Application of Plant-Derived Nanoparticles (PDNP) in Food-Producing Animals as a Bio-Control Agent against Antimicrobial-Resistant Pathogens
Abstract
1. Introduction
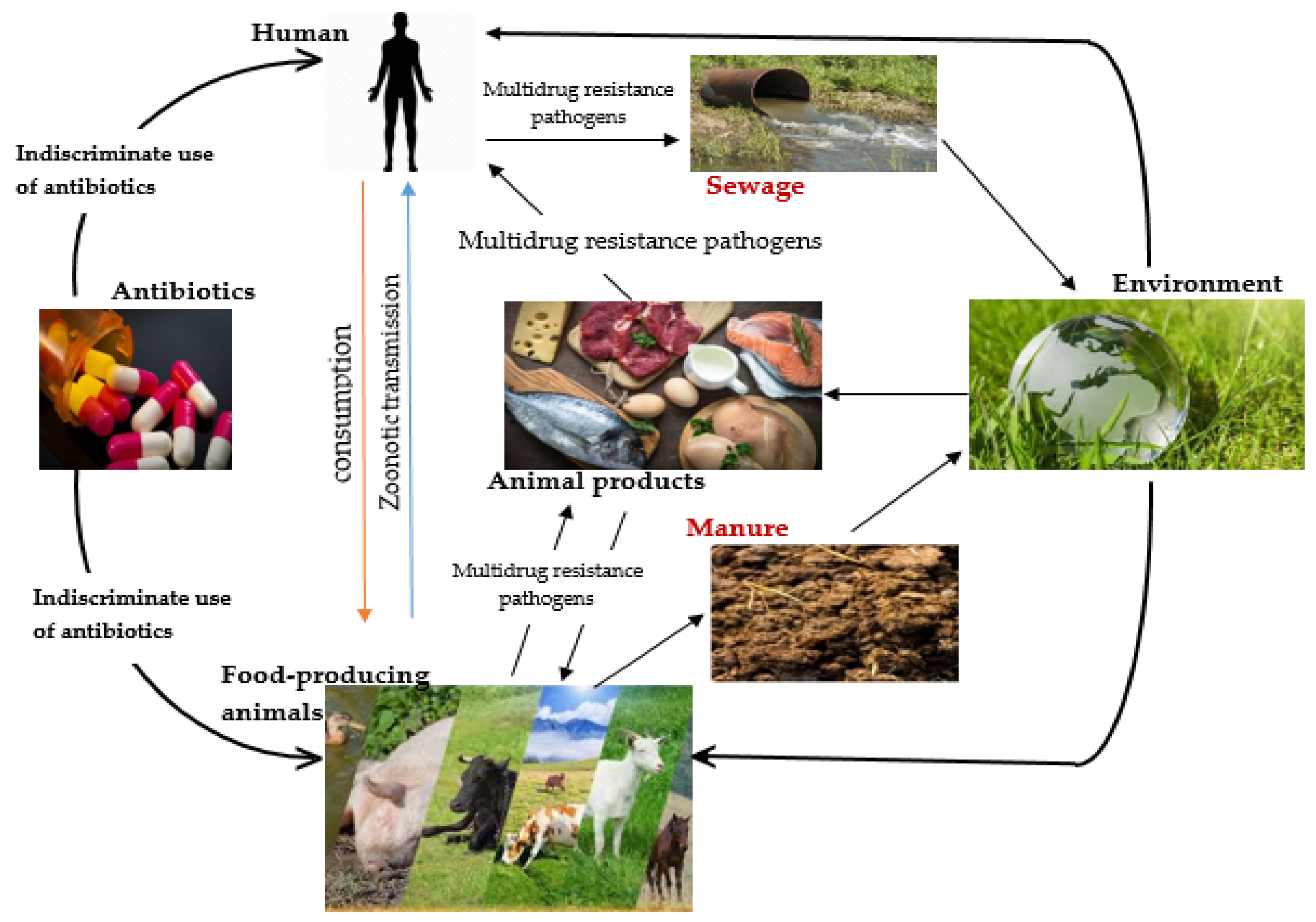
2. Emergence of Multidrug Resistance (MDR) Pathogens in the Food Chain
Use of Antibiotics in Animal Agriculture, Their Mode of Action and Resistance Mechanisms
| Antibiotic Family | Mode of Action | Mechanism of Resistance | Reference |
|---|---|---|---|
| β-lactams β-lactamase inhibitors Fluoroquinolones Macrolides, Lincosamides and Streptogamin (MLS) Aminoglycosides Tetracyclines Sulfonamides (Folate pathway inhibitors) | Cell wall synthesis inhibitors. Binds transpeptidase also known as penicillin binding proteins (PBPs) that help form peptidoglycan Inactivates the enzyme; beta-lactamase Hydrolysis of the beta-lactam ring Binds DNA-gyrase or topoisomerase II and topoisomerase IV; enzymes needed for supercoiling, replication and separation of circular bacterial DNA. Binds the bacterial 50S ribosomal subunits; inhibit protein synthesis Bind to the bacterial 30S ribosomal subunit thus inhibit bacterial protein synthesis Bind reversibly to the 30S ribosomal subunit as such blocks the binding of the aminoacyl-tRNA to the acceptor site on the mRNA-ribosome complex Inhibit the bacterial enzyme dihydropteroate synthetase (DPS) in the folic acid pathway, thereby blocking bacterial nucleic acid synthesis | Beta-lactamase production primarily - bla genes, Expression of alternative PBPs Production of extended spectrum beta-lactamases (ESBLs) Target modification, Decreased membrane permeability, Efflux pumps Target site modification, Active drug efflux Target site modification (via the action of 16S rRNA methyltransferases (RMTs)), Enzymatic Drug Modification (adenylation, acetylation and phosphorylation), Efflux systems Efflux systems, Target modification, Inactivating enzymes, Ribosomal protection Excessive bacterial production of dihydrofolate reductase (DHFR), Reduction in the ability of the drug to penetrate the bacterial cell wall, Production of altered forms of the dihydropteroate synthetase (DPS) enzyme with a lower affinity for sulfonamides, Hyperproduction of para-amino benzoic acid (PABA), which overcomes the competitive substitution of the sulfonamides | [25,28,29] [30] [31] [32] [33,34] [35,36] |
3. Annals of One Health Antimicrobial Resistance
3.1. Third-Generation Cephalosporins
3.2. Colistin
| Pathogen | Class of antibiotic Resistance | Transmission Route | Food Product Susceptible to Contamination | Reference |
|---|---|---|---|---|
| Nontyphoidal Salmonella Campylobacter jejuni Escherichia coli Staphylococcus aureus, Methicillin- resistant Staphylococcus aureus (MRSA) and other staphylococci Listeria monocytogenes and other Listeria species | Cephalosporin a,b Fluoroquinolone b Tetracycline b,c Penicillin a,b Sulfonamide b,c Fluoroquinolone b Macrolide a,b Cephalosporin a,b Fluoroquinolone b Carbapenem a Cephalosporin c Methicillin a,b Vancomycin a Cephalosporin a,b Penicillin a,b Fluoroquinolone b Tetracycline b,c Aminoglycoside a,b Carbapenem a Monobactam a Macrolide a,b Lincosamide c,d | Faecal shedding into the environment Waste water, faeces and urine Water Contact with carrier animals; indiscriminate use of antibiotics in animals; negligence resulting in cross-infections within the confines of and amid farms; foreign trade in animal, food or supplementary outputs Sewage, effluent, faeces of man and animal, soil water | Meat and poultry products, fruits and vegetables Meat and poultry products Milk, meat and eggs Bacon, meat, milk and eggs Unpasteurized milk and its derivatives, meat, fish, chicken, poultry products, vegetables and salads | [38,40,50] [43,51] [40,52,53] [40,54] [55,56] |
4. Nanotechnology and One Health in Agriculture (Animal Husbandry)
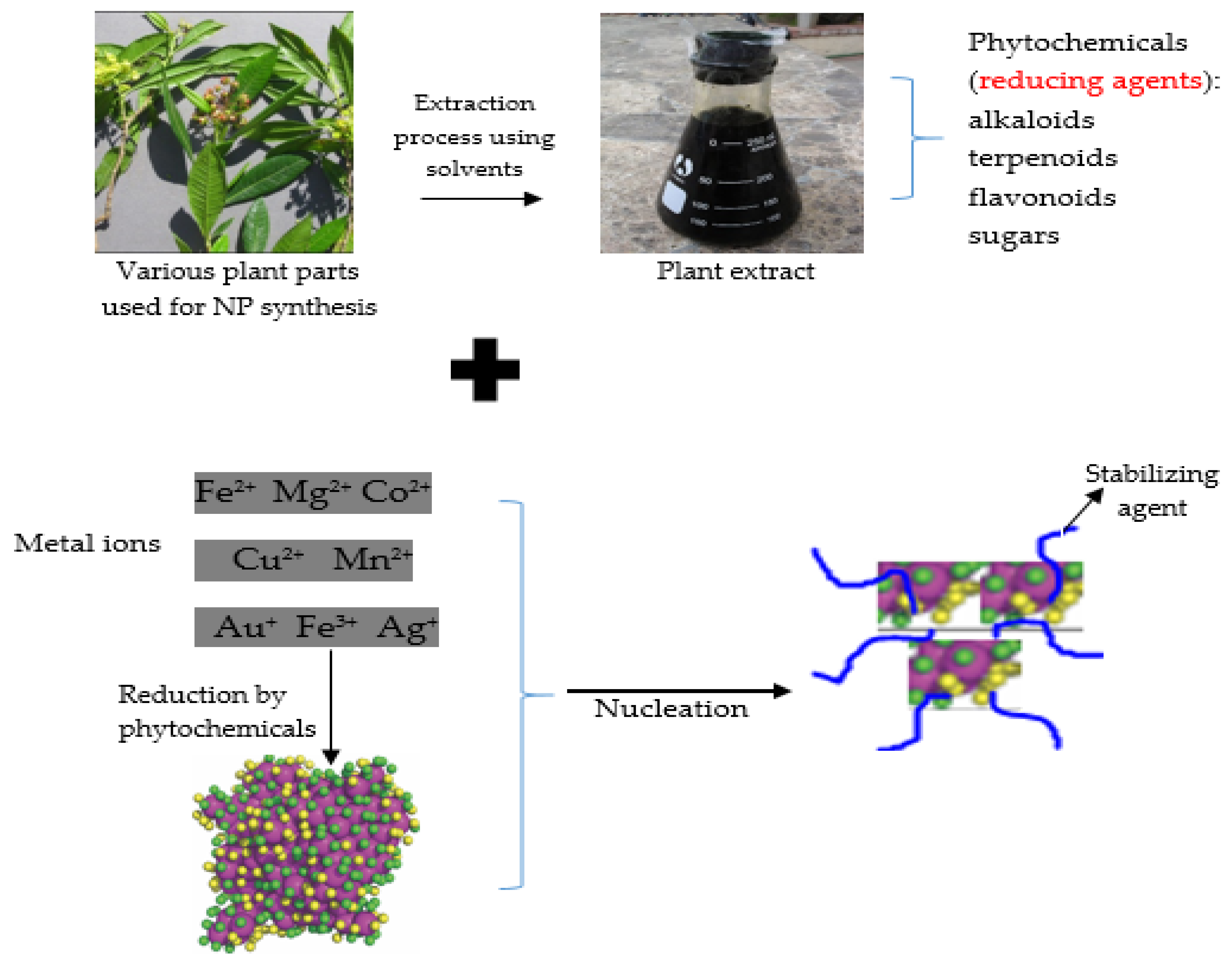
4.1. Synthesis of Nanoparticles
4.1.1. Top-Down Method (Physical Approach)
4.1.2. Bottom-Up (Chemical and Biological Approaches, Green Chemistry or Plant-Mediated Synthesis): An Approach Used for Synthesizing Plant-Derived Nanoparticles
| Green Synthesis of Plant-Derived Nanoparticles (PDNP) | Antibiotics | References |
|---|---|---|
| Efficient uptake of drug owing to their small sizes | Limited uptake of drug | [36,82] |
| Sufficient drug accumulation at target site | Reduced drug accumulation at target site owing to modification in target site | [83,84,85] |
| Pharmacokinetics: protection of encapsulated drug | Active drug efflux | [68,86] |
| Pharmacodynamics: retention of drug at active site increases bioavailability; thus therapeutic efficiency is enhanced and level of drug stability is increased | Inactivation of drug by cellular enzymes | [29,68] |
| Safety and activity: considerably safe and products have antibacterial properties | Resistance; a public health concern has developed on account of the indiscriminate use and the development and/or acquisition of resistant genes by pathogens | [24,87,88] |
| Minimal energy utilization, ecofriendliness, biocompatibility, and the use of renewable resources Cost-effective and easy to produce | Adoption of an organic chemistry method which uses chemicals, some of which may be dangerous and cause environmental concern Capital-intensive | [87,88,89] [70,90] |
4.2. Characterization of Metallic Nanoparticles
5. Applications of Plant-Derived Nanoparticles in the Food Industry
| Plant Used | Plant Part Used for Extraction | Solvent Used for Extraction | Phytochemicals | Nano-Particle | Target Pathogen | Reference |
|---|---|---|---|---|---|---|
| Aegle marmelos | Fruit | Methanol | Tannins, saponins, steroids, alkaloids, flavonoids, glycosides | Ag | Bacillus cereus, Pseudomonas aeruginosa, Salmonella dysentriae | [111] |
| Allium rotundum | Leaf | Deionised water, ethanol | Terpenes, phenol, carvacrol | Ag | Pseudomonas aeruginosa, S. aureus | [112] |
| Aloe vera and Linum usitatissimum | Leaf and seed | Distilled and deionised water | Phenolics, phenolic glycosides | Fe2O3 | S. aureus, Salmonella typhi | [113,114] |
| Annona muricata | Leaf | Deionised water | Flavonoids, terpenoids | Au | S. aureus, Entrococcus faecalis, Klebsiella pneumonia, Clostridium sporogenes | [115] |
| Ashwagandha, bufera | Leaf | Water | Flavonoid, tannin | Se | Bacillus subtilis | [116] |
| Asparagus racemosus | Root | NM | Phenols, tannins, sterols | Pd | S. aureus, E. coli | [117,118] |
| Caesalpinia bonducella | Seed | NM | Citrulline, phytosterinin, flavonoids | CuO | S. aureus, Aeromonas species | [119] |
| Camellia sinensis | Leaf | Water | Polyphenol | NiO | S. epidermidis, Pseudomonas aeruginosa | [120] |
| Catharanthus roseus | Leaf | Water | NM | Ag | Shigella dysenteriae, Klebsiella pneumoniae, Bacillus anthraces, Staphylococcus aureus, Pseudomonas aeruginosa | [121] |
| Chromolaena odorata Clerodendrum inerme | Root Leaf | Coconut sap Fruit juice | Alkaloid Terpenoids, tannins, saponins, alkaloids, phenolics, cardiac glycosides, anthraquinones | Fe3O4 Ag, Au | E. coli, S. aureus S. aureus, B. subtilis, E. coli, Klebsiella species | [122,123] [124] |
| Cocos nucifera | Inflorescence sap | Methanol, chloroform, water | Flavonoids | Ag | Bacillus pumilus | [125] |
| Datura metel | Leaf | Water | Alkaloid, flavonoid | CeO2 | Enterococcus faecalis, S. aureus, Klebsiella pneumonia, E. coli | [126,127] |
| Diospyros kaki | Peel | Methanol | tannins, carotenoids, flavonoids, steroids, lipid, terpenoids, naphthoquinones | MgO | S. aureus, E. coli | [128] |
| Euphorbia heterophylla | Leaf | Water | Alkaloid, flavonoid, saponin, tannin | MnO2 | E. coli, S. aureus, Streptococcus mutans | [129,130] |
| Galphimia glauca | Leaf | Water | Tri-terpenes, galic acids, terpenoids, phenolics | Ag | Pseudomonas aeruginosa | [131] |
| Gardenia jasminoides | Leaf | Water | Polyphenol, flavonoid | Cu | S. aureus, E. coli | [132] |
| Leucaena leucocephala | Leaf | Water | Flavonoids, coumarins, tannin, saponin, phenol, steroid, Cardial glycoside | CdO | Pseudomonas aeruginosa | [133] |
| Musa paradisiaca | Stem | Water | Glycosides, flavonoids and terpenoids | Ag | Bacillus subtilis, E. coli | [134] |
| Tamarix nilotica | Shoot | Water | Phenol | Ag | Listeria monocytogenes | [135] |
| Trigonella foenum-graecum | Leaf | Water | NM | TiO2 | Bacillus subtilis | [136] |
5.1. Function and Significance of Natural Products of Plants in the Activity of Plant-Derived Nanoparticles
5.2. Antibacterial Effects of Plant-Derived Nanoparticles (PDNPs)
5.3. The Inhibition of Biofilm Formation by Plant-Derived Nanoparticles (PDNPs)
5.4. Parameters Affecting the Antibacterial Activity of Plant-Derived Nanoparticle
6. Conclusions and Future Prospect
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Garkovenko, A.; Radchenko, V.; Ilnitskaya, E.; Koshchaev, A.; Shchukina, I.; Bakharev, A.; Sukhanova, S. Polymorphism of cattle microsatellite complexes. J. Pharm. Sci. Res. 2018, 10, 1545–1551. [Google Scholar]
- Koshchaev, A.; Shchukina, I.; Garkovenko, A.; Ilnitskaya, E.; Radchenko, V.; Bakharev, A.; Khrabrova, L. Allelic variation of marker genes of hereditary diseases and economically important traits in dairy breeding cattle population. J. Pharm. Sci. Res. 2018, 10, 1566–1572. [Google Scholar]
- Zhou, K.; Li, C.; Chen, D.; Pan, Y.; Tao, Y.; Qu, W.; Liu, Z.; Wang, X.; Xie, S. A review on nanosystems as an effective approach against infections of Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 7333. [Google Scholar] [CrossRef]
- Sztachanska, M.; Baranski, W.; Janowski, T.; Pogorzelska, J.; Zdunczyk, S. Prevalence and etiological agents of subclinical mastitis at the end of lactation in nine dairy herds in North-East Poland. Pol. J. Vet. Sci. 2016, 19, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, M.H.G.; Tarek, A.M. Clostridium botulinum, A foodborne pathogen and its impact on public health. Ann. Trop. Med. Public Health 2020, 23, 49–62. [Google Scholar] [CrossRef]
- Lúquez, C.; Edwards, L.; Griffin, C.; Sobel, J. Foodborne botulism outbreaks in the United States, 2001–2017. Front. Microbiol. 2021, 1982. [Google Scholar] [CrossRef] [PubMed]
- Chaidoutis, E.; Keramydas, D.; Papalexis, P.; Migdanis, A.; Migdanis, I.; Lazaris, A.C.; Kavantzas, N. Foodborne botulism: A brief review of cases transmitted by cheese products. Biomed. Rep. 2022, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sofos, J.N. Challenges to meat safety in the 21st century. Meat Sci. 2008, 78, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Baydan, E.; Kanbur, M.; Arslanbaş, E.; Aydın, F.G.; Gürbüz, S.; Tekeli, M.Y. Contaminants in animal products. Livest. Sci. 2017, 129. [Google Scholar]
- WHO. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Robbins, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance: Mitigation opportunities in livestock sector development. Animal 2016, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, P.M.; Blaak, H.; de Jong, M.C.; Graat, E.A.; Vandenbroucke-Grauls, C.M.; de Roda Husman, A.M. Role of the environment in the transmission of antimicrobial resistance to humans: A review. Environ. Sci. Technol. 2015, 49, 11993–12004. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sasikala, P. Nanoscience and nanotechnology: Perspectives and overview. First Indian at the South Pole 3 PS Sehra Madame Marie Curie–Radioactivity and 10 Atomic Energy. Science 2006, 44. [Google Scholar]
- Radha, K.; Thomas, A.; Sathian, C. Application of nano technology in dairy industry: Prospects and challenges—A review. Indian J. Dairy Sci. 2014, 67, 367–374. [Google Scholar]
- Neculai-Valeanu, A.S.; Ariton, A.M.; Mădescu, B.M.; Rîmbu, C.M.; Creangă, Ş. Nanomaterials and essential oils as candidates for developing novel treatment options for bovine mastitis. Animals 2021, 11, 1625. [Google Scholar] [CrossRef]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fevre, E.M.; Woolhouse, M.E.; van Bunnik, B.A. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ghafur, A.; Shankar, C.; GnanaSoundari, P.; Venkatesan, M.; Mani, D.; Thirunarayanan, M.; Veeraraghavan, B. Detection of chromosomal and plasmid-mediated mechanisms of colistin resistance in Escherichia coli and Klebsiella pneumoniae from Indian food samples. J. Glob. Antimicrob. Resist. 2019, 16, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, F.; Taban, B.M. A state-of-art review on multi-drug resistant pathogens in foods of animal origin: Risk factors and mitigation strategies. Front. Microbiol. 2019, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic resistance in the food chain: A developing country-perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.; Moulin, G. Antimicrobial resistance at farm level. Rev. Sci. Tech. Int. Off. Epizoot. 2006, 25, 775–792. [Google Scholar] [CrossRef]
- Ajose, D.J.; Oluwarinde, B.O.; Abolarinwa, T.O.; Fri, J.; Montso, K.P.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Combating bovine mastitis in the dairy sector in an era of antimicrobial resistance: Ethnoveterinary medicinal option as a viable alternative approach. Front. Vet. Sci. 2022, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Dowling, A.; O’Dwyer, J.; Adley, C. Antibiotics: Mode of action and mechanisms of resistance. Antimicrob. Res. Nov. Bioknowledge Educ. Programs 2017, 1, 536–545. [Google Scholar]
- Cully, M. Public health: The politics of antibiotics. Nature 2014, 509, S16–S17. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-lactamase inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.A.; Cryer, T.L.; Lafi, S.Q.; Basha, E.-A.; Good, L.; Tarazi, Y.H. Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Vet. Res. 2019, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Patel, P.H.; Hashmi, M.F. Macrolides. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Wendlandt, S.; Feßler, A.T.; Monecke, S.; Ehricht, R.; Schwarz, S.; Kadlec, K. The diversity of antimicrobial resistance genes among staphylococci of animal origin. Int. J. Med. Microbiol. 2013, 303, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Shin, S.W.; Shin, M.K.; Jung, M.; Belaynehe, K.M.; Yoo, H.S. Prevalence of antimicrobial resistance and transfer of tetracycline resistance genes in Escherichia coli isolates from beef cattle. Appl. Environ. Microbiol. 2015, 81, 5560–5566. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L. Pharmacologic principles. Equine Intern. Med. 2018, 4, 79–137. [Google Scholar]
- Jiang, H.; Cheng, H.; Liang, Y.; Yu, S.; Yu, T.; Fang, J.; Zhu, C. Diverse mobile genetic elements and conjugal transferability of sulfonamide resistance genes (sul1, sul2, and sul3) in Escherichia coli isolates from Penaeus vannamei and pork from large markets in Zhejiang, China. Front. Microbiol. 2019, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- WHO, Advisory Group on Integrated Surveillance of Antimicrobial Resistance. Critically Important Antimicrobials for Human Medicine; 4th revision; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- European Medicines Agency. Reflection Paper on the Use of Fluoroquinolones in Food Producing Animals-Precautions for Use in the SPC Regarding Prudent Use Guidance (EMEA/CVMP/416168/2006-FINAL; European Medicines Agency: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Park, S.H. Third-generation cephalosporin resistance in gram-negative bacteria in the community: A growing public health concern. Korean J. Intern. Med. 2014, 29, 27. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Kanwar, N.; Scott, H.M.; Norby, B.; Loneragan, G.H.; Vinasco, J.; McGowan, M.; Cottell, J.L.; Chengappa, M.M.; Bai, J.; Boerlin, P. Effects of ceftiofur and chlortetracycline treatment strategies on antimicrobial susceptibility and on tet (A), tet (B), and bla CMY-2 resistance genes among E. coli isolated from the feces of feedlot cattle. PLoS ONE 2013, 8, e80575. [Google Scholar] [CrossRef]
- De Been, M.; Lanza, V.F.; de Toro, M.; Scharringa, J.; Dohmen, W.; Du, Y.; Hu, J.; Lei, Y.; Li, N.; Tooming-Klunderud, A. dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet. 2014, 10, e1004776. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry# 213, New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI# 209; Center for Veterinary Medicine: Rockville, MD, USA, 2013. Available online: http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM299624.pdf (accessed on 25 July 2022).
- EMA. Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health; EMA: London, UK, 2016. [Google Scholar]
- Fernandes, M.R.; Moura, Q.; Sartori, L.; Silva, K.C.; Cunha, M.P.; Esposito, F.; Lopes, R.; Otutumi, L.K.; Gonçalves, D.D.; Dropa, M. Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Eurosurveillance 2016, 21, 30214. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. OIE Annual Report on the Use of Antimicrobial Agents in Animals. Second Report; World Organisation for Animal Health: Paris, France, 2018; Available online: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/Annual_Report_AMR_2.pdf (accessed on 30 June 2022).
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef]
- Bager, F.; Madsen, M.; Christensen, J.; Aarestrup, F.M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 1997, 31, 95–112. [Google Scholar] [CrossRef]
- Mollenkopf, D.F.; Stull, J.W.; Mathys, D.A.; Bowman, A.S.; Feicht, S.M.; Grooters, S.V.; Daniels, J.B.; Wittum, T.E. Carbapenemase-producing Enterobacteriaceae recovered from the environment of a swine farrow-to-finish operation in the United States. Antimicrob. Agents Chemother. 2017, 61, e01298-16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, B.; Paterson, D.L.; Mollinger, J.L.; Rogers, B.A. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin. Infect. Dis. 2015, 60, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Bou-Antoun, S.; Davies, J.; Guy, R.; Johnson, A.P.; Sheridan, E.A.; Hope, R.J. Descriptive epidemiology of Escherichia coli bacteraemia in England, April 2012 to March 2014. Eurosurveillance 2016, 21, 30329. [Google Scholar] [CrossRef]
- Dorado-García, A.; Dohmen, W.; Bos, M.E.; Verstappen, K.M.; Houben, M.; Wagenaar, J.A.; Heederik, D.J. Dose-response relationship between antimicrobial drugs and livestock-associated MRSA in pig farming. Emerg. Infect. Dis. 2015, 21, 950. [Google Scholar] [CrossRef]
- Escolar, C.; Gómez, D.; del Carmen Rota García, M.; Conchello, P.; Herrera, A. Antimicrobial resistance profiles of Listeria monocytogenes and Listeria innocua isolated from ready-to-eat products of animal origin in Spain. Foodborne Pathog. Dis. 2017, 14, 357–363. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. OIE List of Antimicrobial Agents of Veterinary Importance; OIE: Paris, France, 2015. [Google Scholar]
- Warimwe, G.M.; Francis, M.J.; Bowden, T.A.; Thumbi, S.M.; Charleston, B. Using cross-species vaccination approaches to counter emerging infectious diseases. Nat. Rev. Immunol. 2021, 21, 815–822. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.; Peijs, T. Transparent semi-crystalline polymeric materials and their nanocomposites: A review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in food and agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nutr. 2018, 58, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Kwatra, N.; Abraham, J. Nanoparticles fabrication by plant extracts. In Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–157. [Google Scholar]
- Li, Y.; Duan, X.; Qian, Y.; Yang, L.; Liao, H. Nanocrystalline silver particles: Synthesis, agglomeration, and sputtering induced by electron beam. J. Colloid Interface Sci. 1999, 209, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.; Scurrell, M. Polymer stabilized silver nanoparticles: A photochemical synthesis route. J. Mater. Sci. 2004, 39, 4459–4463. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In Proceedings of the AIP Conference 2016, Rajasthan, India, 24–25 October 2015; p. 020048. [Google Scholar]
- Ali, M.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Ikram, S. Synthesis of gold nanoparticles using plant extract: An overview. Nano Res. 2015, 1, 5. [Google Scholar]
- Ghotekar, S.; Pagar, T.; Pansambal, S.; Oza, R. A review on green synthesis of sulfur nanoparticles via plant extract, characterization and its applications. Adv. J. Chem. Section B 2020, 2, 128–143. [Google Scholar]
- Das, S.K.; Thatoi, H. Mangrove plant–mediated green synthesis of nanoparticles and their pharmaceutical applications: An overview. Biotechnol. Util. Mangrove Resour. 2020, 16, 355–370. [Google Scholar]
- Piwoński, I.; Spilarewicz-Stanek, K.; Kisielewska, A.; Kądzioła, K.; Cichomski, M.; Ginter, J. Examination of Ostwald ripening in the photocatalytic growth of silver nanoparticles on titanium dioxide coatings. Appl. Surf. Sci. 2016, 373, 38–44. [Google Scholar] [CrossRef]
- Pașca, C.; Mărghitaș, L.; Dezmirean, D.; Bobiș, O.; Bonta, V.; Chirilă, F.; Matei, I.; Fiț, N. Medicinal plants based products tested on pathogens isolated from mastitis milk. Molecules 2017, 22, 1473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Pammi, S.; Kollu, P.; Satyanarayana, K.; Shameem, U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind. Crops Prod. 2014, 52, 562–566. [Google Scholar]
- Sadeghi, B.; Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef]
- Orellano, M.S.; Isaac, P.; Breser, M.L.; Bohl, L.P.; Conesa, A.; Falcone, R.D.; Porporatto, C. Chitosan nanoparticles enhance the antibacterial activity of the native polymer against bovine mastitis pathogens. Carbohydr. Polym. 2019, 213, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Esmaeillou, M.; Zarrini, G.; Rezaee, M.A. Vancomycin capped with silver nanoparticles as an antibacterial agent against multi-drug resistance bacteria. Adv. Pharm. Bull. 2017, 7, 479. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Bakuła, Z.; Pleń, M.; Kamiński, M.; Nowakowska, J.; Bielecki, J.; Wolska, K.I.; Grudniak, A.M. The activity of silver nanoparticles against microalgae of the Prototheca genus. Nanomedicine 2018, 13, 1025–1036. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and copper nanoparticles—An alternative in future mastitis treatment and prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef] [PubMed]
- Ostaszewska, T.; Śliwiński, J.; Kamaszewski, M.; Sysa, P.; Chojnacki, M. Cytotoxicity of silver and copper nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Environ. Sci. Pollut. Res. 2018, 25, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.T.; Chrzanowski, W. Nano-enhanced drug delivery and therapeutic ultrasound for cancer treatment and beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance; WHO: Geneva, Switzerland, 2018; Available online: http://www.who.int/newsroom/fact-sheets/detail/antimicrobial-resistance (accessed on 29 June 2022).
- Wintersteiner, O.; Dutcher, J.D. Chemistry of Antibiotics. Annu. Rev. Biochem. 1949, 18, 559–594. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int. J. Microbiol. 2019, 2019, 8642303. [Google Scholar] [CrossRef]
- D’Angelo, K.A.; Schissel, C.K.; Pentelute, B.L.; Movassaghi, M. Total synthesis of himastatin. Science 2022, 375, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Towse, A.; Hoyle, C.K.; Goodall, J.; Hirsch, M.; Mestre-Ferrandiz, J.; Rex, J.H. Time for a change in how new antibiotics are reimbursed: Development of an insurance framework for funding new antibiotics based on a policy of risk mitigation. Health Policy 2017, 121, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef]
- Abbas, Q. Understanding the UV-Vis spectroscopy for nanoparticles. J. Nanomater. Mol. Nanotechnol. 2019, 8, 1–3. [Google Scholar]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Baer, D.R. Guide to making XPS measurements on nanoparticles. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 031201. [Google Scholar] [CrossRef]
- Ealia, S.A.M.; Saravanakumar, M. A review on the classification, characterisation, synthesis of nanoparticles and their application. In Proceedings of the IOP Conference Series: Materials Science and Engineering 2017, Vellore, India, 2–3 May 2017; Volume 263, p. 032019. [Google Scholar]
- Marsalek, R. Particle size and zeta potential of ZnO. APCBEE Procedia 2014, 9, 13–17. [Google Scholar] [CrossRef]
- Bzdek, B.R.; Zordan, C.A.; Luther, G.W., III; Johnston, M.V. Nanoparticle chemical composition during new particle formation. Aerosol Sci. Technol. 2011, 45, 1041–1048. [Google Scholar] [CrossRef]
- Zscherp, C.; Barth, A. Reaction-induced infrared difference spectroscopy for the study of protein reaction mechanisms. Biochemistry 2001, 40, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schlücker, S. Rational design and synthesis of SERS labels. Analyst 2013, 138, 2224–2238. [Google Scholar] [CrossRef]
- Shkilnyy, A.; Soucé, M.; Dubois, P.; Warmont, F.; Saboungi, M.L.; Chourpa, I. Poly (ethylene glycol)-stabilized silver nanoparticles for bioanalytical applications of SERS spectroscopy. Analyst 2009, 134, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Chen, Q.Q.; Chen, X.; Wang, A.; Tian, Z.Q.; Li, J.F. Graphene-coated Au nanoparticle-enhanced Raman spectroscopy. J. Raman Spectrosc. 2021, 52, 439–445. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. [Google Scholar]
- Alshehri, A.A.; Malik, M.A. Phytomediated photo-induced green synthesis of silver nanoparticles using Matricaria chamomilla L. and its catalytic activity against rhodamine B. Biomolecules 2020, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Wahid, I.; Kumari, S.; Ahmad, R.; Hussain, S.J.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Silver nanoparticle regulates salt tolerance in wheat through changes in ABA concentration, ion homeostasis, and defense systems. Biomolecules 2020, 10, 1506. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Nasif, O.; Ali Alharbi, S.; Chinni, S.V.; Reddy, L.V.; Reddy, M.R.V.; Sreeramanan, S. Phytogenic synthesis of Pd-Ag/rGO nanostructures using stevia leaf extract for photocatalytic H2 production and antibacterial studies. Biomolecules 2021, 11, 190. [Google Scholar] [CrossRef]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU J. Pharm. Sci. 2015, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J. Nanobiotechnology 2021, 19, 1–25. [Google Scholar] [CrossRef]
- Laad, M.; Jatti, V.K.S. Titanium oxide nanoparticles as additives in engine oil. J. King Saud Univ. Eng. Sci. 2018, 30, 116–122. [Google Scholar] [CrossRef]
- Sani, I.; Ukwuani-Kwaja, A.N.; Abdulkadir, D. Antibacterial activities of plant-derived metallic nanoparticles on some selected multidrug-resistant clinical isolates. Asian J. Biol. Sci. 2022, 15, 15–26. [Google Scholar]
- Ansari, M.A.; Kalam, A.; Al-Sehemi, A.G.; Alomary, M.N.; AlYahya, S.; Aziz, M.K.; Srivastava, S.; Alghamdi, S.; Akhtar, S.; Almalki, H.D. Counteraction of biofilm formation and antimicrobial potential of Terminalia catappa functionalized silver nanoparticles against Candida albicans and multidrug-resistant Gram-negative and Gram-positive bacteria. Antibiotics 2021, 10, 725. [Google Scholar] [CrossRef]
- Devi, M.; Devi, S.; Sharma, V.; Rana, N.; Bhatia, R.K.; Bhatt, A.K. Green synthesis of silver nanoparticles using methanolic fruit extract of Aegle marmelos and their antimicrobial potential against human bacterial pathogens. J. Tradit. Complement. Med. 2020, 10, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Hekmati, M.; Hasanirad, S.; Khaledi, A.; Esmaeili, D. Green synthesis of silver nanoparticles using extracts of Allium rotundum l, Falcaria vulgaris Bernh, and Ferulago angulate Boiss, and their antimicrobial effects in vitro. Gene Rep. 2020, 19, 100589. [Google Scholar] [CrossRef]
- Rahmani, R.; Gharanfoli, M.; Gholamin, M.; Darroudi, M.; Chamani, J.; Sadri, K.; Hashemzadeh, A. Plant-mediated synthesis of superparamagnetic iron oxide nanoparticles (SPIONs) using aloe vera and flaxseed extracts and evaluation of their cellular toxicities. Ceram. Int. 2020, 46, 3051–3058. [Google Scholar] [CrossRef]
- Sudhakar, C.; Poonkothai, M.; Selvankmuar, T.; Selvam, K.; Rajivgandhi, G.; Siddiqi, M.Z.; Alharbi, N.S.; Kadaikunnan, S.; Vijayakumar, N. Biomimetic synthesis of iron oxide nanoparticles using Canthium coromandelicum leaf extract and its antibacterial and catalytic degradation of Janus green. Inorg. Chem. Commun. 2021, 133, 108977. [Google Scholar] [CrossRef]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostructure Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- Alagesan, V.; Venugopal, S. Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. Bionanoscience 2019, 9, 105–116. [Google Scholar] [CrossRef]
- Anjana, P.; Bindhu, M.; Umadevi, M.; Rakhi, R. Antibacterial and electrochemical activities of silver, gold, and palladium nanoparticles dispersed amorphous carbon composites. Appl. Surf. Sci. 2019, 479, 96–104. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Dadashi, J.; Ghafuri, H. Pd-based nanoparticles: Plant-assisted biosynthesis, characterization, mechanism, stability, catalytic and antimicrobial activities. Adv. Colloid Interface Sci. 2020, 276, 102103. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Rudrasenan, A.; Nambiar, D.P. Green-synthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extract and their applications. ACS Omega 2020, 5, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Kalita, C.; Sarkar, R.D.; Verma, V.; Bharadwaj, S.K.; Kalita, M.C.; Boruah, P.K.; Das, M.R.; Saikia, P. Bayesian modeling coherenced green synthesis of NiO nanoparticles using Camellia sinensis for efficient antimicrobial activity. BioNanoScience 2021, 11, 825–837. [Google Scholar] [CrossRef]
- Ahmad, S.; Tauseef, I.; Haleem, K.S.; Khan, K.; Shahzad, M.; Ali, M.; Sultan, F. Synthesis of silver nanoparticles using leaves of Catharanthus roseus and their antimicrobial activity. Appl. Nanosci. 2020, 10, 4459–4464. [Google Scholar] [CrossRef]
- Taimoory, S.M.; Rahdar, A.; Aliahmad, M.; Sadeghfar, F.; Hajinezhad, M.R.; Jahantigh, M.; Shahbazi, P.; Trant, J.F. The synthesis and characterization of a magnetite nanoparticle with potent antibacterial activity and low mammalian toxicity. J. Mol. Liq. 2018, 265, 96–104. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green synthesis and characterization of magnetite (Fe3O4) nanoparticles using Chromolaena odorata root extract for smart nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Lee, C.-S. Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef]
- Rajesh, M.; Muralikrishna, K.; Nair, S.S.; Krishna, K.B.; Subrahmanya, T.; Sonu, K.; Subaharan, K.; Sweta, H.; Keshava, P.T.; Neeli, C. Facile coconut inflorescence sap mediated synthesis of silver nanoparticles and its diverse antimicrobial and cytotoxic properties. Mater. Sci. Eng. C 2020, 111, 110834. [Google Scholar]
- Sebastiammal, S.; Mariappan, A.; Neyvasagam, K.; Fathima, A.L. Annona muricata inspired synthesis of CeO2 nanoparticles and their antimicrobial activity. Mater. Today Proc. 2019, 9, 627–632. [Google Scholar] [CrossRef]
- Yulizar, Y.; Kusrini, E.; Apriandanu, D.O.B.; Nurdini, N. Datura metel L. Leaves extract mediated CeO2 nanoparticles: Synthesis, characterizations, and degradation activity of DPPH radical. Surf. Interfaces 2020, 19, 100437. [Google Scholar] [CrossRef]
- Ali, R.; Shanan, Z.J.; Saleh, G.M.; Abass, Q. Green synthesis and the study of some physical properties of MgO nanoparticles and their antibacterial activity. Iraqi J. Sci. 2020, 266–276. [Google Scholar] [CrossRef]
- Dewi, N.O.M.; Yulizar, Y. Euphorbia heterophylla L. leaf extract-mediated synthesis of MnO2 nanoparticles and its characterization. Mater. Today Proc. 2020, 22, 199–204. [Google Scholar] [CrossRef]
- Joshi, N.C.; Siddiqui, F.; Salman, M.; Singh, A. Antibacterial activity, characterizations, and biological synthesis of manganese oxide nanoparticles using the extract of aloe vera. Asian Pac. J. Health Sci. 2020, 7, 27–29. [Google Scholar] [CrossRef]
- Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Kotresha, D.; Rudrappa, M.; Pallavi, S.; Hiremath, H.; Perumal, K.; Nayaka, S. Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J. King Saud Univ. Sci. 2021, 33, 101660. [Google Scholar] [CrossRef]
- Nieto-Maldonado, A.; Bustos-Guadarrama, S.; Espinoza-Gomez, H.; Flores-López, L.Z.; Ramirez-Acosta, K.; Alonso-Nuñez, G.; Cadena-Nava, R.D. Green synthesis of copper nanoparticles using different plant extracts and their antibacterial activity. J. Environ. Chem. Eng. 2022, 10, 107130. [Google Scholar] [CrossRef]
- Savale, A.; Ghotekar, S.; Pansambal, S.; Pardeshi, O. Green synthesis of fluorescent CdO nanoparticles using Leucaena leucocephala L. extract and their biological activities. J. Bacteriol. Mycol. Open Access 2017, 5, 00148. [Google Scholar]
- Doan, V.-D.; Le, V.-T.; Phan, T.-L.; Nguyen, T.L.-H.; Nguyen, T.-D. Waste banana stem utilized for biosynthesis of silver and gold nanoparticles and their antibacterial and catalytic properties. J. Clust. Sci. 2021, 32, 1673–1682. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Nadeem, M.; Khan, M.S.; Al-Qurainy, F.; Alyousef, A.A.; Arshad, M.; Khan, A.; Khan, J.M.; Alam, P. Bio-inspired facile fabrication of silver nanoparticles from in vitro grown shoots of Tamarix nilotica: Explication of its potential in impeding growth and biofilms of Listeria monocytogenes and assessment of wound healing ability. RSC Adv. 2020, 10, 30139–30149. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Housaindokht, M.R.; Sadeghnia, H.R.; Bahabadi, S.E.; Amiri, M.S.; Darroudi, M. Assessment of phytochemical components and antioxidant activity of Rheum turkestanicum Janisch. Stud. Med. Sci 2020, 31, 75–81. [Google Scholar]
- Modarres, M.; Taghavizadeh Yazdi, M.E. Elicitation improves phenolic acid content and antioxidant enzymes activity in salvia leriifolia cell cultures. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 849–855. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Anand, K.K.H.; Mandal, B.K. Gold nanoparticles—synthesis by Sterculia acuminata extract and its catalytic efficiency in alleviating different organic dyes. J. Mol. Liq. 2015, 211, 868–875. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Makarov, V.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Rajathi, F.A.A.; Arumugam, R.; Saravanan, S.; Anantharaman, P. Phytofabrication of gold nanoparticles assisted by leaves of Suaeda monoica and its free radical scavenging property. J. Photochem. Photobiol. B Biol. 2014, 135, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Dhamodaran, M.; Prasad, R.; Ganesan, M. Synthesis and characterisation of zinc oxide nanoparticles using terpenoid fractions of Andrographis paniculata leaves. Int. Nano Lett. 2017, 7, 141–147. [Google Scholar] [CrossRef]
- Garg, T.; Rath, G.; Goyal, A.K. Colloidal drug delivery systems: Current status and future directions. Crit. Rev.Ther. Drug Carr. Syst. 2015, 32, 89–147. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Rath, G.; Goyal, A.K. Nanotechnological approaches for the effective management of psoriasis. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Rath, G.; Murthy, R.R.; Gupta, U.D.; Vatsala, P.G.; Goyal, A.K. Current nanotechnological approaches for an effective delivery of bioactive drug molecules to overcome drug resistance tuberculosis. Curr. Pharm. Des. 2015, 21, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Algharib, S.A.; Dawood, A.; Xie, S. Nanoparticles for treatment of bovine Staphylococcus aureus mastitis. Drug Deliv. 2020, 27, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.; Mobaraki, M.; Ghaffari, M.; Zarebkohan, A.; Omrani, V.F.; Urbanska, A.M.; Seifalian, A. Targeted drug delivery based on gold nanoparticle derivatives. Curr. Pharm. Des. 2017, 23, 2918–2929. [Google Scholar] [CrossRef]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J. Alloy. Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostructure Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef]
- Chakraborty, N.; Gandhi, S.; Verma, R.; Roy, I. Emerging prospects of nanozymes for antibacterial and anticancer applications. Biomedicines 2022, 10, 1378. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Yacaman, M.J.; Lopez-Ribot, J. Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Qayyum, S.; Khan, A.U. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: A comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 2017, 103, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, M.B.; Balachandranath, R.; Srinivasulu, Y.G.; Kannaiyan, S.K.; Subbiahdoss, G. The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens. Int. Sch. Res. Not. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Adaptive Antimicrobial Nanocarriers for the Control of Infectious Biofilms. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2019. [Google Scholar]
- Singh, B.N.; Prateeksha, N.; Upreti, D.K.; Singh, B.R.; Defoirdt, T.; Gupta, V.K.; De Souza, A.O.; Singh, H.B.; Barreira, J.C.; Ferreira, I.C. Bactericidal, quorum quenching and anti-biofilm nanofactories: A new niche for nanotechnologists. Crit. Rev. Biotechnol. 2017, 37, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shang, F.; Chen, X.; Ni, J.; Yu, L.; Zhang, M.; Sun, D.; Xue, T. The anti-biofilm effect of silver-nanoparticle-decorated quercetin nanoparticles on a multi-drug resistant Escherichia coli strain isolated from a dairy cow with mastitis. PeerJ 2018, 6, e5711. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, C.; Rajasekharan, S.K.; Reifen, R.; Shemesh, M. Mitigating milk-associated bacteria through inducing zinc ions antibiofilm activity. Foods 2020, 9, 1094. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 1–17. [Google Scholar]
- Verma, M.L.; Dhanya, B.; Thakur, M.; Jeslin, J.; Jana, A.K. Plant derived nanoparticles and their biotechnological applications. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 94, pp. 331–362. [Google Scholar]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [PubMed]
- Skomorokhova, E.A.; Sankova, T.P.; Orlov, I.A.; Savelev, A.N.; Magazenkova, D.N.; Pliss, M.G.; Skvortsov, A.N.; Sosnin, I.M.; Kirilenko, D.A.; Grishchuk, I.V. Size-dependent bioactivity of silver nanoparticles: Antibacterial properties, influence on copper status in mice, and whole-body turnover. Nanotechnol. Sci. Appl. 2020, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Sohm, B.; Immel, F.; Bauda, P.; Pagnout, C. Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 2015, 15, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, M.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Wagh, H.; Liang, Y.; Yang, S.; Boyer, C. Enhancing the antimicrobial and antibiofilm effectiveness of silver nanoparticles prepared by green synthesis. J. Mater. Chem. B 2018, 6, 4124–4138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, M.; Halder, D.; Mitra, A. Lippia javanica: A cheap natural source for the synthesis of antibacterial silver nanocolloid. Appl. Nanosci. 2016, 6, 1001–1007. [Google Scholar] [CrossRef][Green Version]
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Stelling, J.; Kamal, M.A. A review on nano-antimicrobials: Metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 2017, 18, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Panpaliya, N.P.; Dahake, P.T.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Siddiqi, A.G.; Maggavi, U.R. In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dent. J. 2019, 31, 76–83. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, S.P.; Manhar, A.K.; Saikia, D.; Namsa, N.D.; Konwar, B.K.; Mandal, M. Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilm and virulence by active fraction of Syzygium cumini (L.) Skeels leaf extract: In-vitro and in silico studies. Indian J. Microbiol. 2019, 59, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Velsankar, K.; RM, A.K.; Preethi, R.; Muthulakshmi, V.; Sudhahar, S. Green synthesis of CuO nanoparticles via Allium sativum extract and its characterizations on antimicrobial, antioxidant, antilarvicidal activities. J. Environ. Chem. Eng. 2020, 8, 104123. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.; Jayaprakasha, G.K.; Patil, B.S. Manganese oxide nanoparticles as safer seed priming agent to improve chlorophyll and antioxidant profiles in watermelon seedlings. Nanomaterials 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Junior, V.E.; Targino, A.G.R.; Flores, M.A.P.; Rodríguez-Díaz, J.M.; Teixeira, J.A.; Heimer, M.V.; Pessoa, H.D.L.F.; Galembeck, A.; Rosenblatt, A. Antimicrobial activity of silver nanoparticle colloids of different sizes and shapes against Streptococcus mutans. Res. Chem. Intermed. 2017, 43, 5889–5899. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajose, D.J.; Abolarinwa, T.O.; Oluwarinde, B.O.; Montso, P.K.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Application of Plant-Derived Nanoparticles (PDNP) in Food-Producing Animals as a Bio-Control Agent against Antimicrobial-Resistant Pathogens. Biomedicines 2022, 10, 2426. https://doi.org/10.3390/biomedicines10102426
Ajose DJ, Abolarinwa TO, Oluwarinde BO, Montso PK, Fayemi OE, Aremu AO, Ateba CN. Application of Plant-Derived Nanoparticles (PDNP) in Food-Producing Animals as a Bio-Control Agent against Antimicrobial-Resistant Pathogens. Biomedicines. 2022; 10(10):2426. https://doi.org/10.3390/biomedicines10102426
Chicago/Turabian StyleAjose, Daniel Jesuwenu, Tesleem Olatunde Abolarinwa, Bukola Opeyemi Oluwarinde, Peter Kotsoana Montso, Omolola Esther Fayemi, Adeyemi Oladapo Aremu, and Collins Njie Ateba. 2022. "Application of Plant-Derived Nanoparticles (PDNP) in Food-Producing Animals as a Bio-Control Agent against Antimicrobial-Resistant Pathogens" Biomedicines 10, no. 10: 2426. https://doi.org/10.3390/biomedicines10102426
APA StyleAjose, D. J., Abolarinwa, T. O., Oluwarinde, B. O., Montso, P. K., Fayemi, O. E., Aremu, A. O., & Ateba, C. N. (2022). Application of Plant-Derived Nanoparticles (PDNP) in Food-Producing Animals as a Bio-Control Agent against Antimicrobial-Resistant Pathogens. Biomedicines, 10(10), 2426. https://doi.org/10.3390/biomedicines10102426







