Robust Preanalytical Performance of Soluble PD-1, PD-L1 and PD-L2 Assessed by Sensitive ELISAs in Blood
Abstract
1. Introduction
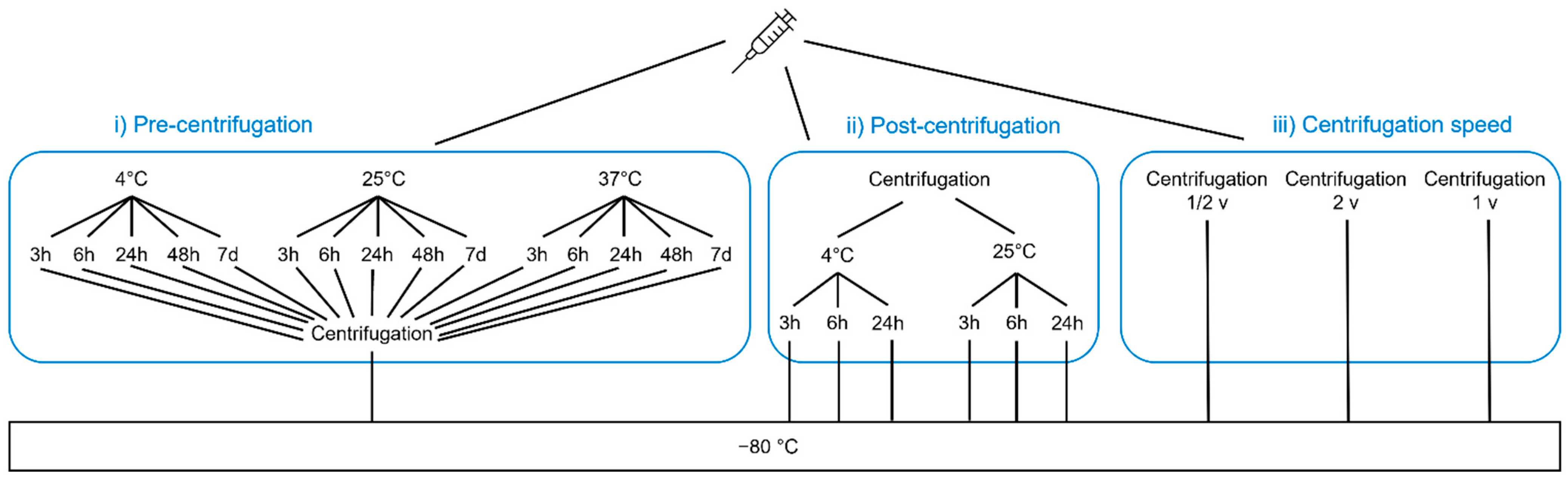
2. Materials and Methods
2.1. Standardization and Quality Control
2.2. Short-Term Stability
2.3. Freeze Effects
2.4. Refreeze Effects
2.5. Sample Preparation before Measurement
2.6. Ethics and Informed Consent
2.7. Statistics and Data Interpretation
3. Results
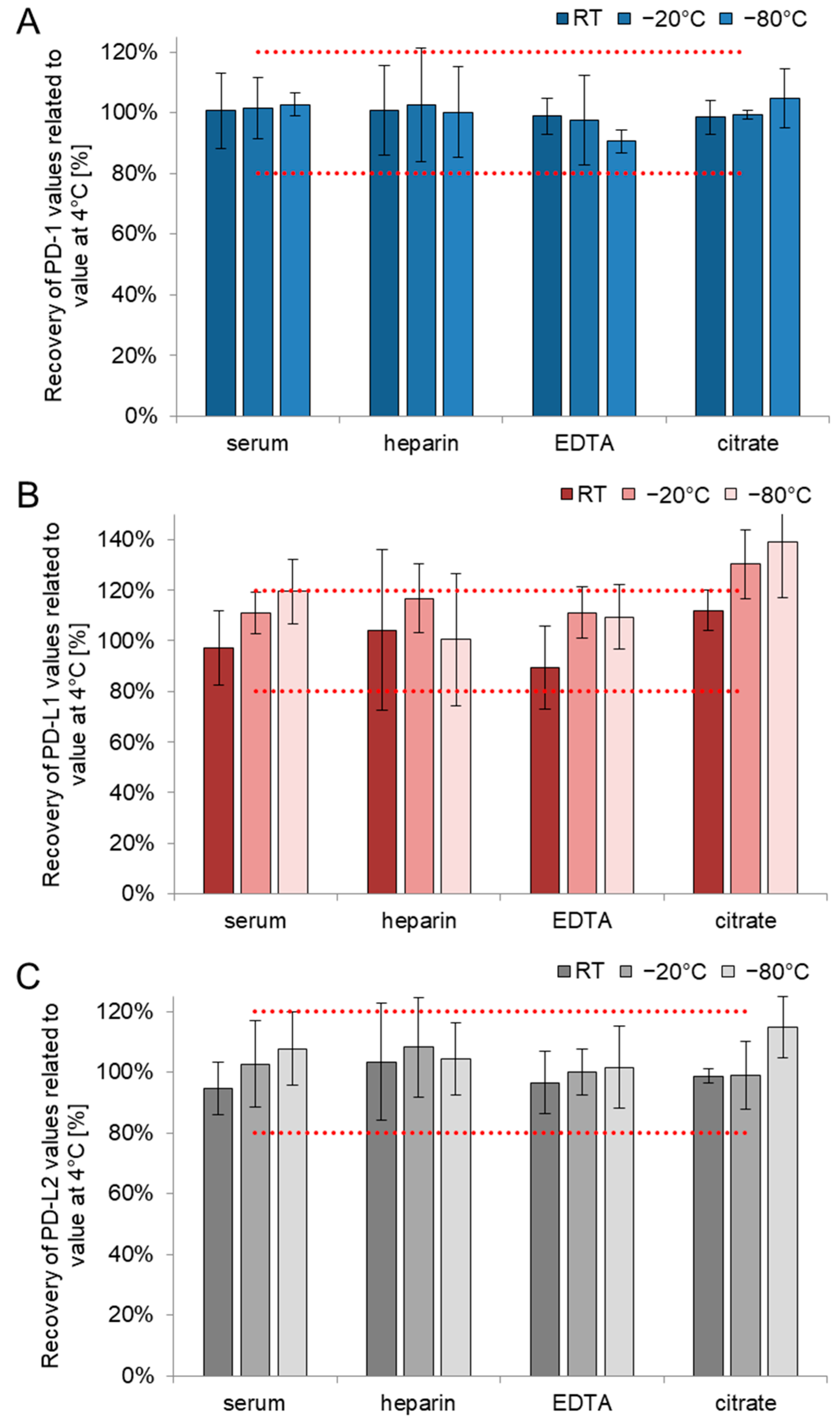
3.1. Short-Term Stability
3.2. Freeze Effects
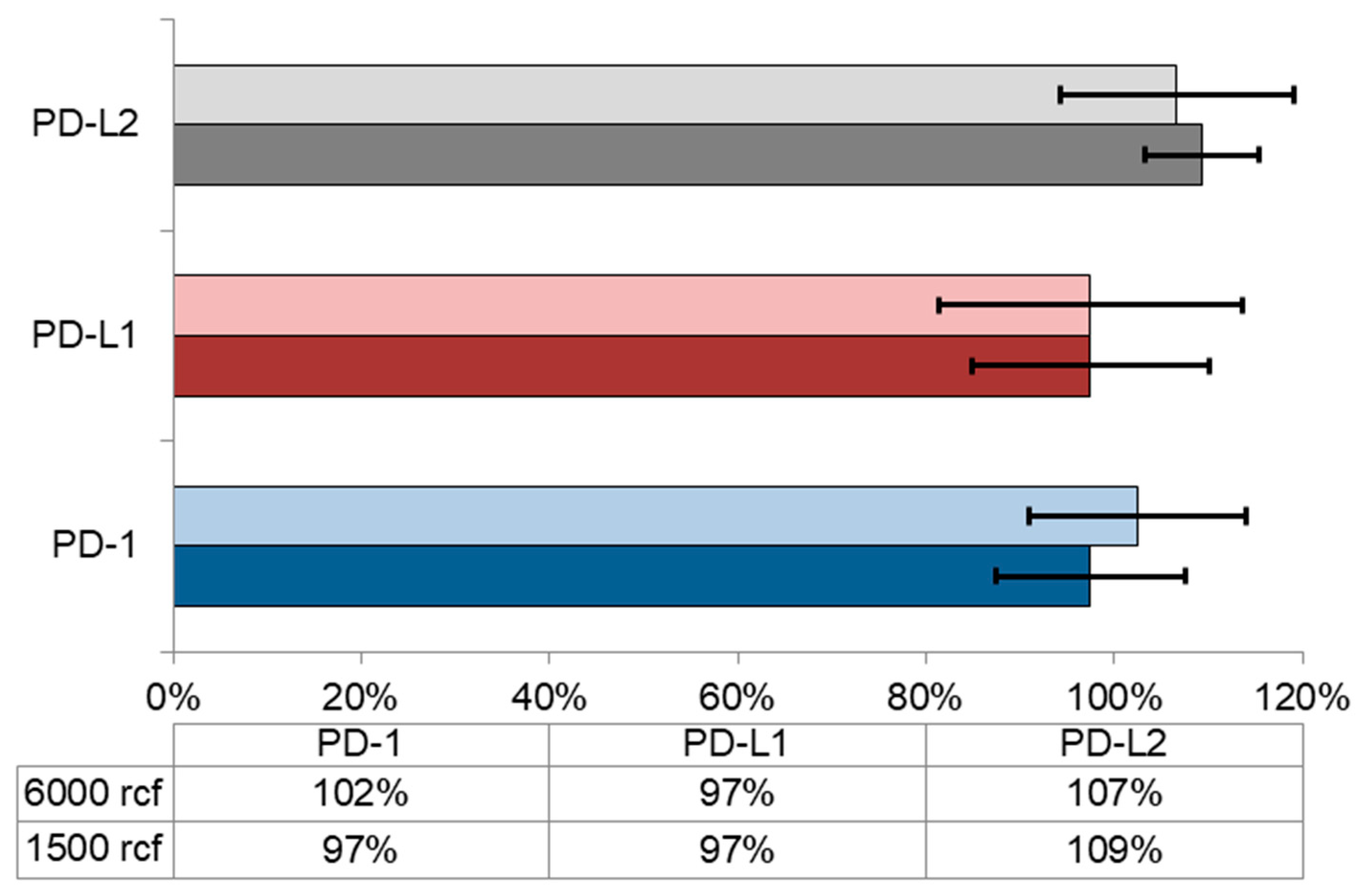
3.3. Refreeze Effects
3.4. Sample Preparation before Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schildberg, F.A.; Klein, S.R.; Freeman, G.J.; Sharpe, A.H. Review coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F.A.P.; Sadelain, M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell 2015, 27, 439–449. [Google Scholar] [CrossRef]
- Man, J.; Millican, J.; Mulvey, A.; Gebski, V.; Hui, R. Response rate and survival at key timepoints with PD-1 blockade vs chemotherapy in PD-L1 subgroups: Meta-analysis of metastatic NSCLC trials. JNCI Cancer Spectr. 2021, 5, pkab012v. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Hua, W.; Li, Z.; Xu, Z.; Qian, Q. Monitoring checkpoint inhibitors: Predictive biomarkers in immunotherapy. Front. Med. 2019, 13, 32–44. [Google Scholar] [CrossRef]
- Guirgis, H.M. Costs of extended use of the immune checkpoint inhibitors in first-line non-small cell lung cancer. J. Clin. Pathw. 2021, 7, 32–36. [Google Scholar] [CrossRef]
- Krueger, K.; Mayer, Z.; Gerckens, M.; Boeck, S.; Luppa, P.; Holdenrieder, S. High quality performance of novel immunoassays for the sensitive quantification of soluble PD-1, PD-L1 and PD-L2 in blood. Biomedicines 2022, 10, 2405. [Google Scholar] [CrossRef]
- Dempke, W.C.M.; Fenchel, K.; Dale, S.P. Programmed cell death ligand-1 (PD-L1) as a biomarker for non- small cell lung cancer (NSCLC) treatment—Are we barking up the wrong tree? Transl. Lung Cancer Res. 2018, 7, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Yin, L.; Zhou, X.; Lei, C.; Tong, R.; Huang, M.; Gong, Y.; Ding, Z.; Xue, J.; Zhu, J.; et al. Assessment of programmed cell death ligand-1 expression with multiple immunohistochemistry antibody clones in non-small cell lung cancer. J. Thorac. Dis. 2018, 10, 816–824. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Ross-Macdonald, P.; Yuan, L.; Song, L.; Veras, E.; Wind-Rotolo, M.; McDermott, D.F.; Hodi, X.F.S.; Choueiri, T.K.; Freeman, G.J. Soluble PD-L1 as an early marker of progressive disease on nivolumab. J. Immunother. Cancer 2022, 10, e003527. [Google Scholar] [CrossRef] [PubMed]
- Duchemann, B.; Remon, J.; Naigeon, M.; Mezquita, L.; Ferrara, R.; Cassard, L.; Jouniaux, J.; Boselli, L.; Grivel, J.; Auclin, E.; et al. Integrating circulating biomarkers in the immune checkpoint inhibitor treatment in lung cancer. Cancers 2020, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Lista, S.; Faltraco, F.; Hampel, H. Biological and methodical challenges of blood-based proteomics in the field of neurological research. Prog. Neurobiol. 2013, 101–102, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Sturgeon, C.M.; Sölétormos, G.; Barak, V.; Molina, R.; Hayes, D.F.; Diamandis, E.P.; Bossuyt, P.M. Validation of new cancer biomarkers: A position statement from the European group on tumor markers. Clin. Chem. 2015, 61, 809–820. [Google Scholar] [CrossRef]
- Pasella, S.; Baralla, A.; Canu, E.; Pinna, S.; Vaupel, J.; Deiana, M.; Franceschi, C.; Baggio, G.; Zinellu, A.; Sotgia, S.; et al. Pre-analytical stability of the plasma proteomes based on the storage temperature. Proteome Sci. 2013, 11, 1–10. [Google Scholar] [CrossRef]
- Hsieh, S.Y.; Chen, R.K.; Pan, Y.H.; Lee, H.L. Systematical evaluation of the effects of sample collection procedures on low-molecular-weight serum/plasma proteome profiling. Proteomics 2006, 6, 3189–3198. [Google Scholar] [CrossRef]
- Grizzle, W.E.; Semmes, O.J.; Bigbee, W.; Zhu, L.; Malik, G.; Oelschlager, D.K.; Manne, B.; Manne, U. CThe need for review and understanding of SELDI/MALDI mass spectroscopy data prior to analysis. Cancer Inform. 2005, 1, 86–97. [Google Scholar] [CrossRef]
- Mitchell, B.L.; Yasui, Y.; Li, C.I.; Fitzpatrick, A.L.; Lampe, P.D. Impact of freeze-thaw cycles and storage time on plasma samples used in mass spectrometry based biomarker discovery projects. Cancer Inform. 2005, 1, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Roche Diagnostics GmbH. Elecsys LDH; Roche Diagnostics GmbH: Basel, Switzerland, 2017. [Google Scholar]
- Roche Diagnostics GmbH. Elecsys ISE Indirect Na, K, Cl; Roche Diagnostics GmbH: Basel, Switzerland, 2019. [Google Scholar]
- Roche Diagnostics GmbH. Elecsys NSE Manual; Roche Diagnostics GmbH: Basel, Switzerland, 2017. [Google Scholar]
- Roche Diagnostics GmbH. Elecsys IL-6; Roche Diagnostics GmbH: Basel, Switzerland, 2019. [Google Scholar]
- Roche Diagnostics GmbH. Cobas Vitamin D Total; Roche Diagnostics GmbH: Basel, Switzerland, 2019. [Google Scholar]
- Shimizu, Y.; Ichihara, K. Elucidation of stability profiles of common chemistry analytes in serum stored at six graded temperatures. Clin. Chem. Lab. Med. 2019, 57, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Cuhadar, S.; Koseoglu, M.; Atay, A.; Dirican, A. The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem. Med. 2013, 23, 70–77. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation; Vol. EMEA/CHMP/; Committee for Medicinal Products for Human Use: London, UK, 2012; pp. 1–23. [Google Scholar]
- Andreasson, U.; Perret-Liaudet, A.; van Waalwijk van Doorn, L.J.C.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, H.B.; et al. A practical guide to immunoassay method validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. C31-A2 Ionized Calcium Determinations: Precollection Variables, Specimen Choice, Collection and Handling: Approved Guideline, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne County, MI, USA, 2001; pp. 1–12. [Google Scholar]
- Guo, Q.; Malloy, M.W.; Roweth, H.G.; Italiano, J.E.; Battinelli, E. Platelets up-regulate tumor cell programmed death-ligand 1 through epidermal growth factor receptor signal transduction. Blood 2021, 138, 1006. [Google Scholar] [CrossRef]
- Aziz, N.; Detels, R.; Quint, J.J.; Li, Q.; Gjertson, D.; Butch, A.W. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine 2016, 84, 17–24. [Google Scholar] [CrossRef]
- Wittwer, C.; Lehner, J.; Fersching, D.; Siegele, B.; Stoetzer, O.J.; Holdenrieder, S. Methodological and preanalytical evaluation of a RAGE immunoassay. Anticancer Res. 2012, 32, 2075–2078. [Google Scholar]
- Lehner, J.; Wittwer, C.; Fersching, D.; Siegele, B.; Holdenrieder, S.; Stoetzer, O.J. Methodological and preanalytical evaluation of an HMGB1 immunoassay. Anticancer Res. 2012, 32, 2075–2078. [Google Scholar]


| Assay | Condition | Time | ||||
|---|---|---|---|---|---|---|
| 3 h | 6 h | 24 h | 48 h | 7 days | ||
| PD-1 | BC 4 °C | 100% (9%) | 102% (12%) | 97% (11%) | 93% (17%) | 114% (10%) |
| BC 25 °C | 104% (11%) | 100% (10%) | 97% (7%) | 95% (15%) | 84% (34%) | |
| BC 37 °C | 97% (9%) | 87% (12%) | 97% (17%) | 83% (26%) | - | |
| AC 4 °C | 90% (7%) | 102 (23%) | 96% (7%) | - | - | |
| AC 25 °C | 97% (14%) | 99% (10%) | 96% (12%) | - | - | |
| PD-L1 | BC 4 °C | 109% (27%) | 107 (28%) | 100% (32%) | 110% (19%) | 119% (17%) |
| BC 25 °C | 107% (17%) | 104% (16%) | 92% (22%) | 103% (18%) | 109% (13%) | |
| BC 37 °C | 101% (28%) | 90% (25%) | 117% (16%) | 103% (18%) | - | |
| AC 4 °C | 89% (17%) | 92% (31%) | 89% (26%) | - | - | |
| AC 25 °C | 88% (25%) | 78% (29%) | 93% (28%) | - | - | |
| PD-L2 | BC 4 °C | 100% (8%) | 104% (9%) | 103 (14%) | 97% (28%) | 120% (16%) |
| BC 25 °C | 102% (6%) | 104% (16%) | 109% (14%) | 106% (17%) | 97% (35%) | |
| BC 37 °C | 97% (16%) | 97% (21%) | 124% (15%) | 104% (27%) | - | |
| AC 4 °C | 96% (14%) | 94% (19%) | 96% (9%) | - | - | |
| AC 25 °C | 97% (14%) | 97% (19%) | 95% (17%) | - | - | |
| PD-1 | PD-L1 | PD-L2 | |
|---|---|---|---|
| Material | Serum, Heparin, EDTA, Citrate | Serum, Heparin, EDTA | Serum, Heparin, EDTA, Citrate |
| Time whole blood | |||
| 4 °C | Up to 7 d | Up to 7 d | Up to 7 d |
| 25 °C | Up to 7 d, 3 h | Up to 7 d, 3 h | Up to 7 d, 3 h |
| 37 °C | Up to 7 d | Up to 24 h | Up to 6 h |
| Centrifugation | 1500,3000,6000 rcf | 1500,3000,6000 rcf | 1500,3000,6000 rcf |
| Time after plasma | |||
| 4 °C | Up to 24 h, 3 h | Up to 24 h, 3 h | Up to 24 h, 3 h |
| 25 °C | Up to 24 h, 1 h | Up to 3 h, 1 h | Up to 24 h, 1 h |
| Storage tubes | Cryotube | Sample tube, cryotube | Cryotube |
| Plate application | Mixing (pipette or vortexer), centrifugation | Mixing (pipette or vortexer), centrifugation | Mixing (pipette), centrifugation |
| Freezing process | |||
| Serum | Stable, −80 °C | Stable, −80 °C | Stable, −80 °C |
| Heparin plasma | Stable, −80 °C | Stable, −80 °C | Stable, −80 °C |
| EDTA plasma | Stable, −80 °C | Stable, −80 °C | Stable, −80 °C |
| Citrate plasma | Stable, −80 °C | Not stable | Stable, −80 °C |
| Freeze–thaw cycles | |||
| Serum | 3 | 3 | 3 |
| Heparin plasma | 1 | 1 | 3 |
| EDTA plasma | 3 | 3 | 3 |
| Citrate plasma | 3 | Not stable | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krueger, K.; Mayer, Z.; Kottmaier, M.; Gerckens, M.; Boeck, S.; Luppa, P.; Holdenrieder, S. Robust Preanalytical Performance of Soluble PD-1, PD-L1 and PD-L2 Assessed by Sensitive ELISAs in Blood. Biomedicines 2022, 10, 2534. https://doi.org/10.3390/biomedicines10102534
Krueger K, Mayer Z, Kottmaier M, Gerckens M, Boeck S, Luppa P, Holdenrieder S. Robust Preanalytical Performance of Soluble PD-1, PD-L1 and PD-L2 Assessed by Sensitive ELISAs in Blood. Biomedicines. 2022; 10(10):2534. https://doi.org/10.3390/biomedicines10102534
Chicago/Turabian StyleKrueger, Kimberly, Zsuzsanna Mayer, Marc Kottmaier, Miriam Gerckens, Stefan Boeck, Peter Luppa, and Stefan Holdenrieder. 2022. "Robust Preanalytical Performance of Soluble PD-1, PD-L1 and PD-L2 Assessed by Sensitive ELISAs in Blood" Biomedicines 10, no. 10: 2534. https://doi.org/10.3390/biomedicines10102534
APA StyleKrueger, K., Mayer, Z., Kottmaier, M., Gerckens, M., Boeck, S., Luppa, P., & Holdenrieder, S. (2022). Robust Preanalytical Performance of Soluble PD-1, PD-L1 and PD-L2 Assessed by Sensitive ELISAs in Blood. Biomedicines, 10(10), 2534. https://doi.org/10.3390/biomedicines10102534






