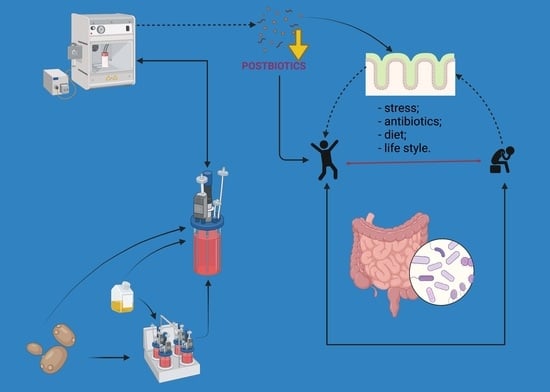
Mineral-Enriched Postbiotics: A New Perspective for Microbial Therapy to Prevent and Treat Gut Dysbiosis
Abstract
:1. Introduction
2. The Health Benefit of Postbiotics and Their Applications in Microbial Therapy to Prevent and Treat Gut Dysbiosis
2.1. Postbiotic Concept
- Postbiotic—“compounds derived from microbial metabolism synthesized by cells or produced in the matrix by enzymatic action”;
- Paraprobiotic—“inactivated microbial cells (non-viable), specifically, cells as a whole, including both structural components and synthesized or excreted metabolites that confer a health benefit to the consumer” [7].
2.2. Postbiotic Advantages and Beneficial Effects on Human and Animal Health
3. The Health Benefit of Mineral-Enriched Biomass and Their Applications in Microbial Therapy to Maintain Eubiosis and Improve Mineral Bioavailability
3.1. The Impact of Micronutrients on Human Health
3.2. Mineral-Enriched Biomass Obtaining and Advantages
3.3. Minerals—Gut Microbiome Relationship and the Beneficial Effects of Mineral-Enriched Biomass on Human and Animal Health
4. Mineral-Enriched Postbiotics and Their Applications in Microbial Therapy to Prevent and Treat Gut Dysbiosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, S.; Thukral, S.K.; Kaur, P.; Samota, M.K. Perturbations Associated with Hungry Gut Microbiome and Postbiotic Perspectives to Strengthen the Microbiome Health. Future Foods 2021, 4, 100043. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step beyond Pre- and Probiotics. Nutrients 2020, 23, 2189. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Available online: www.marketsandmarkets.com (accessed on 23 August 2022).
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef]
- Koleilat, A. Beyond probiotics the Postbiotics. Gastroenterol. Hepatol. 2019, 10, 2189. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Reply to: Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, G.H.; Cho, H. Postbiotics for cancer prevention and treatment. Korean J. Microbiol. 2021, 57, 142–153. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Wu, C. Microbiota and Tuberculosis: A Potential Role of Probiotics, and Postbiotics. Front. Nutr. 2021, 8, 626254. [Google Scholar] [CrossRef] [PubMed]
- Mantziari, A.; Salminen, S.; Szajewska, H.; Malagón-Rojas, J.N. Postbiotics against pathogens commonly involved in pediatric infectious diseases. Microorganisms 2020, 8, 1510. [Google Scholar] [CrossRef]
- Mack, I.; Schwille-Kiuntke, J.; Mazurak, N.; Niesler, B.; Zimmermann, K.; Mönnikes, H.; Enck, P. A Nonviable Probiotic in Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1039–1047. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.J.; Shin, H.Y.; Suh, H.J.; Choi, H.S. Lactobacillus plantarum K8-based paraprobiotics suppress lipid accumulation during adipogenesis by the regulation of JAK/STAT and AMPK signaling pathways. J. Funct. Foods 2021, 87, 104824. [Google Scholar] [CrossRef]
- Chang, H.M.; Foo, H.L.; Loh, T.C.; Lim, E.T.C.; Abdul Mutalib, N.E. Comparative Studies of Inhibitory and Antioxidant Activities, and Organic Acids Compositions of Postbiotics Produced by Probiotic Lactiplantibacillus plantarum Strains Isolated from Malaysian Foods. Front. Vet. Sci. 2021, 7, 602280. [Google Scholar] [CrossRef]
- Sevin, S.; Karaca, B.; Haliscelik, O.; Kibar, H.; OmerOglou, E.; Kiran, F. Postbiotics secreted by Lactobacillus sakei EIR/CM-1 isolated from cow milk microbiota, display antibacterial and antibiofilm activity against ruminant mastitis-causing pathogens. Ital. J. Anim. Sci. 2021, 20, 1302–1316. [Google Scholar] [CrossRef]
- Moradi, M.; Mardani, K.; Tajik, H. Characterization and application of postbiotics of Lactobacillus spp. on Listeria monocytogenes in vitro and in food models. LWT 2021, 111, 457–464. [Google Scholar] [CrossRef]
- Haileselassie, Y.; Navis, M.; Vu, N.; Qazi, K.R.; Rethi, B.; Sverremark-Ekström, E. Postbiotic modulation of retinoic acid imprinted mucosal-like dendritic cells by probiotic Lactobacillus reuteri 17,938 in vitro. Front. Immunol. 2016, 7, 96. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; Xiao, L.; Wehkamp, T.; van Ark, I.; Hoogendoorn, E.J.; Leusink-Muis, T.; Folkerts, G.; Garssen, J.; Willemsen, L.E.M.; Van’T Land, B. A fermented milk matrix containing postbiotics supports th1-and th17-type immunity in vitro and modulates the influenza-specific vaccination response in vivo in association with altered serum galectin ratios. Vaccines 2021, 9, 254. [Google Scholar] [CrossRef]
- Trindade, L.M.; Torres, L.; Matos, I.D.; Miranda, V.C.; de Jesus, L.C.L.; Cavalcante, G.; de Souza Oliveira, J.J.; Cassali, G.D.; Mancha-Agresti, P.; de Carvalho Azevedo, V.A.; et al. Paraprobiotic Lacticaseibacillus rhamnosus Protects Intestinal Damage in an Experimental Murine Model of Mucositis. Prob. Antimicrob. Prot. 2021. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.M.; Lescura, C.M.; Milhan, N.V.M.; Ribeiro, J.L.; Silva, F.A.; Anbinder, A.L. Live and heat-killed Lactobacillus reuteri reduce alveolar bone loss on induced periodontitis in rats. Archiv. Oral Biol. 2020, 119, 104894. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Kogut, M.H.; Genovese, K.; He, H.; Kazemi, S.; Arsenault, R.J. Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms 2019, 7, 268. [Google Scholar] [CrossRef]
- Rigo-Adrover, M.D.M.; Knipping, K.; Garssen, J.; van Limpt, K.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Prevention of rotavirus diarrhea in suckling rats by a specific fermented milk concentrate with prebiotic mixture. Nutrients 2019, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M. Postbiotic L. plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Samsudin, A.A. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Asmara, S.A.; Akit, H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 2017, 96, 966–975. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary supplementation of postbiotics mitigates adverse impacts of heat stress on antioxidant enzyme activity, total antioxidant, lipid peroxidation, physiological stress indicators, lipid profile and meat quality in broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [Green Version]
- Montazeri-Najafabady, N.; Ghasemi, Y.; Dabbaghmanesh, M.H.; Ashoori, Y.; Talezadeh, P.; Koohpeyma, F.; Abootalebi, S.N.; Gholami, A. Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Complement. Med. Therap. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Almada, C.N.; Almada-Érix, C.N.; Bonatto, M.S.; Pradella, F.; dos Santos, P.; Abud, Y.K.D.; Farias, A.S.; Martínez, J.; Sant’Anna Filho, C.B.; Lollo, P.C.; et al. Obtaining paraprobiotics from Lactobacilus acidophilus, Lacticaseibacillus casei and Bifidobacterium animalis using six inactivation methods: Impacts on the cultivability, integrity, physiology, and morphology. J. Funct. Foods 2021, 87, 104826. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Olmo, M.; Araña, M.; Urtasun, R.; Encio, I.J.; Barajas, M. Role of postbiotics in diabetes mellitus: Current knowledge and future perspectives. Foods 2021, 10, 1590. [Google Scholar] [CrossRef]
- Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Zavoshti, H.F.; Abbasi, A. Postbiotics as promising tools for cancer adjuvant therapy. Adv. Pharm. Bull. 2021, 11, 1. [Google Scholar] [CrossRef]
- Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Zavoshti, H.F.; Abbasi, A. Postbiotics as novel health-promoting ingredients in functional foods. Health Promot. Perspect. 2020, 10, 3–4. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: Modulation of the brain–gut–microbiome axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- De Brito, L.P.; da Silva Júnior, J.N.; de Barros, P.D.S.; da Silva, E.C.; Calaça, P.R.d.A.; Soares, M.T.C.V.; Porto, A.L.F. Can postbiotics show antiviral effects against Sars-CoV-2? Res. Soc. Dev. 2021, 10, e14610817259. [Google Scholar] [CrossRef]
- Hadadi, N.; Berweiler, V.; Wang, H.; Trajkovski, M. Intestinal microbiota as a route for micronutrient bioavailability. Curr. Opin. Endocr. Metab. Res. 2021, 20, 100285. [Google Scholar] [CrossRef]
- van den Broek, T.J.; Kremer, B.; Marcondes Rezende, M.; Hoevenaars, F.; Weber, P.; Hoeller, U.; van Ommen, B.; Wopereis, S. The impact of micronutrient status on health: Correlation network analysis to understand the role of micronutrients in metabolic-inflammatory processes regulating homeostasis and phenotypic flexibility. Genes Nutr. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Peña-Rosas, J.P.; Mithra, P.; Unnikrishnan, B.; Kumar, N.; De-Regil, L.M.; Nair, N.S.; Garcia-Casal, M.N.; Solon, J.A. Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition. Cochrane Database Syst. Rev. 2019, 10, CD009902. [Google Scholar] [CrossRef] [PubMed]
- Field, M.S.; Mithra, P.; Peña-Rosas, J.P. Wheat flour fortification with iron and other micronutrients for reducing anaemia and improving iron status in populations. Cochrane Database Syst. Rev. 2021, 1, CD011302. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Jefferds, M.; Peña-Rosas, J.P. Point-of-use fortification of foods with micronutrient powders containing iron in children of preschool and school-age. Cochrane Database Syst. Rev. 2017, 11, CD009666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, L.; Man, Q.; Wang, J.; Zhao, W.; Zhang, J. Dietary Micronutrients Intake Status among Chinese Elderly People Living at Home: Data from CNNHS 2010–2012. Nutrients 2019, 11, 1787. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S.A. Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Arruda de Souza Monnerat, J.; Ribeiro de Souza, P.; Monteiro da Fonseca Cardoso, L.; Dario Mattos, J.; de Souza Rocha, G.; Frauches Medeiros, R. Micronutrients and bioactive compounds in the immunological pathways related to SARS-CoV-2 (adults and elderly). Eur. J. Nutr. 2021, 60, 559–579. [Google Scholar] [CrossRef]
- Keflie, T.S.; Biesalski, H.K. Micronutrients and bioactive substances: Their potential roles in combating COVID-19. Nutrition 2021, 84, 111103. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Mejía-Caballero, A.; Salas-Villagrán, V.A.; Jiménez-Serna, A.; Farrés, A. Challenges in the production and use of probiotics as therapeuticals in cancer treatment or prevention. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab052. [Google Scholar] [CrossRef]
- Mörschbächer, A.P.; Dullius, A.; Dullius, C.H.; Brandt, C.R.; Kuhn, D.; Brietzke, D.T.; José Malmann Kuffel, F.; Etgeton, H.P.; Altmayer, T.; Gonçalves, T.E.; et al. Assessment of selenium bioaccumulation in lactic acid bacteria. J. Dairy Sci. 2018, 101, 10626–10635, Erratum in J. Dairy Sci. 2019, 102, 1883. [Google Scholar] [CrossRef]
- Kitamura, D.H.; Porto de Souza Vandenberghe, L.; Rodrigues, C. Selenium-Enriched Probiotic Saccharomyces boulardii CCT 4308 Biomass Production Using Low-Cost Sugarcane Molasses Medium. Braz. Arch. Biol. Technol. 2021, 64S. [Google Scholar] [CrossRef]
- Rajashree, K.; Muthukumar, T. Selection of culture medium and conditions for the production of selenium enriched Saccharomyces cerevisiae. Afr. J. Biotechnol. 2013, 12, 2972–2977. [Google Scholar]
- Alidee, T.; Habbal, H.; Tohla, M. Optimization of selenium enriched Saccharomyces cerevisiae by Response Surface Methodology (RSM). Int. J. Chem. Tech. Res. 2016, 9, 221–226. Available online: https://www.sphinxsai.com/2016/ch_vol9_no2/abstracts/A(221-226)V9N2CT.pdf (accessed on 21 September 2022).
- Amorim Mota, L.; Maria da Silva, A.P.; Alberto da Silva, E.; Maria Ferreira Lima Leite, G.; Perez Calegari, R.; Sampaio Baptista, A. Ability of the Saccharomyces cerevisiae Y904 to tolerate and adapt to high concentrations of selenium. Rev. Bras. Eng. Biossistemas 2022, 16. Available online: https://assets.researchsquare.com/files/rs-499880/v1/fcf8b8fb-5a63-468c-8bb9-8b2eee9e234f.pdf?c=1637245050 (accessed on 21 September 2022). [CrossRef]
- Meng, Y.; Liang, Z.; Yi, M.; Tan, Y.; Li, Z.; Du, P.; Li, A.; Li, C.; Liu, L. Enrichment of zinc in Lactobacillus plantarum DNZ-4: Impact on its characteristics, metabolites and antioxidant activity. LWT-Food Sci Tech. 2022, 153, 112462. [Google Scholar] [CrossRef]
- Kamran, A.S.; Shariatmadari, F.; Torshizi, M.K. Production of zinc-enriched biomass of Saccharomyces cerevisiae. J. Elem. 2012, 19, 313–326. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-aa51ebbb-8104-4006-b184-d958de650bb5 (accessed on 21 September 2022).
- Forough, S.; Amini, K.; Haddadi, A. Application of S. cerevisiae isolated from industrial effluent for zinc biosorption and zinc-enriched SCP production. Authorea 2020. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.L. Characteristics of Zn2+ biosorption by Saccharomyces cerevisiae. Biomed. Environ. Sci. 2007, 20, 478–482. [Google Scholar]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut microbiome-micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F.; Pelinescu, D.R. Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods 2020, 9, 1281. [Google Scholar] [CrossRef]
- Bohn, L.; Josefsen, L.; Meyer, A.S.; Rassmusen, S.K. Quantitative analysis of phytate globoids isolated from wheat bran and characterization of their sequential dephosphorylation by wheat phytase. J. Agric. Food Chem. 2007, 55, 7547–7552. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, U.; Camarinha-Silva, A.; Jahreis, G.; Lorkowski, S.; Glei, M. High phosphorus intake and gut-related parameters—Results of a randomized placebo-controlled human intervention study. Nutr. J. 2018, 17, 23. [Google Scholar] [CrossRef]
- Sauer, A.K.; Grabrucker, A.M. Zinc deficiency during pregnancy leads to altered microbiome and elevated inflammatory markers in mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, Gut Microbiome, and Bone Health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020, 31, 115–130.e6. [Google Scholar] [CrossRef]
- Qi, B.; Han, M. Microbial Siderophore Enterobactin Promotes Mitochondrial Iron Uptake and Development of the Host via Interaction with ATP Synthase. Cell 2018, 175, 571–582. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef] [PubMed]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2010, 12, 381. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; Hemphill, N.O.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Gnoni, A.; De Vito, D.; Dipalma, G.; Cantore, S.; Gargiulo Isacco, C. Effect of probiotics on the occurrence of nutrition absorption capacities in healthy children: A randomized double-blinded placebo-controlled pilot study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Huang, K. Selenium-enriched Bacillus subtilis yb-114246 improved growth and immunity of broiler chickens through modified ileal bacterial composition. Sci. Rep. 2021, 11, 21690. [Google Scholar] [CrossRef]
- Kang, S.; Li, R.; Jin, H.; You, H.J.; Ji, G.E. Effects of Selenium- and Zinc-Enriched Lactobacillus plantarum SeZi on Antioxidant Capacities and Gut Microbiome in an ICR Mouse Model. Antioxidants 2020, 9, 1028. [Google Scholar] [CrossRef]
- Martínez, F.G.; Moreno-Martin, G.; Pescuma, M.; Madrid-Albarrán, Y.; Mozzi, F. Biotransformation of selenium by lactic acid bacteria: Formation of seleno-nanoparticles and seleno-amino acids. Front. Bioeng. Biotechnol. 2020, 8, 506. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, Z.; Wang, Y.; Huang, K. Preparation of selenium/zinc-enriched probiotics and their effect on blood selenium and zinc concentrations, antioxidant capacities, and intestinal microflora in canine. Biol. Trace Elem. Res. 2011, 141, 170–183. [Google Scholar] [CrossRef]
- Rinninella, E.; Mele, M.C.; Merendino, N.; Cintoni, M.; Anselmi, G.; Caporossi, A.; Gasbarrini, A.; Minnella, A.M. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut–Retina Axis. Nutrients 2018, 10, 1677. [Google Scholar] [CrossRef]
- Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells 2022, 11, 1362. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef]
- Zhai, Q.; Cen, S.; Li, P.; Tian, F.; Al, E. Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ. Sci. Technol. Lett. 2018, 5, 724–730. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Chen, P.; Xie, H.; Tao, Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Sig Transduct. Target Ther. 2019, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Mego, M.; Májek, J.; Konceková, R.; Ebringer, L.; Cierniková, S.; Rauko, P. Intramucosal bacteria in colon cancer and their elimination by probiotic strain Enterococcus faecium M-74 with organic selenium. Folia Microbiol. 2005, 50, 443–447. [Google Scholar] [CrossRef]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef]
- Hatayama, H.; Iwashita, J.; Kuwajima, A.; Abe, T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem. Biophys. Res. Commun. 2007, 356, 599–603. [Google Scholar] [CrossRef]
- Nakkarach, A.; Foo, H.L.; Song, A.A.; Mutalib, N.E.A.; Nitisinprasert, S.; withayagiat, U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb. Cell Factories 2021, 20, 36. [Google Scholar] [CrossRef]
- Meng, Z.; Ye, Z.; Zhu, P.; Zhu, J.; Fang, S.; Qiu, T.; Li, Y.; Meng, L. New Developments and Opportunities of Microbiota in Treating Breast Cancers. Front. Microbiol. 2022, 13, 818793. [Google Scholar] [CrossRef]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the Interaction Between Selenium and Zinc on DNA Repair in Association with Cancer Prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M. Role of Zinc and Selenium in Oxidative Stress and Immunosenescence: Implications for Healthy Aging and Longevity. In Handbook on Immunosenescence; Springer: Berlin/Heidelberg, Germany, 2019; Volume 11, pp. 2539–2573. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Huang, X.; Yi, S.; Pan, S.; Zhang, Y.; Yuan, G.; Cao, Q.; Ye, X.; Li, H. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep. 2021, 36, 109726. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile Acid–Gut Microbiota Axis in Inflammatory Bowel Disease: From Bench to Bedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; El-Gazzar, N.; Almanaa, T.N.; El-Hadary, A.; Sitohy, M. Lipolytic Postbiotic from Lactobacillus paracasei Manages Metabolic Syndrome in Albino Wistar Rats. Molecules 2021, 26, 472. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Ceppa, F.A.; Izzo, L.; Sardelli, L.; Raimondi, I.; Tunesi, M.; Albani, D.; Giordano, C. Human Gut-Microbiota Interaction in Neurodegenerative Disorders and Current Engineered Tools for Its Modeling. Front. Cell Infect. Microbiol. 2020, 10, 297. [Google Scholar] [CrossRef]
- Schwarz, S.; Lehmbecker, A.; Tongtako, W. Neurotrophic effects of GM1 ganglioside, NGF, and FGF2 on canine dorsal root ganglia neurons in vitro. Sci. Rep. 2020, 10, 5380. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017, 7, 124–136. [Google Scholar] [CrossRef] [Green Version]


| Model | Effect | Components | Reference |
|---|---|---|---|
| Clinical study | Potentially reduced abdominal pain and modification of stool consistency for patients with irritable bowel syndrome | Non-viable probiotic lysate of Escherichia coli DSM 17252 and Enterococcus faecalis DSM 16440 | [15] |
| In vitro | Inhibition of lipid accumulation during adipocyte differentiation | Cell lysate of Lactobacillus plantarum K8 | [16] |
| In vitro | Antibacterial and antioxidant activity | The cell-free supernatant from L. plantarum RG11, RG14, RI11, RS5, TL1, and UL4 | [17] |
| In vitro | Antibacterial and antibiofilm activity | The cell-free supernatant from L. sakei EIR/CM-1 | [18] |
| In vitro | Antimicrobial activity | Metabolic products of L. acidophilus LA5, L. casei 431, and L. salivarius | [19] |
| In vitro | Increased interleukin IL10 concentration and expression CD103 and CD1d Downregulated expression of NFκB1, RELB, and TNF genes Influenced retinoic acid—driven mucosal-like dendritic cells | The cell-free supernatant from L. reuteri DSM 17938 | [20] |
| In vitro and animal model mice | Immunomodulatory effect | Milk fermentation product using Brevibacterium breve C50 and Streptococcus thermophiles 065 | [21] |
| Animal—mice | Improving the parameters associated with intestinal mucositis induced by chemotherapy | Heat inactivated cells of L. rhamnosus | [22] |
| Animal—rat | Preventing periodontitis by reducing alveolar bone loss and ameliorating the bone microarchitecture parameters | Heat inactivated cells of L. reuteri | [23] |
| Animal—broiler chickens | Immunomodulatory effect on jejunal tissue Decreased Clostridium perfringens colony counts, decreased lesions scores, and mortality | Fermented product produced from a consortium containing Pediococcus acidilactici, L. reuter, Enterococcus faecium, and L. acidophilus | [24] |
| Animal—suckling rat | Protection against rotavirus infection | Cell fragments and metabolites of probiotic microorganisms and prebiotics | [25] |
| Animal—post-weaning lambs | Immunomodulatory effect (increase of IL-6, decrease of IL1 and TNF) Pathogenic bacteria inhibition | The cell-free supernatant from L. plantarum RG14 | [26] |
| Animal—broiler chickens | Immunomodulatory effect Reduce cell number of Enterobacteria and E. coli | The cell-free supernatant from L. plantarum RG14 probiotic microorganisms and inulin | [27,28] |
| Animal—broiler chickens | Improving growth performance Reduced number of Enterobacteriaceae Increase expression of hepatic IGF-1 and GHR mRNA and plasma immunoglobulins (IgG and IgM) | The cell-free supernatant from L. plantarum RI11, L. plantarum RS5 and L. plantarum UL4 | [29,30] |
| Animal—neonatale rat | Promoting mucin secretion and the epithelial tight junction protein expression | Components of the cell-free supernatant from L. rhamnosus GG | [31] |
| Animal—rat | Increase the global bone mineral density | Bacterial lysate and supernatant from L. acidophilus, L. casei, L. reuteri, Bifidobacterium longum, and Bacillus coagulans | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, L.-D.; Avram, I.; Pelinescu, D.-R.; Vamanu, E. Mineral-Enriched Postbiotics: A New Perspective for Microbial Therapy to Prevent and Treat Gut Dysbiosis. Biomedicines 2022, 10, 2392. https://doi.org/10.3390/biomedicines10102392
Dinu L-D, Avram I, Pelinescu D-R, Vamanu E. Mineral-Enriched Postbiotics: A New Perspective for Microbial Therapy to Prevent and Treat Gut Dysbiosis. Biomedicines. 2022; 10(10):2392. https://doi.org/10.3390/biomedicines10102392
Chicago/Turabian StyleDinu, Laura-Dorina, Ionela Avram, Diana-Roxana Pelinescu, and Emanuel Vamanu. 2022. "Mineral-Enriched Postbiotics: A New Perspective for Microbial Therapy to Prevent and Treat Gut Dysbiosis" Biomedicines 10, no. 10: 2392. https://doi.org/10.3390/biomedicines10102392
APA StyleDinu, L.-D., Avram, I., Pelinescu, D.-R., & Vamanu, E. (2022). Mineral-Enriched Postbiotics: A New Perspective for Microbial Therapy to Prevent and Treat Gut Dysbiosis. Biomedicines, 10(10), 2392. https://doi.org/10.3390/biomedicines10102392