulse Width = 180 msec, freq = 40 to 60 Hz and Amplitude from 0.5 to 2 mA | 23 (10 underwent permanent implantation and were analyzed for efficacy) | 12 months | 60% of patients who underwent permanent device implantation showed a pain reduction of 50% or greater (75% reduction on average), and 20% showed a 30% reduction in pain. | There was an improvement in quality of life and a return to engagement in the activities of daily life (no % reported). |
| Sokal 2017 [25] | Prospective clinical trial study | No funding | 59.3 | Permanent PNS | Intermittent stimulation with a pulse width of up to 800 μs, freq up to 40 Hz, and amplitude of up to 18 mA. | 6 | 6 months | Average VAS score 2.6, 1.6 and 1.3 at 1, 3 and 6 months, respectively, down from 7.5 at baseline. Average short-form McGill pain questionnaire score was 11, 6.3 and 4.5 at 1, 3 and 6 months, respectively, down from 23.8 at baseline. | N/A |
| Deer 2016 [26] | Prospective, Multicenter, Randomized, Double-Blinded, Partial Crossover Study | Industry funding | 53 | Permanent PNS | Pulse width = 200 μs; Freq = 100 Hz, with amplitude set for paresthesia. | 147 (94 underwent implantation and were analyzed for efficacy) | 3 months for efficacy and 1 year for safety | 27% reduction in pain in treatment group compared to 2.3% reduction in control group at 3 months follow up. Treatment group had significant improvement in worst pain score. | Treatment group had significant improvement in BPI score for general activity, mood, walking, normal work, relations to other people, sleep, and enjoyment in life, overall quality of life related to the painful condition and better global impression of degree of satisfaction. |
| Voorbrood 2015 [27] | Prospective study | Industry funding | 53 | Permanent PNS | N/A | 37 (7 patients received PNS) | 3 months | Reduction of pain on the NRS scale from 8 to 2. | N/A |
| Wilson 2014 [28] | Case series | Industry and non-industry funding | 52.2 | Temporary PNS | Pulse width range of 20–200 μs, freq 12 Hz, and amplitude of 20 V. | 10 | 3 months | 36.6% reduction in pain at end of treatment, 35.4% reduction at 5-week follow up, 40.2% reduction at 8-week follow up, and 48.8% reduction at 16-week follow up. | 45.5% reduction in shoulder related disability at end of treatment (EOT), 37.4% reduction at 5-week follow up, 53.7% reduction at 8-week follow up, and 47.5% reduction at 16-week follow up. 52% reduction in pain Interference with ADL at EOT, 46% reduction at 5-week follow up, 60% reduction at 8-week follow up, and 58% reduction at 16-week follow up. 47.8% increase in range of Motion (ROM) at 8-week follow up, and 48.6% increase at 16-week follow up. Improvement in quality of Quality of life (PGIC scale) for 8/10 patients (80%) at EOT and 5/8 (62.5%) at week 16 |
| Stevanato 2014 [29] | Open label trial | No funding | 46 | Permanent PNS | Pulse width = 250, freq = 50 Hz, and amplitude ranging from 0.15 to 0.30 V | 7 | 12 months | Reduction of NRS pain score from 9 before surgery to 2.14 at the 6-month follow-up and to 2.57 at the 12-month follow-up. | N/A |
| Rauck 2014 [30] | Case series | No funding | 47 | Temporary PNS | Pulse width = 10–40 μs, freq 50–100 Hz, and amplitude of 1–20 mA | 16 (9 analyzed for efficacy) | 1 month | 56% reduction in the mean of worst daily post-amputation pain in the second week and forth week of stimulation. 8/9 patients (89%) reported clinically significant relief during the second week of stimulation, and 7/9 (78%) reported significant relief during the fourth week of follow-up. Significant decrease in average pain, pain interference and pain disability Index (PDI) scores in the second week and fourth week of follow up. | Small non-significant decreases in depression scores (BDI-II). Improvement in quality of life with the assessment of the patient global impression of change in the second week and fourth week of follow up. |
| Deer 2010 [31] | Single-center open-label prospective feasibility trial | No funding | 53.7 | Temporary PNS | Pulse width (100 to 300 ms), freq 20 to 45 Hz). amplitude (<80 mA) | 8 patients (10 implants, i.e., each implant was considered a separate patient) | 1–2 weeks | 2/10 patients (20%) have a >30% decrease in pain. Reduction of mean average pain score pain to 6.7 preimplant to 6.2 at the post explant follow-up visit V5. 9/10 (90%) experienced 17–100% reduction in pain intensity on day 5 (at the end of stimulation) versus baseline, with an average pain reduction of 44.2%. 1/10 (10%) experienced a 17% increase in pain intensity on day 5 (at the end of stimulation) versus baseline. N.B After explant, pain returned to baseline, increasing 36.8% to 45.6% relative to average reduced pain with daily stimulation. | Overall satisfaction score with the study was 9.6 cm, on a scale from 0 to 10 cm. All patients (100%) responded by selecting 10/10 (with 10 meaning ‘‘complete likelihood’’) as to their likelihood for wanting to undergo similar treatment with a permanent device. |
| Hassenbusch 1996 [32] | Prospective, consecutive series | No funding | N/A | Permanent PNS | N/A | 32 (30 underwent permanent PNS placement were analyzed for efficacy) | 2–4 years | 19/30 patients (63%) experienced Long-term pain relief. 10/19 patients (52.6%) had good long-term relief and 9/19 (47.43%) had fair relief. Pain decreased from 8.3 preimplantation to 3.5 at the latest follow up on verbal digital scale. 60.9% reduction in allodynia. | Marked improvement in patient activity levels and vascular motor tone; however, less improvement in motor weakness and trophic changes. Activity levels increased by 63.3% in the success group between preimplantation and last follow-up evaluations. (i.e., success group are patients who experienced pain relief). 6/30 patients (20%) returned to part-time or full-time work after being unemployed before stimulator implantation. |
| Author | Year | Selection | Comparability | Outcome |
|---|---|---|---|---|
| Peripheral Nerve Stimulation Studies | ||||
| Frederico et al. [14] | 2020 | *** | - | *** |
| Oswold et al. [15] | 2019 | *** | - | ** |
| Freitas et al. [17] | 2019 | *** | - | ** |
| Sokal et al. [18] | 2017 | *** | - | *** |
| Wilson et al. [20] | 2014 | *** | - | *** |
| Stevanato et al. [21] | 2014 | *** | - | *** |
| Deer et al. [23] | 2010 | *** | - | ** |
| Hassenbusch et al. [24] | 1996 | *** | - | ** |
| Certainty Assessment | Impact | Certainty | ||||||
|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | ||
| CRPS Pain | ||||||||
| 3 | observational studies | serious a | not serious | not serious | not serious | strong association | All 3 studies reported improvements in pain caused by CRPS with avergage reductions in pain scores ranging from 56% to 83% | ⨁⨁◯◯ Low |
| Shoulder Pain | ||||||||
| 2 | observational studies | not serious | not serious | not serious | serious b | strong association | Both studies reported improvements in pain, ranging from 48.8% to 80% reductions. | ⨁⨁◯◯ Low |
| Phantom Limb Pain | ||||||||
| 3 | observational studies (2 RCTs) | not serious | not serious | not serious | not serious | strong association | All three studies reported reductions in pain. Average reductions were greater than 50%. In the RCT and its follow up, more patients in the PNS group experienced significant long term pain relief. | ⨁⨁⨁◯ Moderate |
| Post-Surgical Pain | ||||||||
| 2 | observational studies (1 RCT) | not serious | not serious | not serious | not serious | none | Both studies reported improvement in pain. Average pain score reductions ranged from 27% to 75%. In one RCT, the PNS group had greater reductions in pain scores than the control (27% compared to 2.3%) | ⨁⨁◯◯ Low |
| Mononeuropathy Pain | ||||||||
| 5 | observational studies | serious a | not serious | not serious | not serious | strong association | All 5 studies reported improvements in pain caused by mononeuropathy. Average reductions in pain scores ranged from 36–71% | ⨁⨁◯◯ Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Char, S.; Jin, M.Y.; Francio, V.T.; Hussain, N.; Wang, E.J.; Morsi, M.; Orhurhu, V.; Prokop, L.J.; Fink, A.; D’Souza, R.S. Implantable Peripheral Nerve Stimulation for Peripheral Neuropathic Pain: A Systematic Review of Prospective Studies. Biomedicines 2022, 10, 2606. https://doi.org/10.3390/biomedicines10102606
Char S, Jin MY, Francio VT, Hussain N, Wang EJ, Morsi M, Orhurhu V, Prokop LJ, Fink A, D’Souza RS. Implantable Peripheral Nerve Stimulation for Peripheral Neuropathic Pain: A Systematic Review of Prospective Studies. Biomedicines. 2022; 10(10):2606. https://doi.org/10.3390/biomedicines10102606
Chicago/Turabian StyleChar, Steven, Max Y. Jin, Vinicius Tieppo Francio, Nasir Hussain, Eric J. Wang, Mahmoud Morsi, Vwaire Orhurhu, Larry J. Prokop, Adam Fink, and Ryan S. D’Souza. 2022. "Implantable Peripheral Nerve Stimulation for Peripheral Neuropathic Pain: A Systematic Review of Prospective Studies" Biomedicines 10, no. 10: 2606. https://doi.org/10.3390/biomedicines10102606
APA StyleChar, S., Jin, M. Y., Francio, V. T., Hussain, N., Wang, E. J., Morsi, M., Orhurhu, V., Prokop, L. J., Fink, A., & D’Souza, R. S. (2022). Implantable Peripheral Nerve Stimulation for Peripheral Neuropathic Pain: A Systematic Review of Prospective Studies. Biomedicines, 10(10), 2606. https://doi.org/10.3390/biomedicines10102606





