Comparable Outcomes in Early Hepatocellular Carcinomas Treated with Trans-Arterial Chemoembolization and Radiofrequency Ablation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Selection of Primary Treatment Modality
2.3. Trans-Arterial Chemoembolization
2.4. Radiofrequency Ablation
2.5. Patient Follow-Up
2.6. Evaluation of Data
2.7. Statistical Analysis
3. Results
3.1. Baseline Charateristics
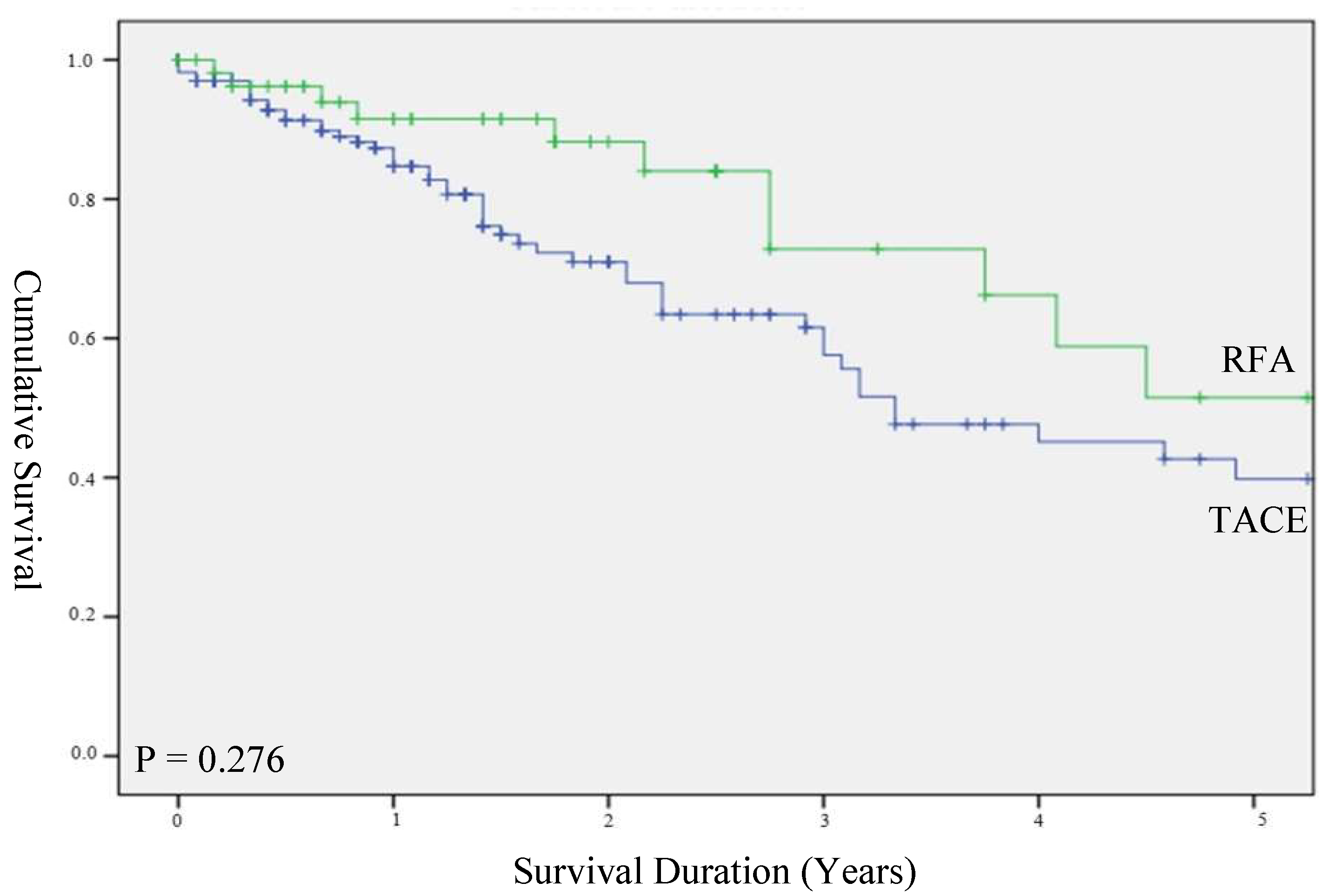
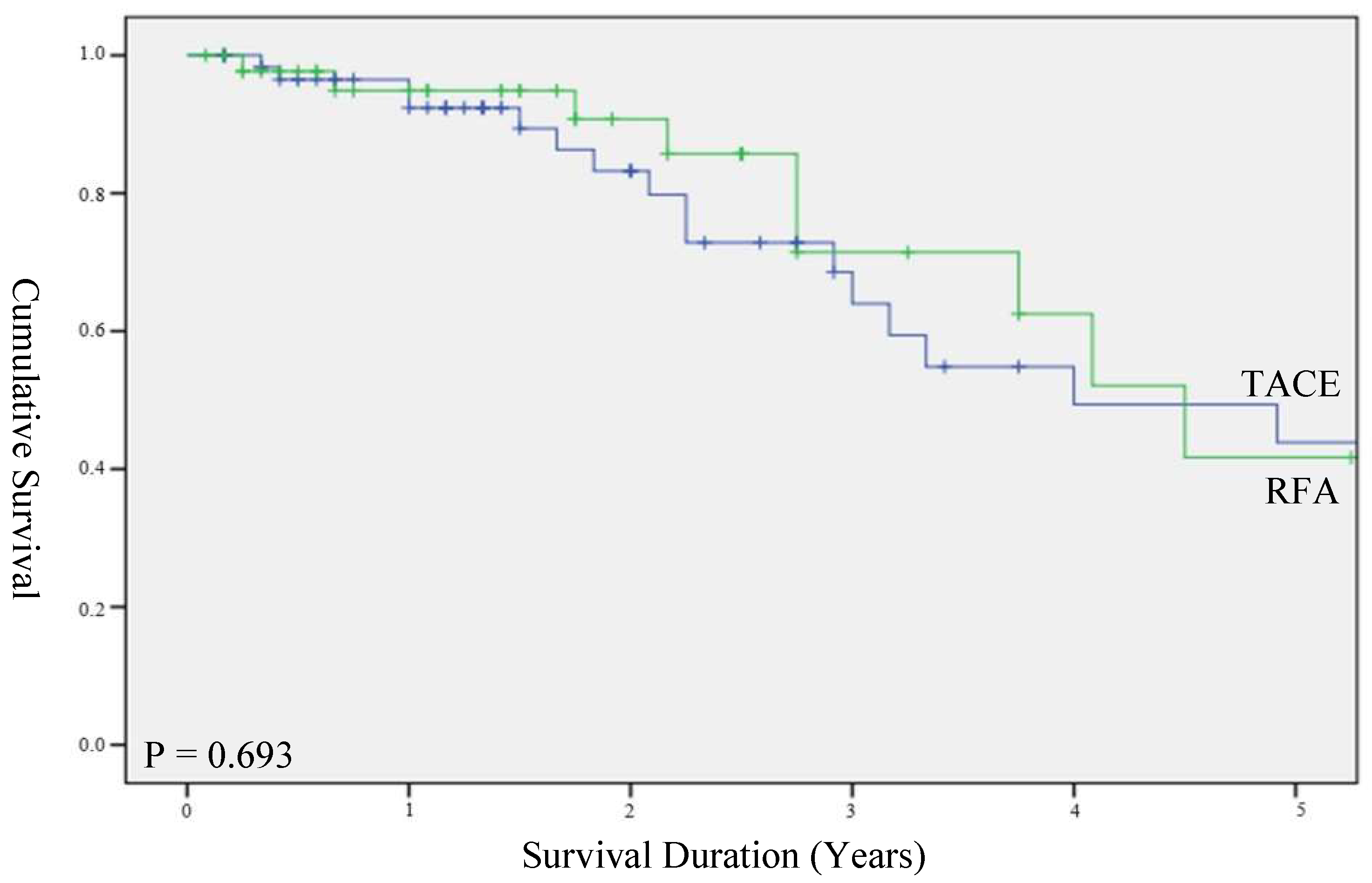
3.2. Survival
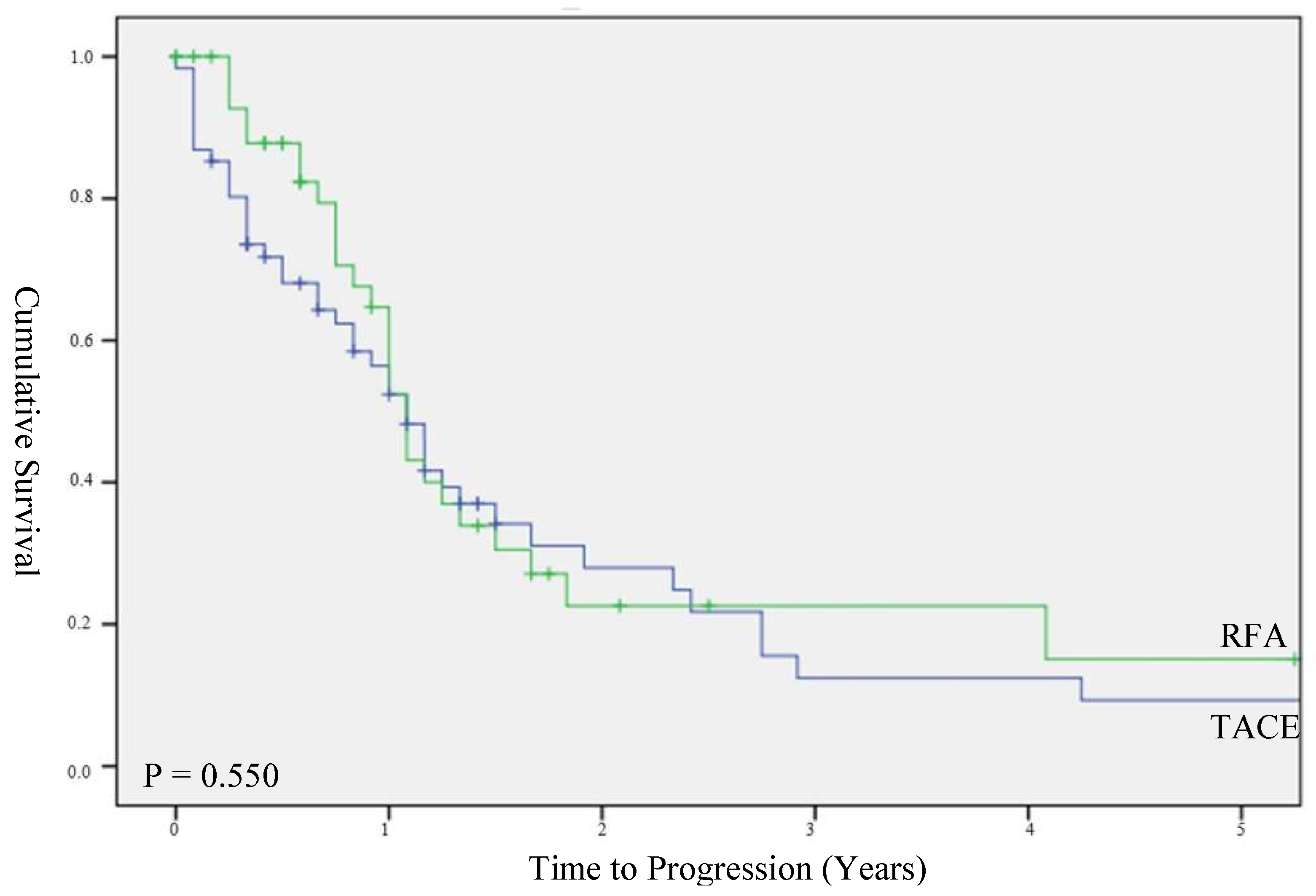
3.3. Time to Progression
3.4. Chance of Complete Response
3.5. Recurrence
3.6. Number of Treatments
3.7. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 14 September 2022).
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 14 September 2022).
- Reveron-Thornton, R.F.; Teng, M.L.P.; Lee, E.Y.; Tran, A.; Vajanaphanich, S.; Tan, E.X.; Nerurkar, S.N.; Ng, R.X.; Teh, R.; Tripathy, D.P.; et al. Global and regional long-term survival following resection for HCC in the recent decade: A meta-analysis of 110 studies. Hepatol. Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Tan, D.J.H.; Ong, Y.; Lim, W.H.; Ng, C.H.; Tay, P.W.L.; Yong, J.N.; Muthiah, M.D.; Tan, E.X.; Pang, N.Q.; et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: A meta-analysis of 18,421 patients. Hepatobiliary Surg. Nutr. 2022, 11, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Lim, W.; Yong, J.N.; Ng, C.H.; Muthiah, M.D.; Tan, E.X.; Xiao, J.; Lim, S.Y.; Tang, A.S.P.; Pan, X.H.; et al. UNOS Down-Staging Criteria for Liver Transplantation of Hepatocellular Carcinoma: Systematic Review and Meta-Analysis of 25 Studies. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef]
- Huang, D.Q.; Muthiah, M.D.; Zhou, L.; Jumat, H.; Tan, W.X.; Lee, G.H.; Lim, S.G.; Kow, A.; Bonney, G.; Shridhar, I.; et al. Predicting HCC Response to Multikinase Inhibitors With In Vivo Cirrhotic Mouse Model for Personalized Therapy. Cell. Mol. Gastroenterol. Hepatol. 2020, 11, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, H.; Huang, D.Q.; Xu, C.; Zheng, H.; Maeda, M.; Zhao, X.; Wang, L.; Xiao, F.; Lv, H.; et al. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. Eur. Radiol. 2020, 30, 6357–6368. [Google Scholar] [CrossRef]
- Teratani, T.; Yoshida, H.; Shiina, S.; Obi, S.; Sato, S.; Tateishi, R.; Mine, N.; Kondo, Y.; Kawabe, T.; Omata, M. Radiofrequency Ablation for Hepatocellular Carcinoma in So-Called High-Risk Locations. Hepatology 2006, 43, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Komorizono, Y.; Oketani, M.; Sako, K.; Yamasaki, N.; Shibatou, T.; Maeda, M.; Kohara, K.; Shigenobu, S.; Ishibashi, K.; Arima, T. Risk Factors for Local Recurrence of Small Hepatocellular Carcinoma Tumors after a Single Session, Single Application of Percutaneous Radiofrequency Ablation. Cancer 2003, 97, 1253–1262. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Huang, Y.-H.; Chiou, Y.-Y.; Su, C.-W.; Lin, H.-C.; Lee, R.-C.; Chiang, J.-H.; Huo, T.-I.; Lee, F.-Y.; Lee, S.-D. Comparison of Radiofrequency Ablation and Transarterial Chemoembolization for Hepatocellular Carcinoma within the Milan Criteria: A Propensity Score Analysis. Liver Transpl. 2011, 17, 556–566. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.H.; Sung, K.-B.; Ko, H.-K.; Shin, J.H.; Kim, P.N.; Choi, H.-K.; Ko, G.-Y.; Yoon, H.-K.; Chun, S.-Y.; et al. Transarterial Chemoembolization vs. Radiofrequency Ablation for the Treatment of Single Hepatocellular Carcinoma 2 Cm or Smaller. Am. J. Gastroenterol. 2014, 109, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, N.H.; Kim, J.D.; Kim, Y.N.; Kim, Y.J.; Kim, E.J.; Yoo, K.D.; Ryu, C.H.; Song, H.H.; Kim, H. Transarterial Chemoembolization Using Drug-Eluting Bead Compared with Radiofrequency Ablation for Treatment of Single Small Hepatocellular Carcinoma: A Pilot Non-Randomized Trial. J. Liver Cancer 2021, 21, 146–154. [Google Scholar] [CrossRef]
- Attwa, M.H.; El-Etreby, S.A. Guide for Diagnosis and Treatment of Hepatocellular Carcinoma. World J. Hepatol. 2015, 7, 1632–1651. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.A.; Marra, P.; Beaty, K.; Ellis, L.M.; Vauthey, J.N.; Abdalla, E.K.; Scaife, C.; Raut, C.; Wolff, R.; Choi, H.; et al. Early and Late Complications After Radiofrequency Ablation of Malignant Liver Tumors in 608 Patients. Ann. Surg. 2004, 239, 450–458. [Google Scholar] [CrossRef]
- Huang, H.-W. Influence of Blood Vessel on the Thermal Lesion Formation during Radiofrequency Ablation for Liver Tumors. Med. Phys. 2013, 40, 073303. [Google Scholar] [CrossRef]
- Llovet, J.M.; Vilana, R.; Brú, C.; Bianchi, L.; Salmeron, J.M.; Boix, L.; Ganau, S.; Sala, M.; Pagès, M.; Ayuso, C.; et al. Increased Risk of Tumor Seeding after Percutaneous Radiofrequency Ablation for Single Hepatocellular Carcinoma. Hepatology 2001, 33, 1124–1129. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-Analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Park, J.-W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.-J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global Patterns of Hepatocellular Carcinoma Management from Diagnosis to Death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef]
- Liu, K.; Min, X.-L.; Peng, J.; Yang, K.; Yang, L.; Zhang, X.-M. The Changes of HIF-1α and VEGF Expression After TACE in Patients With Hepatocellular Carcinoma. J. Clin. Med. Res. 2016, 8, 297–302. [Google Scholar] [CrossRef]
- Sumie, S.; Kuromatsu, R.; Okuda, K.; Ando, E.; Takata, A.; Fukushima, N.; Watanabe, Y.; Kojiro, M.; Sata, M. Microvascular Invasion in Patients with Hepatocellular Carcinoma and Its Predictable Clinicopathological Factors. Ann. Surg. Oncol. 2008, 15, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Greig, P.D.; Gallinger, S.; Cattral, M.S.; Dixon, E.; Kim, R.D.; Taylor, B.R.; Grant, D.R.; Vollmer, C.M. Factors Associated with Early Recurrence after Resection for Hepatocellular Carcinoma and Outcomes. J. Am. Coll Surg. 2006, 202, 275–283. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, C.; Feng, K.; Zhang, Y.; Chen, M.; Guo, R.; Lin, X.; Li, J. Micrometastasis distribution in liver tissue surrounding hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2002, 24, 257–260. [Google Scholar] [PubMed]
- Chen, M.-S.; Li, J.-Q.; Zheng, Y.; Guo, R.-P.; Liang, H.-H.; Zhang, Y.-Q.; Lin, X.-J.; Lau, W.Y. A Prospective Randomized Trial Comparing Percutaneous Local Ablative Therapy and Partial Hepatectomy for Small Hepatocellular Carcinoma. Ann. Surg. 2006, 243, 321–328. [Google Scholar] [CrossRef]
- Dehn, T. Optimal Treatment for Hepatocellular Carcinoma in the Cirrhotic Liver. Ann. R Coll Surg. Engl. 2009, 91, 545–550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk Factors Contributing to Early and Late Phase Intrahepatic Recurrence of Hepatocellular Carcinoma after Hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Turon, F.; Berzigotti, A.; Villanueva, A. Management of Small Hepatocellular Carcinoma in Cirrhosis: Focus on Portal Hypertension. World J. Gastroenterol. 2013, 19, 1193–1199. [Google Scholar] [CrossRef]

| TACE | RFA | p Value | |||
|---|---|---|---|---|---|
| Number of patients | 168 | 56 | |||
| Age, median (IQR) | 68 | (57–75) | 65 | (59–70) | 0.173 |
| Gender (%) | |||||
| Male | 123 | (73.2%) | 43 | (76.8%) | 0.597 |
| Female | 45 | (26.8%) | 13 | (23.2%) | |
| Ethnicity (%) | |||||
| Chinese | 95 | (56.5%) | 45 | (80.4%) | 0.012 |
| Malay | 9 | (5.4%) | 2 | (3.5%) | |
| Indian | 6 | (3.6%) | 0 | - | |
| Others | 58 | (34.5%) | 9 | (16.1%) | |
| Cirrhosis (%) | |||||
| Yes | 167 | (86.8%) | 47 | (83.9%) | 0.564 |
| No | 22 | (13.1%) | 8 | (14.3%) | |
| Unknown | 1 | (0.6%) | 1 | (1.8%) | |
| Etiology of underlying liver disease (% of total etiologies) | |||||
| 213 cases have a single etiology while 11 cases have multiple etiologies. | |||||
| Hepatitis B | 78 | 23 | 0.002 | ||
| Hepatitis C | 34 | 13 | |||
| Alcoholic Cirrhosis | 11 | 12 | |||
| NAFLD | 12 | 8 | |||
| Autoimmune Hepatitis | 1 | 1 | |||
| Wilson’s disease | 2 | 0 | |||
| Primary Biliary Cirrhosis | 0 | 2 | |||
| Etiology not known | 35 | 4 | |||
| Biochemistry | |||||
| Alpha-fetoprotein (ng/mL) | 18.0 | (5.0–122.0) | 12.5 | (7.0–58.0) | 0.460 |
| Prothrombin Time (s) | 14.0 | (13.0–16.0) | 15.0 | (14.0–16.0) | 0.193 |
| Platelet × 109/L | 128.0 | (91.0–211.0) | 132.5 | (82.5–171.3) | 0.339 |
| Total Bilirubin (umol/L) | 16.0 | (10.0–26.25) | 17.0 | (11.0–33.75) | 0.283 |
| Albumin (g/L) | 36.0 | (31.0–40.0) | 36.0 | (31.0–40.0) | 0.870 |
| Child-Pugh Score (%) | |||||
| A | 124 | (73.8%) | 41 | (73.2%) | 0.930 |
| B | 44 | (26.2%) | 15 | (26.8%) | |
| Tumor Nodularity | |||||
| Uninodular | 95 | (56.5%) | 34 | (60.7%) | 0.748 |
| Multinodular | 71 | (43.5%) | 22 | (39.3%) | |
| Diffuse | 2 | (1.2%) | 0 | - | |
| Primary nodule characteristics | |||||
| Median Size (cm) | 3.8 | (2.2–6.2) | 2.10 | (1.5–2.7) | <0.001 |
| TACE | RFA | p Value | |||
|---|---|---|---|---|---|
| Number of patients | 62 | 45 | 0.100 | ||
| Age | 64 | (57–72) | 65 | (59–70) | 0.880 |
| Gender (%) | |||||
| Male | 40 | (64.5%) | 34 | (75.6%) | 0.222 |
| Female | 22 | (35.5%) | 11 | (24.4%) | |
| Ethnicity (%) | |||||
| Chinese | 34 | (54.8%) | 37 | (82.2%) | 0.018 |
| Malay | 3 | (4.8%) | 2 | (4.4%) | |
| Indian | 3 | (4.8%) | 0 | - | |
| Others | 22 | (35.5%) | 6 | (13.3%) | |
| Etiology of underlying liver disease (% of total etiologies) 101 cases had single etiology while 6 cases had multiple etiologies | |||||
| Hepatitis B | 24 | 19 | 0.2184 | ||
| Hepatitis C | 18 | 10 | |||
| Alcoholic Cirrhosis | 7 | 10 | |||
| NAFLD | 6 | 6 | |||
| Autoimmune Hepatitis | 0 | 1 | |||
| Wilson’s disease | 1 | 0 | |||
| Primary Biliary Cirrhosis | 0 | 2 | |||
| Etiology not known | 6 | 1 | |||
| Biochemistry | |||||
| Alpha-fetoprotein (ng/mL) | 16.5 | (5.8–99.0) | 12.0 | (7.0–46.0) | 0.781 |
| Prothrombin Time (s) | 14.0 | (13.0–15.0) | 15.0 | (14.0–15.0) | 0.229 |
| Platelet × 109/L | 108.0 | (73.5–160.5) | 137.0 | (91.0–169.5) | 0.100 |
| Total Bilirubin (umol/L) | 15.0 | (11.75–27.0) | 15.0 | (10.0–27.0) | 0.865 |
| Albumin (g/L) | 37.0 | (31.5–40.5) | 36.4 | (32.5–40.0) | 0.597 |
| Tumor Nodularity | |||||
| Uninodular | 34 | (54.8%) | 26 | (57.8%) | 0.762 |
| Multinodular | 28 | (45.2%) | 19 | (42.2%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, B.W.R.; Huang, D.Q.; Mark, M.; Thong, N.W.; Guan Huei, L.; Gee, L.S.; Cheng, L.H.; Mei, L.Y.; Thurairajah, P.; Chen, L.J.; et al. Comparable Outcomes in Early Hepatocellular Carcinomas Treated with Trans-Arterial Chemoembolization and Radiofrequency Ablation. Biomedicines 2022, 10, 2361. https://doi.org/10.3390/biomedicines10102361
Tay BWR, Huang DQ, Mark M, Thong NW, Guan Huei L, Gee LS, Cheng LH, Mei LY, Thurairajah P, Chen LJ, et al. Comparable Outcomes in Early Hepatocellular Carcinomas Treated with Trans-Arterial Chemoembolization and Radiofrequency Ablation. Biomedicines. 2022; 10(10):2361. https://doi.org/10.3390/biomedicines10102361
Chicago/Turabian StyleTay, Benjamin Wei Rong, Daniel Q. Huang, Muthiah Mark, Neo Wee Thong, Lee Guan Huei, Lim Seng Gee, Low How Cheng, Lee Yin Mei, Prem Thurairajah, Lim Jia Chen, and et al. 2022. "Comparable Outcomes in Early Hepatocellular Carcinomas Treated with Trans-Arterial Chemoembolization and Radiofrequency Ablation" Biomedicines 10, no. 10: 2361. https://doi.org/10.3390/biomedicines10102361
APA StyleTay, B. W. R., Huang, D. Q., Mark, M., Thong, N. W., Guan Huei, L., Gee, L. S., Cheng, L. H., Mei, L. Y., Thurairajah, P., Chen, L. J., Ng, C. H., Lim, W. H., Tan, D. J. H., Maureen, D. C., Alfred, K. W. C., Ganpathi, I. S., Seng, T. P., & Young, D. Y. (2022). Comparable Outcomes in Early Hepatocellular Carcinomas Treated with Trans-Arterial Chemoembolization and Radiofrequency Ablation. Biomedicines, 10(10), 2361. https://doi.org/10.3390/biomedicines10102361






