Early Clinical Remission Is a Predictor of Long-Term Remission with the Use of Vedolizumab for Ulcerative Colitis
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Patients
2.3. Treatment
2.4. Definition of Response
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
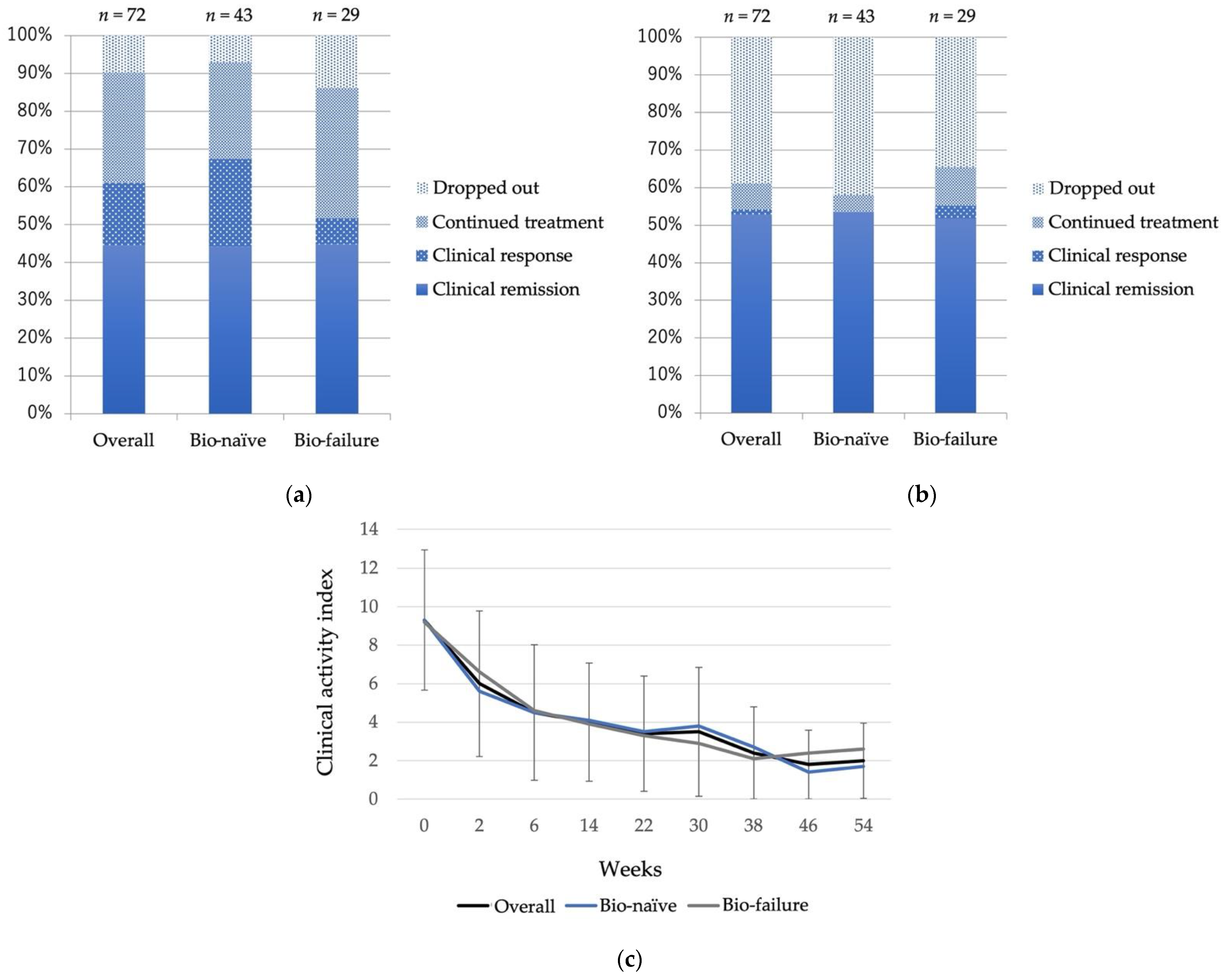
3.2. Treatment Efficacy
3.3. Treatment Efficacy in Bio-Failure Cases
3.4. Analysis of VDZ Dropout Patients
3.5. Early Clinical Remission Can Be a Predictor of Long-Term Remission
3.6. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, B.P.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Toshifumi, H.; Makoto, N.; Tetsuji, K.; Fukunori, K.; Takashi, S. Low dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative Colitis. J. Gastroenterol. 2003, 38, 740–746. [Google Scholar]
- McLean, L.P.; Shea-Donohue, T.; Cross, K.R. Vedolizumab for the treatment of ulcerative colitis and Crohn’s disease. Immunotherapy 2012, 4, 883–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedyk, E.R.; Wyant, T.; Yang, L.L.; Csizmadia, V.; Burke, K.; Yang, H.; Kadambi, V.J. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm. Bowel Dis. 2012, 18, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Soler, D.; Chapman, T.; Yang, L.L.; Wyant, T.; Egan, R.; Fedyk, E.R. The binding specificity and selective antagonism of vedolizumab, an anti-α4β7 integrin therapeutic antibody in development for inflammatory bowel diseases. J. Pharmacol. Exp. Ther. 2009, 330, 864–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesterberg, P.E.; Winsor-Hines, D.; Briskin, M.J.; Soler-Ferran, D.; Merrill, C.; Mackay, C.R.; Newman, W.; Ringler, D.J. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin α4β7. Gastroenterology 1996, 111, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Pouillon, L.; Stappan, J.V.; Bossuyt, P.; Danese, S.; Peyrin-Biroulet, L. Should we use anti-tumor necrosis factor agents or vedolizumab as first-line biological therapy in ulcerative colitis? Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeers, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Assche, G.V.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Feagan, B.G.; Patel, H.; Colombel, J.-F.; Rubin, D.T.; Jamed, A.; Mody, R.; Lasch, K. Effect of vedolizumab on health-related quality of life in patients with ulcerative colitis: Results from the randomized GEMINI 1 trial. Aliment. Pharmacol. Ther. 2017, 45, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Colombel, J.-F.; Feagan, B.G.; Vermeire, S.; Sandborn, W.J.; Sands, B.E.; Danese, S.; D’Haens, G.R.; Kaser, A.; Panaccione, R.; et al. Long-term efficacy of vedolizumab for ulcerative colitis. J. Crohns Colitis 2017, 11, 400–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoshi, M.; Kenji, W.; Haruhiko, O.; Takanori, K.; Toshiyuki, M.; Yasuo, S.; Mitsuhiro, S.; Kenkichi, S.; Kazunori, O.; Tetsuharu, H.; et al. Vedolizumab in Japanese patients with ulcerative colitis: A phase 3, randomized, double-blind, placebo-controlled study. PLoS ONE 2019, 14, e0212989. [Google Scholar]
- Kenji, W.; Satoshi, M.; Haruhiko, O.; Takanori, K.; Toshiyuki, M.; Yasuo, S.; Mitsuhiro, S.; Kenkichi, S.; Kazunori, O.; Tetsuharu, H.; et al. Effects of vedolizumab in Japanese patients with Crohn’s disease:a prospective, multicenter, randomized, placebo-controlled Phase 3 trial with exploratory analyses. J. Gastroenterol. 2020, 55, 291–306. [Google Scholar]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.-F.; Vermeire, S.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J. Crohns Colitis 2012, 6, 965–990. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Gotz, M.; Katsanos, K.H.; KieBlich, R.; et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis 2013, 7, 982–1018. [Google Scholar] [CrossRef] [Green Version]
- Baars, J.E.; Nuij, V.J.; Oldenburg, B.; Kuipers, E.J.; Woude, C.J. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm. Bowel Dis. 2012, 18, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; Lawlor, G.O.; Zenlea, T.; Goldsmith, J.D.; Gifford, A.; Falchuk, K.R.; Wolf, J.L.; Cheifetz, A.S.; Robson, S.C.; Moss, A.C. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm. Bowel Dis. 2013, 19, 779–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiho, T.; Jun, K.; Sakiko, H.; Asuka, N.; Daisuke, T.; Toshihiro, I.; Yuusaku, S.; Masahiro, T.; Keita, H.; Hiroyuki, O.; et al. Evaluation of Mucosal Healing in Ulcerative Colitis by Fecal Calprotectin Vs. Fecal Immunochemical Test. Am. J. Gastroenterol. 2015, 110, 873–880. [Google Scholar]
- Martine, D.V.; De Vos, M.; Louis, E.J.; Jahnsen, J.; Vandervoort, J.G.; Noman, M.; Dewit, O.; D’haens, G.R.; Franchimont, D.; Baert, F.J.; et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm. Bowel Dis. 2013, 19, 2111–2117. [Google Scholar]
- Shinazaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J. Crohns Colitis 2017, 11, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinisch, W.; Bressler, B.; Curtis, R.; Parikh, A.; Yang, H.; Rosario, M.; Røseth, A.; Danese, S.; Feagan, B.; Sands, B.E.; et al. Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: A post hoc analysis of GEMINI 1. Inflamm. Bowel Dis. 2019, 25, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Eliadou, E.; Day, A.S.; Thompson-Fawcett, M.W.; Gearry, R.B.; Rowbotham, D.S.; Walmsley, R.; Schultz, M.; Inns, S.J. New Zealand Society of Gastroenterology Guidelines for the Management of Refractory Ulcerative Colitis. N. Z. Med. J. 2015, 128, 63–76. [Google Scholar] [PubMed]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; D’haens, G.; Noman, M.; Vermeire, S.; Hiele, M.; Asnong, K.; Arts, J.; D’Hoore, A.; Penninckx, F.; Rutgeerts, P. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003, 125, 1025–1031. [Google Scholar] [CrossRef]
- Yoko, Y.; Mikio, K.; Ken, F.; Koji, K.; Kazuko, N.; Koji, N.; Tomoaki, K.; Yoshio, O.; Masaki, I.; Nobuyuki, H.; et al. Looking for predictive factors of clinical response to adsorptive granulocyte and monocyte apheresis in patients with ulcerative colitis: Markers of response to GMA. BMC Gastroenterol. 2013, 13, 27. [Google Scholar]
- Mohammed Vashist, N.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2018, 1, CD011450. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Boal Carvalho, P.; Cotter, J. Mucosal Healing in Ulcerative Colitis: A Comprehensive Review. Drugs 2017, 77, 159–173. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: From basic science to clinical practice. J. Crohns Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Jiang, X.; Peerani, F.; Narula, N.; Chaudrey, K.; Whitehead, D.; Hudesman, D.; Lukin, D.; Swaminath, A.; et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: Results from the US VICTORY consortium. Am. J. Gastroenterol. 2016, 111, 1147–1155. [Google Scholar] [CrossRef]
- Wils, P.; Bouhnik, Y.; Michetti, P.; Michetti, P.; Flourie, B.; Brixi, H.; Bourrier, A.; Allez, M.; Duclos, B.; Grimaud, J.C.; et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents. Clin. Gastroenterol. Hepatol. 2016, 14, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Mavroudis, G.; Magnusson, M.K.; Isaksson, S.; Sundin, J.; Semiren, M.; Ohman, L.; Strid, H. Mucosal and systemic immune profiles differ during early and late phases of the disease in patients with active ulcerative colitis. J. Crohns Colitis 2019, 13, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Shelton, E.; Allegretti, J.R.; Stevens, B.; Lucci, M.; Khalili, H.; Nguyen, D.D.; Yajnik, V. Efficacy of vedolizumab as induction therapy in refractory IBD patients: A multicenter cohort. Inflamm. Bowel Dis. 2015, 21, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Danese, S.; Colombel, J.F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef]
- Singh, S.; Murad, M.H.; Fumery, M.; Dulai, P.S.; Sandborn, W.J. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: An updated network meta-analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 2179–2191. [Google Scholar] [CrossRef]
- Neeraj, N.; Wong, E.C.L.; Marshall, J.K.; Colombel, J.F.; Dulai, P.S.; Reinisch, W. Comparative Efficacy for Infliximab Vs Vedolizumab in Biologic Naive Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2022, 20, 1588–1597. [Google Scholar]
- Makoto, N.; Kenji, W.; Satoshi, M.; Haruhiko, O.; Toshiyuki, M.; Yasuo, S.; Ursos, L.; Shigeru, S.; Mitsuhiro, S.; Tetsuharu, H.; et al. Potential benefits of immunomodulator use with vedolizumab for maintenance of remission in ulcerative colitis. J. Gastroenterol. Hepatol. 2022, 37, 81–88. [Google Scholar]
- Stallmach, A.; Langbein, C.; Atreya, R.; Bruns, T.; Dignass, A.; Ende, K.; Hampe, J.; Hartmann, F.; Neurath, M.F.; Maul, J.; et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease a prospective multicenter observational study. Aliment. Pharmacol. Ther. 2016, 44, 1199–1212. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rubin, D.T.; Danese, S.; Vermeire, S.; Abhyankar, B.; Sankoh, S.; James, A.; Smyth, M. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin. Gastroenterol. Hepatol. 2017, 15, 229–239. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Bokemeyer, B.; Drabik, A.; Stallmach, A.; Schreiber, S. Vedolizumab Germany Consortium. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice—A nationwide consecutive German cohort study. Aliment. Pharmacol. Ther. 2016, 43, 1090–1102. [Google Scholar] [CrossRef]
- Kopylov, U.; Ron, Y.; Avni-Biron, I.; Koslowsky, B.; Waterman, M.; Daher, S.; Dotan, I. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease the Israeli real-world experience. Inflamm. Bowel Dis. 2017, 23, 404–408. [Google Scholar] [CrossRef]
- Lenti, M.V.; Levison, S.; Avni-Biron, I.; Koslowsky, B.; Waterman, M.; Daher, S.; Ungar, B.; Yanai, H.; Maharshak, N.; Ben-Bassat, O.; et al. A real-world, long-term experience on effectiveness and safety of vedolizumab in adult patients with inflammatory bowel disease: The Cross Pennine study. Dig. Liver Dis. 2018, 50, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J.; Colombel, J.F.; Panaccione, R.; Dulai, P.S.; Rosario, M.; Cao, C.; Barocas, M.; Lasch, K. Deep remission with vedolizumab in patients with moderately to severely active ulcerative colitis: A GEMINI 1 post hoc analysis. J. Crohns Colitis 2018, 13, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arijs, I.; Hertogh, G.D.; Lemmens, B.; Van Lommel, L.; de Bruyn, M.; Vanhove, W.; Cleynen, I.; Machiels, K.; Ferrante, M.; Schuit, F.; et al. Effect of vedolizumab (anti-α4β7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut 2017, 67, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahori, M.; Watanabe, K.; Satoshi, M.; Haruhiko, O.; Takanori, K.; Toshiyuki, M.; Yasuo, S.; Philippe, P.; Lyann, U.; Shigeru, S.; et al. Symptomatic response with vedolizumab as a predictive factor in Japanese Anti-TNFα-naive patients with ulcerative colitis: A post hoc analysis of a randomized, placebo-controlled phase 3 trial. Digestion 2021, 102, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Daisuke, S.; Minoru, M.; Ryo, O.; Sotao, T.; Shintaro, M.; Tatsuya, M.; Miki, M.; Akihito, S.; Mari, H.; Jun, M.; et al. Clinical response of vedolizumab at week 6 predicted endoscopic remission at week 24 in ulcerative colitis. JGH Open 2021, 5, 1056–1062. [Google Scholar]





| (n = 87) | |||
|---|---|---|---|
| Age, Mean ± SD (range) | 45.2 ± 14.0 (20–86) | ||
| Sex, male: female (n) | 47:40 | ||
| Disease duration in years, mean ± SD (range) | 9.4 ± 7.5 (1–33) | ||
| Remission induction: maintenance (n) | 72:15 | ||
| Clinical Activity, mean ± SD (range) | Lichtiger CAI 1 | 7.7 ± 4.6 (0–17) | |
| Location of colitis, n (%) | Pancolitis | 56 (64.4%) | |
| Left-sided colitis | 31 (35.6%) | ||
| Mayo endoscopic subscore, n (%) | 0 | 13 (14.9%) | |
| 1 | 11 (12.6%) | ||
| 2 | 33 (41.3%) | ||
| 3 | 30 (37.5%) | ||
| Baseline data, mean ± SD | C-reactive protein (mg/dL) | 1.5 ± 1.2 | |
| Hemoglobin (g/dL) | 12.3 ± 2.3 | ||
| Bio-naïve, n (%) | 53 (60.9%) | ||
| Bio-failure, n (%): Prior bio use | 34 (39.1%) | 1 | 22 (25.3%) |
| 2 | 11 (12.6%) | ||
| 3 | 1 (1.2%) | ||
| Concomitant drug, n (%) | 5-Aminosalicylate | 78 (89.7%) | |
| Prednisolone | 36 (41.4%) | ||
| Azathioprine | 33 (37.9%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haga, K.; Shibuya, T.; Osada, T.; Sato, S.; Fukuo, Y.; Kobayashi, O.; Yamada, T.; Asaoka, D.; Ito, K.; Nomura, K.; et al. Early Clinical Remission Is a Predictor of Long-Term Remission with the Use of Vedolizumab for Ulcerative Colitis. Biomedicines 2022, 10, 2526. https://doi.org/10.3390/biomedicines10102526
Haga K, Shibuya T, Osada T, Sato S, Fukuo Y, Kobayashi O, Yamada T, Asaoka D, Ito K, Nomura K, et al. Early Clinical Remission Is a Predictor of Long-Term Remission with the Use of Vedolizumab for Ulcerative Colitis. Biomedicines. 2022; 10(10):2526. https://doi.org/10.3390/biomedicines10102526
Chicago/Turabian StyleHaga, Keiichi, Tomoyoshi Shibuya, Taro Osada, Shunsuke Sato, Yuka Fukuo, Osamu Kobayashi, Toshio Yamada, Daisuke Asaoka, Kentaro Ito, Kei Nomura, and et al. 2022. "Early Clinical Remission Is a Predictor of Long-Term Remission with the Use of Vedolizumab for Ulcerative Colitis" Biomedicines 10, no. 10: 2526. https://doi.org/10.3390/biomedicines10102526
APA StyleHaga, K., Shibuya, T., Osada, T., Sato, S., Fukuo, Y., Kobayashi, O., Yamada, T., Asaoka, D., Ito, K., Nomura, K., Haraikawa, M., Nomura, O., Fukushima, H., Murakami, T., Ishikawa, D., Hojo, M., & Nagahara, A. (2022). Early Clinical Remission Is a Predictor of Long-Term Remission with the Use of Vedolizumab for Ulcerative Colitis. Biomedicines, 10(10), 2526. https://doi.org/10.3390/biomedicines10102526








