Cannabinoid Therapeutic Effects in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
1.1. Background
1.2. Study Rationale and Hypothesis
1.3. Objectives
2. Methods
2.1. Stakeholder Involvement (PPI)
2.2. Eligibility Criteria
- Study design:
- ○
- Randomized Controlled Trial (RCT);
- ○
- Prospective case-control study;
- Exposure:
- ○
- Treatment with cannabinoids, by either inhalation or oral administration.
- Outcome:
- ○
- Clinical evaluation of the patient, either via standard-use scale or via other validated clinical measurement.
- Publication type:
- ○
- Primary studies published in peer-reviewed journals;
- ○
- Non-peer reviewed publications and grey literature articles (e.g., trial results), as long as they are publicly available.
- Study design:
- ○
- Cross-sectional (prevalence) studies;
- ○
- Case reports and case series;
- ○
- Prospective and retrospective studies without measures of association and confidence intervals (or data enabling to calculate them);
- ○
- Studies with no comparison.
- Publication type:
- ○
- Abstracts with no data available (i.e., conference abstracts, unless providing data for analysis);
- ○
- Studies reporting results that have been superseded by subsequent reports from the same study (note: this includes conditions when that reports include a mix of results that have or have not been updated).
2.3. Information Sources and Search Strategies
2.4. Addressing Bias
- It was chosen to include only prospective controlled studies (both RCT and NRS) in the selection process, since they provide the least biased results while still permitting reasonable statistical precision;
- Risk of Bias was rated using a specific, validated tool in our protocol;
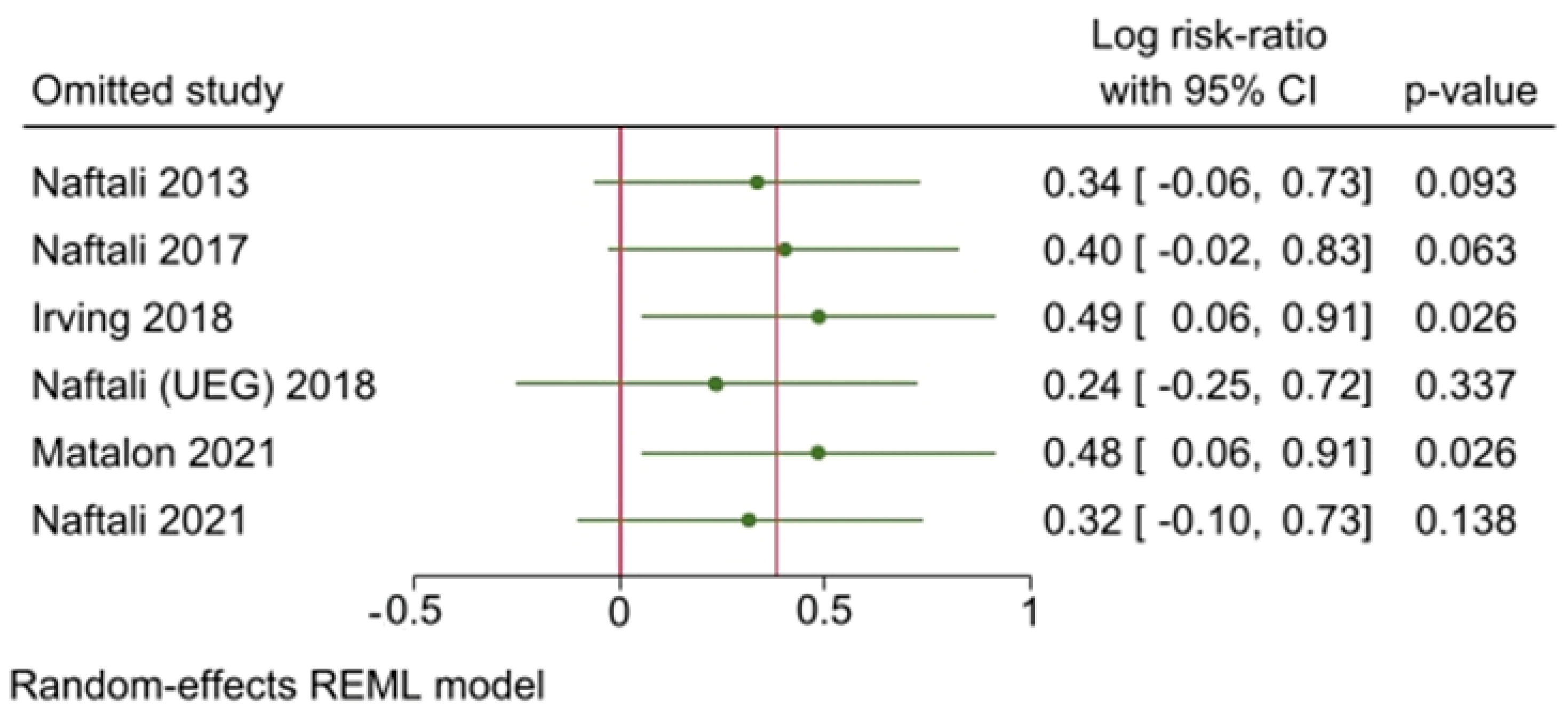
- Sensitivity analyses were performed by executing a leave-one-out analysis, consisting in performing multiple meta-analyses by excluding one study at each step, and a meta-regression [31].
2.5. Selection Process
2.6. Data Collection Process
2.7. Data Items
- Publication year;
- Involved country;
- Disease type;
- Treatment type and duration;
- Control type;
- Follow-up time;
- Study effect measure;
- Number of patients in each cohort;
- Therapeutic successes in each cohort;
- Therapeutic failures in each cohort.
- When available, variation in disease activity index in each cohort was also retrieved.
2.8. Study Risk of Bias Assessment
2.9. Effect Measures
2.10. Synthesis Methods
2.11. Reporting Bias Assessment
2.12. Certainty Assessment
2.13. Reporting
3. Results
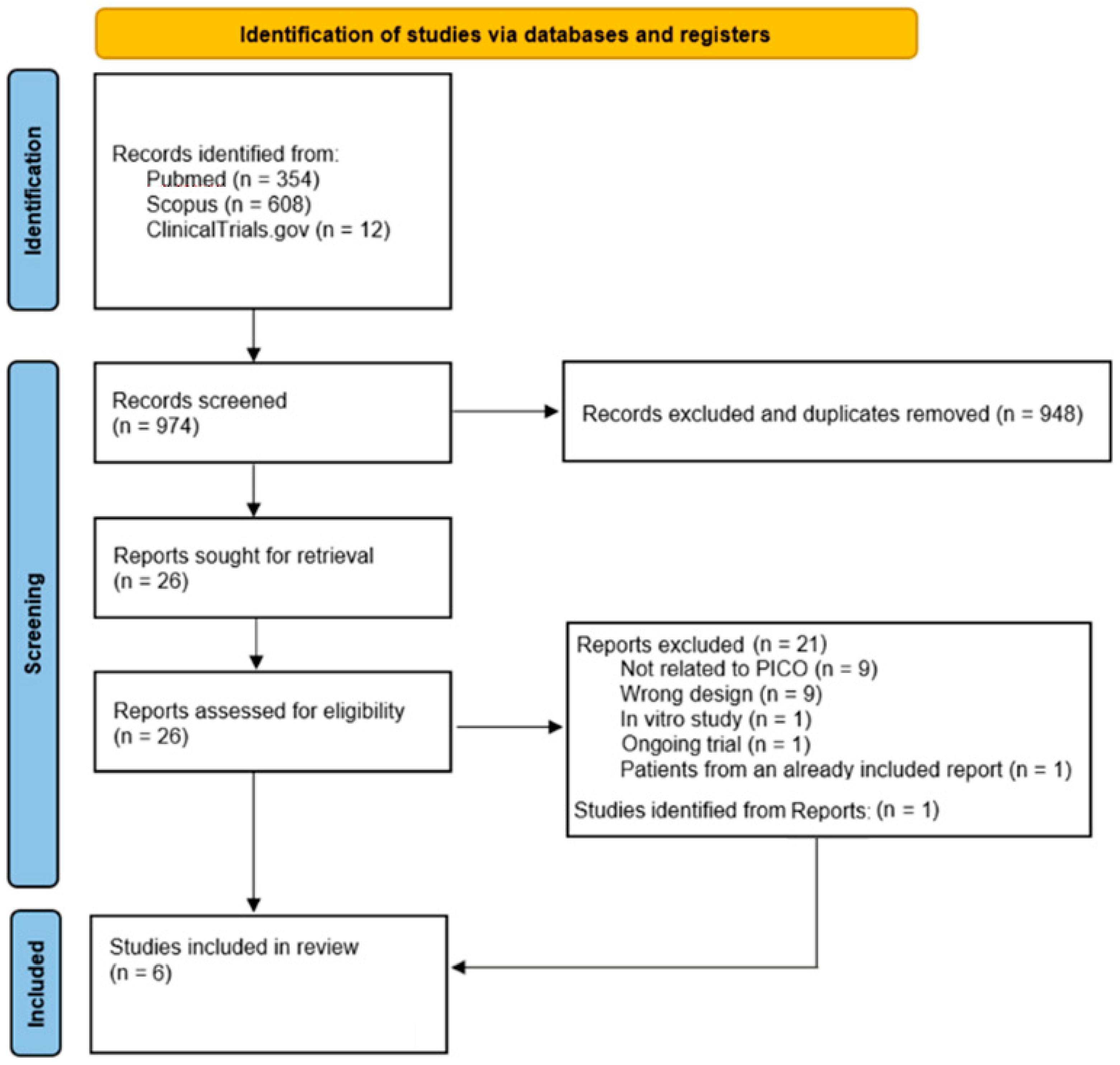
3.1. Study Selection
3.2. Study Characteristics
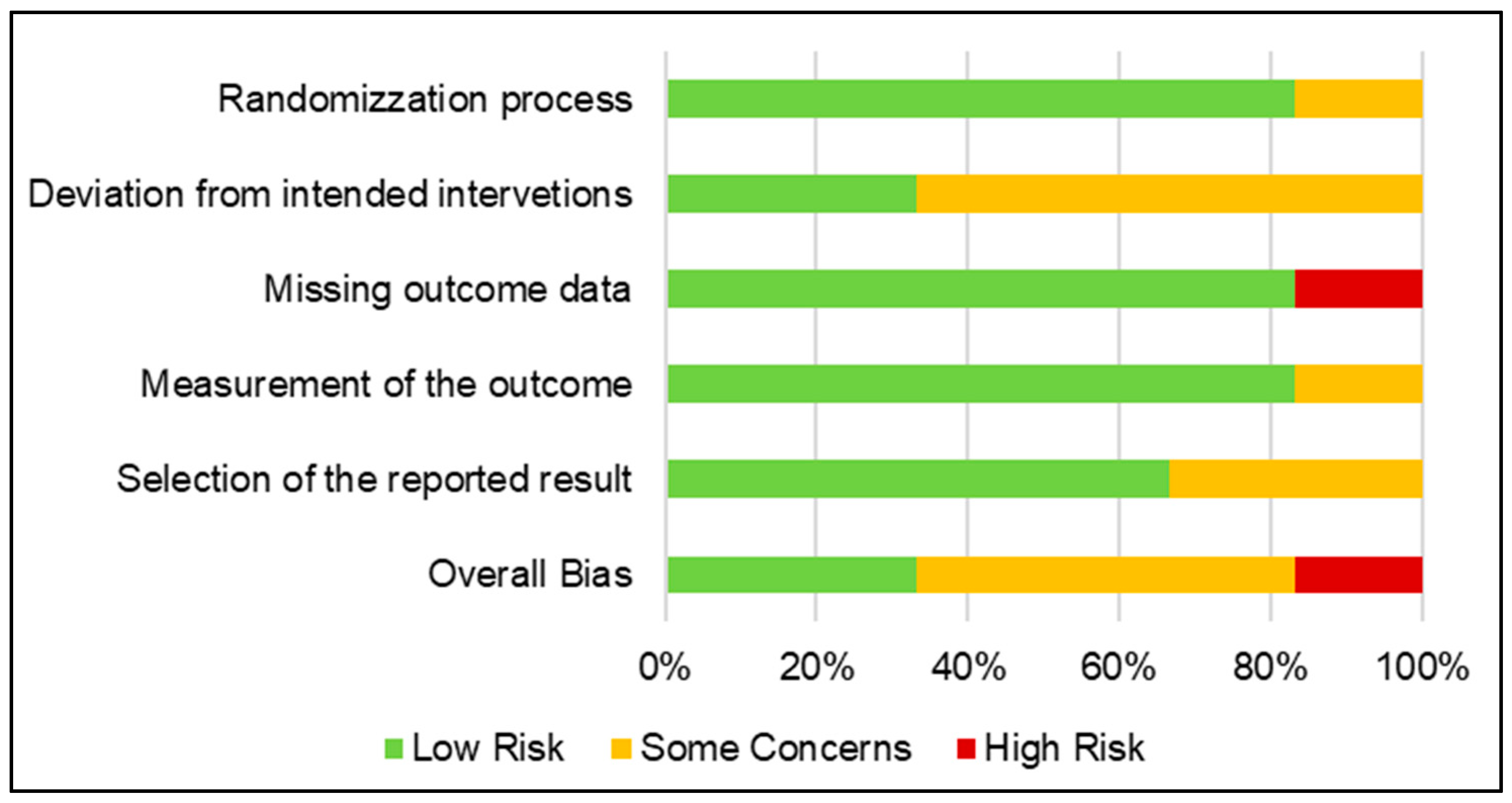
3.3. Risk of Bias in Studies
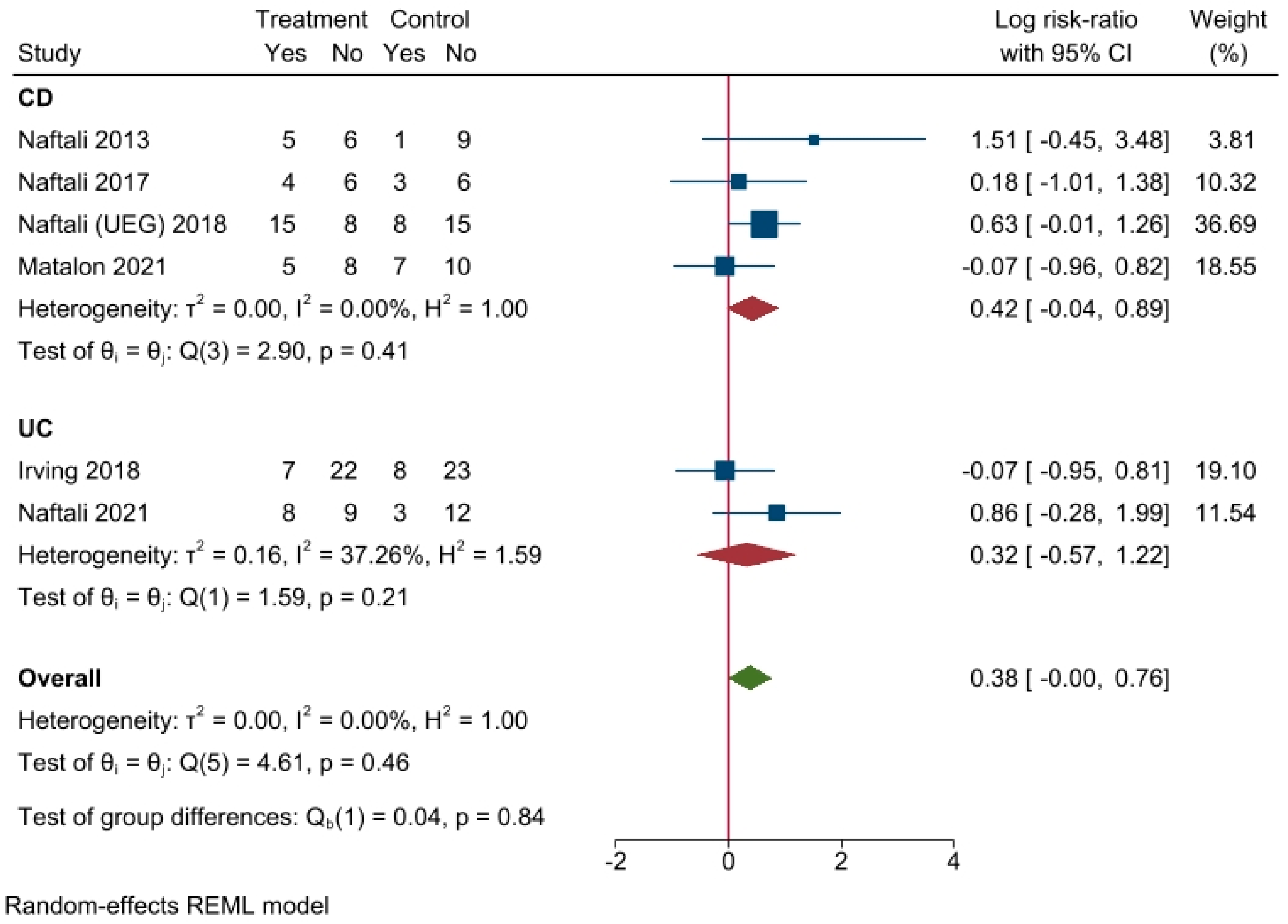
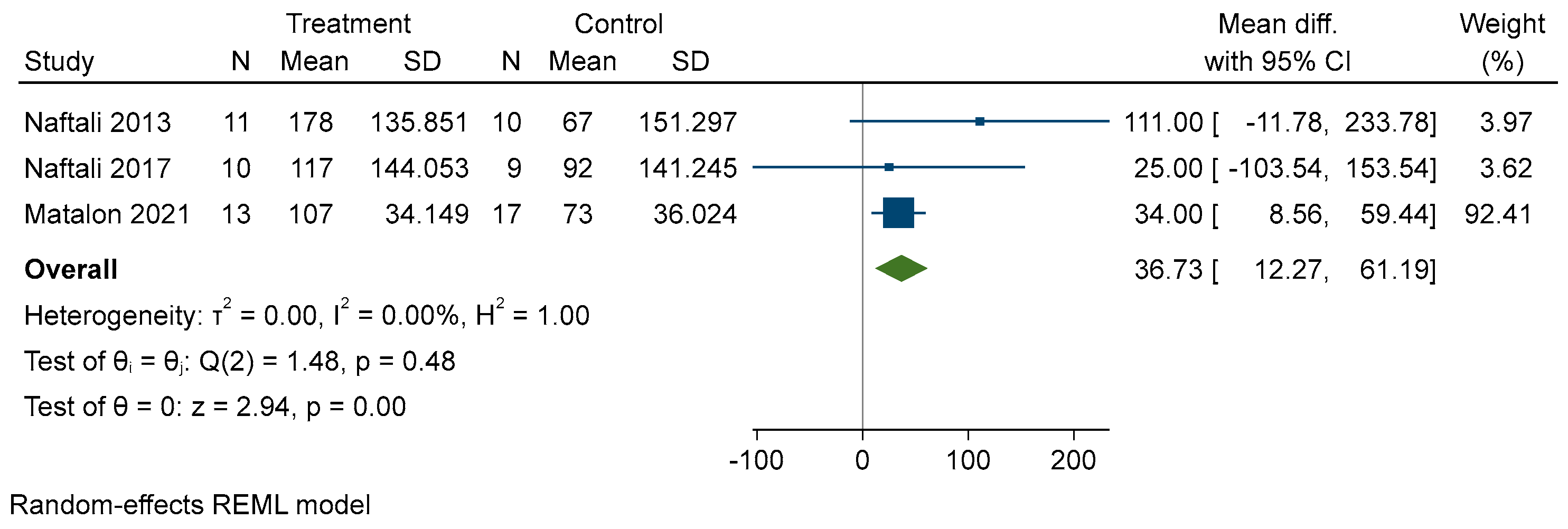
3.4. Results of Individual Studies
- Effectiveness of cannabinoid supplementation on clinical outcome
- Effectiveness of cannabinoid supplementation on disease activity reduction, quality of life and other variables
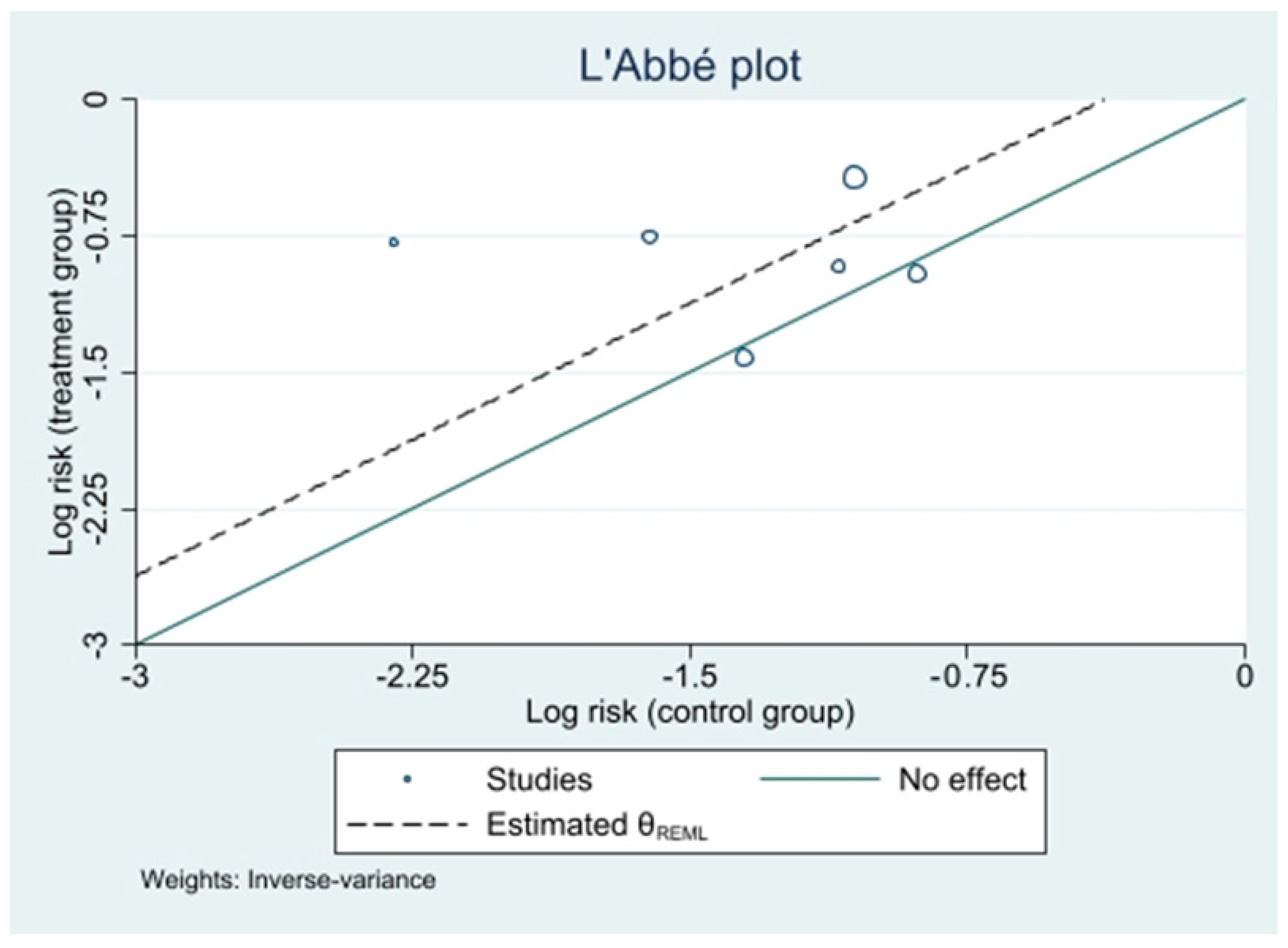
3.5. Results of Synthesis
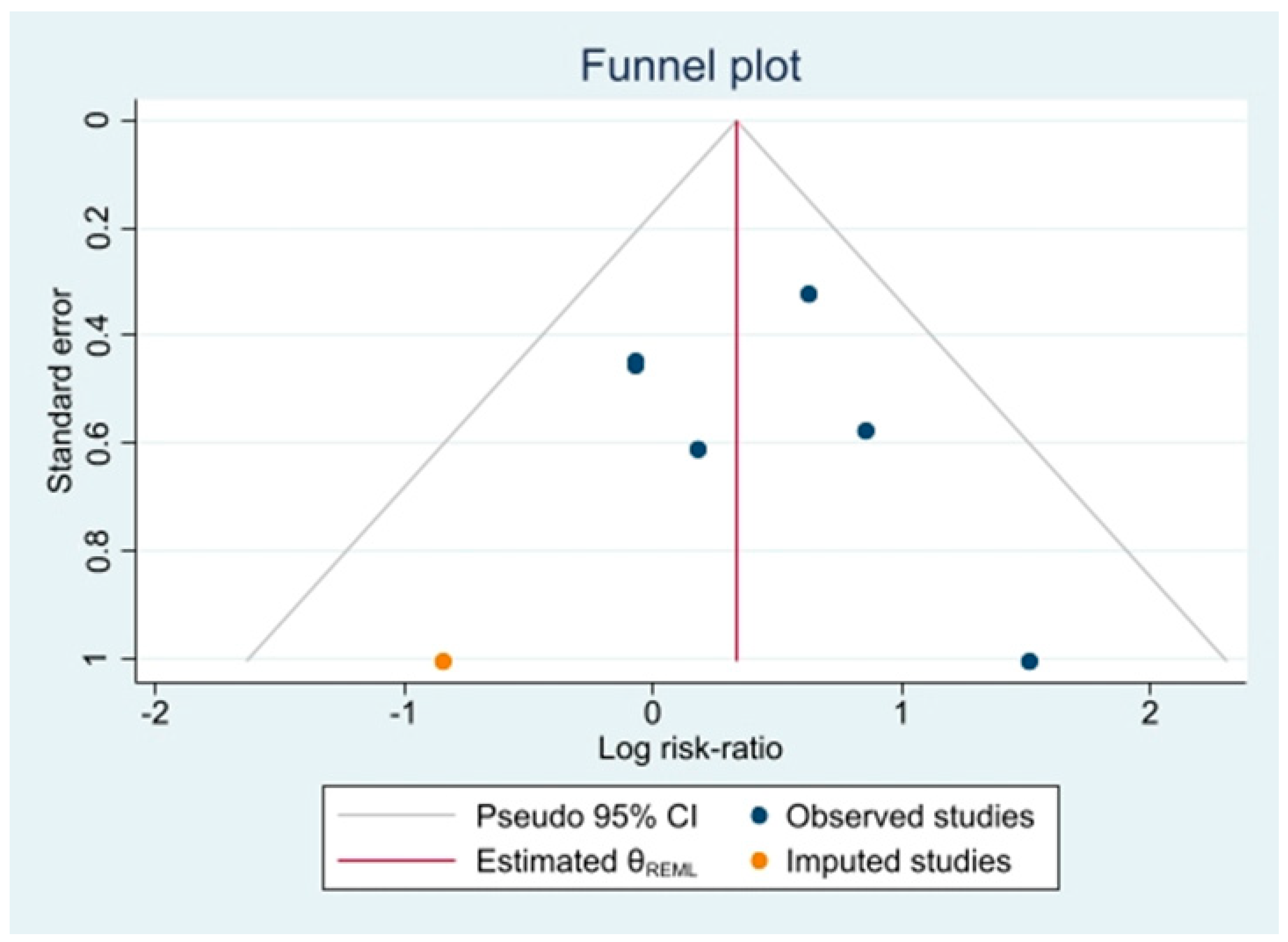
3.6. Risk of Reporting Bias in Syntheses
3.7. Certainty of Evidence
4. Discussion
4.1. Results in Context
4.2. Limitations of Included Studies
4.3. Limitations of the Review Methods
4.4. Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Registration and Protocol
References
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet Lond. Engl. 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet Lond. Engl. 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. WJG 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; Van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohn’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Park, S.; Abdi, T.; Gentry, M.; Laine, L. Histological Disease Activity as a Predictor of Clinical Relapse among Patients With Ulcerative Colitis: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2016, 111, 1692–1701. [Google Scholar] [CrossRef]
- Viscido, A.; Valvano, M.; Stefanelli, G.; Capannolo, A.; Castellini, C.; Onori, E.; Ciccone, A.; Vernia, F.; Latella, G. Systematic review and meta-analysis: The advantage of endoscopic Mayo score 0 over 1 in patients with ulcerative colitis. BMC Gastroenterol. 2022, 22, 92. [Google Scholar] [CrossRef]
- Zaami, S.; Di Luca, A.; Di Luca, N.M.; Vergallo, G.M. Medical use of cannabis: Italian and European legislation. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 1161–1167. [Google Scholar]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Raad, M.A.; Chams, N.H.; Sharara, A.I. New and Evolving Immunotherapy in Inflammatory Bowel Disease. Inflamm. Intest. Dis. 2016, 1, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, S.; Zhang, W.; Wu, D.; Yang, G. Probiotic Escherichia coli NISSLE 1917 for inflammatory bowel disease applications. Food Funct. 2022, 13, 5914–5924. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M.; Magistroni, M.; Mancusi, A.; D’Ascenzo, D.; Longo, S.; Stefanelli, G.; Vernia, F.; Viscido, A.; Necozione, S.; Latella, G. The Usefulness of Serum Vitamin D Levels in the Assessment of IBD Activity and Response to Biologics. Nutrients 2021, 13, 323. [Google Scholar] [CrossRef]
- Naftali, T.; Bar Lev, L.; Yablecovitch, D.; Yablekovitz, D.; Half, E.; Konikoff, F.M. Treatment of Crohn’s disease with cannabis: An observational study. Isr. Med Assoc. J. IMAJ 2011, 13, 455–458. [Google Scholar]
- Wynne, J.; Kozuch, P. Medical marijuana for inflammatory bowel disease: The highs and lows. Scand. J. Gastroenterol. 2022, 57, 197–205. [Google Scholar] [CrossRef]
- Capasso, R.; Borrelli, F.; Aviello, G.; Romano, B.; Scalisi, C.; Capasso, F.; Izzo, A.A. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice: Cannabidiol and intestinal motility. Br. J. Pharmacol. 2008, 154, 1001–1008. [Google Scholar] [CrossRef]
- Schicho, R.; Storr, M. Topical and Systemic Cannabidiol Improves Trinitrobenzene Sulfonic Acid Colitis in Mice. Pharmacology 2012, 89, 149–155. [Google Scholar] [CrossRef]
- Mbachi, C.; Attar, B.; Wang, Y.; Paintsil, I.; Mba, B.; Fugar, S.; Agrawal, R.; Simons-Linares, R.C.; Jaiswal, P.; Trick, W.; et al. Association Between Cannabis Use and Complications Related to Crohn’s Disease: A Retrospective Cohort Study. Dig. Dis. Sci. 2019, 64, 2939–2944. [Google Scholar] [CrossRef]
- Choi, C.; Abougergi, M.; Peluso, H.; Weiss, S.H.; Nasir, U.; Pyrsopoulos, N. Cannabis Use is Associated with Reduced 30-Day All-cause Readmission Among Hospitalized Patients with Irritable Bowel Syndrome: A Nationwide Analysis. J. Clin. Gastroenterol. 2022, 56, 257–265. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. The endocannabinoid system in inflammatory bowel diseases: From pathophysiology to therapeutic opportunity. Trends Mol. Med. 2012, 18, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Zielińska, A.; Scott, R.J.; Słomski, R.; Pławski, A. Endocannabinoid System as a Promising Therapeutic Target in Inflammatory Bowel Disease—A Systematic Review. Front. Immunol. 2021, 12, 790803. [Google Scholar] [CrossRef]
- Li, L.; Xuan, Y.; Zhu, B.; Wang, X.; Tian, X.; Zhao, L.; Wen, N. Protective Effects of Cannabidiol on Chemotherapy-Induced Oral Mucositis via the Nrf2/Keap1/ARE Signaling Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 4619760. [Google Scholar] [CrossRef]
- Klein, M.; de Quadros De Bortolli, J.; Guimarães, F.S.; Salum, F.G.; Cherubini, K.; de Figueiredo, M.A.Z. Effects of cannabidiol, a Cannabis sativa constituent, on oral wound healing process in rats: Clinical and histological evaluation: Cannabidiol in oral ulcers. Phytother. Res. 2018, 32, 2275–2281. [Google Scholar] [CrossRef]
- Storr, M.; Devlin, S.; Kaplan, G.G.; Panaccione, R.; Andrews, C.N. Cannabis Use Provides Symptom Relief in Patients with Inflammatory Bowel Disease but Is Associated with Worse Disease Prognosis in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 472–480. [Google Scholar] [CrossRef]
- Kafil, T.S.; Nguyen, T.M.; Macdonald, J.K.; Chande, N. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst. Rev. 2018, 11, CD012853. [Google Scholar] [CrossRef]
- Kafil, T.S.; Nguyen, T.M.; Macdonald, J.K.; Chande, N. Cannabis for the treatment of ulcerative colitis. Cochrane Database Syst. Rev. 2018, 11, CD012954. [Google Scholar] [CrossRef]
- Doeve, B.H.; van de Meeberg, M.M.; van Schaik, F.D.M.; Fidder, H.H. A Systematic Review with Meta-Analysis of the Efficacy of Cannabis and Cannabinoids for Inflammatory Bowel Disease: What Can We Learn from Randomized and Nonrandomized Studies? J. Clin. Gastroenterol. 2021, 55, 798–809. [Google Scholar] [CrossRef]
- Mathur, M.B.; VanderWeele, T.J. Methods to Address Confounding and Other Biases in Meta-Analyses: Review and Recommendations. Annu. Rev. Public Health 2022, 43, 19–35. [Google Scholar] [CrossRef]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice: Statistical validity of meta-analysis and meta-regression estimates. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; West, S.L.; Mansfield, A.J.; Poole, C.; Tant, E.; Lux, L.J.; Lohr, K.N. Clinical heterogeneity in systematic reviews and health technology assessments: Synthesis of guidance documents and the literature. Int. J. Technol. Assess. Health Care 2012, 28, 36–43. [Google Scholar] [CrossRef]
- Higgins, J.; Li, T.; Deek, J. (Eds.) Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2022; Chapter 6. [Google Scholar]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Yoon, H.R. Rapid screening for acidic non-steroidal anti-inflammatory drugs in urine by gas chromatography-mass spectrometry in the selected-ion monitoring mode. J. Chromatogr. B Biomed. Sci. Appl. 1996, 682, 55–66. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Harbord, R.M. Funnel Plots in Meta-analysis. Stata J. 2004, 4, 127–141. [Google Scholar] [CrossRef]
- Kirmayr, M.; Quilodrán, C.; Valente, B.; Loezar, C.; Garegnani, L.; Franco, J.V.A. The GRADE approach, Part 1: How to assess the certainty of the evidence. Medwave 2021, 21, e8109. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M. GRADE guidelines: Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews|The BMJ. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 26 September 2022).
- Naftali, T.; Schleider, L.B.-L.; Dotan, I.; Lansky, E.P.; Benjaminov, F.S.; Konikoff, F.M. Cannabis Induces a Clinical Response in Patients with Crohn’s Disease: A Prospective Placebo-Controlled Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280.e1. [Google Scholar] [CrossRef] [PubMed]
- Naftali, T.; Mechulam, R.; Marii, A.; Gabay, G.; Stein, A.; Bronshtain, M.; Konikoff, F.M. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, a Randomized Controlled Trial. Dig. Dis. Sci. 2017, 62, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S.T.; Azar, S.; Meiri, D.; Hadar, R.; Nemirovski, A.; Abu Jabal, N.; Konikoff, F.M.; Drucker, L.; Tam, J.; Naftali, T. Endocannabinoid Levels in Ulcerative Colitis Patients Correlate with Clinical Parameters and Are Affected by Cannabis Consumption. Front. Endocrinol. 2021, 12, 685289. [Google Scholar] [CrossRef] [PubMed]
- Week, U.E.G. Oral Presentations. United Eur. Gastroenterol. J. 2018, 6, A1–A134. [Google Scholar]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A.; et al. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 714–724. [Google Scholar] [CrossRef]
- Naftali, T.; Schleider, L.B.-L.; Benjaminov, F.S.; Konikoff, F.M.; Matalon, S.T.; Ringel, Y. Cannabis is associated with clinical but not endoscopic remission in ulcerative colitis: A randomized controlled trial. PLoS ONE 2021, 16, e0246871. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Kanters, S. Fixed- and Random-Effects Models. Methods Mol. Biol. Clifton NJ 2022, 2345, 41–65. [Google Scholar]
- Katsidoni, V.; Kastellakis, A.; Panagis, G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int. J. Neuropsychopharmacol. 2013, 16, 2273–2284. [Google Scholar] [CrossRef]
- Dalal, R.S.; Palchaudhuri, S.; Snider, C.K.; Lewis, J.D.; Mehta, S.J.; Lichtenstein, G.R. Preadmission Cannabis Use Is Positively Correlated with Inpatient Opioid Dose Exposure in Hospitalized Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lauro, R.; Mannino, F.; Irrera, N.; Squadrito, F.; Altavilla, D.; Squadrito, G.; Pallio, G.; Bitto, A. Pharmacogenetics of Biological Agents Used in Inflammatory Bowel Disease: A Systematic Review. Biomedicines 2021, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Lucaroni, F.; Modica, D.C.; Macino, M.; Palombi, L.; Abbondanzieri, A.; Agosti, G.; Biondi, G.; Morciano, L.; Vinci, A. Can risk be predicted? An umbrella systematic review of current risk prediction models for cardiovascular diseases, diabetes and hypertension. BMJ Open 2019, 9, e030234. [Google Scholar] [CrossRef] [PubMed]
- Burke, W. Utility and Diversity: Challenges for Genomic Medicine. Annu. Rev. Genom. Hum. Genet. 2021, 22, 1–24. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Lakatos, P.L.; Szamosi, T.; Lakatos, L. Smoking in inflammatory bowel diseases: Good, bad or ugly? World J. Gastroenterol. WJG 2007, 13, 6134–6139. [Google Scholar] [CrossRef]
- Goyal, H.; Singla, U.; Gupta, U.; May, E. Role of cannabis in digestive disorders. Eur. J. Gastroenterol. Hepatol. 2017, 29, 135–143. [Google Scholar] [CrossRef]

| Population | Patients with IBD |
| Intervention | Cannabinoid administration |
| Comparison | Standard treatment |
| Outcome | Improvement in patient’s clinical condition |
| Database | Search String(s) | Filters |
|---|---|---|
| MEDLINE | ((ulcerative colitis) OR (crohn) OR (inflammatory bowel disease) OR colitis) and (Cannabis or cannabinoids or THC or cannabidiol) | Publication year: >2000 |
| Scopus | ((ulcerative AND colitis) OR (crohn) OR (inflammatory AND bowel AND disease) OR colitis) AND (cannabis OR cannabinoids OR thc OR cannabidiol) PUBYEAR > 2002 AND PUBYEAR > 2001 AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (EXACTKEYWORD, “Controlled Study”) OR EXCLUDE (EXACTKEYWORD, “Animals”) OR EXCLUDE (EXACTKEYWORD, “Animal Experiment”)) | Not Applicable (already included in string search) |
| ClinicalTrials.gov (accessed on 10 August 2022) | Disease Type: Inflammatory Bowel Disease (automatic field) Additional keywords: Cannabis | Not Applicable |
| Authors | Year of Publication | Country | Included Population | Intervention | Control | Effect Measure | Treatment Duration | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Naftali et al. [43] | 2013 | Israel | 21 CD | Cigarette smoking: 0.5 g of dried cannabis flowers, equivalent to 11.5 mg of THC twice daily. | Cigarette smoking w/o cannabis after THC extraction. | CDAI | 8 weeks | 2 weeks |
| Naftali et al. [44] | 2017 | Israel | 19 CD | Cannabinoid oil at a concentration of 5 mg/mL (0.3 mg/kg). | Placebo containing pure olive oil | CDAI | 8 weeks | 2 weeks |
| Matalon et al. [45] | 2021 | Israel | 30 CD 19 UC | Cigarette smoking: 0.5 g of dried cannabis flowers, equivalent to 11.5 mg of THC. | Cigarette smoking w/o cannabis after THC extraction. | Endocannabinoid blood level change; CDAI; BM; QOL; immunohistochemistry measures. | 8 weeks | None |
| Naftali et al. (UEG) [46] | 2018 | Israel | 46 CD | Cannabis oil 15% cannabidiol + 4% tetrahydrocannabinol | Placebo | Clinical Remission | 8 weeks | None |
| Irving et al. [47] | 2018 | United Kingdom | 60 UC | Cannabidiol-rich botanical extract capsules, up to 500 mg/day | Placebo capsules | Mayo score; IBDQ score; PGAS score; stool frequency NRS; rectal bleeding NRS pain 0–10 NRS score. | 10 weeks | 1 week baseline + 1 week follow-up |
| Naftali et al. [48] | 2021 | Israel | 32 UC | Cigarette smoking: linear increasing up to 1 g/day of dried cannabis flowers (0.25 g steps), equivalent to 23 mg of THC. | Cigarette smoking w/o cannabis after THC extraction. | Lichtiger index; QOL; Mayo endoscopic score | 8 weeks | 2 weeks |
| Study ID | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Overall |
|---|---|---|---|---|---|---|
| Irving 2018 | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Matalon 2021 | Low Risk | Some Concerns | High Risk | Low Risk | Low Risk | High Risk |
| Naftali 2013 | Low Risk | Some Concerns | Low Risk | Low Risk | Low Risk | Some Concerns |
| Naftali 2017 | Low Risk | Some Concerns | Low Risk | Low Risk | Some Concerns | Some Concerns |
| Naftali 2018 | Some Concerns | Some Concerns | Low Risk | Some Concerns | Some Concerns | Some Concerns |
| Naftali 2021 | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinci, A.; Ingravalle, F.; Bardhi, D.; Cesaro, N.; Frassino, S.; Licata, F.; Valvano, M. Cannabinoid Therapeutic Effects in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomedicines 2022, 10, 2439. https://doi.org/10.3390/biomedicines10102439
Vinci A, Ingravalle F, Bardhi D, Cesaro N, Frassino S, Licata F, Valvano M. Cannabinoid Therapeutic Effects in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomedicines. 2022; 10(10):2439. https://doi.org/10.3390/biomedicines10102439
Chicago/Turabian StyleVinci, Antonio, Fabio Ingravalle, Dorian Bardhi, Nicola Cesaro, Sara Frassino, Francesca Licata, and Marco Valvano. 2022. "Cannabinoid Therapeutic Effects in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Biomedicines 10, no. 10: 2439. https://doi.org/10.3390/biomedicines10102439
APA StyleVinci, A., Ingravalle, F., Bardhi, D., Cesaro, N., Frassino, S., Licata, F., & Valvano, M. (2022). Cannabinoid Therapeutic Effects in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomedicines, 10(10), 2439. https://doi.org/10.3390/biomedicines10102439






