Efficacy and Safety of Fecal Microbiota Transplantation for Clearance of Multidrug-Resistant Organisms under Multiple Comorbidities: A Prospective Comparative Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Donor
2.3. Fecal Sample Collection, Preparation, and Administration
2.4. Outcome
2.5. Stool Bacterial Analysis for Metagenomics Analysis
2.6. Ethical Considerations
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
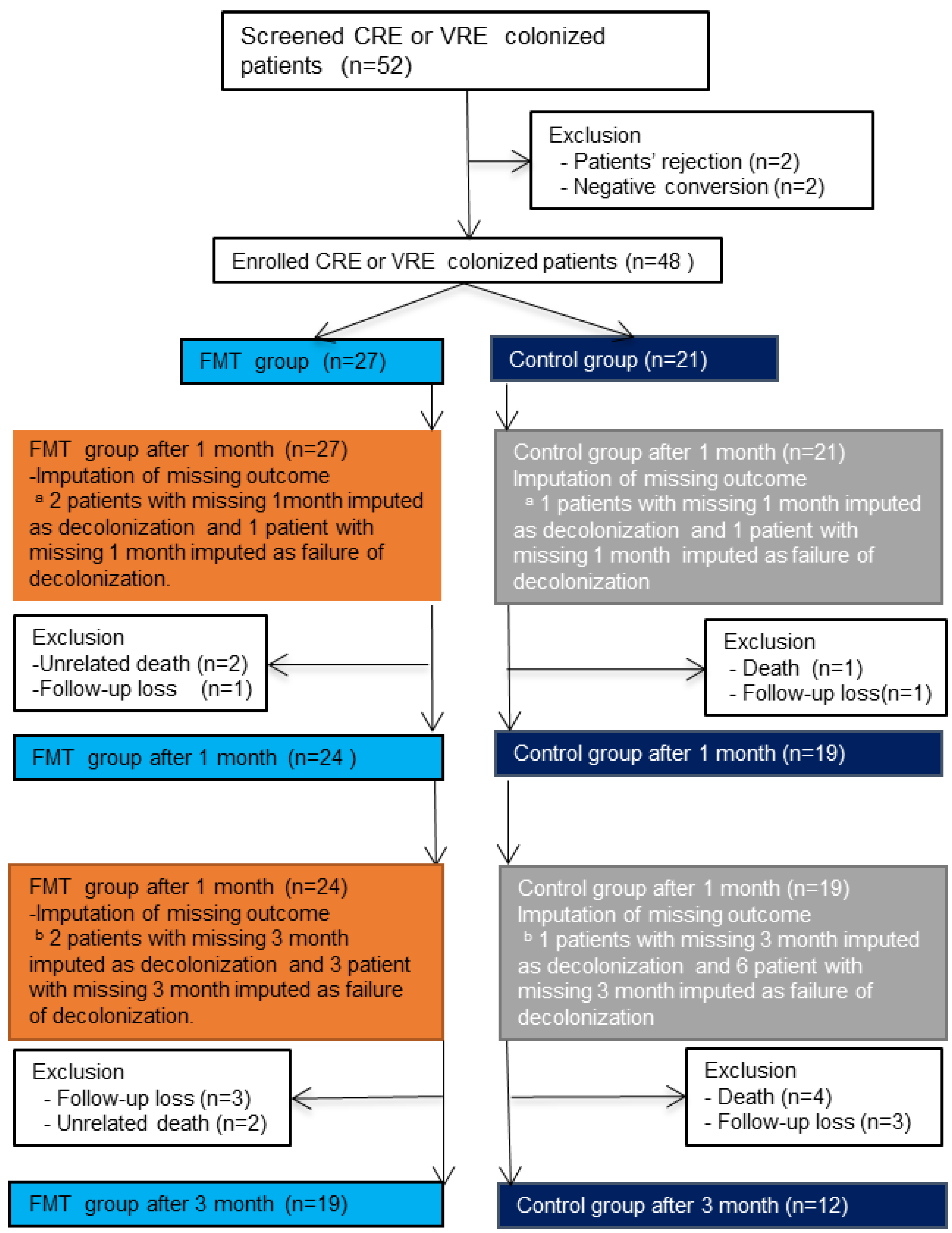
3.2. Withdrawal, Loss to Follow-Up, and Missing Data
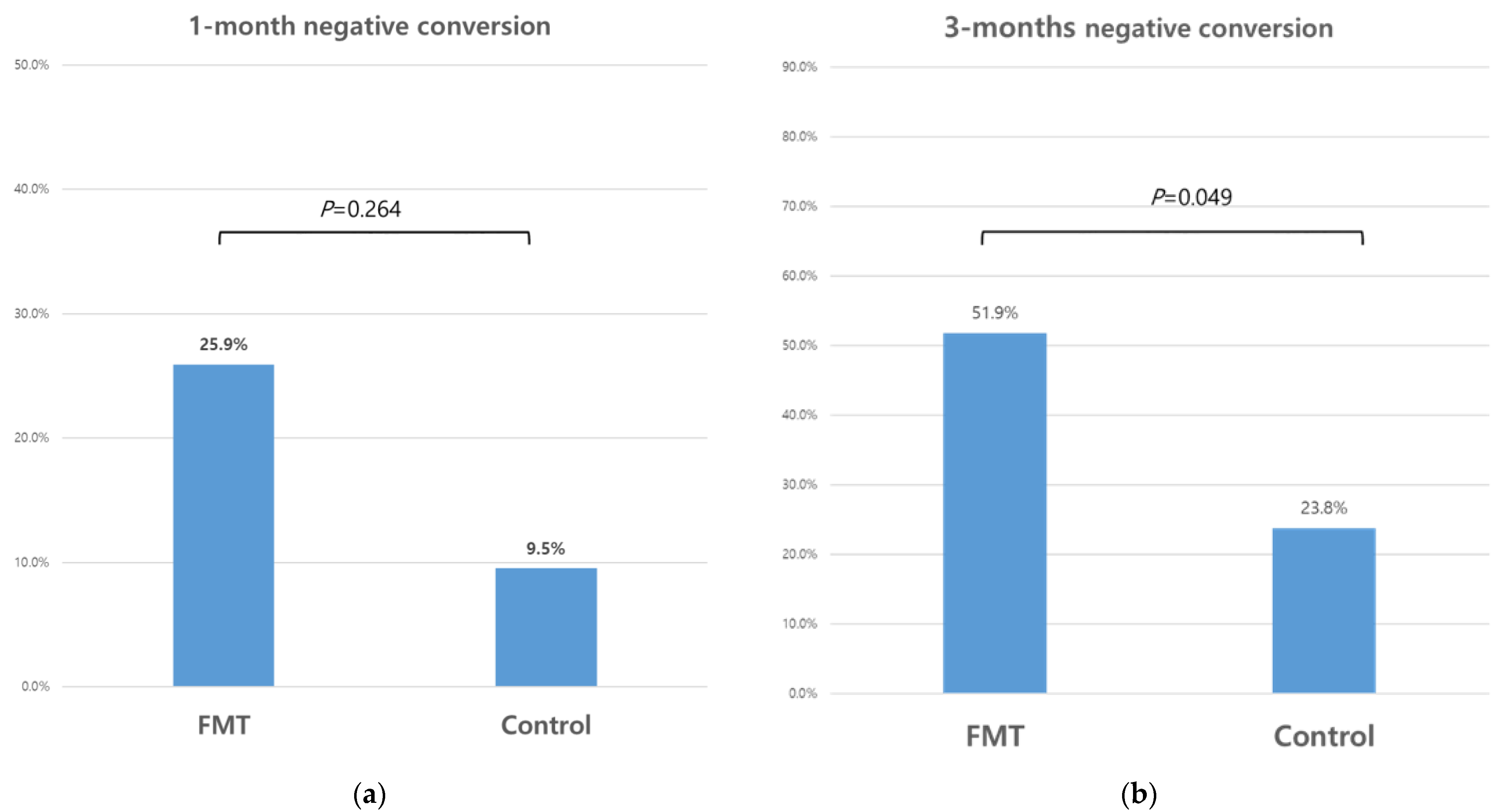
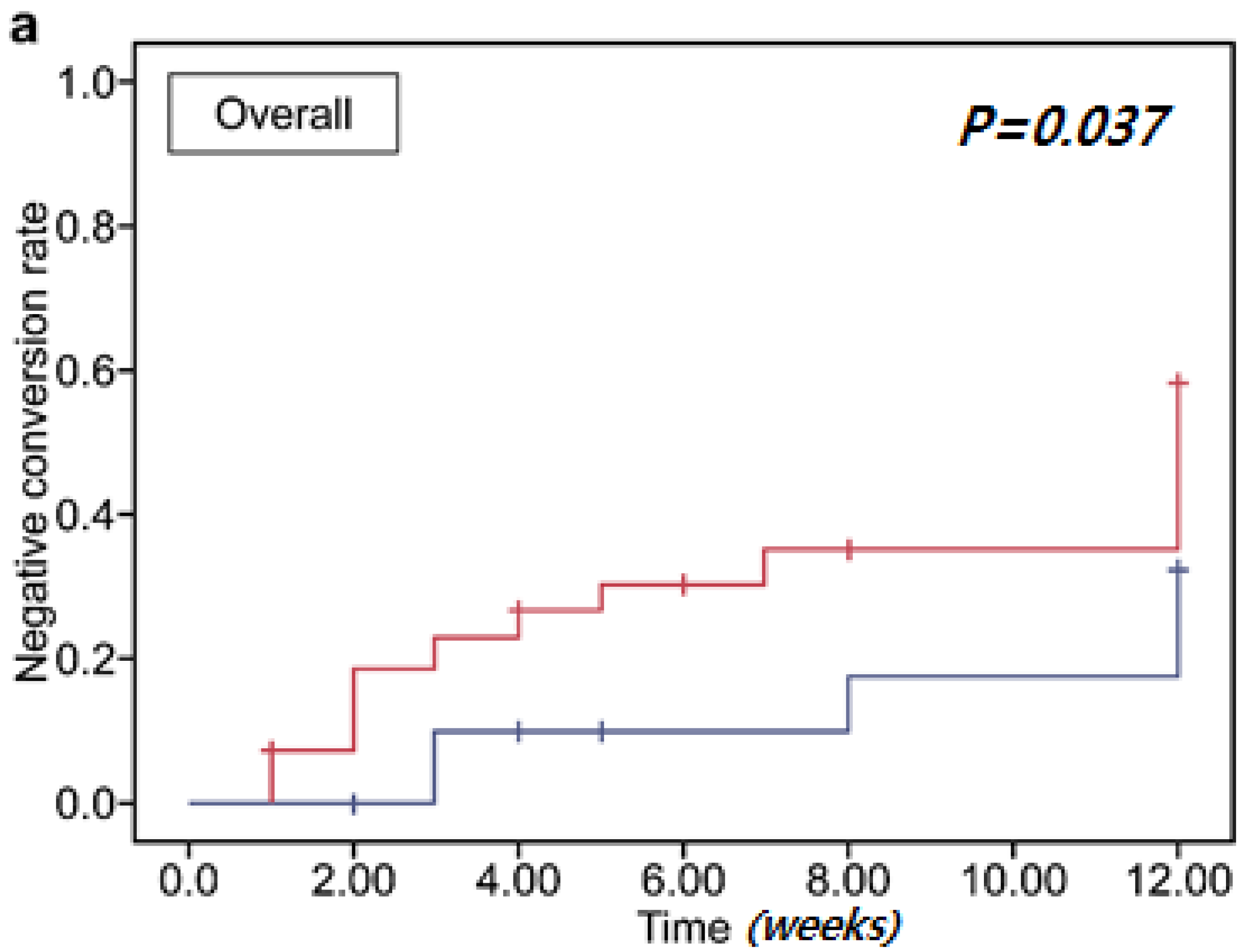
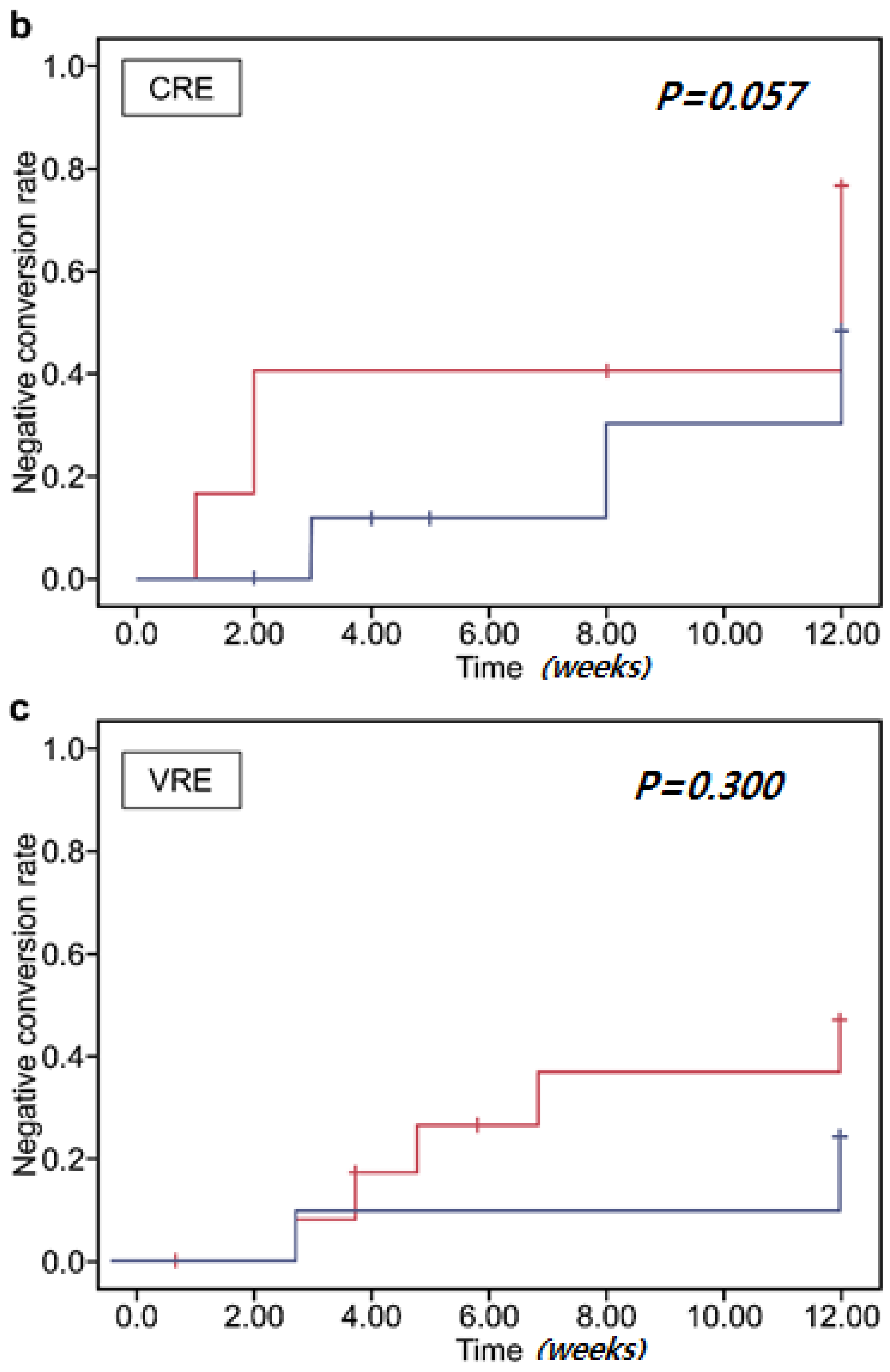
3.3. Clearance of Multidrug-Resistant Organisms
3.4. Clearance of Multidrug-Resistant Organisms Associated with Antibiotics
3.5. Adverse Events
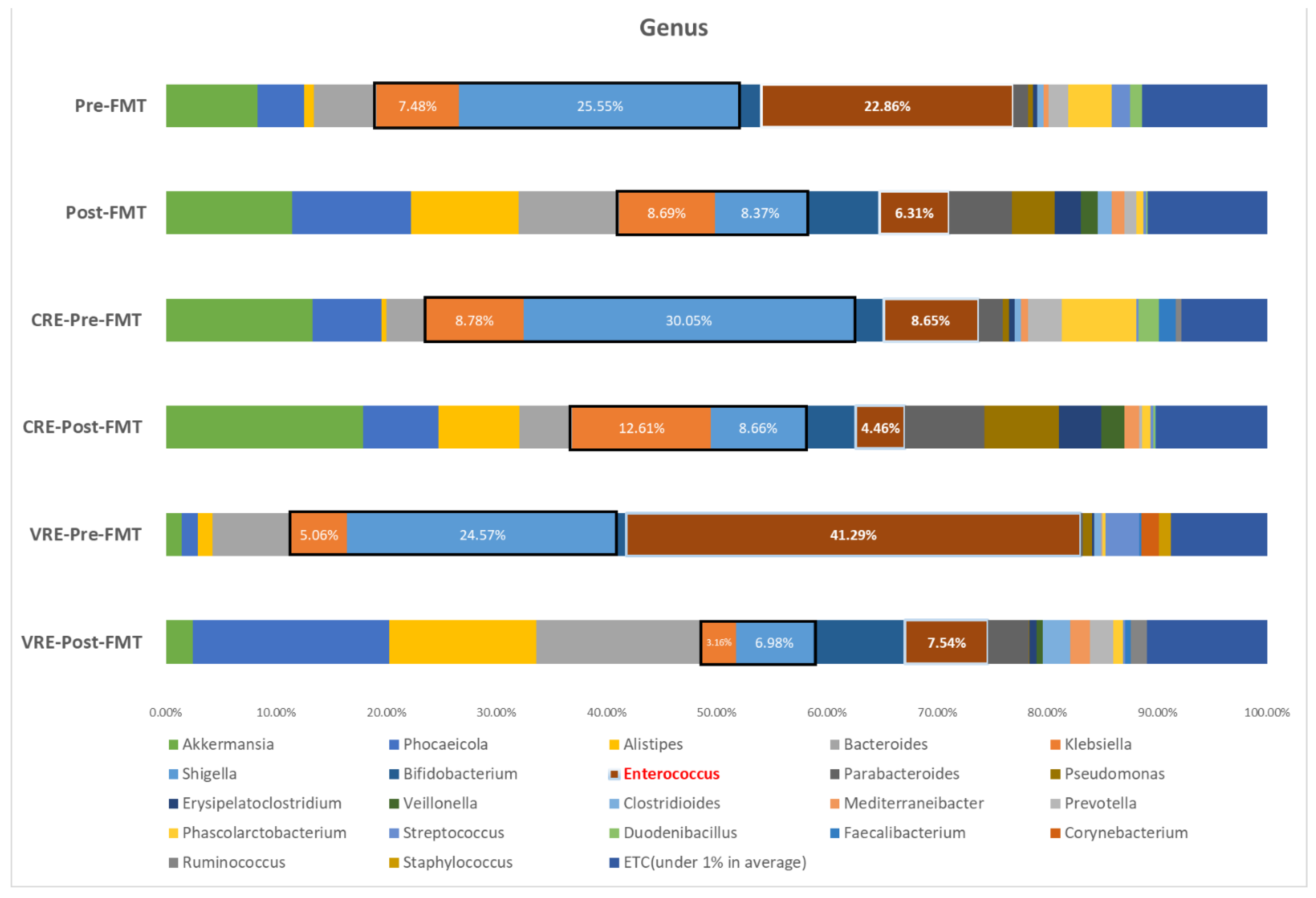
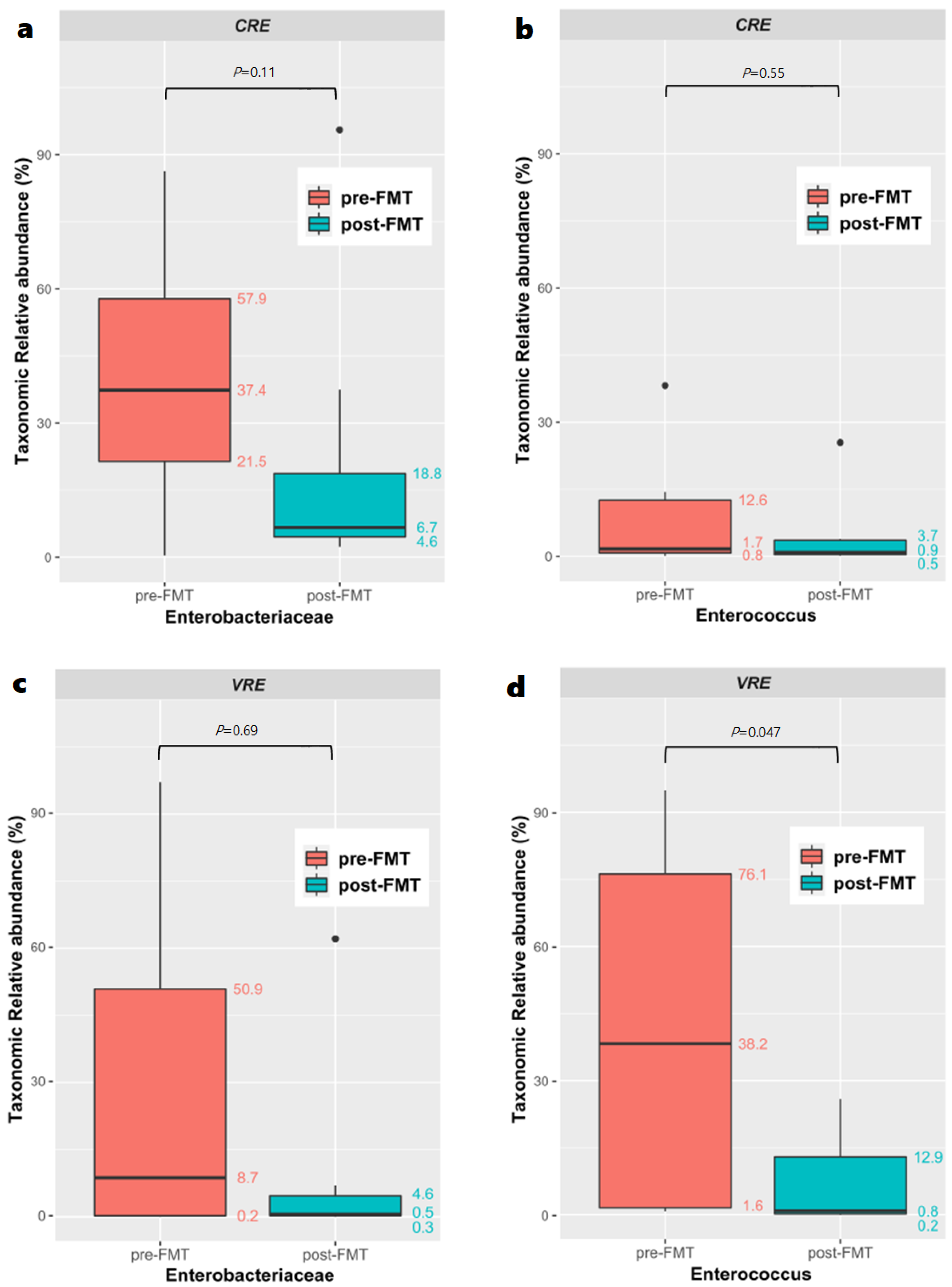
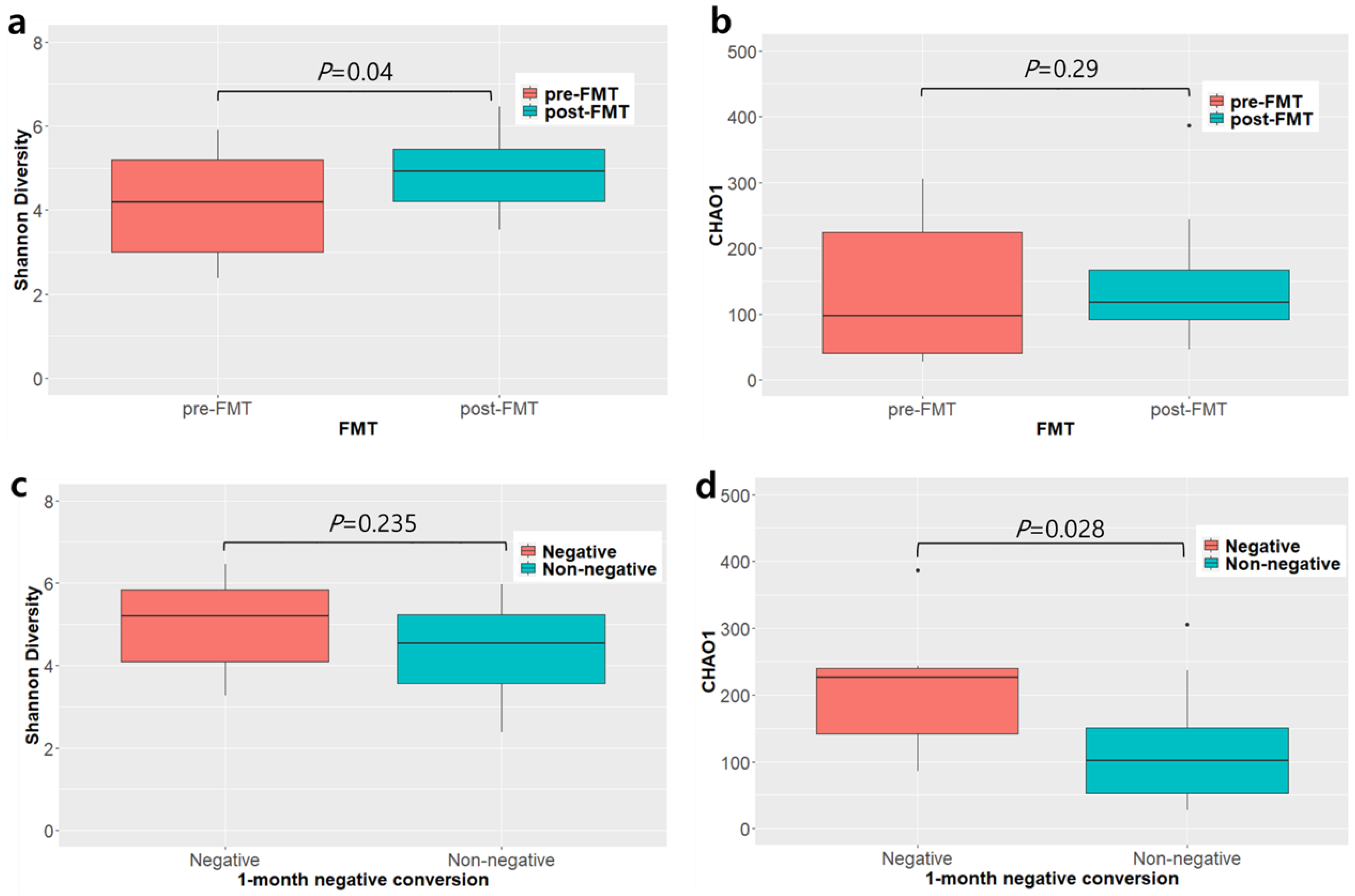
3.6. Microbiome Analysis before and after Fecal Microbiota Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance: 2014 Summary; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Mody, L.; Washer, L.L.; Kaye, K.S.; Gibson, K.; Saint, S.; Reyes, K.; Cassone, M.; Mantey, J.; Cao, J.; Altamimi, S.; et al. Multidrug-resistant organisms in hospitals: What is on patient hands and in their rooms? Clin. Infect. Dis. 2019, 69, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vital signs: Carbapenem-resistant Enterobacteriaceae. Morb. Mortal. Wkly. Rep. 2013, 62, 165–170. [Google Scholar]
- Shi, H.J.; Lee, J.S.; Cho, Y.K.; Eom, J.S. Predictors of mortality in patients with carbapenem-resistant gram-negative bacilli or vancomycin-resistant enterococci bacteremia. Infect. Drug Resist. 2020, 13, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control. Korean Antimicrobial Resistance Monitoring System; 2014 Annual Report; Korea Centers for Disease Control: Cheongju, Korea, 2016.
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef]
- Huttner, B.D.; de Lastours, V.; Wassenberg, M.; Maharshak, N.; Mauris, A.; Galperine, T.; Zanichelli, V.; Kapel, N.; Bellanger, A.; Olearo, F.; et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A randomized clinical trial. Clin. Microbiol. Infect. 2019, 25, 830–838. [Google Scholar] [CrossRef]
- Dinh, A.; Fessi, H.; Duran, C.; Batista, R.; Michelon, H.; Bouchand, F.; Lepeule, R.; Vittecoq, D.; Escaut, L.; Sobhani, I.; et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: A prospective comparative study. J. Hosp. Infect. 2018, 99, 481–486. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Suh, J.W.; Kang, E.J.; Kim, J.Y. Efficacy and safety of fecal microbiota transplantation for decolonization of intestinal multidrug-resistant microorganism carriage: Beyond Clostridioides difficile infection. Ann. Med. 2019, 51, 379–389. [Google Scholar] [CrossRef]
- Davido, B.; Batista, R.; Fessi, H.; Michelon, H.; Escaut, L.; Lawrence, C.; Denis, M.; Perronne, C.; Salomon, J.; Dinh, A. Fecal Microbiota Transplantation to eradicate vancomycin-resistant enterococci colonization in case of an Outbreak. Med. Mal. Infect. 2019, 49, 214–218. [Google Scholar] [CrossRef]
- Saha, S.; Tariq, R.; Tosh, P.K.; Pardi, D.S.; Khanna, S. Faecal microbiota transplantation for eradicating carriage of multidrug-resistant organisms: A systematic review. Clin. Microbiol. Infect. 2019, 25, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; de Groot, P.F.; Geerlings, S.E.; Pardi, D.S.; Khanna, S.; Berge, I.J.M.T.; de Vos, W.M.; Bemelman, F.J.; Nieuwdorp, M. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: A proof of principle study. BMC Res. Notes 2018, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Grzesiowski, P.; Sorensen, N.; Madry, K.; Muszynski, J.; Robak, K.; Wroblewska, M.; Dzieciatkowski, T.; Dulny, G.; Dwilewicz-Trojaczek, J.; et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: Results of a prospective, single-center study. Clin. Infect. Dis. 2017, 65, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Davido, B.; Batista, R.; Michelon, H.; Lepainteur, M.; Bouchand, F.; Lepeule, R.; Salomon, J.; Vittecoq, D.; Duran, C.; Escaut, L.; et al. Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage? J. Hosp. Infect. 2017, 95, 433–437. [Google Scholar] [CrossRef]
- Lee, J.J.; Yong, D.; Suk, K.T.; Kim, D.J.; Woo, H.J.; Lee, S.S.; Kim, B.S. Alteration of gut microbiota in carbapenem-resistant Enterobacteriaceae carriers during fecal microbiota transplantation according to decolonization periods. Microorganisms 2021, 9, 352. [Google Scholar] [CrossRef]
- Seong, H.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Jeong, W.Y.; Lee, W.J.; Sohn, Y.; Cho, Y.; et al. Fecal microbiota transplantation for multidrug-resistant organisms: Efficacy and response prediction. J. Infect. 2020, 81, 719–725. [Google Scholar] [CrossRef]
- Shin, J.Y.; Ko, E.J.; Lee, S.H.; Shin, J.B.; Kim, S.I.; Kwon, K.S.; Kim, H.G.; Shin, Y.W.; Bang, B.W. Refractory pseudomembranous colitis that was treated successfully with colonoscopic fecal microbial transplantation. Intest. Res. 2016, 14, 83–88. [Google Scholar] [CrossRef]
- Manges, A.R.; Steiner, T.S.; Wright, A.J. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: A review. Infect. Dis. 2016, 48, 587–592. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Li, W.; Fu, L.; Niu, B.; Wu, S.; Wooley, J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 2012, 13, 656–668. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Hagerty, S.L.; Hutchison, K.E.; Lowry, C.A.; Bryan, A.D. An empirically derived method for measuring human gut microbiome alpha diversity: Demonstrated utility in predicting health-related outcomes among a human clinical sample. PLoS ONE 2020, 15, e0229204. [Google Scholar] [CrossRef] [PubMed]
- Davido, B.; Moussiegt, A.; Dinh, A.; Bouchand, F.; Matt, M.; Senard, O.; Deconinck, L.; Espinasse, F.; Lawrence, C.; Fortineau, N.; et al. Germs of thrones-spontaneous decolonization of Carbapenem-Resistant Enterobacteriaceae (CRE) and Vancomycin-Resistant Enterococci (VRE) in Western Europe: Is this myth or reality? Antimicrob. Resist. Infect. Control 2018, 7, 100. [Google Scholar] [CrossRef]
- Bar-Yoseph, H.; Hussein, K.; Braun, E.; Paul, M. Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2016, 71, 2729–2739. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, E.S.; Paras, M.L.; Noubary, F.; Walensky, R.P.; Hooper, D.C. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): A systematic review. BMC Infect. Dis. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Drinka, P.; Niederman, M.S.; El-Solh, A.A.; Crnich, C.J. Assessment of risk factors for multi-drug resistant organisms to guide empiric antibiotic selection in long term care: A dilemma. J. Am. Med. Dir. Assoc. 2011, 12, 321–325. [Google Scholar] [CrossRef]
- Buke, C.; Armand-Lefevre, L.; Lolom, I.; Guerinot, W.; Deblangy, C.; Ruimy, R.; Andremont, A.; Lucet, J.C. Epidemiology of multidrug-resistant bacteria in patients with long hospital stays. Infect. Control Hosp. Epidemiol. 2007, 28, 1255–1260. [Google Scholar] [CrossRef]
- Tariq, R.; Hayat, M.; Pardi, D.; Khanna, S. Predictors of failure after fecal microbiota transplantation for recurrent Clostridioides difficile infection: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1383–1392. [Google Scholar] [CrossRef]
- Sohn, K.M.; Cheon, S.; Kim, Y.S. Can fecal microbiota transplantation (FMT) eradicate fecal colonization with vancomycin-resistant enterococci (VRE)? Infect. Control Hosp. Epidemiol. 2016, 37, 1519–1521. [Google Scholar] [CrossRef]
- Saïdani, N.; Lagier, J.C.; Cassir, N.; Million, M.; Baron, S.; Dubourg, G.; Eldin, C.; Kerbaj, J.; Valles, C.; Raoult, D.; et al. Faecal microbiota transplantation shortens the colonisation period and allows re-entry of patients carrying carbapenamase-producing bacteria into medical care facilities. Int. J. Antimicrob. Agents 2019, 53, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2021, 53, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Wang, W.; Cao, X.; Piao, M.; Khan, S.; Yan, F.; Cao, H.; Wang, B. Systematic review: Adverse events of fecal microbiota transplantation. PLoS ONE 2016, 11, e0161174. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Araos, R.; Montgomery, V.; Ugalde, J.A.; Snyder, G.M.; D’Agata, E.M.C. Microbial disruption indices to detect colonization with multidrug-resistant organisms. Infect. Control Hosp. Epidemiol. 2017, 38, 1312–1318. [Google Scholar] [CrossRef]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef]
- Aira, A.; Fehér, C.; Rubio, E.; Soriano, A. The intestinal microbiota as a reservoir and a therapeutic target to fight multi-drug-resistant bacteria: A narrative review of the literature. Infect. Dis. Ther. 2019, 8, 469–482. [Google Scholar] [CrossRef]
- Chanderraj, R.; Brown, C.A.; Hinkle, K.; Falkowski, N.; Ranjan, P.; Dickson, R.P.; Woods, R.J. Gut microbiota predict Enterococcus expansion but not vancomycin-resistant Enterococcus acquisition. mSphere 2020, 5, e00537-20. [Google Scholar] [CrossRef]
- Korach-Rechtman, H.; Hreish, M.; Fried, C.; Gerassy-Vainberg, S.; Azzam, Z.S.; Kashi, Y.; Berger, G. Intestinal dysbiosis in carriers of carbapenem-resistant Enterobacteriaceae. mSphere 2020, 5, e00173-20. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Eysenbach, L.; Allegretti, J.; Aroniadis, O.; Brandt, L.J.; Fischer, M.; Grinspan, A.; Kelly, C.; Morrow, C.; Rodriguez, M.; et al. Microbiome predictors of dysbiosis and VRE decolonization in patients with recurrent C. difficile infections in a multi-center retrospective study. AIMS Microbiol. 2019, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, O.V.; Olekhnovich, E.I.; Sidorenko, S.V.; Moiseev, I.S.; Kucher, M.A.; Fedorov, D.E.; Pavlenko, A.V.; Manolov, A.I.; Gostev, V.V.; Veselovsky, V.A.; et al. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol. 2019, 19, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| FMT Group (N = 27) | Control Group (N = 21) | p-Value | |
|---|---|---|---|
| Age, year, median (IQR) | 73 (63–81) | 80 (73–84) | 0.03 * |
| Sex | 0.422 | ||
| Male | 11 (41) | 11 (52) | |
| Female | 16 (59) | 10 (48) | |
| Multidrug-resistant organism | 0.444 | ||
| CRE | 12 (44) | 10 (48) | |
| VRE | 13 (48) | 11 (52) | |
| CRE + VRE | 2 (7) | 0 (0) | |
| Disease (at admission) | |||
| Pneumonia | 12 (44) | 13 (62) | 0.23 |
| Urinary tract infection | 12 (44) | 7 (33) | 0.435 |
| Clostridium difficile colitis | 11 (41) | 7 (33) | 0.599 |
| Comorbidity | |||
| Diabetes | 0.922 | ||
| Uncomplicated | 5 (19) | 3 (14) | |
| Complicated | 7 (26) | 6 (29) | |
| Liver disease | 0.507 | ||
| Mild | 0 (0) | 1 (5) | |
| Moderate to severe | 1 (4) | 1 (5) | |
| Malignancy | 1 (4) | 3 (14) | 0.188 |
| Chronic kidney disease | 3 (11) | 1 (5) | 0.43 |
| Congestive heart failure | 9 (33) | 6 (29) | 0.724 |
| Peripheral arterial disease | 1 (4) | 0 (0) | 0.373 |
| Chronic obstructive pulmonary disease | 5 (19) | 8 (38) | 0.13 |
| Cerebral vascular accident | 7 (26) | 8 (38) | 0.367 |
| Hemiplegia | 10 (37) | 9 (43) | 0.683 |
| Rheumatic disease | 1 (4) | 1 (5) | 0.856 |
| Dementia | 5 (19) | 3 (14) | 0.696 |
| Peptic ulcer | 2 (7) | 1 (5) | 0.707 |
| Charlson Comorbidity Index, median (IQR) | 2 (1–5) | 3 (2–5) | 0.378 |
| FMT delivery method | |||
| Colonoscopy | 14 (52) | ··· | |
| Duodenoscopy | 1 (4) | ··· | |
| Duodenoscopy + colonoscopy | 12 (44) | ··· | |
| Bowel preparation | 10 (42) | ··· |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Lee, J.-H.; Park, S.-H.; Cha, B.; Kwon, K.S.; Kim, H.; Shin, Y.W. Efficacy and Safety of Fecal Microbiota Transplantation for Clearance of Multidrug-Resistant Organisms under Multiple Comorbidities: A Prospective Comparative Trial. Biomedicines 2022, 10, 2404. https://doi.org/10.3390/biomedicines10102404
Shin J, Lee J-H, Park S-H, Cha B, Kwon KS, Kim H, Shin YW. Efficacy and Safety of Fecal Microbiota Transplantation for Clearance of Multidrug-Resistant Organisms under Multiple Comorbidities: A Prospective Comparative Trial. Biomedicines. 2022; 10(10):2404. https://doi.org/10.3390/biomedicines10102404
Chicago/Turabian StyleShin, Jongbeom, Jung-Hwan Lee, Soo-Hyun Park, Boram Cha, Kye Sook Kwon, Hyungkil Kim, and Yong Woon Shin. 2022. "Efficacy and Safety of Fecal Microbiota Transplantation for Clearance of Multidrug-Resistant Organisms under Multiple Comorbidities: A Prospective Comparative Trial" Biomedicines 10, no. 10: 2404. https://doi.org/10.3390/biomedicines10102404
APA StyleShin, J., Lee, J.-H., Park, S.-H., Cha, B., Kwon, K. S., Kim, H., & Shin, Y. W. (2022). Efficacy and Safety of Fecal Microbiota Transplantation for Clearance of Multidrug-Resistant Organisms under Multiple Comorbidities: A Prospective Comparative Trial. Biomedicines, 10(10), 2404. https://doi.org/10.3390/biomedicines10102404






