Actualities in the Morphology and Immunohistochemistry of Cutaneous and Ocular Melanoma: What Lies Ahead? A Single-Centre Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Epidemiologic Data and Tumor Classification
3.2. Histology and IHC
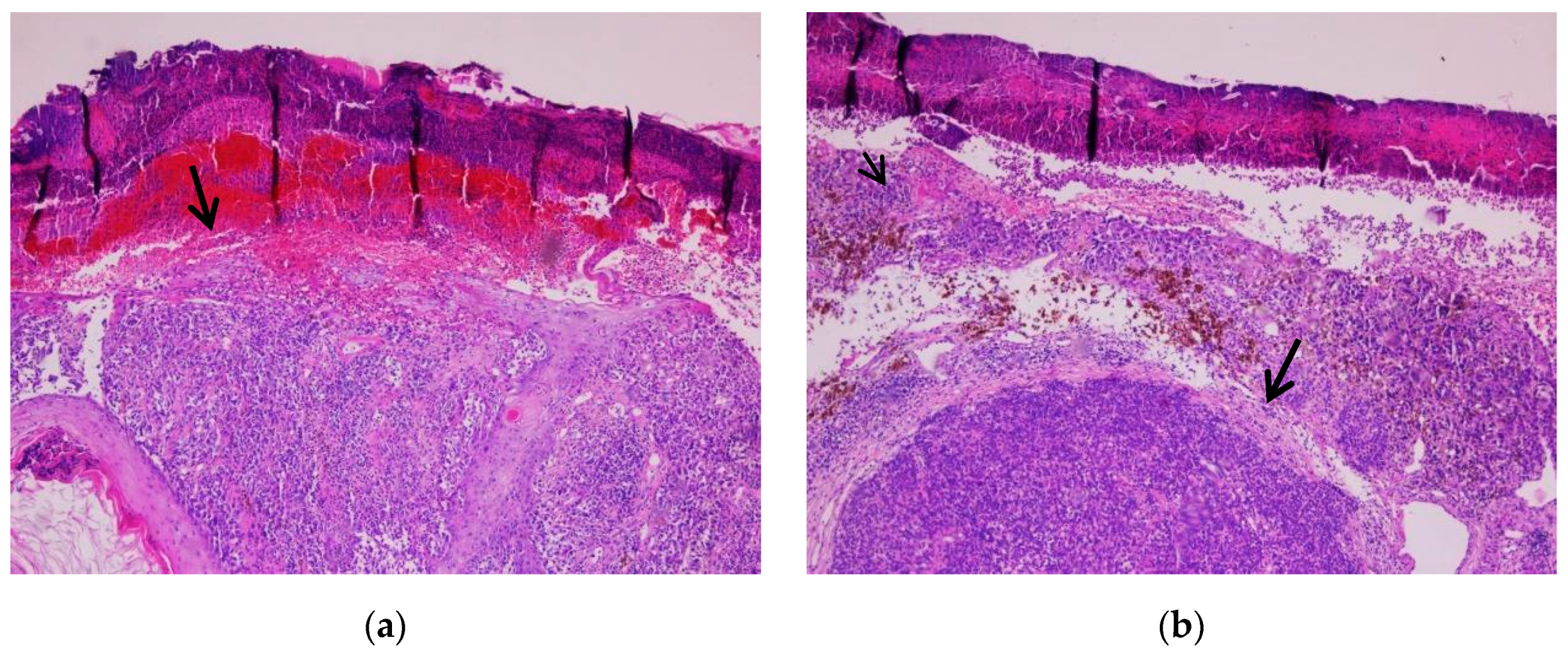
3.2.1. Cutaneous Melanoma
- Nodular melanoma
- Superficial spreading melanoma
- Nevoid melanoma
3.2.2. Ocular Melanoma
- Conjunctival melanoma
- Uveal melanoma
3.2.3. Sentinel Lymph Nodes and Capsular Nevi
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobos, M. Histopathologic classification and prognostic factors of melanoma: A 2021 update. Ital. J. Dermatol. Venereol. 2021, 156, 300–321. [Google Scholar] [CrossRef]
- Reed, R.J.; Martin, P. Variants of melanoma. Semin. Cutan. Med. Surg. 1997, 16, 137–158. [Google Scholar] [CrossRef]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.-J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Prim. 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.; Shields, J. Uveal melanoma: Estimating prognosis. Indian J. Ophthalmol. 2015, 63, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Parravano, M.; Gatta, G.; Capocaccia, R.; Mazzini, C.; Mallone, S.; Botta, L.; RARECAREnet Working Group. Incidence and Survival of Patients with Conjunctival Melanoma in Europe. JAMA Ophthalmol. 2020, 138, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ghazawi, F.M.; Darwich, R.; Le, M.; Jfri, A.; Rahme, E.; Burnier, J.V.; Sasseville, D.; Burnier, M.N., Jr.; Litvinov, I.V. Incidence trends of conjunctival malignant melanoma in Canada. Br. J. Ophthalmol. 2019, 104, 23–25. [Google Scholar] [CrossRef]
- Tuffaha, M.S.A.; Guski, H.; Kristiansen, G. Immunohistochemistry in Tumor Diagnostics; Springer: Cham, Switzerland, 2018; ISBN 9783319535760. [Google Scholar]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef]
- Dinehart, M.S.; Dinehart, S.M.; Sukpraprut-Braaten, S.; High, W.A. Immunohistochemistry utilization in the diagnosis of melanoma. J. Cutan. Pathol. 2020, 47, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.-J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, O.; Lyons, T.; Murphy, S.; Feeley, L.; Power, D.; Heffron, C.C.B.B. BRAF V600 mutation detection in melanoma: A comparison of two laboratory testing methods. J. Clin. Pathol. 2017, 70, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma—An Update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamoto, K.; Al-Habsi, M.; Honjo, T. Role of PD-1 in Immunity and Diseases. Curr. Top. Microbiol. Immunol. 2017, 410, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Morales-Betanzos, C.A.; Lee, H.; Gonzalez Ericsson, P.I.; Balko, J.M.; Johnson, D.B.; Zimmerman, L.J.; Liebler, D.C. Quantita-tive Mass Spectrometry Analysis of PD-L1 Protein Expression, N-glycosylation and Expression Stoichiometry with PD-1 and PD-L2 in Human Melanoma. Mol. Cell. Proteom. 2017, 16, 1705–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.J.E.; Carlos, G.; Wakade, D.; Byth, K.; Kong, B.Y.; Chou, S.; Carlino, M.S.; Kefford, R.; Fernandez-Penas, P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J. Am. Acad. Dermatol. 2016, 74, 455–461.e1. [Google Scholar] [CrossRef] [Green Version]
- Tinca, A.C.; Cocuz, I.G.; Șincu, M.C.; Niculescu, R.; Sabău, A.H.; Chiorean, D.M.; Szőke, A.R.; Cotoi, O.S. VISTA, PDL-L1, and BRAF—A Review of New and Old Markers in the Prognosis of Melanoma. Medicina 2022, 58, 74. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.R.; Knecht, M.; Mollaee, M.; Zhong, Z.; Erkes, D.A.; McCue, P.A.; Chervoneva, I.; Berger, A.C.; Lo, J.A.; Fisher, D.E.; et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020, 30, 510–524.e6. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, Y.J.; Yun, K.A.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E.; Lee, W.J. The prognostic significance of VISTA and CD33-positive myeloid cells in cutaneous melanoma and their relationship with PD-1 expression. Sci. Rep. 2020, 10, 14372. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Tucker, M.A. Genetic Epidemiology of Familial Melanoma. Dermatol. Clin. 1995, 13, 605–612. [Google Scholar] [CrossRef]
- Ward, W.H.; Lambreton, F.; Goel, N.; Yu, J.Q.; Farma, J.M. Clinical Presentation and Staging of Melanoma. Available online: https://www.ncbi.nlm.nih.gov/books/NBK481857/ (accessed on 20 July 2022).
- Brenn, T. Histologisches Spektrum des malignen Melanoms. Der Pathol. 2015, 36, 53–61. [Google Scholar] [CrossRef]
- Xavier, M.H.; Drummond-Lage, A.P.; Baeta, C.; Rocha, L.; Almeida, A.M.; Wainstein, A.J. Delay in cutaneous melanoma diagnosis: Sequence analyses from suspicion to diagnosis in 211 patients. Medicine 2016, 95, e4396. [Google Scholar] [CrossRef]
- Barnhill, R.L.; Mihm, M.C., Jr. The histopathology of cutaneous malignant melanoma. Semin. Diagn. Pathol. 1993, 10, 47–75. [Google Scholar] [PubMed]
- Greenwald, H.S.; Friedman, E.B.; Osman, I. Superficial spreading and nodular melanoma are distinct biological entities. Melanoma Res. 2012, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osella-Abate, S.; Ribero, S.; Sanlorenzo, M.; Maule, M.M.; Richiardi, L.; Merletti, F.; Tomasini, C.; Marra, E.; Macripò, G.; Fierro, M.T.; et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int. J. Cancer 2014, 136, 2453–2457. [Google Scholar] [CrossRef]
- Zhang, A.J.; Rush, P.S.; Tsao, H.; Duncan, L.M. BRCA1-associated protein (BAP1)-inactivated melanocytic tumors. J. Cutan. Pathol. 2019, 46, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalised” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Wong, S.L.; Faries, M.B.; Kennedy, E.B.; Agarwala, S.S.; Akhurst, T.J.; Ariyan, C.; Balch, C.M.; Berman, B.S.; Cochran, A.; Delman, K.A.; et al. Sentinel Lymph Node Biopsy and Management of Regional Lymph Nodes in Melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Szumera-Ciećkiewicz, A.; Bosisio, F.; Teterycz, P.; Antoranz, A.; Delogu, F.; Koljenović, S.; van de Wiel, B.A.; Blokx, W.; van Kempen, L.; Rutkowski, P.; et al. SOX10 is as specific as S100 protein in detecting metastases of melanoma in lymph nodes and is recommended for sentinel lymph node assessment. Eur. J. Cancer 2020, 137, 175–182. [Google Scholar] [CrossRef]
- Prieto, V.G. Sentinel Lymph Nodes in Cutaneous Melanoma. Clin. Lab. Med. 2017, 37, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Patil, J.; Aydin, N.; Mishra, A.; Misra, S. Capsular nevus versus metastatic malignant melanoma—A diagnostic dilemma. Int. J. Surg. Case Rep. 2016, 29, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tardelli, E.; Mazzarri, S.; Rubello, D.; Gennaro, M.; Fantechi, L.; Duce, V.; Romanini, A.; Chondrogiannis, S.; Volterrani, D.; Colletti, P.M.; et al. Sentinel Lymph Node Biopsy in Cutaneous Melanoma. Clin. Nucl. Med. 2016, 41, e498–e507. [Google Scholar] [CrossRef]
- Lezcano, C.; Pulitzer, M.; Moy, A.P.; Hollmann, T.J.; Jungbluth, A.A.; Busam, K.J. Immunohistochemistry for PRAME in the Distinction of Nodal Nevi From Metastatic Melanoma. Am. J. Surg. Pathol. 2019, 44, 503–508. [Google Scholar] [CrossRef]
- Carr, M.J.; Monzon, F.A.; Zager, J.S. Sentinel lymph node biopsy in melanoma: Beyond histologic factors. Clin. Exp. Metastasis 2021, 39, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Read, R.L.; Haydu, L.; Saw, R.P.M.; Quinn, M.J.; Shannon, K.; Spillane, A.J.; Stretch, J.R.; Scolyer, R.A.; Thompson, J.F. In-transit Melanoma Metastases: Incidence, Prognosis, and the Role of Lymphadenectomy. Ann. Surg. Oncol. 2014, 22, 475–481. [Google Scholar] [CrossRef]
- Tie, E.N.; Henderson, M.A.; Gyorki, D.E. Management of in-transit melanoma metastases: A review. ANZ J. Surg. 2018, 89, 647–652. [Google Scholar] [CrossRef]
- Mohamed, A.; Gonzalez, R.S.; Lawson, D.; Wang, J.; Cohen, C. SOX10 expression in malignant melanoma, carcinoma, and normal tissues. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Kazlouskaya, V.; Kochoumian, E.; Mangold, A.; Lal, K.; Maia-Cohen, S.; Elston, D.M. Tumor with the features of both squamous cell carcinoma and melanoma (melanocarcinoma). Indian Dermatol. Online J. 2015, 6, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-A.; Madigan, M.C.; Conway, R.M. Conjunctival melanoma: A review of conceptual and treatment advances. Clin. Ophthalmol. 2013, 6, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Chandravanshi, S.L.; Rathore, M.K.; Goyal, P.; Jain, S.C. Huge malignant melanoma of caruncle with extensive involve-ment of conjunctiva. J Indian Med Assoc. 2012, 110, 115–117. [Google Scholar]
- Tuomaala, S.; Eskelin, S.; Tarkkanen, A.; Kivelä, T. Population-Based Assessment of Clinical Characteristics Predicting Out-come of Conjunctival Melanoma in Whites. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3399–3408. [Google Scholar]
- Bronkhorst, I.H.; Jager, M.J. Uveal Melanoma: The Inflammatory Microenvironment. J. Innate Immun. 2012, 4, 454–462. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Al, E. WHO Classification of Tumours of the Eye; International Agency for Research on Cancer: Lyon, France, 2018; ISBN 9789283244974. [Google Scholar]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef]
- de Lange, M.J.; Nell, R.J.; Lalai, R.N.; Versluis, M.; Jordanova, E.S.; Luyten, G.P.; Jager, M.J.; van der Burg, S.H.; Zoutman, W.H.; van Hall, T.; et al. Digital PCR-Based T-cell Quantification–Assisted Deconvolution of the Microenvironment Reveals that Activated Macrophages Drive Tumor Inflammation in Uveal Melanoma. Mol. Cancer Res. 2018, 16, 1902–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaštelan, S.; Antunica, A.G.; Oresković, L.B.; Pelčić, G.; Kasun, E.; Hat, K. Immunotherapy for Uveal Melanoma—Current Knowledge and Perspectives. Curr. Med. Chem. 2020, 27, 1350–1366. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, M.; Giunta, E.; Tortora, M.; Curvietto, M.; Attademo, L.; Bosso, D.; Cardalesi, C.; Rosanova, M.; De Placido, P.; Pietroluongo, E.; et al. BRAF Gene and Melanoma: Back to the Future. Int. J. Mol. Sci. 2021, 22, 3474. [Google Scholar] [CrossRef]
- Fauviaux, E.; Promelle, V.; Boucenna, V.; Jany, B.; Errera, M.; Delbarre, M. Toxicité oculaire des thérapies ciblées anti-MEK et anti-BRAF dans le traitement des mélanomes cutanés métastatiques [Ocular toxicity of targeted therapies with MEK inhibitors and BRAF inhibitors in the treatment of metastatic cutaneous melanoma]. J. Fr. Ophtalmol. 2022, 45, 612–618. [Google Scholar] [CrossRef]
- Jackett, L.A.; McCarthy, S.W.; Scolyer, R.A. SOX10 expression in cutaneous scars: A potential diagnostic pitfall in the evaluation of melanoma re-excision specimens. Pathology 2016, 48, 626–628. [Google Scholar] [CrossRef]
- Blochin, E.; Nonaka, D. Diagnostic value of Sox10 immunohistochemical staining for the detection of metastatic melanoma in sentinel lymph nodes. Histopathology 2009, 55, 626–628. [Google Scholar] [CrossRef] [PubMed]














| Clone | Control and Expression | Stain Type |
|---|---|---|
| SOX10 rabbit monoclonal primary antibody, CELL MARQUE (SP267) | Control on melanocytes (basement layer), staining melanoma cells | Nuclear |
| S100 polyclonal primary antibody, VENTANA | Control on melanocytes (basement layer), staining melanoma cells | Nuclear |
| Anti-melanosome (HMB45) mouse monoclonal primary antibody, VENTANA | Control on melanocytes (basement layer), staining melanoma cells | Cytoplasmic |
| Melan-A A103, CONFIRM Anti-MART-1/Melan-A (A103) mouse monoclonal primary antibody, VENTANA | Control on melanocytes (basement layer), staining melanoma cells | Cytoplasmic |
| CONFIRM Anti-Ki-67 (30–9) rabbit monoclonal primary antibody, VENTANA | Keratinoblasts; expressed in >10% of melanoma cells | Nuclear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinca, A.C.; Moraru, R.; Cocuz, I.G.; Șincu, M.C.; Niculescu, R.; Sabău, A.H.; Chiorean, D.M.; Szoke, A.R.; Morariu, S.-H.; Cotoi, O.S. Actualities in the Morphology and Immunohistochemistry of Cutaneous and Ocular Melanoma: What Lies Ahead? A Single-Centre Study. Biomedicines 2022, 10, 2500. https://doi.org/10.3390/biomedicines10102500
Tinca AC, Moraru R, Cocuz IG, Șincu MC, Niculescu R, Sabău AH, Chiorean DM, Szoke AR, Morariu S-H, Cotoi OS. Actualities in the Morphology and Immunohistochemistry of Cutaneous and Ocular Melanoma: What Lies Ahead? A Single-Centre Study. Biomedicines. 2022; 10(10):2500. https://doi.org/10.3390/biomedicines10102500
Chicago/Turabian StyleTinca, Andreea Cătălina, Raluca Moraru, Iuliu Gabriel Cocuz, Mihaela Cornelia Șincu, Raluca Niculescu, Adrian Horațiu Sabău, Diana Maria Chiorean, Andreea Raluca Szoke, Silviu-Horia Morariu, and Ovidiu Simion Cotoi. 2022. "Actualities in the Morphology and Immunohistochemistry of Cutaneous and Ocular Melanoma: What Lies Ahead? A Single-Centre Study" Biomedicines 10, no. 10: 2500. https://doi.org/10.3390/biomedicines10102500
APA StyleTinca, A. C., Moraru, R., Cocuz, I. G., Șincu, M. C., Niculescu, R., Sabău, A. H., Chiorean, D. M., Szoke, A. R., Morariu, S.-H., & Cotoi, O. S. (2022). Actualities in the Morphology and Immunohistochemistry of Cutaneous and Ocular Melanoma: What Lies Ahead? A Single-Centre Study. Biomedicines, 10(10), 2500. https://doi.org/10.3390/biomedicines10102500







