Plasma Cytokines Level and Spinal Cord MRI Predict Clinical Outcome in a Rat Glial Scar Cryoinjury Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Surgical Approach to Spinal Trauma
2.3. Treadmill Exercise Test
2.4. Magnetic Resonance Imaging of the Spine
2.5. Computed Tomography Scans of the Spine
2.6. Plasma Cytokine Measurement
2.7. Histological Analysis
2.8. Statistical Analysis
3. Results
3.1. Spinal Cord Cryoinjury Model in SD Rats
3.2. Histological Analysis of the Spinal Cord Cryoinjury
3.3. Magnetic Resonance Imaging of the Spinal Cord Cryoinjury
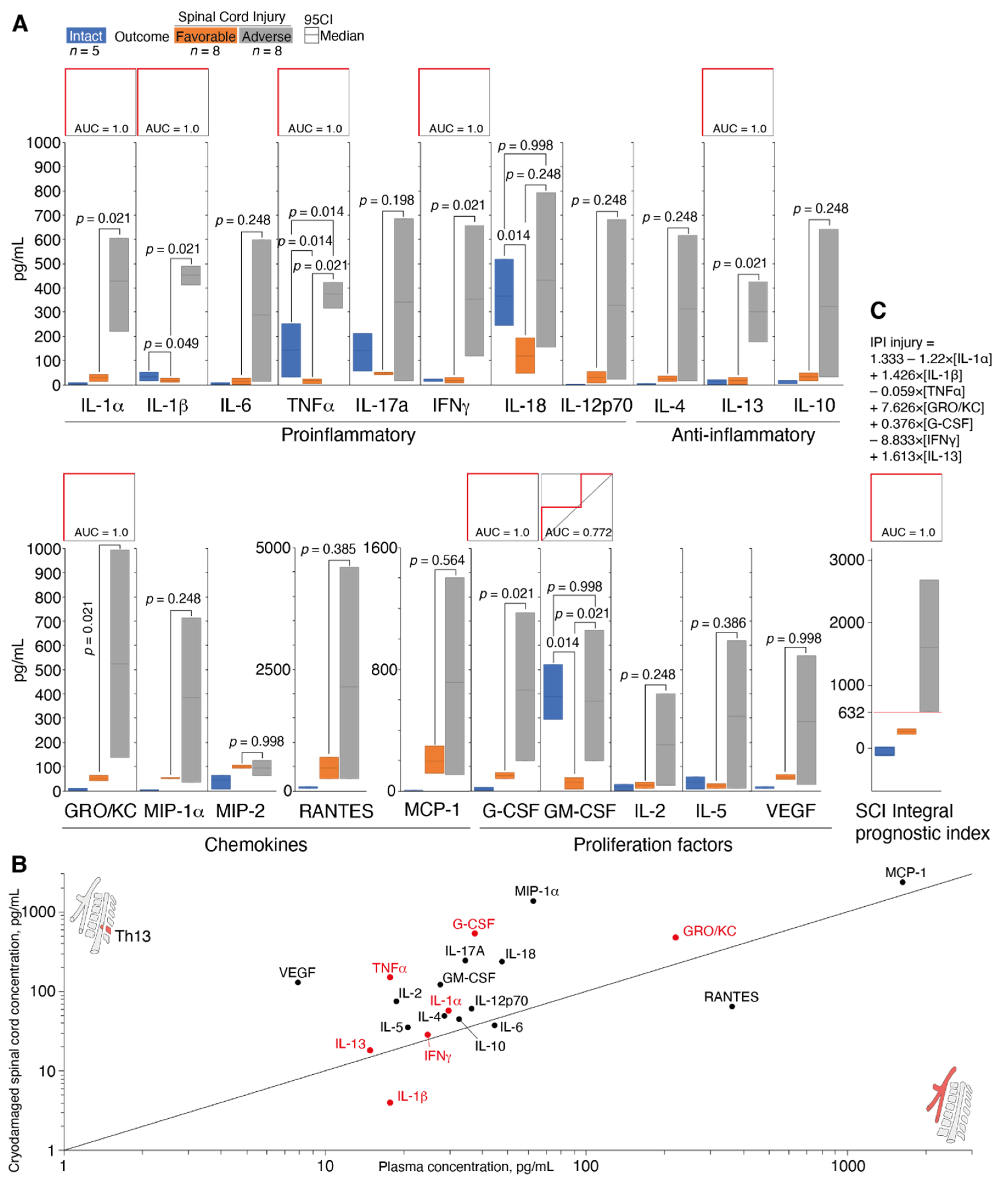
3.4. Determination of Cytokine Status during Spinal Cord Cryoinjury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oña, A.; Strøm, V.; Lee, B.S.; Le Fort, M.; Middleton, J.; Gutenbrunner, C.; Pacheco Barzallo, D. Health inequalities and income for people with spinal cord injury. A comparison between and within countries. SSM Popul. Health 2021, 15, 100854. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, M.; Schwartz, O.S.; Kasch, H.; Rasmussen, M.M. Early decompressive surgery in patients with traumatic spinal cord injury improves neurological outcome. Acta Neurochir. 2019, 161, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Damianakis, E.I.; Benetos, I.S.; Evangelopoulos, D.S.; Kotroni, A.; Vlamis, J.; Pneumaticos, S.G. Stem Cell Therapy for Spinal Cord Injury: A Review of Recent Clinical Trials. Cureus 2022, 14, e24575. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Perret, I.; Bayart, A.; Lackner, P.; Binder, H.; Freundl, B.; Minassian, K. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience 2020, 24, 101930. [Google Scholar] [CrossRef]
- de Paleville, D.G.L.T.; Harkema, S.J.; Angeli, C.A. Epidural stimulation with locomotor training improves body composition in individuals with cervical or upper thoracic motor complete spinal cord injury: A series of case studies. J. Spinal Cord Med. 2019, 42, 32–38. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022, 387, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, S.; Liu, C.; Han, X.; Gu, X.; Zhou, S. Deciphering glial scar after spinal cord injury. Burn. Trauma 2021, 9, tkab035. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J. Psychiatry 2016, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Konsman, J.P. Cytokines in the Brain and Neuroinflammation: We Didn’t Starve the Fire! Pharmaceuticals 2022, 15, 140. [Google Scholar] [CrossRef]
- Ren, H.; Chen, X.; Tian, M.; Zhou, J.; Ouyang, H.; Zhang, Z. Regulation of Inflammatory Cytokines for Spinal Cord Injury Repair Through Local Delivery of Therapeutic Agents. Adv. Sci. 2018, 5, 1800529. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Decker, J.T.; Margul, D.J.; Smith, D.R.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Local Immunomodulation with Anti-inflammatory Cytokine-Encoding Lentivirus Enhances Functional Recovery after Spinal Cord Injury. Mol. Ther. 2018, 26, 1756–1770. [Google Scholar] [CrossRef]
- Telegin, G.B.; Minakov, A.N.; Chernov, A.S.; Manskikh, V.N.; Asyutin, D.S.; Konovalov, N.A.; Gabibov, A.G. Surgical simulation of a posttraumatic spinal cord glial scar in rats. Acta Nat. 2019, 432, 75–81. [Google Scholar] [CrossRef]
- Telegin, G.B.; Minakov, A.N.; Chernov, A.S.; Kazakov, V.A.; Kalabina, E.A.; Manskikh, V.N.; Asyutin, D.S.; Belogurov, A.A., Jr.; Gabibov, A.G.; Konovalov, N.A.; et al. A New Precision Minimally Invasive Method of Glial Scar Simulation in the Rat Spinal Cord Using Cryoapplication. Front. Surg. 2021, 8, 607551. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Martynova, E.V.; Khaiboullina, S.F.; Galieva, L.R.; Rizvanov, A.A. Systemic and Local Cytokine Profile following Spinal Cord Injury in Rats: A Multiplex Analysis. Front. Neurol. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.L.; Farah, M.H. Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders. Neural Regen. Res. 2021, 16, 1901–1910. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Khazaei, M.; Fehlings, M.G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 2015, 218, 15–54. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef]
- Telegin, G.B.; Chernov, A.S.; Konovalov, N.A.; Belogurov, A.A.; Balmasova, I.P.; Gabibov, A.G. Cytokine Profile As a Marker of Cell Damage and Immune Dysfunction after Spinal Cord Injury. Acta Naturae 2020, 12, 92–101. [Google Scholar] [CrossRef]
- Yang, L.; Blumbergs, P.C.; Jones, N.R.; Manavis, J.; Sarvestani, G.T.; Ghabriel, M.N. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine 2004, 29, 966–971. [Google Scholar] [CrossRef]
- Callaway, J.K.; Wood, C.; Jenkins, T.A.; Royse, A.G.; Royse, C.F. Isoflurane in the presence or absence of surgery increases hippocampal cytokines associated with memory deficits and responses to brain injury in rats. Behav. Brain Res. 2016, 303, 44–52. [Google Scholar] [CrossRef]
- Mortazavi, M.M.; Verma, K.; Harmon, O.A.; Griessenauer, C.J.; Adeeb, N.; Theodore, N.; Tubbs, R.S. The microanatomy of spinal cord injury: A review. Clin. Anat. 2015, 28, 27–36. [Google Scholar] [CrossRef]
- Jin, X.; Yamashita, T. Microglia in central nervous system repair after injury. J. Biochem. 2016, 159, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Biglari, B.; Swing, T.; Child, C.; Büchler, A.; Westhauser, F.; Bruckner, T.; Ferbert, T.; Gerner, H.J.; Moghaddam, A. A pilot study on temporal changes in IL-1β and TNF-α serum levels after spinal cord injury: The serum level of TNF-α in acute SCI patients as a possible marker for neurological remission. Spinal Cord 2015, 53, 510–514. [Google Scholar] [CrossRef]
- Yan, P.; Li, Q.; Kim, G.M.; Xu, J.; Hsu, C.Y.; Xu, X.M. Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J. Neurotrauma 2001, 18, 563–568. [Google Scholar] [CrossRef]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [PubMed]
- Paintlia, M.K.; Paintlia, A.S.; Singh, A.K.; Singh, I. Synergistic activity of interleukin-17 and tumor necrosis factor-alpha enhances oxidative stress-mediated oligodendrocyte apoptosis. J. Neurochem. 2011, 116, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, F.X.; Cen, J.S.; Ping, S.N.; Li, Z.Q.; Chen, N.N.; Cui, S.B.; Wan, Y.; Liu, S.Y. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res. 2014, 1575, 87–100. [Google Scholar] [CrossRef]
- Mukaino, M.; Nakamura, M.; Yamada, O.; Okada, S.; Morikawa, S.; Renault-Mihara, F.; Iwanami, A.; Ikegami, T.; Ohsugi, Y.; Tsuji, O. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp. Neurol. 2010, 224, 403. [Google Scholar] [CrossRef]
- Guerrero, A.R.; Uchida, K.; Nakajima, H.; Watanabe, S.; Nakamura, M.; Johnson, W.E.; Baba, H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J. Neuroinflammation 2012, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Ogurcov, S.; Shulman, I.; Garanina, E.; Sabirov, D.; Baichurina, I.; Kuznetcov, M.; Masgutova, G.; Kostennikov, A.; Rizvanov, A.; James, V.; et al. Blood Serum Cytokines in Patients with Subacute Spinal Cord Injury: A Pilot Study to Search for Biomarkers of Injury Severity. Brain Sci. 2021, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, G.; Amin, M.; Shunmugavel, A.; Vazirinejad, R.; Vakilian, A.; Sanji, M.; Shamsizadeh, A.; RafatPanah, H.; Poor, N.M.; Moosavi, S.R.; et al. Temporal expression profile of CXC chemokines in serum of patients with spinal cord injury. Neurochem. Int. 2013, 63, 363–367. [Google Scholar] [CrossRef]
- Aschauer-Wallner, S.; Leis, S.; Bogdahn, U.; Johannesen, S.; Couillard-Despres, S.; Aigner, L. Granulocyte colony-stimulating factor in traumatic spinal cord injury. Drug Discov. Today 2021, 26, 1642–1655. [Google Scholar] [CrossRef] [PubMed]
- Sanli, A.M.; Serbes, G.; Caliskan, M.; Kaptanoglu, E.; Sargon, M.F.; Kilinc, K.; Besalti, O.; Sekerci, Z. Effect of granulocyte-colony stimulating factor on spinal cord tissue after experimental contusion injury. J. Clin. Neurosci. 2010, 17, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Roselli, F.; Chandrasekar, A.; Morganti-Kossmann, M.C. Interferons in Traumatic Brain and Spinal Cord Injury: Current Evidence for Translational Application. Front. Neurol. 2018, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Amo-Aparicio, J.; Garcia-Garcia, J.; Francos-Quijorna, I.; Urpi, A.; Esteve-Codina, A.; Gut, M.; Quintana, A.; Lopez-Nales, R. Interleukin-4 and interleukin-13 induce different metabolic profiles in microglia and macrophages that relate with divergent outcomes after spinal cord injury. Theranostics 2021, 11, 9805–9820. [Google Scholar] [CrossRef]
- Kjell, J.; Olson, L. Rat models of spinal cord injury: From pathology to potential therapies. Dis. Model. Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef]
- Bilgen, M.; Al-Hafez, B.; Malone, T.M.; Smirnova, I.V. Ex vivo magnetic resonance imaging of rat spinal cord at 9.4 T. Magn. Reson. Imaging 2005, 23, 601–605. [Google Scholar] [CrossRef]
- Jirjis, M.B.; Kurpad, S.N.; Schmit, B.D. Ex vivo diffusion tensor imaging of spinal cord injury in rats of varying degrees of severity. J. Neurotrauma 2013, 30, 1577–1586. [Google Scholar] [CrossRef]
- Mogatadakala, K.V.; Narayana, P.A. In vivo diffusion tensor imaging of thoracic and cervical rat spinal cord at 7 T. Magn. Reson. Imaging 2009, 27, 1236–1241. [Google Scholar] [CrossRef] [Green Version]


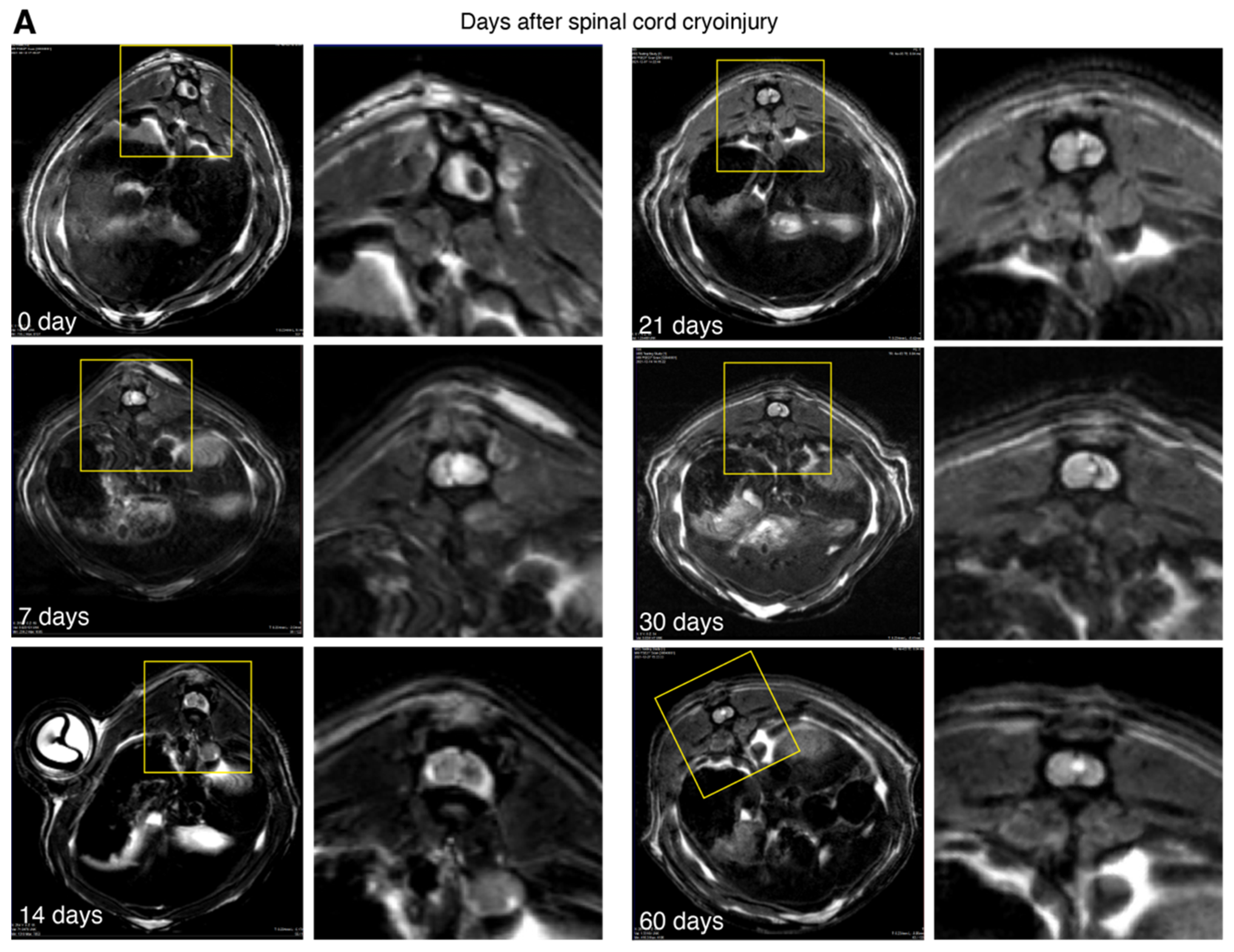
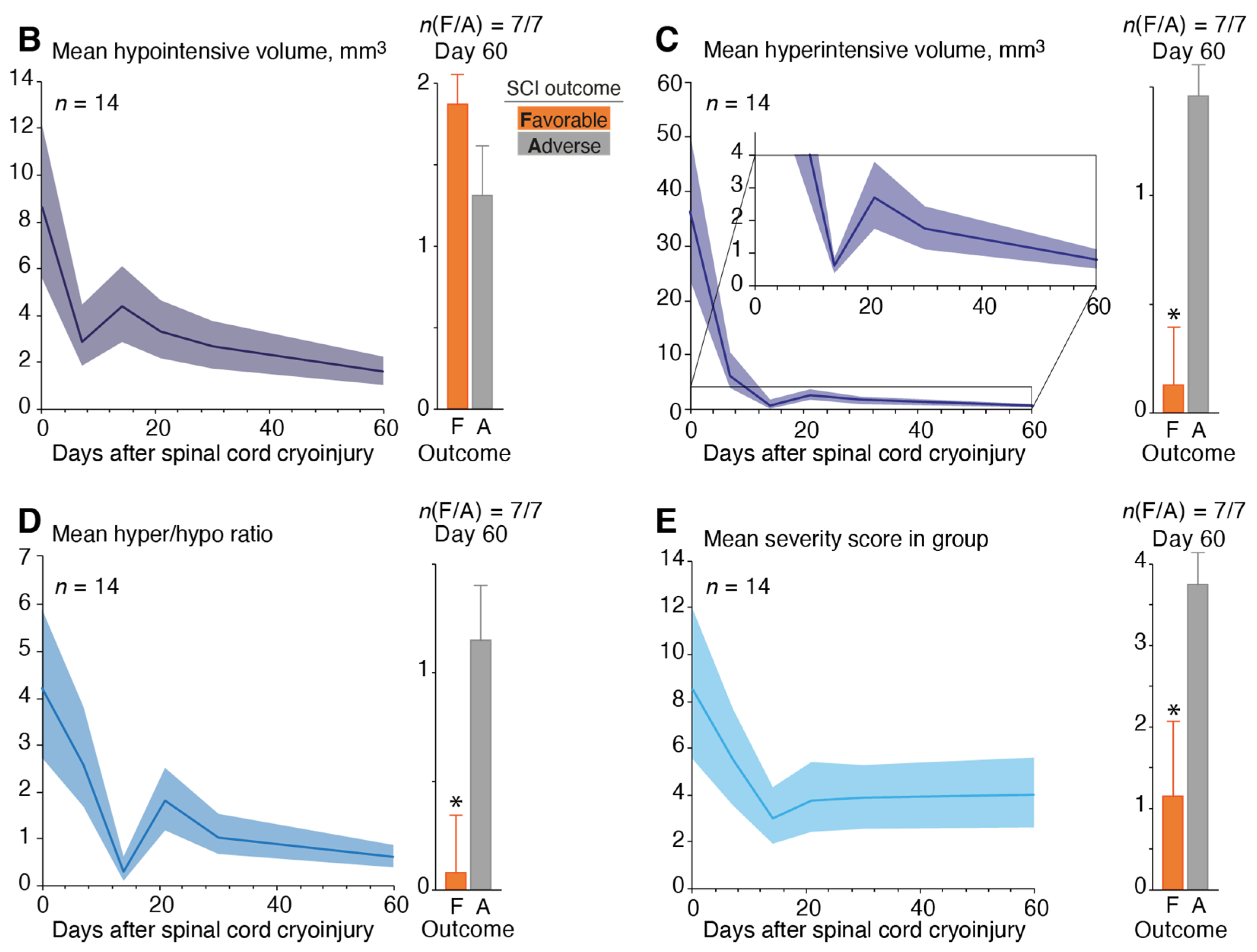
| Criterion | Significant (3 Points) | Moderate (2 Points) | Mild (1 Point) | None (0 Points) |
|---|---|---|---|---|
| Hyperintense area volume, mm3 | >10 | 5–10 | <5 | Not defined |
| Hypointense area volume, mm3 | >5 | 2–5 | <2 | Not defined |
| VHyper/VHypo ratio | >3 | 2–3 | 1–2 | 0–1 |
| Clear signs of syringomyelia cyst | Adds 1 point to final score | |||
| Cytokine/Chemokine Group | Median [Minimum; Maximum] of Plasma Level (pg/mL) | p Value, Mann–Whitney Test | |||||
|---|---|---|---|---|---|---|---|
| Intact n = 5 | 12 h after SCI | Favorable Outcome vs. Intact | Adverse Outcome vs. Intact | Favorable vs. Adverse Outcome | |||
| Favorable Outcome n = 8 | Adverse Outcome n = 8 | ||||||
| Proinflammatory cytokines | IL-1α | 4 [1.5; 7.5] | 28.8 [14.5; 42] | 427.5 [221.5; 605.5] | 0.014 | 0.014 | 0.021 |
| IL-1β | 33.5 [17.5; 50.5] | 17 [10; 25] | 451.5 [413; 491] | 0.049 | 0.014 | 0.021 | |
| IL-6 | 5.5 [2; 9] | 15 [2; 27] | 288 [15; 598] | 0.389 | 0.014 | 0.248 | |
| TNFα | 142.5 [32; 251.5] | 16 [6; 23] | 375 [318; 422] | 0.014 | 0.014 | 0.021 | |
| IL-17A | 140.5 [58.5; 210.5] | 46.5 [43; 52.5] | 341.5 [18; 685.5] | 0.014 | 0.048 | 0.198 | |
| IFNγ | 20 [13; 24] | 18.5 [7; 30.5] | 355 [118; 658] | 0.998 | 0.014 | 0.021 | |
| IL-12p70 | 0.5 [0; 1] | 30 [7; 53] | 330.5 [23; 682] | 0.014 | 0.014 | 0.248 | |
| IL-18 | 366 [247; 518] | 118.8 [49.5; 192] | 430.5 [157; 794] | 0.014 | 0.998 | 0.248 | |
| Chemokines | GRO/KC | 5.5 [2.5; 11.5] | 52.5 [42; 62] | 525.5 [137; 993.5] | 0.014 | 0.014 | 0.021 |
| MCP-1 | 1.2 [0.9; 2] | 196 [114; 294] | 715.5 [105.5; 1402.5] | 0.014 | 0.014 | 0.564 | |
| MIP-1α | 3 [1; 5] | 52.8 [50.5; 55] | 386 [35; 712.5] | 0.014 | 0.014 | 0.248 | |
| MIP-2 | 44 [6.5; 64.5] | 99.5 [95; 108] | 94 [63; 124] | 0.014 | 0.027 | 0.998 | |
| RANTES | 66.5 [47; 89.5] | 467.5 [249; 688] | 2147.5 [250; 4598] | 0.014 | 0.014 | 0.386 | |
| Proliferation factors | G-CSF | 7 [3; 13] | 62 [51; 75] | 416 [126.5; 734] | 0.014 | 0.014 | 0.021 |
| GM-CSF | 389 [294; 522] | 35 [8; 53] | 369 [124; 664] | 0.014 | 0.998 | 0.021 | |
| IL-2 | 16.5 [1.5; 26] | 22.5 [11; 34.5] | 191 [23.5; 401] | 0.327 | 0.049 | 0.248 | |
| IL-5 | 34.5 [6.5; 57.5] | 21 [10; 30.5] | 308 [12; 619] | 0.623 | 0.325 | 0.386 | |
| VEGF | 12 [10; 17] | 58.5 [48.5; 65] | 284.5 [26; 559] | 0.014 | 0.014 | 0.998 | |
| Anti-inflammatory cytokines | IL-4 | 1.5 [0.5; 3.5] | 23 [12.5; 35] | 314.5 [18; 618] | 0.014 | 0.014 | 0.248 |
| IL-13 | 14.5 [2.5; 20.5] | 16.5 [3; 29] | 301 [177.5; 424] | 0.462 | 0.014 | 0.021 | |
| IL-10 | 6 [4; 16] | 33 [16; 49] | 324 [32; 640] | 0.019 | 0.014 | 0.248 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telegin, G.B.; Chernov, A.S.; Minakov, A.N.; Rodionov, M.V.; Kazakov, V.A.; Palikov, V.A.; Balmasova, I.P.; Asyutin, D.S.; Poluektov, Y.M.; Konovalov, N.A.; et al. Plasma Cytokines Level and Spinal Cord MRI Predict Clinical Outcome in a Rat Glial Scar Cryoinjury Model. Biomedicines 2022, 10, 2345. https://doi.org/10.3390/biomedicines10102345
Telegin GB, Chernov AS, Minakov AN, Rodionov MV, Kazakov VA, Palikov VA, Balmasova IP, Asyutin DS, Poluektov YM, Konovalov NA, et al. Plasma Cytokines Level and Spinal Cord MRI Predict Clinical Outcome in a Rat Glial Scar Cryoinjury Model. Biomedicines. 2022; 10(10):2345. https://doi.org/10.3390/biomedicines10102345
Chicago/Turabian StyleTelegin, Georgii B., Aleksandr S. Chernov, Alexey N. Minakov, Maksim V. Rodionov, Vitaly A. Kazakov, Viktor A. Palikov, Irina P. Balmasova, Dmitry S. Asyutin, Yuri M. Poluektov, Nikolay A. Konovalov, and et al. 2022. "Plasma Cytokines Level and Spinal Cord MRI Predict Clinical Outcome in a Rat Glial Scar Cryoinjury Model" Biomedicines 10, no. 10: 2345. https://doi.org/10.3390/biomedicines10102345
APA StyleTelegin, G. B., Chernov, A. S., Minakov, A. N., Rodionov, M. V., Kazakov, V. A., Palikov, V. A., Balmasova, I. P., Asyutin, D. S., Poluektov, Y. M., Konovalov, N. A., Kudriaeva, A. A., Spallone, A., Gabibov, A. G., & Belogurov, A. A., Jr. (2022). Plasma Cytokines Level and Spinal Cord MRI Predict Clinical Outcome in a Rat Glial Scar Cryoinjury Model. Biomedicines, 10(10), 2345. https://doi.org/10.3390/biomedicines10102345