COVID-19 Severity and Thrombo-Inflammatory Response Linked to Ethnicity
Abstract
1. Introduction
2. Results
2.1. Milder COVID-19 Disease Course in Japanese Patients Than in German Patients
2.2. Cardiovascular Comorbidities Predict Severe Disease in the Japanese and German Cohorts
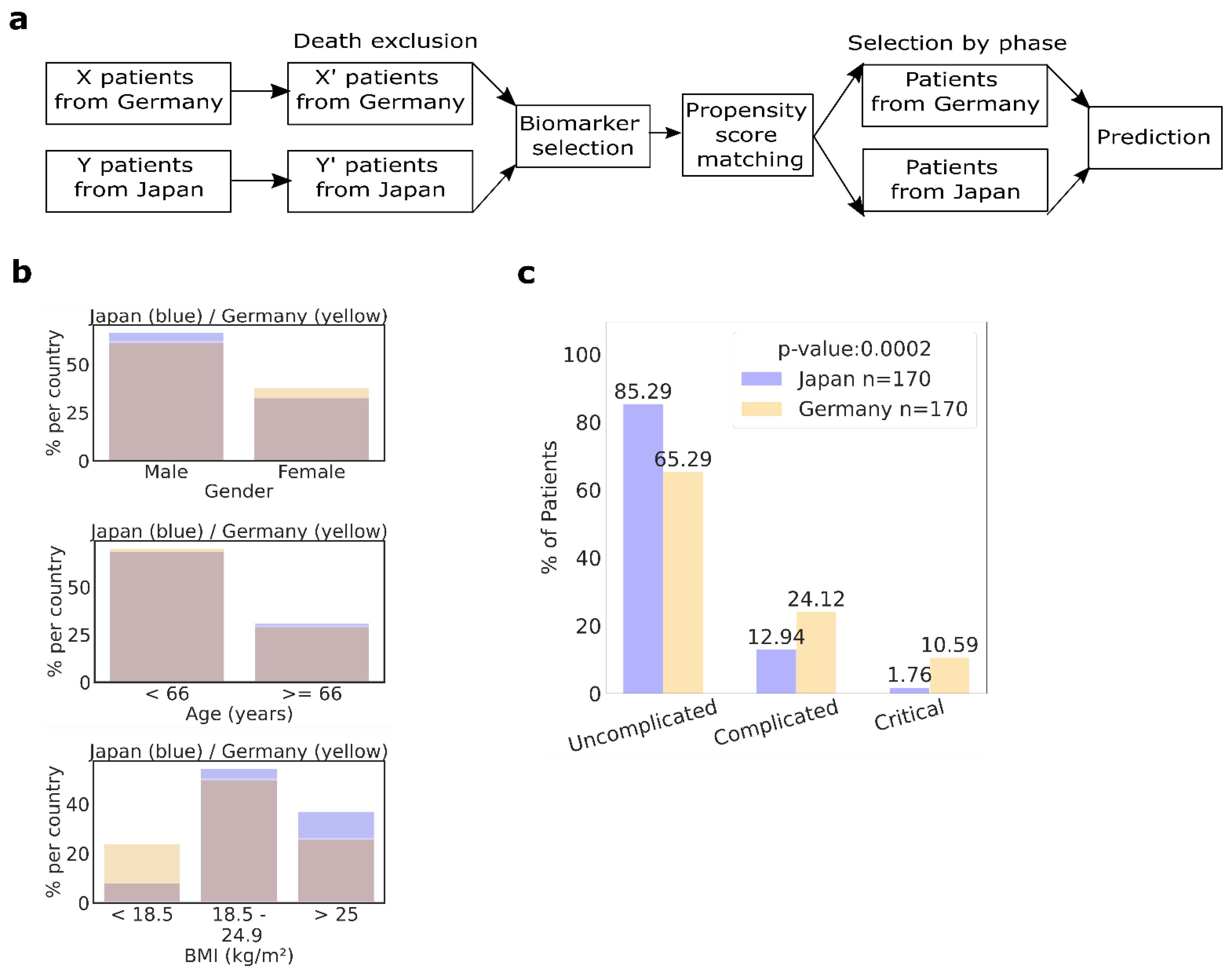
2.3. Fewer Japanese Suffer from Severe Illness According to Propensity Score Matched Data Sets
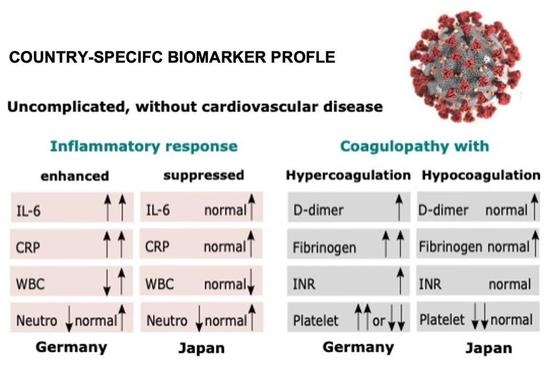
2.4. Japanese, but Not Germans, without Cardiovascular Comorbidities at Diagnosis, Presented a Milder Inflammatory Response
2.5. Coagulopathy with Hypocoagulation/Hyperfibrinolysis in Japanese Patients and Coagulopathy with Hypercoagulation in German Patients without Comorbidities
2.6. No Racial Differences in the Coagulation/Fibrinolysis Response in Patients with Cardiovascular Comorbidities
2.7. Thrombo-Inflammatory Biomarkers as Predictors of Severe COVID-19 Differ between Country Cohorts
3. Discussion
4. Materials and Methods
4.1. Study Design, Participants, and Data Collection
4.2. Statistical Analysis
4.3. PSM Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Consent to Publish
References
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Rentsch, C.T.; Morton, C.E.; Hulme, W.J.; Schultze, A.; MacKenna, B.; Eggo, R.M.; Bhaskaran, K.; Wong, A.Y.S.; Williamson, E.J.; et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: An observational cohort study using the OpenSAFELY platform. Lancet 2021, 397, 1711–1724. [Google Scholar] [CrossRef]
- Go, R.C.; Nyirenda, T.; Bojarian, M.; Hosseini, D.K.; Kim, K.; Rahim, M.; Paleoudis, E.G.; Go, A.C.; Han, Z.; Sperber, S.J.; et al. Racial/ethnic disparities on inflammation and response to methylprednisolone in severe COVID-19 pneumonia. BMC Infect. Dis. 2022, 22, 254. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Stringer, D.; Braude, P.; Myint, P.K.; Evans, L.; Collins, J.T.; Verduri, A.; Quinn, T.J.; Vilches-Moraga, A.; Stechman, M.J.; Pearce, L.; et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021, 50, 420–429. [Google Scholar] [CrossRef]
- Datta, T.; Brunson, A.; Mahajan, A.; Keegan, T.; Wun, T. Racial disparities in cancer-associated thrombosis. Blood Adv. 2022, 6, 3167–3177. [Google Scholar] [CrossRef]
- Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Asai, Y.; Tsuzuki, S.; Suzuki, S.; Toyoda, A.; Suzuki, K.; Endo, M.; et al. Clinical Epidemiology of Hospitalized Patients With Coronavirus Disease 2019 (COVID-19) in Japan: Report of the COVID-19 Registry Japan. Clin. Infect. Dis. 2020, 73, e3677–e3689. [Google Scholar] [CrossRef]
- Heissig, B.; Salama, Y.; Takahashi, S.; Osada, T.; Hattori, K. The multifaceted role of plasminogen in inflammation. Cell Signal. 2020, 75, 109761. [Google Scholar] [CrossRef]
- Brodin, P. Why is COVID-19 so mild in children? Acta Paediatr. 2020, 109, 1082–1083. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.P.; Haverfield, J.; Ursin, R.L.; Tannenbaum, C.; Klein, S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020, 20, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Cremer, S.; Jakob, C.; Berkowitsch, A.; Borgmann, S.; Pilgram, L.; Tometten, L.; Classen, A.; Wille, K.; Weidlich, S.; Gruener, B.; et al. Elevated markers of thrombo-inflammatory activation predict outcome in patients with cardiovascular comorbidities and COVID-19 disease: Insights from the LEOSS registry. Clin. Res. Cardiol. 2021, 110, 1029–1040. [Google Scholar] [CrossRef]
- de Souza, W.M.; Buss, L.F.; Candido, D.D.S.; Carrera, J.P.; Li, S.; Zarebski, A.E.; Pereira, R.H.M.; Prete, C.A., Jr.; de Souza-Santos, A.A.; Parag, K.V.; et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat. Hum. Behav. 2020, 4, 856–865. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Manson, J.J.; Crooks, C.; Naja, M.; Ledlie, A.; Goulden, B.; Liddle, T.; Khan, E.; Mehta, P.; Martin-Gutierrez, L.; Waddington, K.E.; et al. COVID-19-associated hyperinflammation and escalation of patient care: A retrospective longitudinal cohort study. Lancet Rheumatol. 2020, 2, e594–e602. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Zouaoui Boudjeltia, K.; Piagnerelli, M.; Brohée, D.; Guillaume, M.; Cauchie, P.; Vincent, J.-L.; Remacle, C.; Bouckaert, Y.; Vanhaeverbeek, M. Relationship between CRP and hypofibrinolysis: Is this a possible mechanism to explain the association between CRP and outcome in critically ill patients? Thromb. J. 2004, 2, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H.; Townsend, L.; Ni Cheallaigh, C.; Bergin, C.; Martin-Loeches, I.; Browne, P.; Bacon, C.L.; Gaule, R.; Gillett, A.; Byrne, M.; et al. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020, 189, 1044–1049. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ko, J.H.; Park, G.E.; Lee, J.Y.; Lee, J.Y.; Cho, S.Y.; Ha, Y.E.; Kang, C.I.; Kang, J.M.; Kim, Y.J.; Huh, H.J.; et al. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J. Infect. 2016, 73, 468–475. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef]
- Demelo-Rodriguez, P.; Cervilla-Munoz, E.; Ordieres-Ortega, L.; Parra-Virto, A.; Toledano-Macias, M.; Toledo-Samaniego, N.; Garcia-Garcia, A.; Garcia-Fernandez-Bravo, I.; Ji, Z.; de-Miguel-Diez, J.; et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb. Res. 2020, 192, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Morishita, E.; Urano, T.; Yokoyama, K.; the Questionnaire-Survey Joint Team on The COVID-19-Related Thrombosis. COVID-19-Related Thrombosis in Japan: Final Report of a Questionnaire-Based Survey in 2020. J. Atheroscler. Thromb. 2021, 28, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Favaloro, E.J.; Franchini, M.; Guidi, G.C.; Lippi, G. The role of ethnicity, age and gender in venous thromboembolism. J. Thromb. Thrombolysis 2010, 29, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Woulfe, T.; Hyder, S.; Merriman, E.; Simpson, D.; Chunilal, S. Incidence of venous thromboembolism in different ethnic groups: A regional direct comparison study. J. Thromb. Haemost. 2014, 12, 214–219. [Google Scholar] [CrossRef]
- White, R.H.; Keenan, C.R. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb. Res. 2009, 123 (Suppl. 4), S11–S17. [Google Scholar] [CrossRef]
- White, R.H.; Zhou, H.; Murin, S.; Harvey, D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb. Haemost. 2005, 93, 298–305. [Google Scholar]
- Tatsuno, S.Y.; Tatsuno, E.M. Does ethnicity play a role in the dosing of warfarin in Hawai’i? Hawaii J. Med. Public Health 2014, 73, 76–79. [Google Scholar]
- Fajar, J.K. The β fibrinogen gene G-455A polymorphism in Asian subjects with coronary heart disease: A meta analysis. Egypt. J. Med. Hum. Genet. 2017, 18, 19–28. [Google Scholar] [CrossRef][Green Version]
- Long, W.; Yang, J.; Li, Z.; Li, J.; Chen, S.; Chen, D.; Wang, S.; Li, Q.; Hu, D.; Huang, J.; et al. Abnormal Fibrinogen Level as a Prognostic Indicator in Coronavirus Disease Patients: A Retrospective Cohort Study. Front. Med. 2021, 8, 687220. [Google Scholar] [CrossRef]
- Jakob, C.E.M.; Kohlmayer, F.; Meurers, T.; Vehreschild, J.J.; Prasser, F. Design and evaluation of a data anonymization pipeline to promote Open Science on COVID-19. Sci. Data 2020, 7, 435. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers. In Breakthroughs in Statistics; Kotz, S., Johnson, N.L., Eds.; Springer Series in Statistics (Perspectives in Statistics); Springer: New York, NY, USA, 1992. [Google Scholar]
- Mehta, C.R.; Patel, N.R. A Network Algorithm for Performing Fisher’s Exact Test in r × c Contingency Tables. J. Am. Stat. Assoc. 1983, 78, 427–434. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]

| GERMANY | JAPAN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Uncomplicated | Complicated | Critical | p-Value | Total | Uncomplicated | Complicated | Critical | p-Value | |
| Clinical Phase | ||||||||||
| 6059 | 4161.0 (69.0%) | 1513.0(25.0%) | 385.0 (6%) | 174 | 147.0 (84.0%) | 22.0 (13%) | 5.0 (3.0%) | |||
| Age | ||||||||||
| <26 years | 250 | 233.0 (93.2%) | 10.0 (4.0%) | 7.0 (2.8%) | 0 | 15 | 15.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.0005 |
| 26–35 years | 447 | 394.0 (88.14%) | 43.0 (9.62%) | 10.0 (2.24%) | 22 | 22.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | ||
| 36–45 years | 527 | 427.0 (81.02%) | 85.0 (16.13%) | 15.0 (2.85%) | 24 | 24.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | ||
| 46–55 years | 924 | 654.0 (70.78%) | 214.0 (23.16%) | 56.0 (6.06%) | 32 | 30.0 (93.75%) | 2.0 (6.25%) | 0.0 (0.0%) | ||
| 56–65 years | 1112 | 761.0 (68.44%) | 265.0 (23.83%) | 86.0 (7.73%) | 27 | 20.0 (74.07%) | 5.0 (18.52%) | 2.0 (7.41%) | ||
| 66–75 years | 991 | 592.0 (59.74%) | 292.0 (29.47%) | 107.0 (10.8%) | 29 | 23.0 (79.31%) | 5.0 (17.24%) | 1.0 (3.45%) | ||
| 76–85 years | 1281 | 781.0 (60.97%) | 422.0 (32.94%) | 78.0 (6.09%) | 21 | 11.0 (52.38%) | 8.0 (38.1%) | 2.0 (9.52%) | ||
| >85 years | 497 | 295.0 (59.36%) | 177.0 (35.61%) | 25.0 (5.03%) | 4 | 2.0 (50.0%) | 2.0 (50.0%) | 0.0 (0.0%) | ||
| Sex | ||||||||||
| M | 3496 | 2295.0 (65.65%) | 928.0 (26.54%) | 273.0 (7.81%) | 0 | 116 | 95.0 (81.9%) | 18.0 (15.52%) | 3.0 (2.59%) | 0.2668 |
| F | 2549 | 1855.0 (72.77%) | 582.0 (22.83%) | 112.0 (4.39%) | 58 | 52.0 (89.66%) | 4.0 (6.9%) | 2.0 (3.45%) | ||
| BMI | ||||||||||
| <18.5 kg/m2 | 106 | 81.0 (76.42%) | 19.0 (17.92%) | 6.0 (5.66%) | 0 | 14 | 14.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.1044 |
| 18.5–24.9 kg/m2 | 1135 | 807.0 (71.1%) | 275.0 (24.23%) | 53.0 (4.67%) | 93 | 73.0 (78.49%) | 17.0 (18.28%) | 3.0 (3.23%) | ||
| 25–29.9 kg/m2 | 1255 | 826.0 (65.82%) | 350.0 (27.89%) | 79.0 (6.29%) | 52 | 47.0 (90.38%) | 5.0 (9.62%) | 0.0 (0.0%) | ||
| 30–34.9 kg/m2 | 672 | 419.0 (62.35%) | 204.0 (30.36%) | 49.0 (7.29%) | 7 | 7.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | ||
| >34.9 kg/m2 | 381 | 211.0 (55.38%) | 106.0 (27.82%) | 64.0 (16.8%) | 4 | 4.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | ||
| GERMANY | JAPAN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Uncomplicated | Complicated | Critical | p-Value | Total | Uncomplicated | Complicated | Critical | p-Value | |
| Heart | ||||||||||
| Yes | 3355 | 2080.0 (62.0%) | 1011.0 (30.13%) | 264.0 (7.87%) | 0 | 13 | 5.0 (38.46%) | 8.0 (61.54%) | 0.0 (0.0%) | 0 |
| No | 2704 | 2081.0 (76.96%) | 502.0 (18.57%) | 121.0 (4.47%) | 161 | 142.0 (88.2%) | 14.0 (8.7%) | 5.0 (3.11%) | ||
| Hypertension | ||||||||||
| Yes | 2914 | 1800.0 (61.77%) | 879.0 (30.16%) | 235.0 (8.06%) | 0 | 50 | 32.0 (64.0%) | 18.0 (36.0%) | 0.0 (0.0%) | 0 |
| No | 3028 | 2301.0 (75.99%) | 602.0 (19.88%) | 125.0 (4.13%) | 124 | 115.0 (92.74%) | 4.0 (3.23%) | 5.0 (4.03%) | ||
| Diabetes | ||||||||||
| Yes | 1235 | 705.0 (57.09%) | 417.0 (33.77%) | 113.0 (9.15%) | 0 | 27 | 18.0 (66.67%) | 8.0 (29.63%) | 1.0 (3.7%) | 0.0127 |
| No | 4824 | 3456.0 (71.64%) | 1096.0 (22.72%) | 272.0 (5.64%) | 147 | 129.0 (87.76%) | 14.0 (9.52%) | 4.0 (2.72%) | ||
| Dementia | ||||||||||
| Yes | 503 | 295.0 (58.65%) | 178.0 (35.39%) | 30.0 (5.96%) | 0 | 10 | 4.0 (40.0%) | 5.0 (50.0%) | 1.0 (10.0%) | 0.0011 |
| No | 5369 | 3769.0 (70.2%) | 1273.0 (23.71%) | 327.0 (6.09%) | 164 | 143.0 (87.2%) | 17.0 (10.37%) | 4.0 (2.44%) | ||
| Kidney | ||||||||||
| Yes | 843 | 506.0 (60.02%) | 285.0 (33.81%) | 52.0 (6.17%) | 0 | 12 | 8.0 (66.67%) | 4.0 (33.33%) | 0.0 (0.0%) | 0.0944 |
| No | 5047 | 3567.0 (70.68%) | 1175.0 (23.28%) | 305.0 (6.04%) | 162 | 139.0 (85.8%) | 18.0 (11.11%) | 5.0 (3.09%) | ||
| Hemiplegia | ||||||||||
| Yes | 96 | 49.0 (51.04%) | 36.0 (37.5%) | 11.0 (11.46%) | 0 | 4 | 2.0 (50.0%) | 1.0 (25.0%) | 1.0 (25.0%) | 0.1142 |
| No | 5771 | 4012.0 (69.52%) | 1412.0 (24.47%) | 347.0 (6.01%) | 170 | 145.0 (85.29%) | 21.0 (12.35%) | 4.0 (2.35%) | ||
| Cerebrovascular | ||||||||||
| Yes | 505 | 302.0 (59.8%) | 169.0 (33.47%) | 34.0 (6.73%) | 0 | 4 | 2.0 (50.0%) | 2.0 (50.0%) | 0.0 (0.0%) | 0.1142 |
| No | 5554 | 3859.0 (69.48%) | 1344.0 (24.2%) | 351.0 (6.32%) | 170 | 145.0 (85.29%) | 20.0 (11.76%) | 5.0 (2.94%) | ||
| Vascular | ||||||||||
| Yes | 254 | 144.0 (56.69%) | 91.0 (35.83%) | 19.0 (7.48%) | 0 | 4 | 2.0 (50.0%) | 2.0 (50.0%) | 0.0 (0.0%) | 0.1142 |
| No | 5564 | 3893.0 (69.97%) | 1337.0 (24.03%) | 334.0 (6.0%) | 170 | 145.0 (85.29%) | 20.0 (11.76%) | 5.0 (2.94%) | ||
| Respiratory | ||||||||||
| Yes | 862 | 524.0 (60.79%) | 270.0 (31.32%) | 68.0 (7.89%) | 0 | 4 | 3.0 (75.0%) | 1.0 (25.0%) | 0.0 (0.0%) | 0.4938 |
| No | 5197 | 3637.0 (69.98%) | 1243.0 (23.92%) | 317.0 (6.1%) | 170 | 144.0 (84.71%) | 21.0 (12.35%) | 5.0 (2.94%) | ||
| Immunosuppressive | ||||||||||
| Yes | 393 | 268.0 (68.19%) | 105.0 (26.72%) | 20.0 (5.09%) | 0.7526 | 10 | 7.0 (70.0%) | 3.0 (30.0%) | 0.0 (0.0%) | 0.4387 |
| No | 5666 | 3893.0 (68.71%) | 1408.0 (24.85%) | 365.0 (6.44%) | 118 | 94.0 (79.66%) | 19.0 (16.1%) | 5.0 (4.24%) | ||
| Cancer | ||||||||||
| Yes | 766 | 509.0 (66.45%) | 223.0 (29.11%) | 34.0 (4.44%) | 0.1333 | 12 | 11.0 (91.67%) | 1.0 (8.33%) | 0.0 (0.0%) | 0.4583 |
| No | 5293 | 3652.0 (69.0%) | 1290.0 (24.37%) | 351.0 (6.63%) | 116 | 90.0 (77.59%) | 21.0 (18.1%) | 5.0 (4.31%) | ||
| Liver | ||||||||||
| Yes | 152 | 105.0 (69.08%) | 35.0 (23.03%) | 12.0 (7.89%) | 0.9598 | 34 | 24.0 (70.59%) | 9.0 (26.47%) | 1.0 (2.94%) | 0.2534 |
| No | 5907 | 4056.0 (68.66%) | 1478.0 (25.02%) | 373.0 (6.31%) | 94 | 77.0 (81.91%) | 13.0 (13.83%) | 4.0 (4.26%) | ||
| Gastro | ||||||||||
| Yes | 105 | 65.0 (61.9%) | 30.0 (28.57%) | 10.0 (9.52%) | 0 | 0 | 0.0 (nan%) | 0.0 (nan%) | 0.0 (nan%) | 1 |
| No | 5757 | 3993.0 (69.36%) | 1418.0 (24.63%) | 346.0 (6.01%) | 174 | 147.0 (84.48%) | 22.0 (12.64%) | 5.0 (2.87%) | ||
| Inflammation Biomarkers | GERMANY | JAPAN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Uncomplicated | Complicated | Critical | p-Value | Total | Uncomplicated | Complicated | Critical | p-Value | |
| Interleukin-6 | ||||||||||
| <1.8 | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 1.0000 | 1 | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1.0000 |
| 1.8–199 | 19 | 16 (84.21%) | 3 (15.79%) | 0 (0.0%) | 39 | 23 (58.97%) | 16 (41.03%) | 0 (0.0%) | ||
| 200–4999 | 2 | 2 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1 | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | ||
| CRP | ||||||||||
| <3 | 3 | 3 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1.0000 | 20 | 17 (85.0%) | 3 (15.0%) | 0 (0.0%) | 0.0780 |
| 3–29 | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 40 | 24 (60.0%) | 16 (40.0%) | 0 (0.0%) | ||
| >30 | 28 | 24 (85.71%) | 3 (10.71%) | 1 (3.57%) | 0 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| White blood cells | ||||||||||
| <4000 | 8 | 6 (75.0%) | 1 (12.5%) | 1 (12.5%) | 0.2444 | 16 | 13 (81.25%) | 3 (18.75%) | 0 (0.0%) | 0.2284 |
| 4000–7999 | 12 | 12 (100.0%) | 0 (0.0%) | 0 (0.0%) | 36 | 24 (66.67%) | 12 (33.33%) | 0 (0.0%) | ||
| >8000 | 8 | 7 (87.5%) | 1 (12.5%) | 0 (0.0%) | 8 | 4 (50.0%) | 4 (50.0%) | 0 (0.0%) | ||
| Neutrophil | ||||||||||
| <2000 | 2 | 2 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1.0000 | 5 | 3 (60.0%) | 2 (40.0%) | 0 (0.0%) | 1.0000 |
| 2000–8999 | 21 | 19 (90.48%) | 2 (9.52%) | 0 (0.0%) | 39 | 23 (58.97%) | 16 (41.03%) | 0 (0.0%) | ||
| ≥9000 | 2 | 2 (100.0%) | 0 (0.0%) | 0 (0.0%) | 2 | 1 (50.0%) | 1 (50.0%) | 0 (0.0%) | ||
| Thrombosis Biomarkers | GERMANY | JAPAN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Uncomplicated | Complicated | Critical | p-Value | Total | Uncomplicated | Complicated | Critical | p-Value | |
| D-Dimers | ||||||||||
| Normal | 4 | 3 (75.0%) | 0 (0.0%) | 1 (25.0%) | 0.2624 | 12 | 12 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0.008 |
| ULN–2x ULN | 19 | 17 (89.47%) | 2 (10.53%) | 0 (0.0%) | 39 | 25 (64.1%) | 14 (35.9%) | 0 (0.0%) | ||
| >5x ULN | 4 | 3 (75.0%) | 1 (25.0%) | 0 (0.0%) | 8 | 3 (37.5%) | 5 (62.5%) | 0 (0.0%) | ||
| INR | ||||||||||
| <1.25 | 25 | 25 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0.099 | 54 | 36 (66.67%) | 18 (33.33%) | 0 (0.0%) | 1.0000 |
| 1.25–2 | 3 | 2 (66.67%) | 1 (33.33%) | 0 (0.0%) | 2 | 1 (50.0%) | 1 (50.0%) | 0 (0.0%) | ||
| >2 | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 0 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Fibrinogen | ||||||||||
| Normal (LLN–ULN) | 10 | 9 (90.0%) | 0 (0.0%) | 1 (10.0%) | 1.0000 | 12 | 12 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0.0050 |
| >ULN | 9 | 8 (88.89%) | 1 (11.11%) | 0 (0.0%) | 28 | 14 (50.0%) | 14 (50.0%) | 0 (0.0%) | ||
| >2x ULN | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | ||
| Platelets | ||||||||||
| <120,000 | 19 | 18 (94.74%) | 1 (5.26%) | 0 (0.0%) | 0.003 | 6 | 6 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1.0000 |
| 120,000–449,999 | 41 | 23 (56.1%) | 18 (43.9%) | 0 (0.0%) | 0 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| >450,000 | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 23 | 19 (82.61%) | 3 (13.04%) | 1 (4.35%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heissig, B.; Salama, Y.; Iakoubov, R.; Vehreschild, J.J.; Rios, R.; Nogueira, T.; Vehreschild, M.J.G.T.; Stecher, M.; Mori, H.; Lanznaster, J.; et al. COVID-19 Severity and Thrombo-Inflammatory Response Linked to Ethnicity. Biomedicines 2022, 10, 2549. https://doi.org/10.3390/biomedicines10102549
Heissig B, Salama Y, Iakoubov R, Vehreschild JJ, Rios R, Nogueira T, Vehreschild MJGT, Stecher M, Mori H, Lanznaster J, et al. COVID-19 Severity and Thrombo-Inflammatory Response Linked to Ethnicity. Biomedicines. 2022; 10(10):2549. https://doi.org/10.3390/biomedicines10102549
Chicago/Turabian StyleHeissig, Beate, Yousef Salama, Roman Iakoubov, Joerg Janne Vehreschild, Ricardo Rios, Tatiane Nogueira, Maria J. G. T. Vehreschild, Melanie Stecher, Hirotake Mori, Julia Lanznaster, and et al. 2022. "COVID-19 Severity and Thrombo-Inflammatory Response Linked to Ethnicity" Biomedicines 10, no. 10: 2549. https://doi.org/10.3390/biomedicines10102549
APA StyleHeissig, B., Salama, Y., Iakoubov, R., Vehreschild, J. J., Rios, R., Nogueira, T., Vehreschild, M. J. G. T., Stecher, M., Mori, H., Lanznaster, J., Adachi, E., Jakob, C., Tabe, Y., Ruethrich, M., Borgmann, S., Naito, T., Wille, K., Valenti, S., Hower, M., ... on behalf of the LEOSS Study Group. (2022). COVID-19 Severity and Thrombo-Inflammatory Response Linked to Ethnicity. Biomedicines, 10(10), 2549. https://doi.org/10.3390/biomedicines10102549