Modulation of Hedgehog Signaling for the Treatment of Basal Cell Carcinoma and the Development of Preclinical Models
Abstract
:1. Introduction
2. Predominant Treatment Options for BCC
2.1. Surgical Resection
2.2. Radiation Therapy
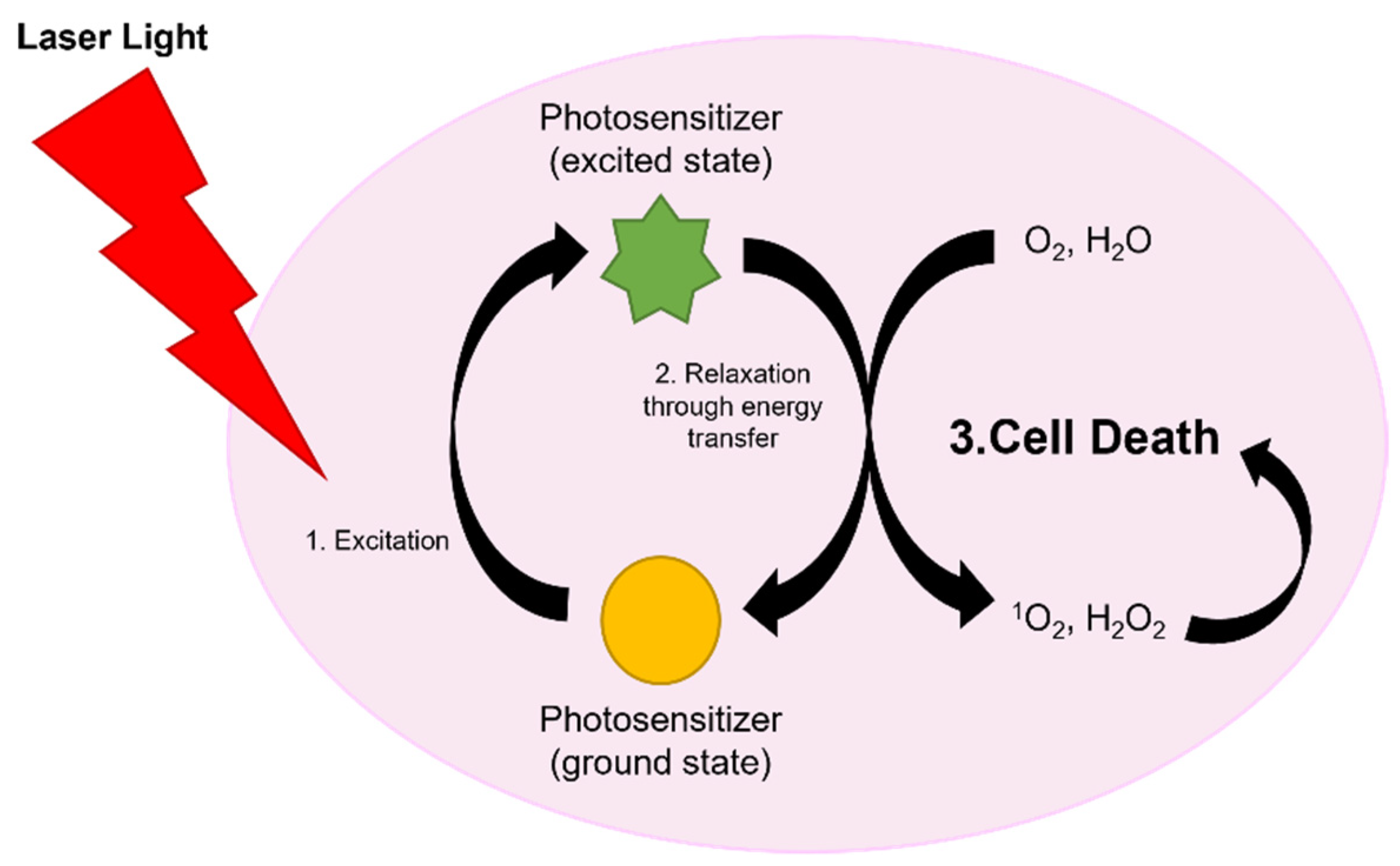
2.3. Laser Therapies
2.4. Imiquimod and 5-Fluorouracil Topical Treatments
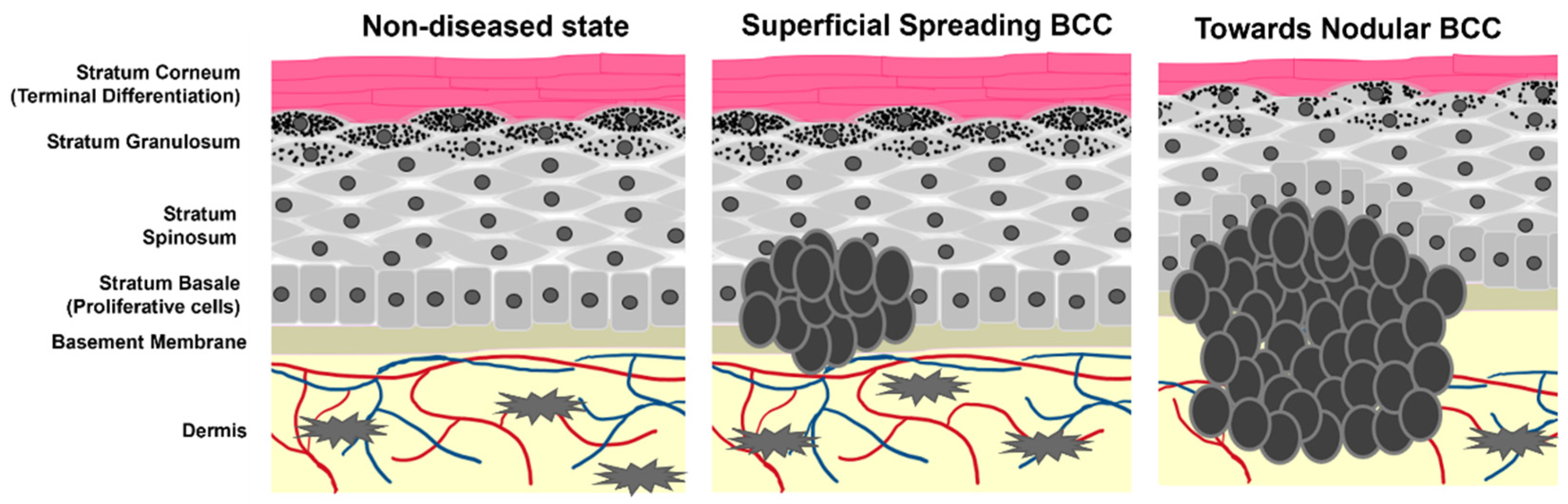
3. The Hedgehog Signaling Cascade in BCC
4. Chemotherapies That Target Hedgehog Signaling
4.1. Smoothened Inhibitors
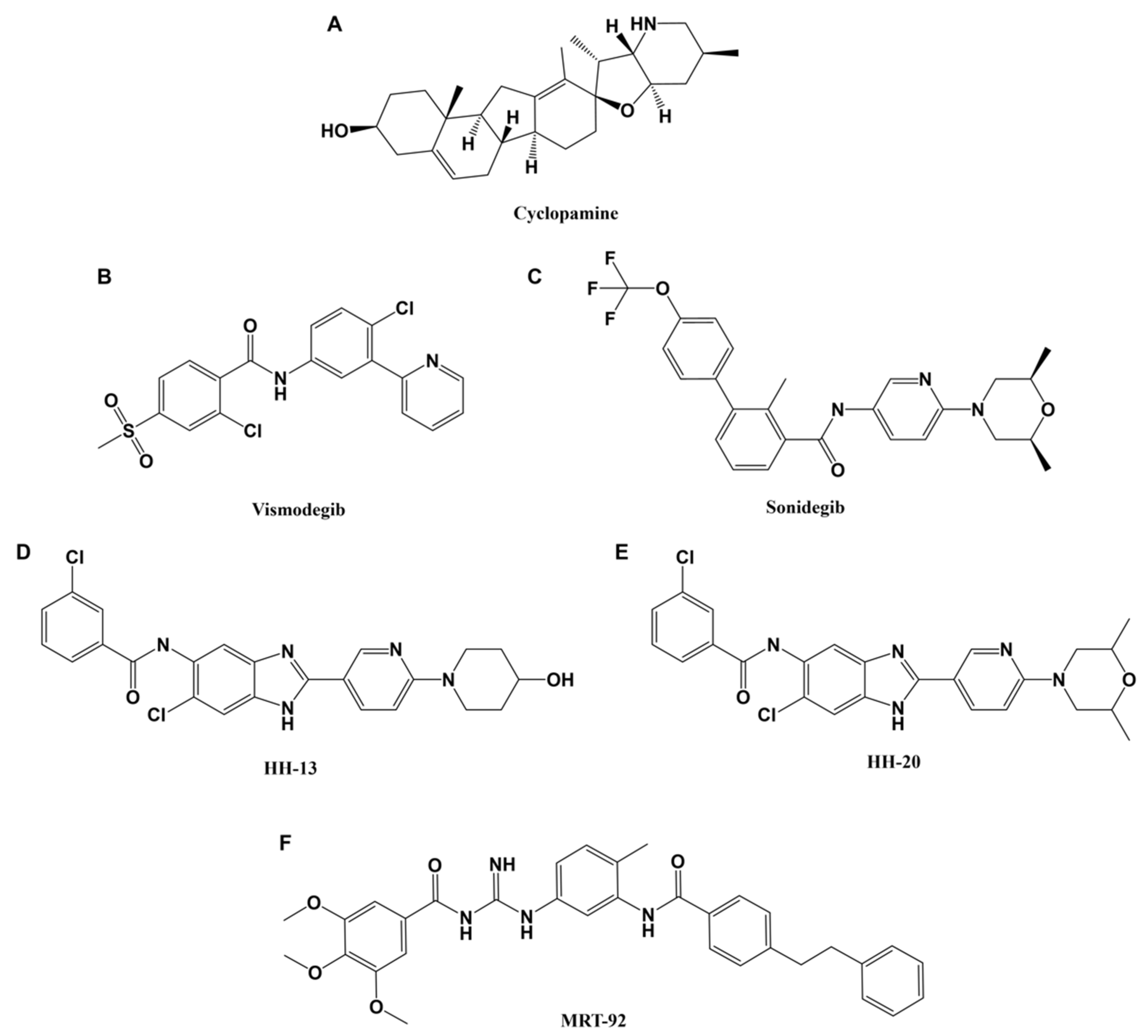
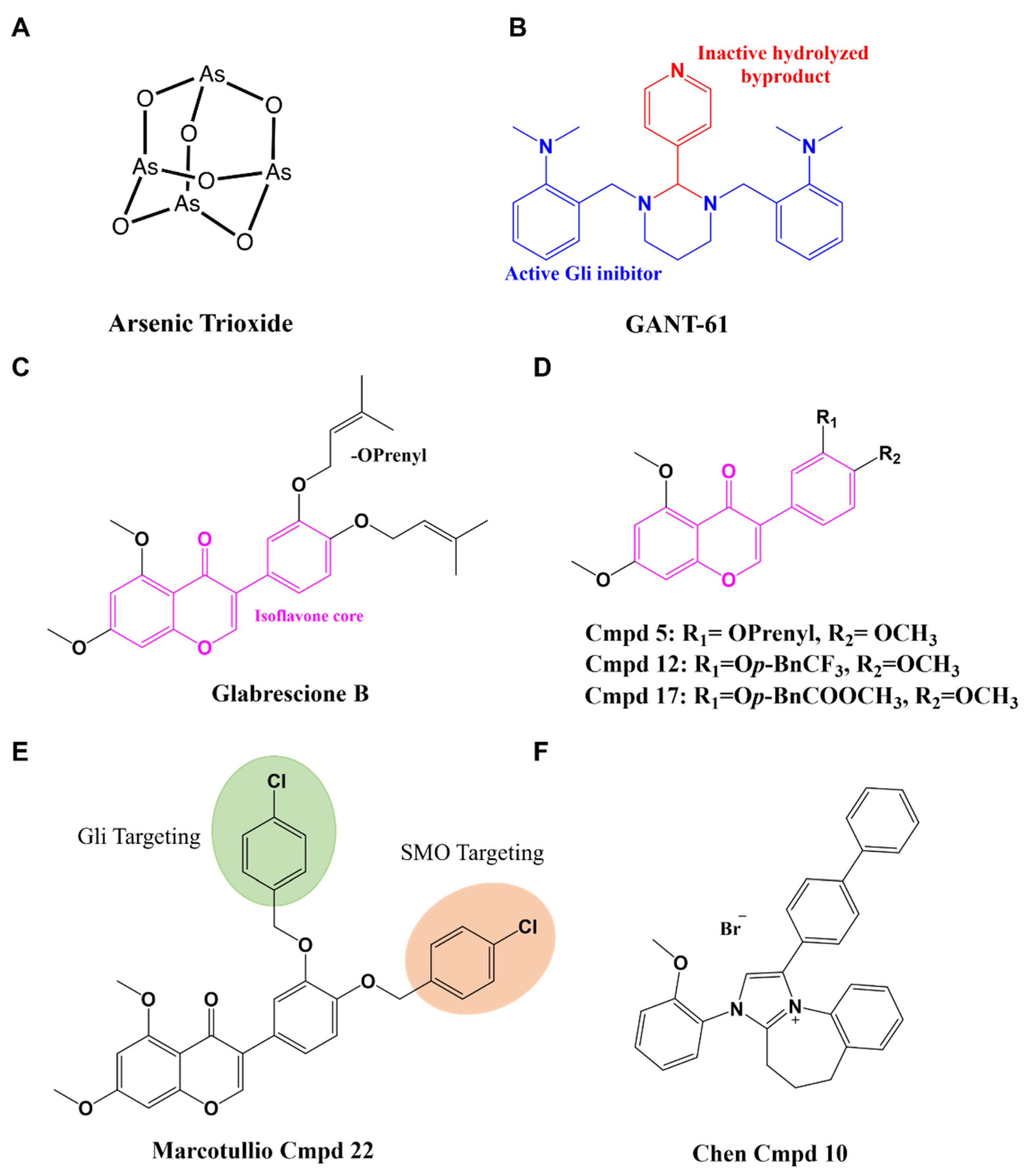
4.2. Gli Inhibitors
5. Preclinical Models for Hedgehog and BCC Research
5.1. Hedgehog Activation in Cellular Assays
5.2. Murine BCC Models
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, V.; Cranwell, W.; Sinclair, R. Epidemiology skin cancer in the mature patient. Clin. Dermatol. 2018, 36, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.R.; Weinstock, M.A. Keratinocyte Carcinoma. CA Cancer J. Clin. 2003, 53, 292–302. [Google Scholar] [CrossRef]
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.M.; Strange, R.C.; Lear, J.T. Basal cell carcinoma. BMJ 2003, 327, 794–798. [Google Scholar] [CrossRef]
- Delishaj, D.; Rembielak, A.; Manfredi, B.; Ursino, S.; Pasqualetti, F.; Laliscia, C.; Orlandi, F.; Morganti, R.; Fabrini, M.G.; Paiar, F. Non-melanoma skin cancer treated with high-dose-rate brachytherapy: A review of literature. J. Contemp. Brachyther. 2016, 8, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, A.; Marinescu, E.-A.; Nica, O.; Ilinoiu, E.; Negrila, A.; Ciurea, M.-E. Basal Cell Carcinoma and its Impact on Different Anatomical Regions. Curr. Health Sci. J. 2021, 47, 75–83. [Google Scholar] [CrossRef]
- Jones, E.A.; Sajid, M.I.; Shenton, A.; Evans, D.G. Basal Cell Carcinomas in Gorlin Syndrome: A Review of 202 Patients. J. Ski. Cancer 2011, 2011, 217378. [Google Scholar] [CrossRef]
- Kiwilsza, M.; Sporniak-Tutak, K. Gorlin-Goltz syndrome—A medical condition requiring a multidisciplinary approach. Med. Sci. Monit. 2012, 18, RA145–RA153. [Google Scholar] [CrossRef]
- Hoban, P.R.; Ramachandran, S.; Strange, R.C. Environment, phenotype and genetics: Risk factors associated with BCC of the skin. Expert Rev. Anticancer Ther. 2002, 2, 570–579. [Google Scholar] [CrossRef]
- Situm, M.; Buljan, M.; Bulat, V.; Lugović Mihić, L.; Bolanca, Z.; Simić, D. The role of UV radiation in the development of basal cell carcinoma. Coll. Antropol. 2008, 32 (Suppl. S2), 167–170. [Google Scholar]
- Wu, S.; Han, J.; Li, W.-Q.; Li, T.; Qureshi, A.A. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am. J. Epidemiol. 2013, 178, 890–897. [Google Scholar] [CrossRef]
- Berlin, N.L.; Cartmel, B.; Leffell, D.J.; Bale, A.E.; Mayne, S.T.; Ferrucci, L.M. Family history of skin cancer is associated with early-onset basal cell carcinoma independent of MC1R genotype. Cancer Epidemiol. 2015, 39, 1078–1083. [Google Scholar] [CrossRef]
- Puig, S.; Berrocal, A. Management of high-risk and advanced basal cell carcinoma. Clin. Transl. Oncol. 2015, 17, 497–503. [Google Scholar] [CrossRef]
- Chung, S. Basal cell carcinoma. Arch. Plast. Surg. 2012, 39, 166–170. [Google Scholar] [CrossRef]
- Bøgelund, F.S.; Philipsen, P.A.; Gniadecki, R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm. Venereol. 2007, 87, 330–334. [Google Scholar] [CrossRef]
- Marcil, I.; Stern, R.S. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: A critical review of the literature and meta-analysis. Arch. Dermatol. 2000, 136, 1524–1530. [Google Scholar] [CrossRef]
- Levi, F.; Randimbison, L.; Maspoli, M.; Te, V.C.; La Vecchia, C. High incidence of second basal cell skin cancers. Int. J. Cancer 2006, 119, 1505–1507. [Google Scholar] [CrossRef]
- Bartos, V. Development of Multiple-Lesion Basal Cell Carcinoma of the Skin: A Comprehensive Review. Sisli Etfal Hast. Tip Bülteni 2019, 53, 323–328. [Google Scholar] [CrossRef]
- Villani, R.; Murigneux, V.; Alexis, J.; Sim, S.-L.; Wagels, M.; Saunders, N.; Soyer, H.P.; Parmentier, L.; Nikolaev, S.; Fink, J.L.; et al. Subtype-Specific Analyses Reveal Infiltrative Basal Cell Carcinomas Are Highly Interactive with their Environment. J. Investig. Dermatol. 2021, 141, 2380–2390. [Google Scholar] [CrossRef]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, L.T.D.; Magnusson, M.R.; Guppy, M.P.B. Risk factors for recurrence of facial basal cell carcinoma after surgical excision: A follow-up analysis. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Fania, L.; Didona, D.; Morese, R.; Campana, I.; Coco, V.; Di Pietro, F.R.; Ricci, F.; Pallotta, S.; Candi, E.; Abeni, D.; et al. Basal Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2020, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.D., Jr.; Parlette, H.L. Duplicitous growth of infiltrative basal cell carcinoma: Analysis of clinically undetected tumor extent in a paired case-control study. Dermatol. Surg. 1996, 22, 535–539. [Google Scholar] [CrossRef]
- Wehner, M.R.; Cidre Serrano, W.; Nosrati, A.; Schoen, P.M.; Chren, M.-M.; Boscardin, J.; Linos, E. All-cause mortality in patients with basal and squamous cell carcinoma: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 78, 663–672.e663. [Google Scholar] [CrossRef]
- Kim, D.P.; Kus, K.J.B.; Ruiz, E. Basal Cell Carcinoma Review. Hematol. Oncol. Clin. N. Am. 2019, 33, 13–24. [Google Scholar] [CrossRef]
- Quazi, S.J.; Aslam, N.; Saleem, H.; Rahman, J.; Khan, S. Surgical Margin of Excision in Basal Cell Carcinoma: A Systematic Review of Literature. Cureus 2020, 12, e9211. [Google Scholar] [CrossRef]
- Ceilley, R.I.; Del Rosso, J.Q. Current modalities and new advances in the treatment of basal cell carcinoma. Int. J. Dermatol. 2006, 45, 489–498. [Google Scholar] [CrossRef]
- Mohs, F.E. Chemosurgery: A microscopically controlled method of cancer excision. Arch. Surg. 1941, 42, 279–295. [Google Scholar] [CrossRef]
- Tolkachjov, S.N.; Brodland, D.G.; Coldiron, B.M.; Fazio, M.J.; Hruza, G.J.; Roenigk, R.K.; Rogers, H.W.; Zitelli, J.A.; Winchester, D.S.; Harmon, C.B. Understanding Mohs Micrographic Surgery: A Review and Practical Guide for the Nondermatologist. Mayo Clin. Proc. 2017, 92, 1261–1271. [Google Scholar] [CrossRef]
- Smeets, N.W.J.; Kuijpers, D.I.M.; Nelemans, P.; Ostertag, J.U.; Verhaegh, M.E.J.M.; Krekels, G.A.M.; Neumann, H.A.M. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face—Results of a retrospective study and review of the literature. Br. J. Dermatol. 2004, 151, 141–147. [Google Scholar] [CrossRef]
- Bittner, G.C.; Cerci, F.B.; Kubo, E.M.; Tolkachjov, S.N. Mohs micrographic surgery: A review of indications, technique, outcomes, and considerations. Bras. Dermatol. 2021, 96, 263–277. [Google Scholar] [CrossRef]
- Rowe, D.E.; Carroll, R.J.; Day, C.L., Jr. Mohs Surgery Is the Treatment of Choice for Recurrent (Previously Treated) Basal Cell Carcinoma. J. Dermatol. Surg. Oncol. 1989, 15, 424–431. [Google Scholar] [CrossRef]
- Lawrence, C.M. Mohs surgery of basal cell carcinoma—A critical review. Br. J. Plast. Surg. 1993, 46, 599–606. [Google Scholar] [CrossRef]
- Likhacheva, A.; Awan, M.; Barker, C.A.; Bhatnagar, A.; Bradfield, L.; Brady, M.S.; Buzurovic, I.; Geiger, J.L.; Parvathaneni, U.; Zaky, S.; et al. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 8–20. [Google Scholar] [CrossRef]
- Silverman, M.K.; Kopf, A.W.; Gladstein, A.H.; Bart, R.S.; Grin, C.M.; Levenstein, M.J. Recurrence rates of treated basal cell carcinomas. Part 4: X-ray therapy. J. Dermatol. Surg. Oncol. 1992, 18, 549–554. [Google Scholar] [CrossRef]
- Baheti, A.D.; Tirumani, S.H.; Giardino, A.; Rosenthal, M.H.; Tirumani, H.; Krajewski, K.; Ramaiya, N.H. Basal Cell Carcinoma: A Comprehensive Review for the Radiologist. Am. J. Roentgenol. 2015, 204, W132–W140. [Google Scholar] [CrossRef]
- Baker, S.; Joseph, K.; Tai, P. Radiotherapy in Gorlin Syndrome: Can It Be Safe and Effective in Adult Patients? J. Cutan. Med. Surg. 2016, 20, 159–162. [Google Scholar] [CrossRef]
- Lo Muzio, L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J. Rare Dis. 2008, 3, 32. [Google Scholar] [CrossRef]
- Salavastru, C.; Tiplica, G.S.; Fritz, K. Lasertherapie des Basalzellkarzinoms. Der Hautarzt 2018, 69, 10–16. [Google Scholar] [CrossRef]
- Moskalik, K.; Kozlov, A.; Demin, E.; Boiko, E. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed. Laser Surg. 2009, 27, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Avram, M.M.; Ortiz, A.E. The Evolving Story of Laser Therapeutics for Basal Cell Carcinoma. Dermatol. Surg. 2020, 46, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.H.; Morton, C.A.; Collier, N.; Haylett, A.; Ibbotson, S.; McKenna, K.E.; Mallipeddi, R.; Moseley, H.; Seukeran, D.C.; Rhodes, L.E.; et al. British Association of Dermatologists and British Photodermatology Group guidelines for topical photodynamic therapy 2018. Br. J. Dermatol. 2019, 180, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Collier, N.J.; Rhodes, L.E. Photodynamic Therapy for Basal Cell Carcinoma: The Clinical Context for Future Research Priorities. Molecules 2020, 25, 5398. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Ibbotson, S.; Murrell, D.F.; Rubel, D.; Frambach, Y.; De Berker, D.; Dummer, R.; Kerrouche, N.; Villemagne, H.; on behalf of the Excilight Study Group. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Basset-Seguin, N.; Bissonnette, R.; Girard, C.; Haedersdal, M.; Lear, J.T.; Paul, C.; Piaserico, S. Consensus recommendations for the treatment of basal cell carcinomas in Gorlin syndrome with topical methylaminolaevulinate-photodynamic therapy. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 626–632. [Google Scholar] [CrossRef]
- Marous, M.; Mueller, K.; Tausk, F. Multiple basal cell carcinomas in Gorlin Syndrome treated with pulsed dye laser. J. Cosmet. Laser Ther. 2020, 22, 230–231. [Google Scholar] [CrossRef]
- Shumack, S.; Robinson, J.; Kossard, S.; Golitz, L.; Greenway, H.; Schroeter, A.; Andres, K.; Amies, M.; Owens, M. Efficacy of Topical 5% Imiquimod Cream for the Treatment of Nodular Basal Cell Carcinoma: Comparison of Dosing Regimens. Arch. Dermatol. 2002, 138, 1165–1171. [Google Scholar] [CrossRef]
- Dummer, R.; Urosevic, M.; Kempf, W.; Hoek, K.; Hafner, J.; Burg, G. Imiquimod in basal cell carcinoma: How does it work? Br. J. Dermatol. 2003, 149 (Suppl. S66), 57–58. [Google Scholar] [CrossRef]
- Gross, K.; Kircik, L.; Kricorian, G. 5% 5-Fluorouracil Cream for the Treatment of Small Superficial Basal Cell Carcinoma: Efficacy, Tolerability, Cosmetic Outcome, and Patient Satisfaction. Dermatol. Surg. 2007, 33, 433–440. [Google Scholar] [CrossRef]
- Epstein, E. Fluorouracil paste treatment of thin basal cell carcinomas. Arch. Dermatol. 1985, 121, 207–213. [Google Scholar] [CrossRef]
- Marks, R.; Gebauer, K.; Shumack, S.; Amies, M.; Bryden, J.; Fox, T.L.; Owens, M.L. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: Results of a multicenter 6-week dose-response trial. J. Am. Acad. Dermatol. 2001, 44, 807–813. [Google Scholar] [CrossRef]
- Torres, A.; Niemeyer, A.; Berkes, B. Treatment of basal cell carcinoma using imiquimod 5% cream as an adjuvant therapy to Mohs micrographic surgery. J. Eur. Acad. Dermatol. Venereol. 2004, 30, 1462–1469. [Google Scholar]
- Gailani, M.R.; Bale, S.J.; Leffell, D.J.; DiGiovanna, J.J.; Peck, G.L.; Poliak, S.; Drum, M.A.; Pastakia, B.; McBride, O.W.; Kase, R.; et al. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell 1992, 69, 111–117. [Google Scholar] [CrossRef]
- Hahn, H.; Wicking, C.; Zaphiropoulous, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996, 85, 841–851. [Google Scholar] [CrossRef]
- Johnson, R.L.; Rothman, A.L.; Xie, J.; Goodrich, L.V.; Bare, J.W.; Bonifas, J.M.; Quinn, A.G.; Myers, R.M.; Cox, D.R.; Epstein, E.H., Jr.; et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996, 272, 1668–1671. [Google Scholar] [CrossRef]
- Klein, R.D.; Dykas, D.J.; Bale, A.E. Clinical testing for the nevoid basal cell carcinoma syndrome in a DNA diagnostic laboratory. Genet. Med. 2005, 7, 611–619. [Google Scholar] [CrossRef]
- Gupta, S.; Takebe, N.; Lorusso, P. Targeting the Hedgehog pathway in cancer. Ther. Adv. Med. Oncol. 2010, 2, 237–250. [Google Scholar] [CrossRef]
- Ming, J.E.; Nanni, L.; Muenke, M.; Meinecke, P.; Pierpont, M.E.M.; Robin, N.H.; Young, I.D.; Roessler, E.; Steinhaus, K.; Bocian, M.; et al. The Mutational Spectrum of the Sonic Hedgehog Gene in Holoprosencephaly: SHH Mutations Cause a Significant Proportion of Autosomal Dominant Holoprosencephaly. Hum. Mol. Genet. 1999, 8, 2479–2488. [Google Scholar] [CrossRef]
- Di Magliano, M.P.; Hebrok, M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer 2003, 3, 903–911. [Google Scholar] [CrossRef]
- Fujii, K.; Miyashita, T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): Update and literature review. Pediatrics Int. 2014, 56, 667–674. [Google Scholar] [CrossRef]
- Sahebjam, S.; Siu, L.L.; Razak, A.A. The Utility of Hedgehog Signaling Pathway Inhibition for Cancer. Oncologist 2012, 17, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Chahal, K.K.; Parle, M.; Abagyan, R. Hedgehog pathway and smoothened inhibitors in cancer therapies. Anti-Cancer Drugs 2018, 29, 387–401. [Google Scholar] [CrossRef]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754. [Google Scholar] [CrossRef]
- Ouhtit, A.; Nakazawa, H.; Armstrong, B.K.; Kricker, A.; Tan, E.; Yamasaki, H.; English, D.R. UV-radiation-specific p53 mutation frequency in normal skin as a predictor of risk of basal cell carcinoma. J. Natl. Cancer Inst. 1998, 90, 523–531. [Google Scholar] [CrossRef]
- Furth, N.; Aylon, Y.; Oren, M. p53 shades of Hippo. Cell Death Differ. 2018, 25, 81–92. [Google Scholar] [CrossRef]
- Mancuso, M.; Pazzaglia, S.; Tanori, M.; Hahn, H.; Merola, P.; Rebessi, S.; Atkinson, M.J.; Di Majo, V.; Covelli, V.; Saran, A. Basal Cell Carcinoma and Its Development: Insights from Radiation-Induced Tumors in Ptch1-Deficient Mice. Cancer Res. 2004, 64, 934–941. [Google Scholar] [CrossRef]
- Mercurio, L.; Albanesi, C.; Madonna, S. Recent Updates on the Involvement of PI3K/AKT/mTOR Molecular Cascade in the Pathogenesis of Hyperproliferative Skin Disorders. Front. Med. 2021, 8, 665647. [Google Scholar] [CrossRef]
- Chakrabarti, J.; Holokai, L.; Syu, L.; Steele, N.G.; Chang, J.; Wang, J.; Ahmed, S.; Dlugosz, A.; Zavros, Y. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget 2018, 9, 37439–37457. [Google Scholar] [CrossRef]
- Lipson, E.J.; Lilo, M.T.; Ogurtsova, A.; Esandrio, J.; Xu, H.; Brothers, P.; Schollenberger, M.; Sharfman, W.H.; Taube, J.M. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J. ImmunoTherapy Cancer 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.J.; Korn, R.L. Metastatic basal cell carcinoma in the era of hedgehog signaling pathway inhibitors. Cancer 2012, 118, 5310–5319. [Google Scholar] [CrossRef] [PubMed]
- Ozgediz, D.; Smith, E.B.; Zheng, J.; Otero, J.; Tabatabai, Z.L.; Corvera, C.U. Basal cell carcinoma does metastasize. Derm. Online J. 2008, 14, 5. [Google Scholar] [CrossRef]
- Von Domarus, H.; Stevens, P.J. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J. Am. Acad. Dermatol. 1984, 10, 1043–1060. [Google Scholar] [CrossRef]
- Lee, S.T.; Welch, K.D.; Panter, K.E.; Gardner, D.R.; Garrossian, M.; Chang, C.-W.T. Cyclopamine: From Cyclops Lambs to Cancer Treatment. J. Agric. Food Chem. 2014, 62, 7355–7362. [Google Scholar] [CrossRef]
- Binns, W.; Shupe, J.L.; Keeler, R.F.; James, L.F. Chronologic evaluation of teratogenicity in sheep fed Veratrum californicum. J. Am. Vet. Med. Assoc. 1965, 147, 839–842. [Google Scholar]
- James, L.F. Teratological research at the USDA-ARS poisonous plant research laboratory. J. Nat. Toxins 1999, 8, 63–80. [Google Scholar]
- Chiang, C.; Litingtung, Y.; Lee, E.; Young, K.E.; Corden, J.L.; Westphal, H.; Beachy, P.A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 1996, 383, 407–413. [Google Scholar] [CrossRef]
- Litingtung, Y.; Lei, L.; Westphal, H.; Chiang, C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998, 20, 58–61. [Google Scholar] [CrossRef]
- Cooper, M.K.; Porter, J.A.; Young, K.E.; Beachy, P.A. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 1998, 280, 1603–1607. [Google Scholar] [CrossRef]
- Incardona, J.P.; Gaffield, W.; Kapur, R.P.; Roelink, H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 1998, 125, 3553–3562. [Google Scholar] [CrossRef]
- Sanchez, P.; i Altaba, A.R. In vivo inhibition of endogenous brain tumors through systemic interference of Hedgehog signaling in mice. Mech. Dev. 2005, 122, 223–230. [Google Scholar] [CrossRef]
- Berman, D.M.; Karhadkar, S.S.; Hallahan, A.R.; Pritchard, J.I.; Eberhart, C.G.; Watkins, D.N.; Chen, J.K.; Cooper, M.K.; Taipale, J.; Olson, J.M. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 2002, 297, 1559–1561. [Google Scholar] [CrossRef]
- Clement, V.; Sanchez, P.; de Tribolet, N.; Radovanovic, I.; Ruiz i Altaba, A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007, 17, 165–172. [Google Scholar] [CrossRef]
- Stecca, B.; Mas, C.; Clement, V.; Zbinden, M.; Correa, R.; Piguet, V.; Beermann, F.; Ruiz i Altaba, A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 5895–5900. [Google Scholar] [CrossRef]
- Varnat, F.; Duquet, A.; Malerba, M.; Zbinden, M.; Mas, C.; Gervaz, P.; Ruiz i Altaba, A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol. Med. 2009, 1, 338–351. [Google Scholar] [CrossRef]
- Feldmann, G.; Dhara, S.; Fendrich, V.; Bedja, D.; Beaty, R.; Mullendore, M.; Karikari, C.; Alvarez, H.; Iacobuzio-Donahue, C.; Jimeno, A. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res. 2007, 67, 2187–2196. [Google Scholar] [CrossRef]
- Karhadkar, S.S.; Steven Bova, G.; Abdallah, N.; Dhara, S.; Gardner, D.; Maitra, A.; Isaacs, J.T.; Berman, D.M.; Beachy, P.A. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004, 431, 707–712. [Google Scholar] [CrossRef]
- Frampton, J.E.; Basset-Séguin, N. Vismodegib: A Review in Advanced Basal Cell Carcinoma. Drugs 2018, 78, 1145–1156. [Google Scholar] [CrossRef]
- Jain, S.; Song, R.; Xie, J. Sonidegib: Mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco. Targets Ther. 2017, 10, 1645–1653. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Solomon, J.A.; Hainsworth, J.D.; Goldberg, L.; McKenna, E.; Day, B.-m.; Chen, D.M.; Weiss, G.J. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J. Am. Acad. Dermatol. 2014, 70, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; Oro, A.E. Initial assessment of tumor regrowth after vismodegib in advanced basal cell carcinoma. Arch. Dermatol. 2012, 148, 1324–1325. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, H.J.; Pau, G.; Dijkgraaf, G.J.; Basset-Seguin, N.; Modrusan, Z.; Januario, T.; Tsui, V.; Durham, A.B.; Dlugosz, A.A.; Haverty, P.M. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 2015, 27, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Atwood, S.X.; Sarin, K.Y.; Whitson, R.J.; Li, J.R.; Kim, G.; Rezaee, M.; Ally, M.S.; Kim, J.; Yao, C.; Chang, A.L.S. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell 2015, 27, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Ridky, T.W.; Cotsarelis, G. Vismodegib resistance in basal cell carcinoma: Not a smooth fit. Cancer Cell 2015, 27, 315–316. [Google Scholar] [CrossRef]
- Danial, C.; Sarin, K.Y.; Oro, A.E.; Chang, A.L.S. An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib. Clin. Cancer Res. 2016, 22, 1325–1329. [Google Scholar] [CrossRef]
- Gutzmer, R.; Solomon, J.A. Hedgehog Pathway Inhibition for the Treatment of Basal Cell Carcinoma. Target. Oncol. 2019, 14, 253–267. [Google Scholar] [CrossRef]
- Li, Q.-r.; Zhao, H.; Zhang, X.-s.; Lang, H.; Yu, K. Novel-smoothened inhibitors for therapeutic targeting of naïve and drug-resistant hedgehog pathway-driven cancers. Acta Pharmacol. Sin. 2019, 40, 257–267. [Google Scholar] [CrossRef]
- Zárate, A.M.; Espinosa-Bustos, C.; Guerrero, S.; Fierro, A.; Oyarzún-Ampuero, F.; Quest, A.F.G.; Di Marcotullio, L.; Loricchio, E.; Caimano, M.; Calcaterra, A.; et al. A New Smoothened Antagonist Bearing the Purine Scaffold Shows Antitumour Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 8372. [Google Scholar] [CrossRef]
- Hoch, L.; Faure, H.; Roudaut, H.; Schoenfelder, A.; Mann, A.; Girard, N.; Bihannic, L.; Ayrault, O.; Petricci, E.; Taddei, M.; et al. MRT-92 inhibits Hedgehog signaling by blocking overlapping binding sites in the transmembrane domain of the Smoothened receptor. FASEB J. 2015, 29, 1817–1829. [Google Scholar] [CrossRef]
- Pietrobono, S.; Santini, R.; Gagliardi, S.; Dapporto, F.; Colecchia, D.; Chiariello, M.; Leone, C.; Valoti, M.; Manetti, F.; Petricci, E.; et al. Targeted inhibition of Hedgehog-GLI signaling by novel acylguanidine derivatives inhibits melanoma cell growth by inducing replication stress and mitotic catastrophe. Cell Death Dis. 2018, 9, 142. [Google Scholar] [CrossRef]
- Švenda, J.; Sheremet, M.; Kremer, L.; Maier, L.; Bauer, J.O.; Strohmann, C.; Ziegler, S.; Kumar, K.; Waldmann, H. Biology-Oriented Synthesis of a Withanolide-Inspired Compound Collection Reveals Novel Modulators of Hedgehog Signaling. Angew. Chem. Int. Ed. 2015, 54, 5596–5602. [Google Scholar] [CrossRef]
- Bonandi, E.; Mori, M.; Infante, P.; Basili, I.; Di Marcotullio, L.; Calcaterra, A.; Catti, F.; Botta, B.; Passarella, D. Design and Synthesis of New Withaferin A Inspired Hedgehog Pathway Inhibitors. Chem.—Eur. J. 2021, 27, 8350–8357. [Google Scholar] [CrossRef]
- Biehs, B.; Dijkgraaf, G.J.P.; Piskol, R.; Alicke, B.; Boumahdi, S.; Peale, F.; Gould, S.E.; de Sauvage, F.J. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature 2018, 562, 429–433. [Google Scholar] [CrossRef]
- Whitson, R.J.; Lee, A.; Urman, N.M.; Mirza, A.; Yao, C.Y.; Brown, A.S.; Li, J.R.; Shankar, G.; Fry, M.A.; Atwood, S.X.; et al. Noncanonical hedgehog pathway activation through SRF-MKL1 promotes drug resistance in basal cell carcinomas. Nat. Med. 2018, 24, 271–281. [Google Scholar] [CrossRef]
- Infante, P.; Alfonsi, R.; Botta, B.; Mori, M.; Di Marcotullio, L. Targeting GLI factors to inhibit the Hedgehog pathway. Trends Pharmacol. Sci. 2015, 36, 547–558. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Imming, P.; Sinning, C.; Meyer, A. Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006, 5, 821–834. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.J.; Kim, J.; Gardner, D.; Beachy, P.A. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc. Natl. Acad. Sci. USA 2010, 107, 13432–13437. [Google Scholar] [CrossRef]
- Kim, J.; Aftab, B.; Tang, J.; Kim, D.; Lee, A.; Rezaee, M.; Kim, J.; Chen, B.; King, E.; Borodovsky, A.; et al. Itraconazole and Arsenic Trioxide Inhibit Hedgehog Pathway Activation and Tumor Growth Associated with Acquired Resistance to Smoothened Antagonists. Cancer Cell 2013, 23, 23–34. [Google Scholar] [CrossRef]
- Miller, W.H., Jr. Molecular Targets of Arsenic Trioxide in Malignant Cells. Oncologist 2002, 7 (Suppl. S1), 14–19. [Google Scholar] [CrossRef]
- Soignet, S.L.; Maslak, P.; Wang, Z.-G.; Jhanwar, S.; Calleja, E.; Dardashti, L.J.; Corso, D.; DeBlasio, A.; Gabrilove, J.; Scheinberg, D.A.; et al. Complete Remission after Treatment of Acute Promyelocytic Leukemia with Arsenic Trioxide. N. Engl. J. Med. 1998, 339, 1341–1348. [Google Scholar] [CrossRef]
- Cohen, M.H.; Hirschfeld, S.; Honig, S.F.; Ibrahim, A.; Johnson, J.R.; O’Leary, J.J.; White, R.M.; Williams, G.A.; Pazdur, R. Drug Approval Summaries: Arsenic Trioxide, Tamoxifen Citrate, Anastrazole, Paclitaxel, Bexarotene. Oncologist 2001, 6, 4–11. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Lu, L.; Magliato, S.; Wu, T. Hedgehog signaling pathway regulates autophagy in human hepatocellular carcinoma cells. Hepatology 2013, 58, 995–1010. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.; Zeng, C.; Lu, Q.; Huang, D.; Shi, C.; Zhang, W.; Deng, L.; Yan, R.; Rao, H. Down-regulation of Gli transcription factor leads to the inhibition of migration and invasion of ovarian cancer cells via integrin β4-mediated FAK signaling. PLoS ONE 2014, 9, e88386. [Google Scholar] [CrossRef]
- Kebenko, M.; Drenckhan, A.; Gros, S.J.; Jücker, M.; Grabinski, N.; Ewald, F.; Grottke, A.; Schultze, A.; Izbicki, J.R.; Bokemeyer, C. ErbB2 signaling activates the Hedgehog pathway via PI3K–Akt in human esophageal adenocarcinoma: Identification of novel targets for concerted therapy concepts. Cell. Signal. 2015, 27, 373–381. [Google Scholar] [CrossRef]
- Santini, R.; Vinci, M.C.; Pandolfi, S.; Penachioni, J.Y.; Montagnani, V.; Olivito, B.; Gattai, R.; Pimpinelli, N.; Gerlini, G.; Borgognoni, L. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells 2012, 30, 1808–1818. [Google Scholar] [CrossRef]
- Fu, J.; Rodova, M.; Roy, S.K.; Sharma, J.; Singh, K.P.; Srivastava, R.K.; Shankar, S. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013, 330, 22–32. [Google Scholar] [CrossRef]
- Lauth, M.; Bergstrm, s.; Shimokawa, T.; Toftgrd, R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl. Acad. Sci. USA 2007, 104, 8455–8460. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Walter, V.; Hayes, D.N.; Onaitis, M. Hedgehog–GLI signaling inhibition suppresses tumor growth in squamous lung cancer. Clin. Cancer Res. 2014, 20, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, T.; DeVecchio, J.; Agyeman, A.; Shi, T.; Houghton, J.A. Blocking Hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Cancer Res. 2011, 71, 5904–5914. [Google Scholar] [CrossRef] [PubMed]
- Kiran Riaz, S.; Ke, Y.; Wang, F.; Kayani, M.; Malik, F. Influence of SHH/GLI1 axis on EMT mediated migration and invasion of breast cancer cells. Sci. Rep. 2019, 9, 6620. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Zhou, X.; Li, X.; Lu, K.; Li, P.; Wang, X. Gli1 Inhibitor GANT61 Exhibits Antitumor Efficacy in T-Cell Lymphoma Cells through Down-Regulation of p-STAT3 and SOCS3 Pathways. Blood 2015, 126, 1287. [Google Scholar] [CrossRef]
- Calcaterra, A.; Iovine, V.; Botta, B.; Quaglio, D.; D’Acquarica, I.; Ciogli, A.; Iazzetti, A.; Alfonsi, R.; Lospinoso Severini, L.; Infante, P.; et al. Chemical, computational and functional insights into the chemical stability of the Hedgehog pathway inhibitor GANT61. J. Enzym. Inhib. Med. Chem. 2018, 33, 349–358. [Google Scholar] [CrossRef]
- Agyeman, A.; Jha, B.K.; Mazumdar, T.; Houghton, J.A. Mode and specificity of binding of the small molecule GANT61 to GLI determines inhibition of GLI-DNA binding. Oncotarget 2014, 5, 4492–4503. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Ferrandon, S.; Glowacki, K.J.; Houghton, J.A. Targeting GLI by GANT61 involves mechanisms dependent on inhibition of both transcription and DNA licensing. Oncotarget 2016, 7, 80190–80207. [Google Scholar] [CrossRef]
- Borah, A.; Palaninathan, V.; Girija, A.R.; Balasubramanian, S.; Rochani, A.K. Poly-lactic-co-glycolic acid Nanoformulation of Small Molecule Antagonist GANT61 for Cancer Annihilation by Modulating Hedgehog Pathway. Nano World J. 2017, 3, 10. [Google Scholar] [CrossRef]
- Infante, P.; Mori, M.; Alfonsi, R.; Ghirga, F.; Aiello, F.; Toscano, S.; Ingallina, C.; Siler, M.; Cucchi, D.; Po, A.; et al. Gli1/DNA interaction is a druggable target for Hedgehog-dependent tumors. EMBO J. 2015, 34, 200–217. [Google Scholar] [CrossRef]
- Infante, P.; Malfanti, A.; Quaglio, D.; Balducci, S.; De Martin, S.; Bufalieri, F.; Mastrotto, F.; Basili, I.; Garofalo, M.; Lospinoso Severini, L.; et al. Glabrescione B delivery by self-assembling micelles efficiently inhibits tumor growth in preclinical models of Hedgehog-dependent medulloblastoma. Cancer Lett. 2021, 499, 220–231. [Google Scholar] [CrossRef]
- Berardozzi, S.; Bernardi, F.; Infante, P.; Ingallina, C.; Toscano, S.; De Paolis, E.; Alfonsi, R.; Caimano, M.; Botta, B.; Mori, M.; et al. Synergistic inhibition of the Hedgehog pathway by newly designed Smo and Gli antagonists bearing the isoflavone scaffold. Eur. J. Med. Chem. 2018, 156, 554–562. [Google Scholar] [CrossRef]
- Lospinoso Severini, L.; Quaglio, D.; Basili, I.; Ghirga, F.; Bufalieri, F.; Caimano, M.; Balducci, S.; Moretti, M.; Romeo, I.; Loricchio, E.; et al. A Smo/Gli Multitarget Hedgehog Pathway Inhibitor Impairs Tumor Growth. Cancers 2019, 11, 1518. [Google Scholar] [CrossRef]
- Hom, M.E.; Ondrus, A.E.; Sakata-Kato, T.; Rack, P.G.; Chen, J.K. Bicyclic Imidazolium Inhibitors of Gli Transcription Factor Activity. ChemMedChem 2020, 15, 1044–1049. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. On the discovery, biological effects, and use of Cisplatin and metallocenes in anticancer chemotherapy. Bioinorg. Chem. Appl. 2012, 2012, 140284. [Google Scholar] [CrossRef] [Green Version]
- Orvig, C.; Abrams, M.J. Medicinal Inorganic Chemistry: Introduction. Chem. Rev. 1999, 99, 2201–2204. [Google Scholar] [CrossRef]
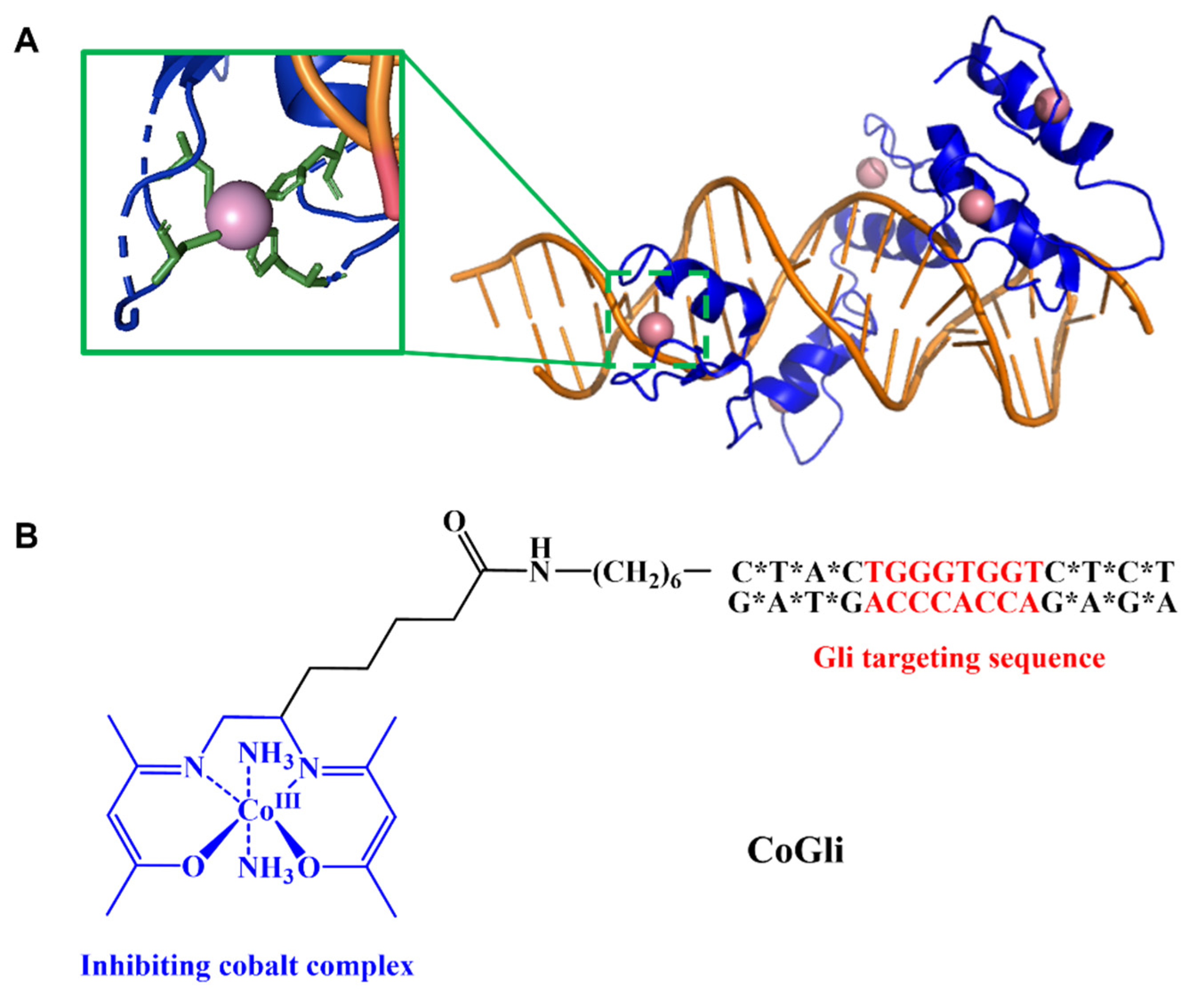
- Dukes, M.W.; Bajema, E.A.; Whittemore, T.J.; Holmgren, R.A.; Meade, T.J. Delivery of Targeted Co(III)–DNA Inhibitors of Gli Proteins to Disrupt Hedgehog Signaling. Bioconjug. Chem. 2022, 33, 643–653. [Google Scholar] [CrossRef]
- Brue, C.R.; Dukes, M.W.; Masotti, M.; Holmgren, R.; Meade, T.J. Functional Disruption of Gli1-DNA Recognition via a Cobalt(III) Complex. ChemMedChem 2022, 17, e202200025. [Google Scholar] [CrossRef]
- Manus, L.M.; Holbrook, R.J.; Atesin, T.A.; Heffern, M.C.; Harney, A.S.; Eckermann, A.L.; Meade, T.J. Axial Ligand Exchange of N-heterocyclic Cobalt(III) Schiff Base Complexes: Molecular Structure and NMR Solution Dynamics. Inorg. Chem. 2013, 52, 1069–1076. [Google Scholar] [CrossRef]
- Harney, A.S.; Lee, J.; Manus, L.M.; Wang, P.; Ballweg, D.M.; LaBonne, C.; Meade, T.J. Targeted inhibition of Snail family zinc finger transcription factors by oligonucleotide-Co(III) Schiff base conjugate. Proc. Natl. Acad. Sci. USA 2009, 106, 13667–13672. [Google Scholar] [CrossRef]
- Harney, A.S.; Meade, T.J.; LaBonne, C. Targeted Inactivation of Snail Family EMT Regulatory Factors by a Co(III)-Ebox Conjugate. PLoS ONE 2012, 7, e32318. [Google Scholar] [CrossRef]
- Hurtado, R.R.; Harney, A.S.; Heffern, M.C.; Holbrook, R.J.; Holmgren, R.A.; Meade, T.J. Specific Inhibition of the Transcription Factor Ci by a Cobalt(III) Schiff Base–DNA Conjugate. Mol. Pharm. 2012, 9, 325–333. [Google Scholar] [CrossRef]
- Sasaki, H.; Nishizaki, Y.; Hui, C.-c.; Nakafuku, M.; Kondoh, H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: Implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 1999, 126, 3915–3924. [Google Scholar] [CrossRef]
- Yun, T.; Wang, J.; Yang, J.; Huang, W.; Lai, L.; Tan, W.; Liu, Y. Discovery of Small Molecule Inhibitors Targeting the Sonic Hedgehog. Front. Chem. 2020, 8, 498. [Google Scholar] [CrossRef]
- Riobó, N.A.; Lu, K.; Ai, X.; Haines, G.M.; Emerson, C.P., Jr. Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 4505–4510. [Google Scholar] [CrossRef] [Green Version]
- Gordon, R.E.; Zhang, L.; Peri, S.; Kuo, Y.-M.; Du, F.; Egleston, B.L.; Ng, J.M.Y.; Andrews, A.J.; Astsaturov, I.; Curran, T.; et al. Statins Synergize with Hedgehog Pathway Inhibitors for Treatment of Medulloblastoma. Clin. Cancer Res. 2018, 24, 1375–1388. [Google Scholar] [CrossRef]
- Bijlsma, M.F.; Spek, C.A.; Zivkovic, D.; van de Water, S.; Rezaee, F.; Peppelenbosch, M.P. Repression of Smoothened by Patched-Dependent (Pro-)Vitamin D3 Secretion. PLoS Biol. 2006, 4, e232. [Google Scholar] [CrossRef]
- Kinto, N.; Iwamoto, M.; Enomoto-Iwamoto, M.; Noji, S.; Ohuchi, H.; Yoshioka, H.; Kataoka, H.; Wada, Y.; Yuhao, G.; Takahashi, H.E.; et al. Fibroblasts expressing Sonic hedgehog induce osteoblast differentiation and ectopic bone formation. FEBS Lett. 1997, 404, 319–323. [Google Scholar] [CrossRef]
- Nakamura, T.; Aikawa, T.; Iwamoto-Enomoto, M.; Iwamoto, M.; Higuchi, Y.; Maurizio, P.; Kinto, N.; Yamaguchi, A.; Noji, S.; Kurisu, K.; et al. Induction of Osteogenic Differentiation by Hedgehog Proteins. Biochem. Biophys. Res. Commun. 1997, 237, 465–469. [Google Scholar] [CrossRef]
- Spinella-Jaegle, S.; Rawadi, G.; Kawai, S.; Gallea, S.; Faucheu, C.; Mollat, P.; Courtois, B.; Bergaud, B.; Ramez, V.; Blanchet, A.M.; et al. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J. Cell Sci. 2001, 114 Pt 11, 2085–2094. [Google Scholar] [CrossRef]
- Ingram, W.J.; Wicking, C.A.; Grimmond, S.M.; Forrest, A.R.; Wainwright, B.J. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene 2002, 21, 8196–8205. [Google Scholar] [CrossRef]
- Oro, A.E.; Higgins, K.M.; Hu, Z.; Bonifas, J.M.; Epstein, E.H.; Scott, M.P. Basal Cell Carcinomas in Mice Overexpressing Sonic Hedgehog. Science 1997, 276, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Murone, M.; Luoh, S.-M.; Ryan, A.; Gu, Q.; Zhang, C.; Bonifas, J.M.; Lam, C.-W.; Hynes, M.; Goddard, A.; et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 1998, 391, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Aszterbaum, M.; Epstein, J.; Oro, A.; Douglas, V.; LeBoit, P.E.; Scott, M.P.; Epstein, E.H. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat. Med. 1999, 5, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Nitzki, F.; Becker, M.; Frommhold, A.; Schulz-Schaeffer, W.; Hahn, H. Patched knockout mouse models of Basal cell carcinoma. J. Ski. Cancer 2012, 2012, 907543. [Google Scholar] [CrossRef] [Green Version]
- Goodrich Lisa, V.; Milenković, L.; Higgins Kay, M.; Scott Matthew, P. Altered Neural Cell Fates and Medulloblastoma in Mouse patched Mutants. Science 1997, 277, 1109–1113. [Google Scholar] [CrossRef]
- Hahn, H.; Wojnowski, L.; Zimmer, A.M.; Hall, J.; Miller, G.; Zimmer, A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat. Med. 1998, 4, 619–622. [Google Scholar] [CrossRef]
- Collins, A.T.; Lang, S.H. A systematic review of the validity of patient derived xenograft (PDX) models: The implications for translational research and personalised medicine. PeerJ 2018, 6, e5981. [Google Scholar] [CrossRef]
- Pawlowski, A.; Haberman, H.F. Heterotransplantation of human basal cell carcinomas in “nude” mice. J. Investig. Dermatol. 1979, 72, 310–313. [Google Scholar] [CrossRef]
- Grimwood, R.E.; Johnson, C.A.; Ferris, C.F.; Mercill, D.B.; Mellette, J.R.; Huff, J.C. Transplantation of human basal cell carcinomas to athymic mice. Cancer 1985, 56, 519–523. [Google Scholar] [CrossRef]
- Grimwood, R.E.; Tharp, M.D. Growth of Human Basal Cell Carcinomas Transplanted to C57/Balb/C bgJ/bgJ-nu/nu (Beige-Nude) Mice. J. Dermatol. Surg. Oncol. 1991, 17, 661–666. [Google Scholar] [CrossRef]
- Carlson, J.A.; Combates, N.J.; Stenn, K.S.; Prouty, S.M. Anaplastic neoplasms arising from basal cellcarcinoma xenotransplants into SCID-beige mice. J. Cutan. Pathol. 2002, 29, 268–278. [Google Scholar] [CrossRef]
- Wang, G.Y.; So, P.-L.; Wang, L.; Libove, E.; Wang, J.; Epstein, E.H. Establishment of Murine Basal Cell Carcinoma Allografts: A Potential Model for Preclinical Drug Testing and for Molecular Analysis. J. Investig. Dermatol. 2011, 131, 2298–2305. [Google Scholar] [CrossRef]
- Jiang, L.P.; Shen, Q.S.; Yang, C.P.; Chen, Y.B. Establishment of basal cell carcinoma animal model in Chinese tree shrew (Tupaia belangeri chinensis). Zool. Res. 2017, 38, 180–190. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Liang, B.; Lü, L.B.; Chen, C.S.; Chen, Y.B.; Zhou, J.M.; Yao, Y.G. Tree shrews under the spot light: Emerging model of human diseases. Dongwuxue Yanjiu 2013, 34, 59–69. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, R.C.; Man, X.Y.; Lv, L.B.; Yao, Y.G.; Zheng, M. The anatomy of the skin of the Chinese tree shrew is very similar to that of human skin. Zool. Res. 2020, 41, 208. [Google Scholar] [CrossRef]
- Gaviria Agudelo, C.; Restrepo, L.M. Human Skin Cancer: An Overview of Animal, Ex Vivo, and In Vitro Models. Curr. Dermatol. Rep. 2022, 11, 168–177. [Google Scholar] [CrossRef]



| Treatment | Advantages | Disadvantages | Patient Restrictions |
|---|---|---|---|
| Wide Local Excision (WLE) | Low recurrence rates upon complete excision Short procedure times | Excision likely to be incomplete and lead to higher recurrence rate Highly invasive | Elderly patients where surgery is considered risky When loss of anatomical function is a risk |
| Mohs Micrographic Surgery (MMS) | Promotes excision of poorly defined tumor margins Suggested for more aggressive/high-risk tumors Minimizes harm to non-diseased tissue | Long treatment times Requires highly trained physicians, and access may be limited to patients in underdeveloped areas | Elderly patients where surgery is considered risky When loss of anatomical function is a risk |
| Radiation Therapy | Can treat tumors in locations where the loss of anatomical function is a risk Can be used in higher-risk BCC Boosts the efficacy of incomplete surgical resection when used in tandem | Use of ionizing radiation Increases risk of melanoma Not as effective in larger Tumors Cannot determine the complete clearance of tumor tissue | Not advised for patients with Gorlin Syndrome Not advised for younger patients due to the long-term impact of ionizing radiation |
| Ablative Laser Therapy | Locally delivered, less destructive to non-diseased tissue Low recurrence rates Favorable cosmetic outcomes | Not applicable to larger, deeper tissues May induce increased sensitivity to the sun | Not recommended for patients with high sunlight sensitivity Not applicable for patients with nodular or infiltrative BCC |
| Photodynamic Therapy (PDT) | Well characterized mechanisms of cytotoxicity Local administration of non-harmful laser light Safe for patients with Gorlin Syndrome | High variability of treatment efficacy Multiple treatment sessions Increased sensitivity to Sunlight Severely limited depth penetration | Not recommended for patients with high sunlight sensitivity Not applicable for patients with nodular or infiltrative BCC |
| Imiquimod Topical Therapy | Well-characterized induction of immune response Topical, localized application reduces harm to healthy tissue | Only approved for small superficial BCCs Many patients report skin Irritation Recurrence rates understudied | Not applicable for patients with nodular or infiltrative BCC |
| 5-Fluorouracil Topical Therapy | Well-characterized inhibition of DNA synthesis Topical, localized application reduces harm to healthy tissue High cure rate | Not specific to tumor tissue and may cause harm to non-diseased skin Many patients report skin Irritation Recurrence rates understudied | Not applicable for patients with nodular or infiltrative BCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dukes, M.W.; Meade, T.J. Modulation of Hedgehog Signaling for the Treatment of Basal Cell Carcinoma and the Development of Preclinical Models. Biomedicines 2022, 10, 2376. https://doi.org/10.3390/biomedicines10102376
Dukes MW, Meade TJ. Modulation of Hedgehog Signaling for the Treatment of Basal Cell Carcinoma and the Development of Preclinical Models. Biomedicines. 2022; 10(10):2376. https://doi.org/10.3390/biomedicines10102376
Chicago/Turabian StyleDukes, Meghan W., and Thomas J. Meade. 2022. "Modulation of Hedgehog Signaling for the Treatment of Basal Cell Carcinoma and the Development of Preclinical Models" Biomedicines 10, no. 10: 2376. https://doi.org/10.3390/biomedicines10102376
APA StyleDukes, M. W., & Meade, T. J. (2022). Modulation of Hedgehog Signaling for the Treatment of Basal Cell Carcinoma and the Development of Preclinical Models. Biomedicines, 10(10), 2376. https://doi.org/10.3390/biomedicines10102376








