1. Introduction
Prostate cancer (PCa) continues to be the second most common oncological diagnosis in men worldwide, and the fifth leading cause of cancer-related deaths. However, up to 50% of diagnosed patients do not require immediate interventional treatment. Randomized clinical trials have shown that active surveillance (AS) does not compromise survival [
1], while it significantly improves quality of life (QoL) and functional outcomes [
2,
3] in patients with localized disease. Current clinical guidelines recommend AS as the preferred method of treatment for patients with low-risk (LR) cancer, and as a viable option for favourable intermediate-risk group (IR) PCa [
4]. Despite these findings, the clinical utilization of AS remains low, and coordinated initiatives are vital in improving the standards of care [
5].
The scepticism can be associated with the observation that despite no (disease-specific) survival benefit of surgery or radiotherapy (RT) over AS, interventional treatment may be associated with a lower rate of progression and metastatic disease [
1,
6]. The high average lifespan conveys a need for both an efficient and effective modality in a long perspective and might contribute to a minor difference in clinical progression, eventually translating into significant survival difference over a longer course of follow-up (FU) [
7]. This hypothetical equilibrium is challenged by the emerging utilization of stereotactic body radiotherapy (SBRT) for oligometastatic disease [
8], especially with the aid of PET-PSMA [
9], and multiple local salvage modalities [
10], which can allow for definitive treatment in the setting of limited cancer recurrence or dissemination.
Among the available interventional approaches toward PCa management, ultra-hypofractionated RT (UHRT) could be an alternative for AS in LR, a standard of care in IR, and possibly even a therapeutic option in selected patients with more advanced PCa [
11]. The five-fraction delivery of 36.25–40 Gy combines surgery-like short overall treatment time, high precision, and exceptional cost-effectiveness while remaining generally non-invasive (except for biopsy and optional fiducial placement). Compared to conventionally fractionated RT, UHRT also takes advantage of the low α/β ratio of PCa, which signalizes a high sensitivity to increases in fraction doses [
12,
13,
14,
15]. Prospective randomized clinical trials have shown non-inferiority of UHRT [
16,
17], which is supported by the real-world data showing an excellent toxicity profile, and as few as 3% clinical failures at a median FU of 31.3 months [
18]. Moreover, as metastases-directed therapy and local salvage methods for local–regional recurrences have been considered a standard of care at our institution for over a decade, we recorded a significant subset of clinical failures treated with ‘second-line definitive treatment’.
In this publication, we aimed to analyze the patterns of recurrence, feasibility, and efficacy of local salvage therapies, and assess the competing oncological survival risk in patients treated with CyberKnifeTM UHRT for localized PCa.
2. Materials and Methods
This retrospective analysis included 650 patients consecutively treated between 2011 and 2018 at a single tertiary institution for localized PCa with primary CyberKnifeTM UHRT and androgen deprivation therapy (ADT) in applicable cases. The patients were treated and monitored according to a board-approved institutional protocol. The treatment was considered to be a standard of care; however, due to its novelty at the time of introduction (2011), the patients were treated and monitored in a manner similar to a prospective trial, and the outcomes were periodically assessed.
Patients considered for the treatment had to fulfil the following inclusion criteria: histopathological diagnosis of previously untreated prostate cancer, International Society of Urological Pathology (ISUP) grade of 2 or lower, TNM T stage of T2c or lower, TNM N and M stage 0, maximum prostate-specific antigen (PSA) concentration < 20 ng/mL, <50 mm maximum two-dimensional prostate measurement, and feasibility of implanting fiducials.
One patient with ISUP grade 3 was removed from the initial database due to concern for low variability in multivariable analysis. The maximum pre-treatment PSA exceeded 20 ng/mL in 10 patients (1.5%), which resulted in 10 patients being allocated to the high-risk group (HR). However, these patients were retained in the analysis assuming a proportional hazard of increase in PSA concentration.
Three Gold Anchor fiducials were implanted in a triangle-like configuration in each patient before treatment planning with computed tomography (CT) for real-time tracking of image-guided radiotherapy (IGRT), which was used for initial positioning and target tracking during each fraction. The patients were positioned on a vacuum mattress, with a moderately full bladder. The target volume and organs at risk were defined on the treatment planning CT, with the aid of a fused treatment planning Magnetic Resonance Imaging (MRI).
The patients received 36.25 Gy in five equal fractions of 7.25 Gy, delivered every other day on a CyberKnifeTM linear accelerator. Assuming an alpha/beta ratio of 1.5 for prostate cancer, this corresponds to an equivalent dose in 2 Gy fractions (EQD2) of 90.6 Gy, or a biologically effective dose (BED) of 211.5 Gy. The Clinical Target Volume (CTV) included the whole prostate and the proximal 1 cm of the seminal vesicles and was expanded by three mm posteriorly and five mm margin in the remaining directions to form the Planning Target Volume (PTV). None of the patients received pelvic lymph node irradiation. The use of androgen deprivation therapy (ADT) was discouraged in LR patients, while six-month ADT was suggested in IR patients with >50% positive biopsy cores or at least two of the following risk factors: PSA > 10 ng/mL, ISUP grade 2 and/or TNM T2b or T2c.
The clinical follow-up was based on the patients’ medical records. The follow-up visits were scheduled at 1, 4, and 8 months, and every 6 months thereafter. As described below, (18)F-fluorocholine-PET, later superseded by PET-PSMA and/or MRI, were considered as routine diagnostic modalities in case of rising PSA level and/or reasonable presumption of clinical failure. The complete survival data were available for each patient based on the National Cancer Registry. Each of the analyzed endpoints was calculated from the first day of RT until:
- -
Prostate, seminal vesicles or regional pelvic lymph node recurrence for local–regional control (LRC);
- -
Occurrence of distant metastases for freedom from distant metastases (FFDM);
- -
Distant metastases or death for metastases-free survival (MFS);
- -
Death for overall survival.
Or from the diagnosis of clinical failure (LRC, FFDM) to the occurrence of clinical progression or death after metastases-directed local therapy for second-line progression-free survival (sPFS).
Death was defined as all-cause mortality (non-disease-specific). Local–regional and distant metastases were defined according to the eight edition of TNM by AJCC. Biochemical failure was defined according to the Phoenix criterion (>2 ng/mL over nadir).
The Kaplan–Meier method was used for the estimation of survival curves. Cox regression analysis was used for survival analysis. The missing data included maximum PSA in one patient, and was substituted with a mean value for the purpose of Cox regression model. In the case of colinear variables (BC and LRC, BC and FFDM), only the more significant was chosen for the multivariable analysis. The p-value of <0.05 was considered to be statistically significant.
3. Results
The median medical history-based FU was 49.4 months (IQR 28.8–71.2). Complete survival data up to the day of data collection were available for all patients. The clinical characteristics of the study group are described in
Table 1.
3.1. Local–Regional Control
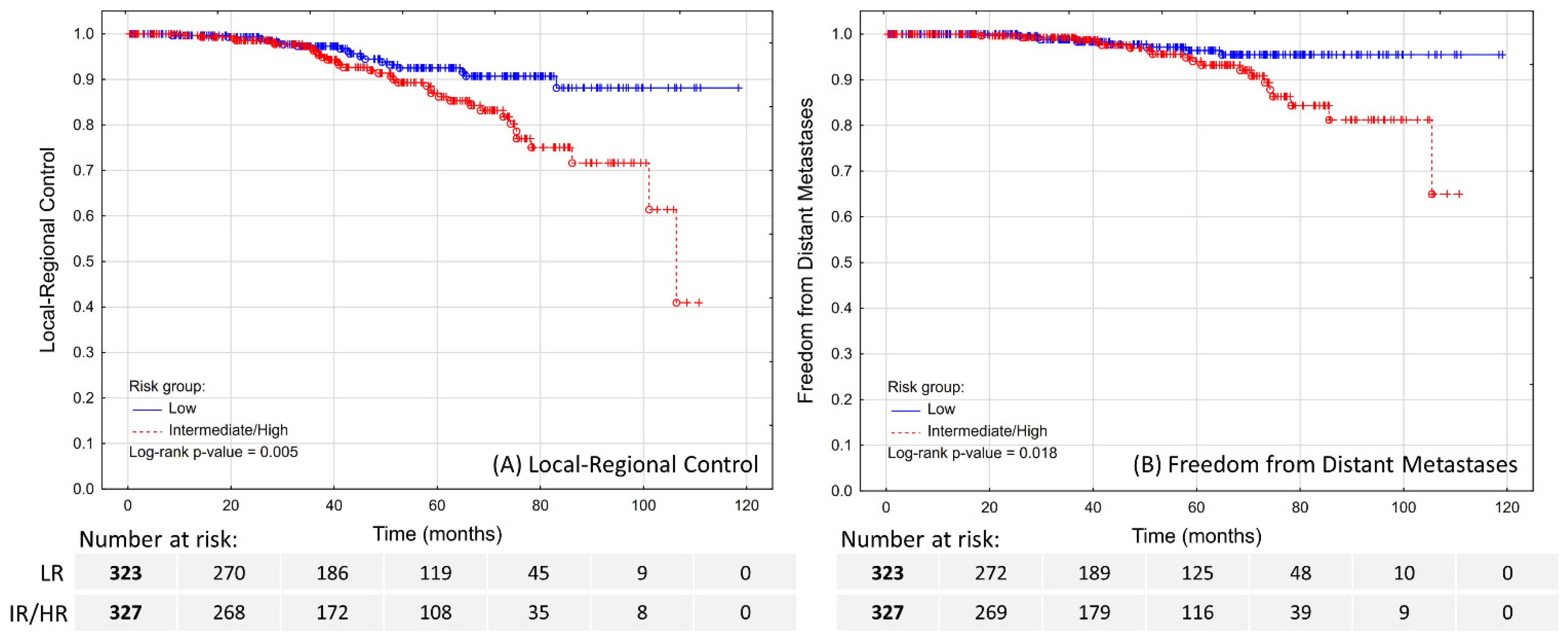
The estimated 5-year LRC was 92.6% in LR patients and 87% in IR/HR patients (
Figure 1A;
p = 0.005). Over the course of FU, a total of 29 patients experienced a local recurrence, 21 had pelvic lymph node recurrence, and four had both. The diagnosis was made by PSMA-PET in the majority of the cases (32; 59.3%), followed by (18)F-fluorocholine-PET (16; 29.6%), MRI (5; 9.3%), and histopathological examination of material obtained through transurethral resection of the prostate in one case. In total, the local recurrence was confirmed histopathologically in 25 out of 33 applicable cases.
The local recurrences were diagnosed simultaneously or preceded by distant metastases in six cases. Out of the 23 isolated local recurrences, 13 patients were treated primarily with salvage brachytherapy (56.5%), 2 patients were treated with salvage radical prostatectomy (8.7%), and 1 patient was treated with salvage SBRT (4.3%). The remaining patients (7; 30.4%) received only ADT.
The regional lymph node recurrences were diagnosed along with distant metastases in six cases. The remaining 15 patients with isolated regional nodal recurrence were treated primarily with SBRT (13; 87%), or with ADT alone (2; 13.3%).
Finally, one of the four patients with simultaneous local and regional recurrence also had a distant failure. The remaining three patients received systemic treatment.
In total, 29 out of 41 patients (70.7%) with isolated local–regional failure received definitive treatment, which was combined with ADT in 11 cases.
3.2. Freedom from Distant Metastases
The estimated 5-year FFDM was 96.4% in LR patients and 94% in IR/HR patients (
Figure 1B;
p = 0.018). A total of 27 patients experienced distant metastases, which were localized in lymph nodes (7; 25.9%), bones (22; 81.5%), or lungs (2; 7.4%), including multifocal metastases in four cases. The distant metastases were diagnosed by PSMA-PET in majority of the cases (16; 59.3%), followed by (18)F-fluorocholine-PET (4; 14.8%), bone scan (4; 14.8%), CT (2; 7.4%), or MRI (1; 3.7%). These patients were treated with systemic therapy, and only four (14.8%) were found to be eligible and received SBRT as salvage treatment.
3.3. Second-Line Progression-Free Survival after Definitive Salvage Treatment
As described above, the first event regarded as a clinical failure was local recurrence in 23 cases, regional lymph node failure in 15 cases, simultaneous local–regional failure in three cases, and distant metastases in 18 cases. Out of these, 16/23 patients with local recurrence, 13/15 patients with regional lymph node recurrence, none with simultaneous local–regional recurrence, and 4/18 patients with distant metastases received definitive local treatment. During a median FU of 27 months (IQR 11.9–34.4), eight patients experienced clinical failure, which was manifested as local progression in the prostate in two cases, and distant metastases to either lymph nodes (n = 2) or bones (n = 4) in the remaining patients. The estimated 1- and 2-year sPFS were 86.3% and 67.8%, respectively.
3.4. Competing Oncological Survival Risk
There were 45 cases of malignant neoplasm (excluding non-melanoma skin cancers) diagnosed (
n = 40) or recurred (
n = 5) after the treatment for PCa, described in
Table 2, resulting in an estimated 7.3% likelihood of a second malignant neoplasm diagnosis at 5-years after treatment. Considering the possible radiotherapy exposure, the time elapsed from PCa treatment to the diagnosis, and prior oncological medical history [
19], 3/45 (6.7%) of these lesions could have been caused by the irradiation (
Table S1), resulting in approximately 0.7% likelihood of possibly radiation-induced cancer at 5-years.
3.5. Metastases-Free Survival and Overall Survival
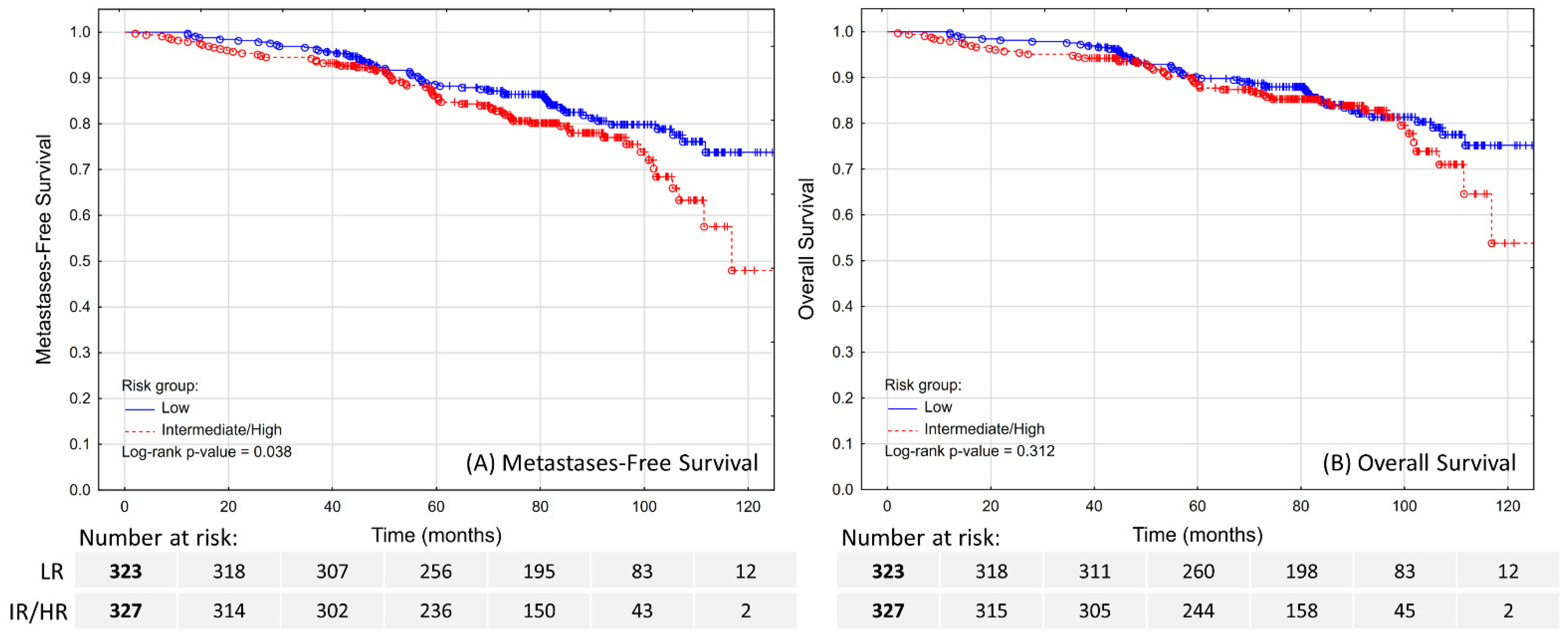
The estimated 5-year MFS was 88.6% in LR patients and 86.2% in IR/HR patients (
Figure 2A;
p = 0.038). No tested clinical features except for age (HR 1.1; 95% CI 1.07–1.14) were independent predictors of MFS (
Table 3).
The estimated 5-year OS was 90.2% in LR patients and 88.8% in IR/HR patients (
Figure 2B;
p = 0.312). In the multivariable model (
Table 3), only age (HR 1.1; 95% CI 1.07–1.14;
p < 0.001) and diagnosis of a second malignant neoplasm (HR 3.67; 95% CI 2.19–6.15;
p < 0.001) were significant prognostic factors for OS. Despite some evidence for negative impact on survival, distant failure did not reach the assumed statistical level of significance (HR 2.82; 95% CI 0.95–8.41;
p = 0.062).
3.6. Exploratory Analysis
To account for the confounding factor of low cancer-specific mortality in LR patients, the survival analysis was repeated for selected subsets of patients including IR (
n = 317) and HR (
n = 10) cases. The results were consistent with the previously described findings, as shown in
Table S2.
4. Discussion
Despite the uncontested impact of PCa on overall mortality, it should be appreciated that overdiagnosis and overtreatment can also be detrimental to public health. In this article, we have shown that both biochemical recurrence and clinical failures in patients treated for localized PCa with UHRT have a limited impact on survival, and local salvage is feasible in the majority of the local–regional recurrences. Most importantly, we have shown that age and competing oncological risks bear a significantly more important impact on survival, even in patients with an existing diagnosis of localized PCa, which can be a crucial consideration when deciding between AS and interventional treatment, and that the subsequent malignancies are, in vast majority of cases, unlikely to be associated with irradiation. Third, our treatment approach is associated with excellent short-term outcomes and could be an alternative if AS is not selected as a therapeutic option.
Mortality in PCa patients can be overshadowed by competing risks [
6,
20]. Despite low absolute rates, RT has been associated with a moderately increased risk of developing a second malignancy [
21], including colon, rectal, and bladder cancers [
22]. This, however, was only true for external beam RT, while odds of developing a second cancer were not increased in patients treated with brachytherapy [
22]. Authors suggest that both exposure and approximately four to five years of delay is necessary to consider a malignancy to be secondary to irradiation [
19,
21]. As shown in
Table S1, these criteria rule out the majority of reported malignancies as possibly secondary to irradiation. Moreover, it must also be considered that the CyberKnife
TM UHRT is a highly conformal local treatment [
23]. Compared to the historic radiotherapy methods, the increased risk of secondary cancers might be lower due to limited exposure to organs at risk. Last but not least, some authors suggested that the incidence of secondary malignancies is comparable if adjusted for patients’ age and history of smoking [
24]. However, a recent large cohort analysis showed that the risk remains increased after adjusting for both [
21].
AS should be considered as the treatment of choice for the majority of PCa patients with localized disease, as it does not compromise survival while significantly improving quality of life [
1,
3]. However, interventional methods of treatment are not exclusive but complementary, as approximately half of the patients will eventually receive active treatment [
1,
25], and some will decline AS to start off with. In general, there are three ultra-short radiotherapy modalities that can be considered in such setting, including low-dose-rate brachytherapy (LDR-BT), high-dose-rate brachytherapy (HDR-BT), and UHRT. LDR-BT provides excellent results through the permanent implantation of radioactive seeds to the prostate [
26]. HDR-BT utilizes a similar concept through the temporary injection of radiation sources to the prostate at the expense of two or more injections necessary to carry out the treatment [
27]. Both are invasive and require anaesthesia, and only UHRT can be truly non-invasive. That said, in the majority of the cases, the treatment will still be associated with a simple minimally-invasive procedure of implanting fiducials to the prostate. It is important to consider that limitations associated with anatomy and comorbidities usually do not apply to UHRT, and patients disqualified from BT can often still be treated with UHRT.
UHRT is generally well tolerated, and in a previous publication regarding side effects, we have observed that over 90% and 75% of patients report no gastro-intestinal and genito–urinary toxicity, respectively [
18]. LDR-BT and HDR-BT have also been shown to present favourable toxicity profiles [
28,
29]. An indirect comparison is difficult due to the differences in reporting low-grade events which constitute the vast majority of localized PCa treatment toxicity. Tsang et al. suggested that HDR BT is associated with less adverse effects compared to UHRT [
30], and Widmar et al. found that UHRT causes more adverse effects compared to conventional fractionation. That said, a high-volume prospective comparison of LDR-BT, HDR-BT, and UHRT toxicity is lacking. Given the available data, the authors believe that all three methods present an acceptable toxicity profile, excellent treatment outcomes, and can be considered as equally viable treatment methods for localized LR or IR PCa patients [
16,
17,
26,
27].
Biochemical factors and local–regional failure are known to have insufficient correlation with survival [
31]. While the excellent feasibility of post-RT local–regional recurrence salvage is confirmed by real-world data, the patients should be informed about a considerable risk of grade 3+ toxicity associated with the treatment of intra-prostatic local recurrences, and lack of clear survival benefit [
10]. It is worth noting that due to the strict inclusion criteria and limited length of ADT as a part of radical treatment, our patients presented almost exclusively hormone-sensitive PCa (HSPC) distant failures. With the aid of rapidly developing systemic treatment options for HSPC, a significant survival benefit was achieved [
32,
33], likely contributing to the low impact of distant failures on survival in such patients.
While the authors believe that very few of the observed cancers could be associated with irradiation, the RT can indirectly interfere with the treatment of subsequent malignancies. For example, pelvic RT can limit the utilization of preoperative irradiation in rectal cancer or a bladder-preserving approach in bladder cancer patients. This could be a consideration when choosing an optimal treatment strategy for patients at increased risk of developing such cancers, for example, due to family history or known risk factors. Additional prognostic data might be useful for an optimal personalized approach while choosing candidates for treatment, including genomic profiling [
34], medical imaging data [
35], and verification of histopathology data at high-reference centres [
36].
The authors acknowledge the limitations of the study. Despite the fact that the analysis was carried out based on a prospectively maintained cohort database, this should be regarded as a retrospective study. The selection of patients for a definitive salvage treatment was significantly biased by the institutional practices and protocols, as described in the article, and in approximately half of the cases, AS would be currently considered a standard of care. A longer observation period and larger study group could provide a more insightful view of the importance of distant failures and second(ary) cancers. Nevertheless, we believe that our findings are an important addition to the existing body of knowledge, and might provide an additional argument for de-escalating treatment in localized PCa patients.
5. Conclusions
The main pattern of failure after ultra-hypofractionated radiotherapy for localized prostate cancer is a local–regional failure, manifested at a similar rate as prostate or regional lymph node recurrence, followed by approximately twice less frequent distant metastases.
Definitive salvage treatment is possible in the majority of such patients with local–regional recurrences, and infrequently in patients with distant metastases. The definitive salvage treatment, however, is significantly less effective compared to primary treatment.
The independent prognostic factors for survival in patients treated with ultra-hypofractionated radiotherapy for localized prostate cancer included age and subsequent oncological diagnosis. There was no statistically significant association between survival and standard prognostic factors, biochemical factors, or local–regional failure. Despite some evidence for negative impact on survival, even distant failure did not reach the assumed level of significance.
Based on our results, competing oncological risks are significantly more important for survival than primary disease in patients treated with ultra-hypofractionated radiotherapy for localized prostate cancer, but very few of the subsequent malignancies can be regarded as possibly secondary to irradiation.
Author Contributions
M.M. (Marcin Miszczyk), G.G. (Gregor Goldner) and P.R.; methodology: M.M. (Matthias Moll), W.M. and P.R.; software and statistical analysis: M.M. (Marcin Miszczyk) and B.T.; investigation: M.S., M.H., A.N.-K., A.N., M.K., M.S.-F., G.W., G.G. (Grzegorz Głowacki), K.K. and Ł.M.; data curation: M.M. (Matthias Moll), M.S., M.H. and B.T.; writing—original draft prepara-tion: M.M. (Marcin Miszczyk), M.S. and M.H.; writing—review and editing: G.G. (Gregor Goldner), P.R., M.M. (Matthias Moll) and W.M.; visualiza-tion: M.M. (Matthias Moll); supervision: G.G. (Grzegorz Głowacki) and W.M.; project administration: M.M. (Marcin Miszczyk). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Maria Sklodowska-Curie National Research Institute of Oncology, Gliwice, Poland (protocol code KB/430-09/22, approved on 27 January 2022).
Informed Consent Statement
All patients were treated according to the protocol approved by the institutional board, and signed an informed consent form for the treatment. Patient consent was waived for this analysis due to the retrospective character of this study.
Data Availability Statement
Anonymized data available on request due to privacy and ethical restrictions.
Acknowledgments
The data presented in this study are a result of the meticulous scientific work of late Leszek Miszczyk and his Radiotherapy Department team, but most importantly, their great concern for the well-being of the patients. We dedicate this work in his memory, as a reminder of our duty to assess the outcomes of our clinical practices.
Conflicts of Interest
Aleksandra Napieralska and Barłomiej Tomasik served as guest editors in the special issue of the journal in which this paper was published. Both of these authors were excluded from the editorial process of this manuscript.
References
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.A.; Donovan, J.L.; Young, G.J.; Davis, M.; Walsh, E.I.; Avery, K.N.L.; Blazeby, J.M.; Mason, M.D.; Martin, R.M.; Peters, T.J.; et al. Functional and Quality of Life Outcomes of Localised Prostate Cancer Treatments (Prostate Testing for Cancer and Treatment [ProtecT] Study). BJU Int. 2022, 130, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; de Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Vince, R.A.; Sun, Y.; Mahal, B.; Ginsburg, K.; George, A.; Cher, M.; Lane, B.; Sarle, R.; Morgan, T.M.; Spratt, D.E. The Impact of a Statewide Active Surveillance Initiative: A Roadmap for Increasing Active Surveillance Utilization Nationwide. Eur. Urol. 2022, S0302–2838, 02405–02408. [Google Scholar] [CrossRef]
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Rider, J.R.; Taari, K.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.-O.; Spångberg, A.; et al. Radical Prostatectomy or Watchful Waiting in Early Prostate Cancer. N. Engl. J. Med. 2014, 370, 932–942. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Mazzola, R.; Cuccia, F.; Figlia, V.; Rigo, M.; Nicosia, L.; Giaj-Levra, N.; Ricchetti, F.; Vitale, C.; Mantoan, B.; di Paola, G.; et al. Stereotactic Body Radiotherapy for Oligometastatic Castration Sensitive Prostate Cancer Using 1.5 T MRI-Linac: Preliminary Data on Feasibility and Acute Patient-Reported Outcomes. La Radiol. Med. 2021, 126, 989–997. [Google Scholar] [CrossRef]
- Valle, L.F.; Lehrer, E.J.; Markovic, D.; Elashoff, D.; Levin-Epstein, R.; Karnes, R.J.; Reiter, R.E.; Rettig, M.; Calais, J.; Nickols, N.G.; et al. A Systematic Review and Meta-Analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur. Urol. 2021, 80, 280–292. [Google Scholar] [CrossRef]
- Foerster, R.; Zwahlen, D.R.; Buchali, A.; Tang, H.; Schroeder, C.; Windisch, P.; Vu, E.; Akbaba, S.; Bostel, T.; Sprave, T.; et al. Stereotactic Body Radiotherapy for High-Risk Prostate Cancer: A Systematic Review. Cancers 2021, 13, 759. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Fractionation and Protraction for Radiotherapy of Prostate Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 1095–1101. [Google Scholar] [CrossRef]
- Fowler, J.; Chappell, R.; Ritter, M. Is Alpha/Beta for Prostate Tumors Really Low? Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1021–1031. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Ritter, M.A. The Alpha/Beta Ratio for Prostate Cancer: What Is It, Really? Radiother. Oncol. 2005, 76, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vogelius, I.R.; Bentzen, S.M. Meta-Analysis of the Alpha/Beta Ratio for Prostate Cancer in the Presence of an Overall Time Factor: Bad News, Good News, or No News? Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 89–94. [Google Scholar] [CrossRef]
- Brand, D.H.; Tree, A.C.; Ostler, P.; van der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; et al. Intensity-Modulated Fractionated Radiotherapy versus Stereotactic Body Radiotherapy for Prostate Cancer (PACE-B): Acute Toxicity Findings from an International, Randomised, Open-Label, Phase 3, Non-Inferiority Trial. Lancet Oncol. 2019, 20, 1531–1543. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-Hypofractionated versus Conventionally Fractionated Radiotherapy for Prostate Cancer: 5-Year Outcomes of the HYPO-RT-PC Randomised, Non-Inferiority, Phase 3 Trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef]
- Miszczyk, L.; Namysł-Kaletka, A.; Napieralska, A.; Kraszkiewicz, M.; Miszczyk, M.; Woźniak, G.; Stąpór-Fudzińska, M.; Głowacki, G.; Tukiendorf, A. Stereotactic Ablative Radiotherapy for Prostate Cancer-The Treatment Results of 500 Patients and Analysis of Failures. Technol. Cancer Res. Treat. 2019, 18, 1533033819870815. [Google Scholar] [CrossRef]
- Murray, L.; Henry, A.; Hoskin, P.; Siebert, F.A.; Venselaar, J. Second Primary Cancers after Radiation for Prostate Cancer: A Systematic Review of the Clinical Data and Impact of Treatment Technique. Radiother. Oncol. 2014, 110, 213–228. [Google Scholar] [CrossRef]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical Prostatectomy versus Observation for Localized Prostate Cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Bagshaw, H.P.; Arnow, K.D.; Trickey, A.W.; Leppert, J.T.; Wren, S.M.; Morris, A.M. Assessment of Second Primary Cancer Risk Among Men Receiving Primary Radiotherapy vs Surgery for the Treatment of Prostate Cancer. JAMA Netw. Open 2022, 5, e2223025. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.D.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second Malignancies after Radiotherapy for Prostate Cancer: Systematic Review and Meta-Analysis. BMJ 2016, 352, i851. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.B.; Naitoh, J.; Lee, C.; Hardy, S.; Jin, H. Virtual HDRSM CyberKnife Treatment for Localized Prostatic Carcinoma: Dosimetry Comparison With HDR Brachytherapy and Preliminary Clinical Observations. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1588–1597. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Pei, X.; Teslova, T.; Kuk, D.; Magsanoc, J.M.; Kollmeier, M.; Cox, B.; Zhang, Z. Secondary Cancers after Intensity-Modulated Radiotherapy, Brachytherapy and Radical Prostatectomy for the Treatment of Prostate Cancer: Incidence and Cause-Specific Survival Outcomes According to the Initial Treatment Intervention. BJU Int. 2012, 110, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, S.; Ramakrishnan, V.; Tamalunas, A.; Hofmann, M.; Vandenhirtz, M.; Vollmer, S.; Hug, J.; Niggli, P.; Nocito, A.; Kubik-Huch, R.A.; et al. Two Decades of Active Surveillance for Prostate Cancer in a Single-Center Cohort: Favorable Outcomes after Transurethral Resection of the Prostate. Cancers 2022, 14, 368. [Google Scholar] [CrossRef]
- Fellin, G.; Mirri, M.A.; Santoro, L.; Jereczek-Fossa, B.A.; Divan, C.; Mussari, S.; Ziglio, F.; la Face, B.; Barbera, F.; Buglione, M.; et al. Low Dose Rate Brachytherapy (LDR-BT) as Monotherapy for Early Stage Prostate Cancer in Italy: Practice and Outcome Analysis in a Series of 2237 Patients from 11 Institutions. Br. J. Radiol. 2016, 89, 20150981. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Kim, S.; Sandler, H.M.; Kamrava, M. High-Dose-Rate Fractionated Brachytherapy Monotherapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis. J. Contemp. Brachyther. 2021, 13, 365–374. [Google Scholar] [CrossRef]
- Aluwini, S.; Busser, W.M.H.; Alemayehu, W.G.; Boormans, J.L.; Kirkels, W.J.; Jansen, P.P.; Praag, J.O.; Bangma, C.H.; Kolkman-Deurloo, I.K.K. Toxicity and Quality of Life after High-Dose-Rate Brachytherapy as Monotherapy for Low- and Intermediate-Risk Prostate Cancer. Radiother. Oncol. 2015, 117, 252–257. [Google Scholar] [CrossRef]
- Lawton, C.A.; Hunt, D.; Lee, W.R.; Gomella, L.; Grignon, D.; Gillin, M.; Morton, G.; Pisansky, T.M.; Sandler, H. Long-Term Results of a Phase II Trial of Ultrasound-Guided Radioactive Implantation of the Prostate for Definitive Management of Localized Adenocarcinoma of the Prostate (RTOG 98-05). Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1–7. [Google Scholar] [CrossRef]
- Tsang, Y.M.; Tharmalingam, H.; Belessiotis-Richards, K.; Armstrong, S.; Ostler, P.; Hughes, R.; Alonzi, R.; Hoskin, P.J. Ultra-Hypofractionated Radiotherapy for Low- and Intermediate Risk Prostate Cancer: High-Dose-Rate Brachytherapy vs Stereotactic Ablative Radiotherapy. Radiother. Oncol. 2021, 158, 184–190. [Google Scholar] [CrossRef]
- Gharzai, L.A.; Jiang, R.; Wallington, D.; Jones, G.; Birer, S.; Jairath, N.; Jaworski, E.M.; McFarlane, M.R.; Mahal, B.A.; Nguyen, P.L.; et al. Intermediate Clinical Endpoints for Surrogacy in Localised Prostate Cancer: An Aggregate Meta-Analysis. Lancet Oncol. 2021, 22, 402–410. [Google Scholar] [CrossRef]
- Mori, K.; Mostafaei, H.; Sari Motlagh, R.; Pradere, B.; Quhal, F.; Laukhtina, E.; Schuettfort, V.M.; Kramer, G.; Abufaraj, M.; Karakiewicz, P.I.; et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: Network meta-analysis. BJU Int. 2022, 129, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Castro, E.; Lozano, R.; Zanardi, E.; Rubagotti, A.; Boccardo, F.; Olmos, D. Current Treatment Options for Metastatic Hormone-Sensitive Prostate Cancer. Cancers 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Nonomura, N. Genomic Profiling of Prostate Cancer: An Updated Review. World J. Mens. Health 2022, 40, 368. [Google Scholar] [CrossRef]
- Miszczyk, M.; Rembak-Szynkiewicz, J.; Magrowski, Ł.; Stawiski, K.; Namysł-Kaletka, A.; Napieralska, A.; Kraszkiewicz, M.; Woźniak, G.; Stąpór-Fudzińska, M.; Głowacki, G.; et al. The Prognostic Value of PI-RADS Score in CyberKnife Ultra-Hypofractionated Radiotherapy for Localized Prostate Cancer. Cancers 2022, 14, 1613. [Google Scholar] [CrossRef]
- Majewski, W.; Lange, D.; Stanek-Widera, A.; Itrych, B.; Krzysztofiak, T.; Jarząb, M.; Oczko-Wojciechowska, M.; Kajor, M.; Tarnawski, R. Grade Migration and Important Prognostic Factors in a Pathology Specimen for Radical Radiotherapy in Prostate Cancer Patients. Pol. J. Pathol. 2022, 73, 27–33. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).