Anti-Müllerian Hormone and Polycystic Ovary Syndrome in Women and Its Male Equivalent
Abstract
:1. Polycystic Ovary Syndrome: Diagnostic Criteria, Main Traits, and Pathophysiology
2. The Anti-Müllerian Hormone System
3. AMH and the PCOS
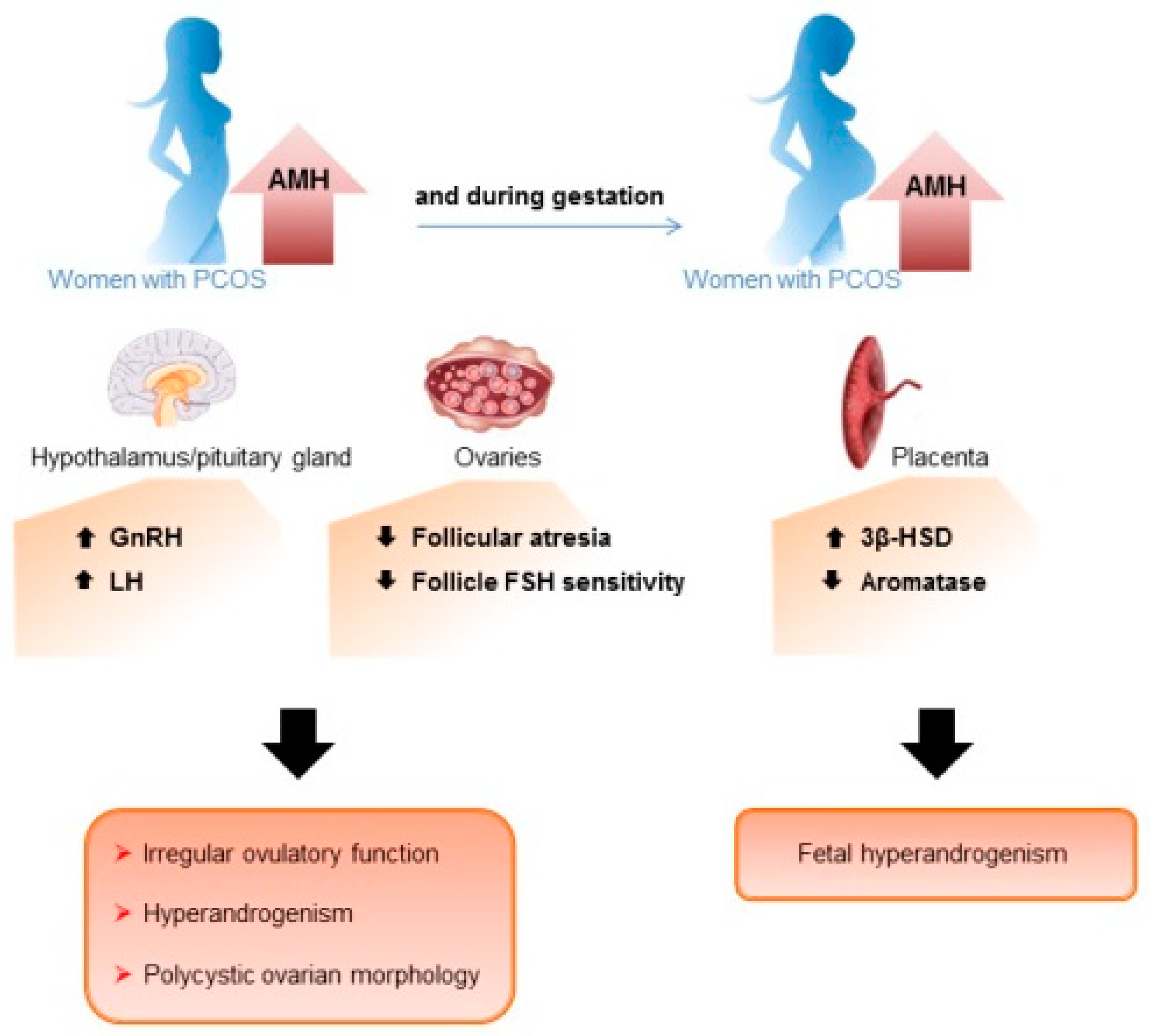
3.1. Overexpression of the AMH/AMHR2 System in Women with PCOS
3.2. Role of the AMH/AMHR2 System in the Reproductive Defects of Women with PCOS
3.3. Involvement of the AMH/AMHR2 System in the Origins of PCOS
4. AMH in Male PCOS Equivalent
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International, PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018, 89, 251–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teede, H.; Misso, M.; Tassone, E.C.; Dewailly, D.; Ng, E.H.; Azziz, R.; Norman, R.J.; Andersen, M.; Franks, S.; Hoeger, K.; et al. Anti-Mullerian Hormone in PCOS: A Review Informing International Guidelines. Trends Endocrinol. Metab. 2019, 30, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Manti, M.; Fornes, R.; Risal, S.; Lu, H.; Benrick, A. Origins and Impact of Psychological Traits in Polycystic Ovary Syndrome. Med. Sci. 2019, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Daolio, J.; Battaglia, F.A.; La Sala, G.B. Pregnancy complications in infertile patients with polycystic ovary syndrome: Updated evidence. Minerva Ginecol. 2018, 70, 754–760. [Google Scholar] [CrossRef]
- Yilmaz, B.; Vellanki, P.; Ata, B.; Yildiz, B.O. Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 523–533.e14. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Dall’Oglio, F.; La Vignera, S.; Mongioi, L.M.; Micali, G.; Calogero, A.E. Increased DHEAS and Decreased Total Testosterone Serum Levels in a Subset of Men with Early-Onset Androgenetic Alopecia: Does a Male PCOS-Equivalent Exist? Int. J. Endocrinol. 2020, 2020, 1942126. [Google Scholar] [CrossRef] [Green Version]
- Di Guardo, F.; Ciotta, L.; Monteleone, M.; Palumbo, M. Male Equivalent Polycystic Ovarian Syndrome: Hormonal, Metabolic, and Clinical Aspects. Int. J. Fertil. Steril. 2020, 14, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, B.; Rodriguez, S.; Cox, J.; Sanchez, S.; Fonseca, A.; Reddy, S.; Clegg, D.; Dwivedi, A.K. A systematic review and meta-analysis of the association between maternal polycystic ovary syndrome and neuropsychiatric disorders in children. Transl. Psychiatry 2021, 11, 569. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioi, L.M.; La Vignera, S.; Calogero, A.E. Does a male polycystic ovarian syndrome equivalent exist? J. Endocrinol. Investig. 2018, 41, 49–57. [Google Scholar] [CrossRef]
- Recabarren, S.E.; Sir-Petermann, T.; Rios, R.; Maliqueo, M.; Echiburu, B.; Smith, R.; Rojas-Garcia, P.; Recabarren, M.; Rey, R.A. Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J. Clin. Endocrinol. Metab. 2008, 93, 3318–3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recabarren, S.E.; Smith, R.; Rios, R.; Maliqueo, M.; Echiburu, B.; Codner, E.; Cassorla, F.; Rojas, P.; Sir-Petermann, T. Metabolic profile in sons of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, N.; Echiburu, B.; Maliqueo, M.; Luchsinger, M.; Rojas, P.; Recabarren, S.; Sir-Petermann, T. Reproductive and metabolic features during puberty in sons of women with polycystic ovary syndrome. Endocr. Connect. 2017, 6, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchen, L.C.; Kumar, A.; Kalra, B.; Savjani, G.; Sisk, R.; Legro, R.S.; Dunaif, A. Increased antimullerian hormone levels and other reproductive endocrine changes in adult male relatives of women with polycystic ovary syndrome. Fertil. Steril. 2016, 106, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Dumesic, D.A.; Hoyos, L.R.; Chazenbalk, G.D.; Naik, R.; Padmanabhan, V.; Abbott, D.H. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction 2020, 159, R1–R13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisosto, N.; de Guevara, A.L.; Echiburu, B.; Maliqueo, M.; Cavada, G.; Codner, E.; Paez, F.; Sir-Petermann, T. Higher luteinizing hormone levels associated with antimullerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil. Steril. 2019, 111, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Deng, Q. Transmission of Polycystic Ovary Syndrome via Epigenetic Inheritance. Trends Mol. Med. 2021, 27, 723–724. [Google Scholar] [CrossRef]
- Palomba, S.; Daolio, J.; Romeo, S.; Battaglia, F.A.; Marci, R.; La Sala, G.B. Lifestyle and fertility: The influence of stress and quality of life on female fertility. Reprod. Biol. Endocrinol. 2018, 16, 113. [Google Scholar] [CrossRef]
- Barrett, E.S.; Hoeger, K.M.; Sathyanarayana, S.; Abbott, D.H.; Redmon, J.B.; Nguyen, R.H.N.; Swan, S.H. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J. Dev. Orig. Health Dis. 2018, 9, 307–314. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.-P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.B.; Giacobini, P. New insights into anti-Mullerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol. Life Sci. 2021, 78, 1–16. [Google Scholar] [CrossRef] [PubMed]
- di Clemente, N.; Racine, C.; Pierre, A.; Taieb, J. Anti-Mullerian hormone in female reproduction. Endocr. Rev. 2021, 42, 753–782. [Google Scholar] [CrossRef] [PubMed]
- Josso, N.; Picard, J.Y. Genetics of anti-Mullerian hormone and its signaling pathway. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101634. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Koishi, K.; McGeachie, A.B.; Kimber, M.; Maclaughlin, D.T.; Donahoe, P.K.; McLennan, I.S. Mullerian inhibiting substance acts as a motor neuron survival factor in vitro. Proc. Natl. Acad. Sci. USA 2005, 102, 16421–16425. [Google Scholar] [CrossRef] [Green Version]
- Lebeurrier, N.; Launay, S.; Macrez, R.; Maubert, E.; Legros, H.; Leclerc, A.; Jamin, S.P.; Picard, J.-Y.; Marret, S.; Laudenbach, V.; et al. Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J. Cell Sci. 2008, 121, 3357–3365. [Google Scholar] [CrossRef] [Green Version]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonardm, S.; Collierm, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef] [Green Version]
- Bedecarrats, G.Y.; O’Neill, F.H.; Norwitz, E.R.; Kaiser, U.B.; Teixeira, J. Regulation of gonadotropin gene expression by Mullerian inhibiting substance. Proc. Natl. Acad. Sci. USA 2003, 100, 9348–9353. [Google Scholar] [CrossRef] [Green Version]
- Ohyama, K.; Ohta, M.; Hosaka, Y.Z.; Tanabe, Y.; Ohyama, T.; Yamano, Y. Expression of anti-Mullerian hormone and its type II receptor in germ cells of maturing rat testis. Endocr. J. 2015, 62, 997–1006. [Google Scholar] [CrossRef]
- Segev, D.L.; Hoshiya, Y.; Hoshiya, M.; Tran, T.T.; Carey, J.L.; Stephen, A.E.; MacLaughlin, D.T.; Donahoe, P.K.; Maheswaran, S. Mullerian-inhibiting substance regulates NF-kappa B signaling in the prostate in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, T.N.; Korobeynikov, V.A.; Kudinov, A.E.; Georgopoulos, R.; Solanki, N.R.; Andrews-Hoke, M.; Kistner, T.M.; Pepin, D.; Donahoe, P.K.; Nicolas, E.; et al. Anti-Mullerian Hormone Signaling Regulates Epithelial Plasticity and Chemoresistance in Lung Cancer. Cell Rep. 2016, 16, 657–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barret, J.M.; Nicolas, A.; Jarry, A.; Dubreuil, O.; Meseure, D.; Passat, T.; Perrial, E.; Deleine, C.; Champenois, G.; Gaillard, S.; et al. The Expression of Anti-Mullerian Hormone Type II Receptor (AMHRII) in Non-Gynecological Solid Tumors Offers Potential for Broad Therapeutic Intervention in Cancer. Biology 2021, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundstrom-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Segev, D.L.; Ha, T.U.; Tran, T.T.; Kenneally, M.; Harkin, P.; Jung, M.; MacLaughlin, D.T.; Donahoe, P.K.; Maheswaran, S. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J. Biol. Chem. 2000, 275, 28371–28379. [Google Scholar] [CrossRef] [Green Version]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Mullerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Victoria, M.; Labrosse, J.; Krief, F.; Cedrin-Durnerin, I.; Comtet, M.; Grynberg, M. Anti Mullerian Hormone: More than a biomarker of female reproductive function. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 19–24. [Google Scholar] [CrossRef]
- Josso, N.; Rey, R.A. What Does AMH Tell Us in Pediatric Disorders of Sex Development? Front. Endocrinol. 2020, 11, 619. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Cannarella, R.; Calogero, A.E.; La Vignera, S. Evaluation of testicular function in prepubertal children. Endocrine 2018, 62, 274–280. [Google Scholar] [CrossRef]
- Benderradji, H.; Prasivoravong, J.; Marcelli, F.; Barbotin, A.-L.; Catteau-Jonard, S.; Marchetti, C.; Guittard, C.; Puech, P.; Mitchell, V.; Rigot, J.-M.; et al. Contribution of serum anti-Mullerian hormone in the management of azoospermia and the prediction of testicular sperm retrieval outcomes: A study of 155 adult men. Basic Clin. Androl. 2021, 31, 15. [Google Scholar] [CrossRef]
- Pellatt, L.; Hanna, L.; Brincat, M.; Galea, R.; Brain, H.; Whitehead, S.; Mason, H. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J. Clin. Endocrinol. Metab. 2007, 92, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Wissing, M.L.; Mikkelsen, A.L.; Kumar, A.; Kalra, B.; Pors, S.E.; Flachs, E.M.; Andersen, C.Y. Associations of different molecular forms of antimullerian hormone and biomarkers of polycystic ovary syndrome and normal women. Fertil. Steril. 2019, 112, 149–155.e1. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Rame, C.; Cornuau, M.; Guerif, F.; Froment, P.; Dupont, J. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int. J. Mol. Sci. 2019, 20, 3778. [Google Scholar] [CrossRef] [Green Version]
- Bourgneuf, C.; Bailbé, D.; Lamazière, A.; Dupont, C.; Moldes, M.; Farabos, D.; Roblot, N.; Gauthier, C.; D’Argent, E.M.; Cohen-Tannoudji, J.; et al. The Goto-Kakizaki rat is a spontaneous prototypical rodent model of polycystic ovary syndrome. Nat. Commun. 2021, 12, 1064. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Yang, Y.J.; Tang, C.L.; Wang, K.; Chen, J.J.; Teng, X.M.; Ruan, Y.C.; Yang, J.Z. Elevation of antimullerian hormone in women with polycystic ovary syndrome undergoing assisted reproduction: Effect of insulin. Fertil. Steril. 2019, 111, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, E.; Kushnir, V.; Ma, X.; Biswas, A.; Prizant, H.; Gleicher, N.; Sen, A. Intra-cellular mechanism of Anti-Mullerian hormone (AMH) in regulation of follicular development. Mol. Cell. Endocrinol. 2016, 433, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Durlinger, A.L.L.; Gruijters, M.J.G.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.J.; Grootegoed, J.A.; et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef]
- Campbell, B.K.; Clinton, M.; Webb, R. The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology 2012, 153, 4533–4543. [Google Scholar] [CrossRef] [Green Version]
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M.; Brown, K.; Simpson, E.R.; Mason, H.D. Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil. Steril. 2011, 96, 1246–1251.e1. [Google Scholar] [CrossRef]
- Sacchi, S.; Marinaro, F.; Xella, S.; Marsella, T.; Tagliasacchi, D.; La Marca, A. The anti-Mullerian hormone (AMH) induces forkhead box L2 (FOXL2) expression in primary culture of human granulosa cells in vitro. J. Assist. Reprod. Genet. 2017, 34, 1131–1136. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Mamsen, L.S.; Jeppesen, J.V.; Botkjaer, J.A.; Pors, S.E.; Borgbo, T.; Ernst, E.; Macklon, K.T.; Andersen, C.Y. Hallmarks of Human Small Antral Follicle Development: Implications for Regulation of Ovarian Steroidogenesis and Selection of the Dominant Follicle. Front. Endocrinol. 2017, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezard, J.; Vigier, B.; Tran, D.; Mauleon, P.; Josso, N. Immunocytochemical study of anti-Mullerian hormone in sheep ovarian follicles during fetal and post-natal development. J. Reprod. Fertil. 1987, 80, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, J.A.; Durlinger, A.L.; Peters, I.J.; van den Heuvel, E.R.; Rose, U.M.; Kramer, P.; de Jong, F.H.; Themmen, A.P. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology 2007, 148, 2301–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racine, C.; Genet, C.; Bourgneuf, C.; Dupont, C.; Plisson-Petit, F.; Sarry, J.; Hennequet-Antier, C.; Vigouroux, C.; Mathieu d’Argent, E.; Pierre, A.; et al. New Anti-Mullerian Hormone Target Genes Involved in Granulosa Cell Survival in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, e1271–e1289. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.S.; Kim, S.K.; Lee, J.; Youm, H.W.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effect of Exogenous Anti-Mullerian Hormone Treatment on Cryopreserved and Transplanted Mouse Ovaries. Reprod. Sci. 2016, 23, 51–60. [Google Scholar] [CrossRef]
- Detti, L.; Fletcher, N.M.; Saed, G.M.; Sweatman, T.W.; Uhlmann, R.A.; Pappo, A.; Peregrin-Alvarez, I. Xenotransplantation of pre-pubertal ovarian cortex and prevention of follicle depletion with anti-Mullerian hormone (AMH). J. Assist. Reprod. Genet. 2018, 35, 1831–1841. [Google Scholar] [CrossRef]
- Xu, J.; Xu, F.; Lawson, M.S.; Tkachenko, O.Y.; Ting, A.Y.; Kahl, C.A.; Park, B.S.; Stouffer, R.R.; Bishop, C.V. Anti-Mullerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture. Biol. Reprod. 2018, 98, 197–207. [Google Scholar] [CrossRef]
- Dilaver, N.; Pellatt, L.; Jameson, E.; Ogunjimi, M.; Bano, G.; Homburg, R.; Homburg, D.M.; Rice, S. The regulation and signalling of anti-Mullerian hormone in human granulosa cells: Relevance to polycystic ovary syndrome. Hum. Reprod. 2019, 34, 2467–2479. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B.; Khanimov, M.; Malter, H.E.; Grazi, R.V.; Leader, B. Characterization of women with elevated antimullerian hormone levels (AMH): Correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am. J. Obstet. Gynecol. 2014, 211, 59.e1–59.e8. [Google Scholar] [CrossRef]
- Sahmay, S.; Atakul, N.; Oncul, M.; Tuten, A.; Aydogan, B.; Seyisoglu, H. Serum anti-Mullerian hormone levels in the main phenotypes of polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 157–161. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.S.B.; Decoster, L.; Trova, S.; Mimouni, N.E.H.; Delli, V.; Chachlaki, K.; Yu, Q.; Boehm, U.; Prevot, V.; Giacobini, P. Female sexual behavior is disrupted in a preclinical mouse model of PCOS via an attenuated hypothalamic nitric oxide pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2203503119. [Google Scholar] [CrossRef]
- Wang, J.; Dicken, C.; Lustbader, J.W.; Tortoriello, D.V. Evidence for a Mullerian-inhibiting substance autocrine/paracrine system in adult human endometrium. Fertil. Steril. 2009, 91, 1195–1203. [Google Scholar] [CrossRef]
- Kevenaar, M.E.; Laven, J.S.; Fong, S.L.; Uitterlinden, A.G.; de Jong, F.H.; Themmen, A.P.; Visser, J.A. A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J. Clin. Endocrinol. Metab. 2008, 93, 1310–1316. [Google Scholar] [CrossRef]
- Gorsic, L.K.; Dapas, M.; Legro, R.S.; Hayes, M.G.; Urbanek, M. Functional Genetic Variation in the Anti-Mullerian Hormone Pathway in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 2855–2874. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, N.E.H.; Paiva, I.; Barbotin, A.-L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V.; et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530.e8. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, P.; Rey, R.; Poggioli, S.; Donzeau, M.; Chevallier, D.; Pointis, G. Anti-Mullerian hormone as a seminal marker for spermatogenesis in non-obstructive azoospermia. Hum. Reprod. 1999, 14, 2020–2024. [Google Scholar] [CrossRef] [Green Version]
- Picard, J.Y.; Josso, N. Persistent Mullerian duct syndrome: An update. Reprod. Fertil. Dev. 2019, 31, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Behringer, R.R.; Cate, R.L.; Froelick, G.J.; Palmiter, R.D.; Brinster, R.L. Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature 1990, 345, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Mishina, Y.; Rey, R.; Finegold, M.J.; Matzuk, M.M.; Josso, N.; Cate, R.L.; Behringer, R.R. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996, 10, 2577–2587. [Google Scholar] [CrossRef]
- Rouiller-Fabre, V.; Carmona, S.; Merhi, R.A.; Cate, R.; Habert, R.; Vigier, B. Effect of anti-Mullerian hormone on Sertoli and Leydig cell functions in fetal and immature rats. Endocrinology 1998, 139, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Messika-Zeitoun, L.; Gouedard, L.; Belville, C.; Dutertre, M.; Lins, L.; Imbeaud, S.; Hughes, I.A.; Picard, J.Y.; Josso, N.; di Clemente, N. Autosomal recessive segregation of a truncating mutation of anti-Mullerian type II receptor in a family affected by the persistent Mullerian duct syndrome contrasts with its dominant negative activity in vitro. J. Clin. Endocrinol. Metab. 2001, 86, 4390–4397. [Google Scholar] [CrossRef] [Green Version]
- Rehman, Z.U.; Worku, T.; Davis, J.S.; Talpur, H.S.; Bhattarai, D.; Kadariya, I.; Hua, G.; Cao, J.; Dad, R.; Farmanullah; et al. Role and mechanism of AMH in the regulation of Sertoli cells in mice. J. Steroid Biochem. Mol. Biol. 2017, 174, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Racine, C.; Rey, R.; Forest, M.G.; Louis, F.; Ferre, A.; Huhtaniemi, I.; Josso, N.; di Clemente, N. Receptors for anti-mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc. Natl. Acad. Sci. USA 1998, 95, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemienowicz, K.J.; Filis, P.; Shaw, S.; Douglas, A.; Thomas, J.; Mulroy, S.; Howie, F.; Fowler, P.A.; Duncan, W.C.; Rae, M.T. Fetal androgen exposure is a determinant of adult male metabolic health. Sci. Rep. 2019, 9, 20195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Garcia, P.P.; Recabarren, M.P.; Sir-Petermann, T.; Rey, R.; Palma, S.; Carrasco, A.; Perez-Marin, C.C.; Padmanabhan, V.; Recabarren, S.E. Altered testicular development as a consequence of increase number of sertoli cell in male lambs exposed prenatally to excess testosterone. Endocrine 2013, 43, 705–713. [Google Scholar] [CrossRef]
- Echiburu, B.; Milagro, F.; Crisosto, N.; Perez-Bravo, F.; Flores, C.; Arpon, A.; Salas-Perez, F.; Recabarren, S.E.; Sir-Petermann, T.; Maliqueo, M. DNA methylation in promoter regions of genes involved in the reproductive and metabolic function of children born to women with PCOS. Epigenetics 2020, 15, 1178–1194. [Google Scholar] [CrossRef]

| Organs | Women | Men | Both | References | |
|---|---|---|---|---|---|
| AMH expression | ovaries | ++++ | Reviewed in [24] | ||
| testes | ++++ | Reviewed in [25] | |||
| nervous tissue | + | [26,27] | |||
| hypothalamus | + | [28] | |||
| pituitary gland | + | [29] | |||
| AMHR2 expression | ovaries | ++++ | Reviewed in [24] | ||
| testes | ++++ | Reviewed in [25] | |||
| uterus | ++ | Reviewed in [24] | |||
| placenta | + | [34] | |||
| breasts | + | [35] | |||
| prostate | + | [31] | |||
| nervous tissue | + | [26,27] | |||
| hypothalamus | + | [28] | |||
| pituitary gland | + | [29] | |||
| lungs | + | [32] | |||
| pancreas | + | [33] | |||
| adrenals | ++ | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Clemente, N.; Racine, C.; Rey, R.A. Anti-Müllerian Hormone and Polycystic Ovary Syndrome in Women and Its Male Equivalent. Biomedicines 2022, 10, 2506. https://doi.org/10.3390/biomedicines10102506
di Clemente N, Racine C, Rey RA. Anti-Müllerian Hormone and Polycystic Ovary Syndrome in Women and Its Male Equivalent. Biomedicines. 2022; 10(10):2506. https://doi.org/10.3390/biomedicines10102506
Chicago/Turabian Styledi Clemente, Nathalie, Chrystèle Racine, and Rodolfo A. Rey. 2022. "Anti-Müllerian Hormone and Polycystic Ovary Syndrome in Women and Its Male Equivalent" Biomedicines 10, no. 10: 2506. https://doi.org/10.3390/biomedicines10102506
APA Styledi Clemente, N., Racine, C., & Rey, R. A. (2022). Anti-Müllerian Hormone and Polycystic Ovary Syndrome in Women and Its Male Equivalent. Biomedicines, 10(10), 2506. https://doi.org/10.3390/biomedicines10102506






