A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Micro-CT Imaging
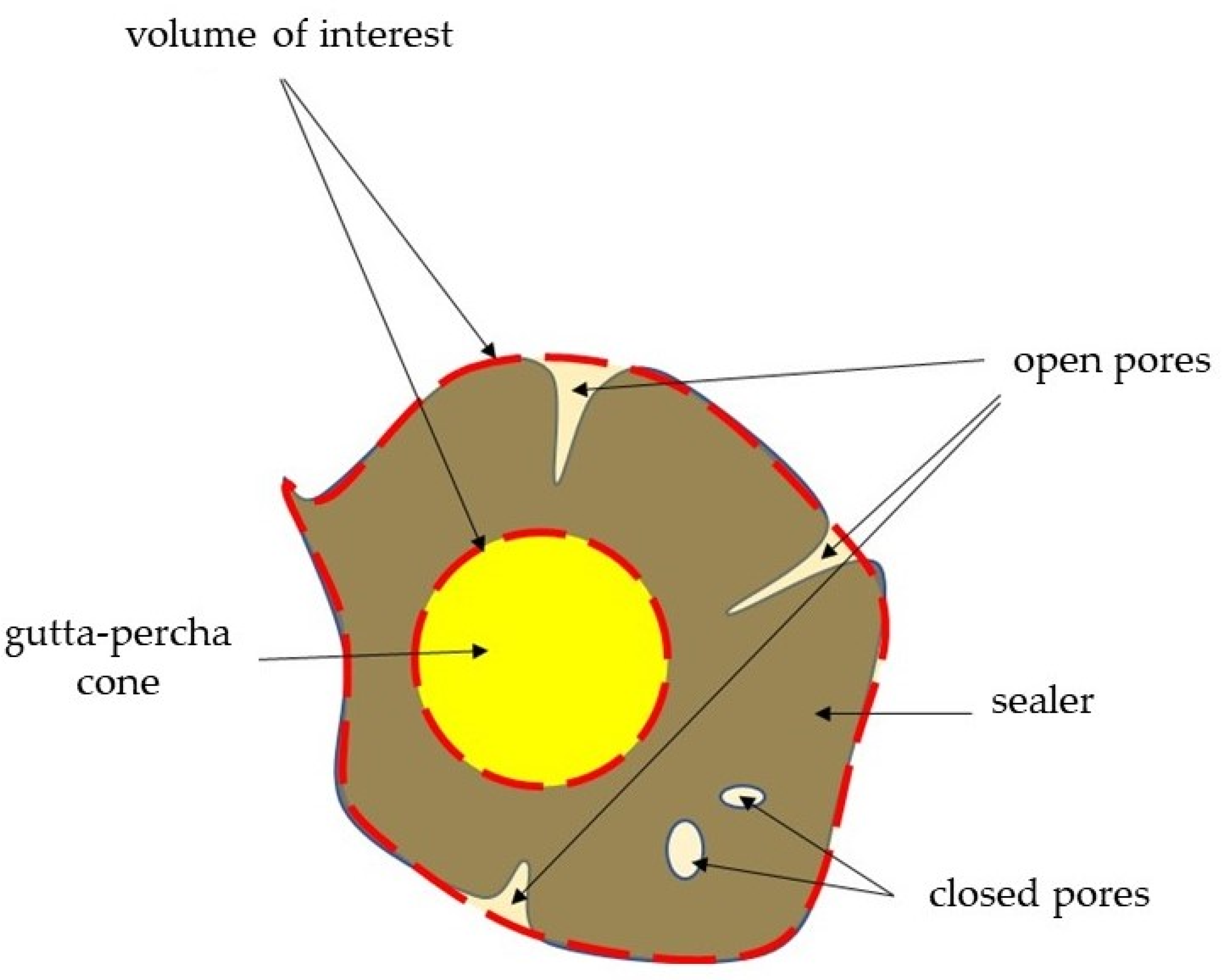
2.2.1. Initial and Long-Term Volume of Pores
2.2.2. Initial and Long-Term Porosity
2.3. Statistical Analysis
3. Results
3.1. Initial and Long-Term Volume of Pores
3.2. Initial and Long-Term Porosity
4. Discussion
5. Conclusions
- The total volume of pores remained unchanged after 6 months of storage.
- GuttaFlow Bioseal exhibited significantly higher long-term volume of open pores than Total Fill BC Sealer.
- The total porosity remained of all investigated sealers unchanged after the 6-month storage except for BioRoot RCS. Total porosity of this materials significantly increased after long-term incubation.
- Initial total porosity of BioRoot RCS was significantly higher in the apical region than in coronal area.
- BioRoot RCS exhibited significantly higher initial total porosity in the apical area than GuttaFlow Bioseal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, M.; Jung, C.; Shin, D.-H.; Cho, Y.-B.; Song, M. Calcium Silicate-Based Root Canal Sealers: A Literature Review. Restor. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef]
- Vishwanath, V.; Rao, H.M. Gutta-Percha in Endodontics—A Comprehensive Review of Material Science. J. Conserv. Dent. 2019, 22, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Alkahtany, M.F.; Almadi, K.H.; Alahmad, F.A.; Alshehri, A.M.; Alswayyed, A.A.; Alzahran, O.M.; Alhadan, A.; Almustafa, A.S.; Vohra, F.; Abduljabbar, T. Influence of Root Canal Sealers and Obturation Techniques on Vertical Root Fracture Resistance. An in Vitro Experiment. Appl. Sci. 2021, 11, 8022. [Google Scholar] [CrossRef]
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef]
- Sfeir, G.; Zogheib, C.; Patel, S.; Giraud, T.; Nagendrababu, V.; Bukiet, F. Calcium Silicate-Based Root Canal Sealers: A Narrative Review and Clinical Perspectives. Materials 2021, 14, 3965. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic Sealers Based on Calcium Silicates: A Systematic Review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef]
- Reszka, P.; Kucharski, Ł.; Klimowicz, A.; Lipski, M. Alkalizing Properties of Selected Calcium-Silicate Root Canal Sealers. An in Vitro Study. Pomeranian J. Life Sci. 2018, 64, 36–41. [Google Scholar]
- Asawaworarit, W.; Pinyosopon, T.; Kijsamanmith, K. Comparison of Apical Sealing Ability of Bioceramic Sealer and Epoxy Resin-Based Sealer Using the Fluid Filtration Technique and Scanning Electron Microscopy. J. Dent. Sci. 2020, 15, 186–192. [Google Scholar] [CrossRef]
- Milanovic, I.; Milovanovic, P.; Antonijevic, D.; Dzeletovic, B.; Djuric, M.; Miletic, V. Immediate and Long-Term Porosity of Calcium Silicate-Based Sealers. J. Endod. 2020, 46, 515–523. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Cardoso, M.L.; Queiroz, T.F.; Silva, E.J.N.L.; Souza, E.M.; De-Deus, G. Suboptimal Push-out Bond Strengths of Calcium Silicate-Based Sealers. Int. Endod. J. 2016, 49, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Peng, B. Assessment of a New Root Canal Sealer’s Apical Sealing Ability. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e79–e82. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Okiji, T. Uptake of Calcium and Silicon Released from Calcium Silicate-Based Endodontic Materials into Root Canal Dentine. Int. Endod. J. 2011, 44, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Festy, F.; Watson, T.F. Dentin-Cement Interfacial Interaction: Calcium Silicates and Polyalkenoates. J. Dent. Res. 2012, 91, 454–459. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial Activity of Endodontic Sealers by Modified Direct Contact Test against Enterococcus Faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.Y.; Aziz, Z. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; de Azevedo Queiroz, Í.O.; Oliveira, P.H.C.D.; Conti, L.C.; Azuma, M.M.; Oliveira, S.H.P.D.; Cintra, L.T.A. Cytotoxicity and Biocompatibility of a New Bioceramic Endodontic Sealer Containing Calcium Hydroxide. Braz. Oral Res. 2019, 33, e042. [Google Scholar] [CrossRef]
- Oh, H.; Kim, E.; Lee, S.; Park, S.; Chen, D.; Shin, S.-J.; Kim, E.; Kim, S. Comparison of Biocompatibility of Calcium Silicate-Based Sealers and Epoxy Resin-Based Sealer on Human Periodontal Ligament Stem Cells. Materials 2020, 13, 5242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Peng, B. Ex Vivo Cytotoxicity of a New Calcium Silicate-Based Canal Filling Material. Int. Endod. J. 2010, 43, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Peng, B. Effects of IRoot SP on Mineralization-Related Genes Expression in MG63 Cells. J. Endod. 2010, 36, 1978–1982. [Google Scholar] [CrossRef]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive Review of Current Endodontic Sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Laranjo, M.; Marto, C.M.; Casalta-Lopes, J.; Serambeque, B.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Carrilho, E.; Botelho, M.F.; Baptista Paula, A.; et al. GuttaFlow(®) Bioseal Cytotoxicity Assessment: In Vitro Study. Molecules 2020, 25, 4297. [Google Scholar] [CrossRef] [PubMed]
- Maciel Pires, P.; Ionescu, A.C.; Pérez-Gracia, M.T.; Vezzoli, E.; Soares, I.P.M.; Brambilla, E.; de Almeida Neves, A.; Sauro, S. Assessment of the Remineralisation Induced by Contemporary Ion-Releasing Materials in Mineral-Depleted Dentine. Clin. Oral Investig. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.S.S.; Ghani, N.R.N.A.; Noorani, T.Y. Physicochemical Properties of Methacrylate Resin, Calcium Hydroxide, Calcium Silicate, and Silicon-Based Root Canal Sealers. J. Stomatol. 2021, 74, 153–159. [Google Scholar] [CrossRef]
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological Aspects of Root Canal Infections and Disinfection Strategies: An Update Review on the Current Knowledge and Challenges. Front. Oral Health 2021, 2, 672887. [Google Scholar] [CrossRef]
- Radwański, M.; Łęski, M.; Puszkarz, A.K.; Krucinska, I. Shaping Ability of ProTaper Next, Hyflex CM, and V-Taper 2H Nickel-Titanium Files in Mandibular Molars: A Micro-Computed Tomographic Study. Iran. Endod. J. 2021, 16, 103–108. [Google Scholar]
- Huang, Y.; Orhan, K.; Celikten, B.; Orhan, A.I.; Tufenkci, P.; Sevimay, S. Evaluation of the Sealing Ability of Different Root Canal Sealers: A Combined SEM and Micro-CT Study. J. Appl. Oral Sci. 2018, 26, e20160584. [Google Scholar] [CrossRef]
- Dammaschke, T. Calcium Silicate-Based Sealers: The End of Thermoplastic Obturation. Dtsch. Zahnärztliche Z. Int. 2021, 3, 71–79. [Google Scholar]
- Atmeh, A.R.; Alharbi, R.; Aljamaan, I.; Alahmari, A.; Shetty, A.C.; Jamleh, A.; Farooq, I. The Effect of Sealer Application Methods on Voids Volume after Aging of Three Calcium Silicate-Based Sealers: A Micro-Computed Tomography Study. Tomography 2022, 8, 778–788. [Google Scholar] [CrossRef]
- Ha, J.-H.; Kim, H.-C.; Kim, Y.K.; Kwon, T.-Y. An Evaluation of Wetting and Adhesion of Three Bioceramic Root Canal Sealers to Intraradicular Human Dentin. Materials 2018, 11, 1286. [Google Scholar] [CrossRef]
- Trope, M.; Bunes, A.; Debelian, G. Root Filling Materials and Techniques: Bioceramics a New Hope? Endod. Top. 2015, 32, 86–96. [Google Scholar] [CrossRef]
- Angerame, D.; De Biasi, M.; Pecci, R.; Bedini, R. Filling Ability of Three Variants of the Single-Cone Technique with Bioceramic Sealer: A Micro-Computed Tomography Study. J. Mater. Sci. Mater. Med. 2020, 31, 91. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.A.; Bakhsh, A. Association between Endodontic Infection, Its Treatment and Systemic Health: A Narrative Review. Medicina 2022, 58, 931. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.W. A Comparison of Canal Preparations in Straight and Curved Root Canals. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef]
- Pirani, C.; Camilleri, J. Effectiveness of Root Canal Filling Materials and Techniques for Treatment of Apical Periodontitis—A Systematic Review. Int. Endod. J. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lyu, W.-J.; Bai, W.; Wang, X.-Y.; Liang, Y.-H. Physicochemical Properties of a Novel Bioceramic Silicone-Based Root Canal Sealer. J. Dent. Sci. 2022, 17, 831–835. [Google Scholar] [CrossRef]
- Ja’apar, S.A.N.; Ichwan, S.J.A.; Mustaffa, M. Biocompatibility of Bioceramic Root Canal Sealers: A Review. J. Biomed. Clin. Sci. 2021, 6, 26–39. [Google Scholar]
- Bianco, E.; Calvelli, C.; Citterio, C.L.; Pellegatta, A.; Venino, P.M.; Maddalone, M. Evaluation with Micro-CT of the Canal Seal Made with Two Different Bioceramic Cements: GuttaFlow Bioseal and BioRoot RCS. J. Contemp. Dent. Pract. 2020, 21, 359–366. [Google Scholar]
- Saygili, G.; Saygili, S.; Tuglu, I.; Davut Capar, I. In Vitro Cytotoxicity of GuttaFlow Bioseal, GuttaFlow 2, AH-Plus and MTA Fillapex. Iran. Endod. J. 2017, 12, 354–359. [Google Scholar] [CrossRef]
- Singla, M.; Panghal, S. Comparative Evaluation of Sealing Ability of Three Bioactive Obturation Materials: A Bacterial Leakage Study. Endodontology 2021, 33, 170–175. [Google Scholar] [CrossRef]
- Uyanik, M.O.; Nagas, E.; Cubukcu, H.E.; Dagli, F.; Cehreli, Z.C. Surface Porosity of Hand-Mixed, Syringe-Mixed and Encapsulated Set Endodontic Sealers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e117–e122. [Google Scholar] [CrossRef]
- Jafari, F.; Jafari, S. Composition and Physicochemical Properties of Calcium Silicate Based Sealers: A Review Article. J. Clin. Exp. Dent. 2017, 9, e1249–e1255. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Jafari, S. Importance and Methodologies of Endodontic Microleakage Studies: A Systematic Review. J. Clin. Exp. Dent. 2017, 9, e812–e819. [Google Scholar] [CrossRef] [PubMed]
- Germain, S.; Meetu, K.; Issam, K.; Alfred, N.; Carla, Z. Impact of the Root Canal Taper on the Apical Adaptability of Sealers Used in a Single-Cone Technique: A Micro-Computed Tomography Study. J. Contemp. Dent. Pract. 2018, 19, 808–815. [Google Scholar] [PubMed]
- Tabassum, S.; Khan, F.R. Failure of Endodontic Treatment: The Usual Suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef]
- Drukteinis, S.; Peciuliene, V.; Shemesh, H.; Tusas, P.; Bendinskaite, R. Porosity Distribution in Apically Perforated Curved Root Canals Filled with Two Different Calcium Silicate Based Materials and Techniques: A Micro-Computed Tomography Study. Materials 2019, 12, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viapiana, R.; Moinzadeh, A.T.; Camilleri, L.; Wesselink, P.R.; Tanomaru Filho, M.; Camilleri, J. Porosity and Sealing Ability of Root Fillings with Gutta-Percha and BioRoot RCS or AH Plus Sealers. Evaluation by Three Ex Vivo Methods. Int. Endod. J. 2016, 49, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Iglecias, E.F.; Freire, L.G.; de Miranda Candeiro, G.T.; Dos Santos, M.; Antoniazzi, J.H.; Gavini, G. Presence of Voids after Continuous Wave of Condensation and Single-Cone Obturation in Mandibular Molars: A Micro-Computed Tomography Analysis. J. Endod. 2017, 43, 638–642. [Google Scholar] [CrossRef]
- Alshehri, M.; Alamri, H.M.; Alshwaimi, E.; Kujan, O. Micro-Computed Tomographic Assessment of Quality of Obturation in the Apical Third with Continuous Wave Vertical Compaction and Single Match Taper Sized Cone Obturation Techniques. Scanning 2016, 38, 352–356. [Google Scholar] [CrossRef]
- Huang, Y.; Celikten, B.; de Faria Vasconcelos, K.; Ferreira Pinheiro Nicolielo, L.; Lippiatt, N.; Buyuksungur, A.; Jacobs, R.; Orhan, K. Micro-CT and Nano-CT Analysis of Filling Quality of Three Different Endodontic Sealers. Dentomaxillofac. Radiol. 2017, 46, 20170223. [Google Scholar] [CrossRef]
- Wang, Z.; Maezono, H.; Shen, Y.; Haapasalo, M. Evaluation of Root Canal Dentin Erosion after Different Irrigation Methods Using Energy-Dispersive X-Ray Spectroscopy. J. Endod. 2016, 42, 1834–1839. [Google Scholar] [CrossRef]
- Simezo, A.P.; da Silveira Bueno, C.E.; Cunha, R.S.; Pelegrine, R.A.; Rocha, D.G.P.; de Martin, A.S.; Kato, A.S. Comparative Analysis of Dentinal Erosion after Passive Ultrasonic Irrigation versus Irrigation with Reciprocating Activation: An Environmental Scanning Electron Study. J. Endod. 2017, 43, 141–146. [Google Scholar] [CrossRef]
- de Camargo, R.V.; Silva-Sousa, Y.T.C.; da Rosa, R.P.F.; Mazzi-Chaves, J.F.; Lopes, F.C.; Steier, L.; Sousa-Neto, M.D. Evaluation of the Physicochemical Properties of Silicone and Epoxy Resin-Based Root Canal Sealers. Braz. Oral Res. 2017, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Amin, A.; Luzi, A.; Giovarruscio, M.; Paolone, G.; Darvizeh, A.; Agulló, V.V.; Sauro, S. Innovative Root-End Filling Materials Based on Calcium-Silicates and Calcium-Phosphates. J. Mater. Sci. Mater. Med. 2017, 28, 31. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Jimeno, E.B. Analysis of the Porosity of Endodontic Sealers through Micro-Computed Tomography: A Systematic Review. J. Conserv. Dent. 2018, 21, 238–242. [Google Scholar] [CrossRef]
- Urban, K.; Neuhaus, J.; Donnermeyer, D.; Schäfer, E.; Dammaschke, T. Solubility and PH Value of 3 Different Root Canal Sealers: A Long-Term Investigation. J. Endod. 2018, 44, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Prüllage, R.-K.; Urban, K.; Schäfer, E.; Dammaschke, T. Material Properties of a Tricalcium Silicate-Containing, a Mineral Trioxide Aggregate-Containing, and an Epoxy Resin-Based Root Canal Sealer. J. Endod. 2016, 42, 1784–1788. [Google Scholar] [CrossRef]
- Sisli, S.N.; Ozbas, H. Comparative Micro-Computed Tomographic Evaluation of the Sealing Quality of ProRoot MTA and MTA Angelus Apical Plugs Placed with Various Techniques. J. Endod. 2017, 43, 147–151. [Google Scholar] [CrossRef]
- Duque, J.A.; Fernandes, S.L.; Bubola, J.P.; Duarte, M.A.H.; Camilleri, J.; Marciano, M.A. The Effect of Mixing Method on Tricalcium Silicate-Based Cement. Int. Endod. J. 2018, 51, 69–78. [Google Scholar] [CrossRef]
- Hammad, M.; Qualtrough, A.; Silikas, N. Evaluation of Root Canal Obturation: A Three-Dimensional in Vitro Study. J. Endod. 2009, 35, 541–544. [Google Scholar] [CrossRef]
- Keleş, A.; Alcin, H.; Kamalak, A.; Versiani, M.A. Micro-CT Evaluation of Root Filling Quality in Oval-Shaped Canals. Int. Endod. J. 2014, 47, 1177–1184. [Google Scholar] [CrossRef]
- Suciu, I.; Dimitriu, B.; Ciocardel, M.; Chirila, M.; Amza, O.; Scarlatescu, S.; Preoteasa, C.; Grigorie, M.; Voiculeanu, M. Evaluation of the Sealer/Gutta-Percha Ratio on Sets of Root Section Surfaces of Some Extracted Teeth Sealed Using the Cold Lateral Condensation Technique. J. Med. Life 2021, 14, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Boutsioukis, C.; Arias-Moliz, M.T. Present Status and Future Directions—Irrigants and Irrigation Methods. Int. Endod. J. 2022, 55 (Suppl. 3), 588–612. [Google Scholar] [CrossRef]




| Root Canal Sealer | Manufacturer | Type of the Sealer | Presentation | Composition |
|---|---|---|---|---|
| GuttaFlow Bioseal | Coltène/Whaledent AG, Altstätten, Switzerland | Silicone-based with calcium silicate | 2 pastes in a double syringe with a self-mixing applicator | Gutta-percha powder particles, polydimethylsiloxane, platinum catalyst, zirconium dioxide, calcium silicate, nano-silver particles, coloring, and bioactive glass ceramic |
| Total Fill BC Sealer | FKG Dentaire, La Chaux-de-Fonds, Switzerland | Calcium–silicate 1 component | Injectable, pre-mixed single syringe | Calcium silicates, calcium phosphate monobasic, zirconium oxide, tantalum oxide, and thickening agents |
| BioRoot RCS | Septodont, Saint Maur Des Fosses, France | Calcium-silicate 2 components | Manually mixed powder and pre-portioned liquid ampules | Powder: Tricalcium silicate, zirconium oxide, and povidone Liquid: Aqueous solution of calcium chloride and polycarboxylate |
| Group | Volume of Open Pores (mm3) | Volume of Closed Pores (mm3) | Total Volume of Pores (mm3) | Percentage Volume of Open Pores (%) | Percentage Volume of Closed Pores (%) |
|---|---|---|---|---|---|
| GuttaFlow Bioseal | 0.032 ± 0.035 | 0.175 ± 0.148 | 0.210 ± 0.150 | 19.620 ± 22.530 | 80.380 ± 22.530 |
| Total Fill BC Sealer | 0.007 ± 0.015 | 0.071 ± 0.046 | 0.078 ± 0.050 | 7.330 ± 12.190 | 92.670 ± 12.190 |
| BioRoot RCS | 0.036 ± 0.052 | 0.091 ± 0.088 | 0.127 ± 0.080 | 27.120 ± 36.500 | 72.880 ± 36.500 |
| Group | Volume of Open Pores (mm3) | Volume of Closed Pores (mm3) | Total Volume of Pores (mm3) | Percentage Volume of Open Pores (%) | Percentage Volume of Closed Pores (%) |
|---|---|---|---|---|---|
| GuttaFlow Bioseal | 0.073 ± 0.070 A | 0.123 ± 0.159 | 0.197 ± 0.136 | 44.370 ± 34.400 | 55.630 ± 34.400 |
| Total Fill BC Sealer | 0.010 ± 0.019 A | 0.047 ± 0.033 | 0.057 ± 0.041 | 11.340 ± 18.290 | 88.660 ± 18.290 |
| BioRoot RCS | 0.055 ± 0.060 | 0.113 ± 0.147 | 0.168 ± 0.160 | 31.270 ± 31.570 | 68.730 ± 31.570 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwanski, M.; Leski, M.; Puszkarz, A.K.; Sokolowski, J.; Hardan, L.; Bourgi, R.; Sauro, S.; Lukomska-Szymanska, M. A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers. Biomedicines 2022, 10, 2403. https://doi.org/10.3390/biomedicines10102403
Radwanski M, Leski M, Puszkarz AK, Sokolowski J, Hardan L, Bourgi R, Sauro S, Lukomska-Szymanska M. A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers. Biomedicines. 2022; 10(10):2403. https://doi.org/10.3390/biomedicines10102403
Chicago/Turabian StyleRadwanski, Mateusz, Michal Leski, Adam K. Puszkarz, Jerzy Sokolowski, Louis Hardan, Rim Bourgi, Salvatore Sauro, and Monika Lukomska-Szymanska. 2022. "A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers" Biomedicines 10, no. 10: 2403. https://doi.org/10.3390/biomedicines10102403
APA StyleRadwanski, M., Leski, M., Puszkarz, A. K., Sokolowski, J., Hardan, L., Bourgi, R., Sauro, S., & Lukomska-Szymanska, M. (2022). A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers. Biomedicines, 10(10), 2403. https://doi.org/10.3390/biomedicines10102403










