Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors
Abstract
:1. Introduction
2. Classification of Brain Tumor Grade
2.1. Types of Low Grade (Grade I and Grade II) Brain Tumors
2.1.1. Craniopharyngiomas
2.1.2. Chordomas
2.1.3. Gangliogliomas and Gangliocytomas
2.1.4. Schwannomas
2.1.5. Pituitary Adenomas and Pineocytomas
2.2. Types of High Grade (Grade III and Grade IV) Brain Tumors
2.2.1. Anaplastic Astrocytomas
2.2.2. Anaplastic Oligodendrogliomas
2.2.3. Glioblastoma Multiforme (GBM)
3. Strategies to Improve Permeability of Nanocarrier through the Blood-Brain Barrier
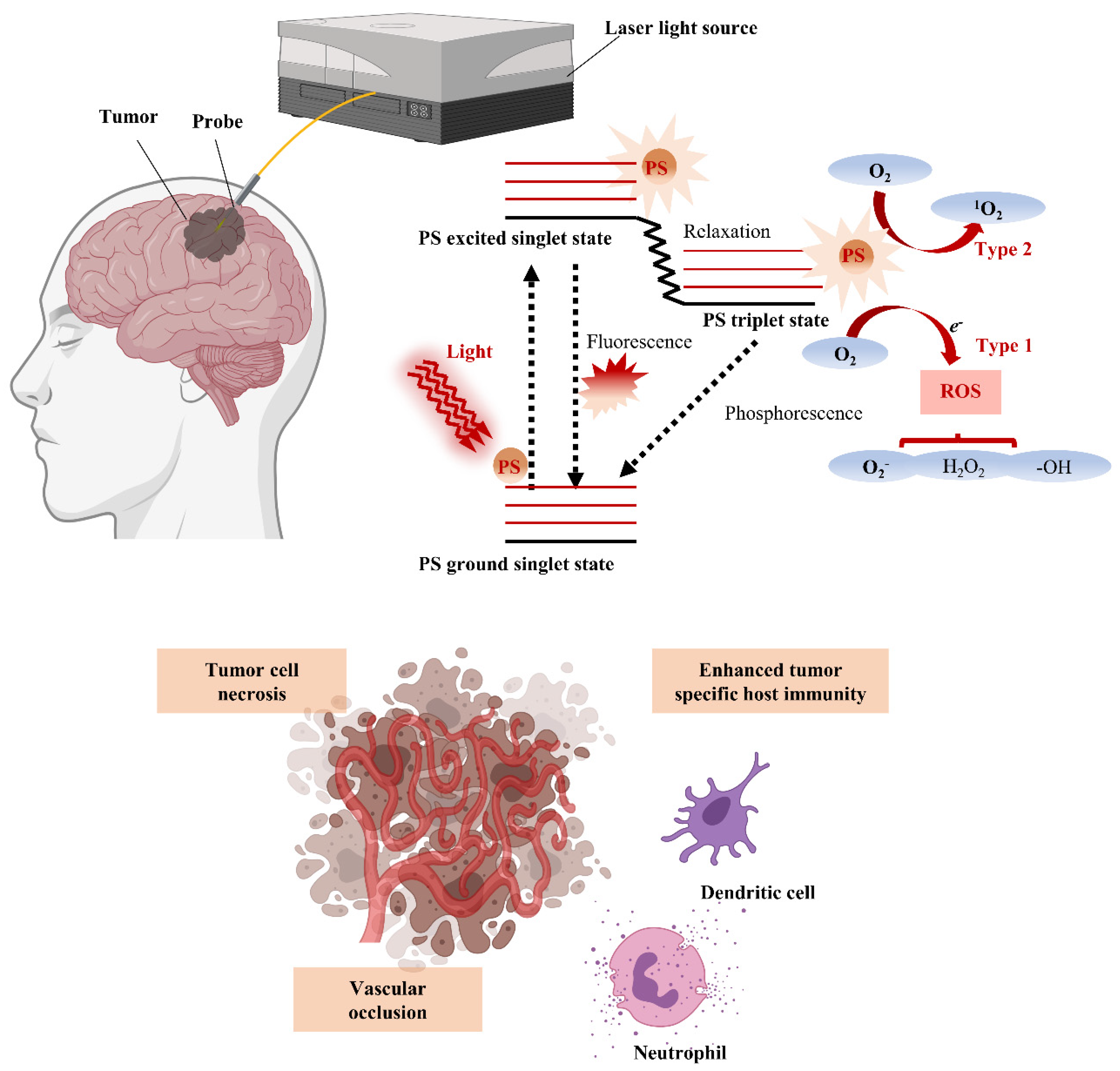
4. Advantages and Clinical Application of PDT for the Treatment of Brain Tumors
4.1. PDT Mechanism and Advantages for Brain Tumor Treatment
4.2. Clinical Trials of PDT for Brain Tumors
5. Nanotechnology for Enhanced Photodynamic Therapy
5.1. Recent Advances in Preclinical Application of Nanocarriers for PDT
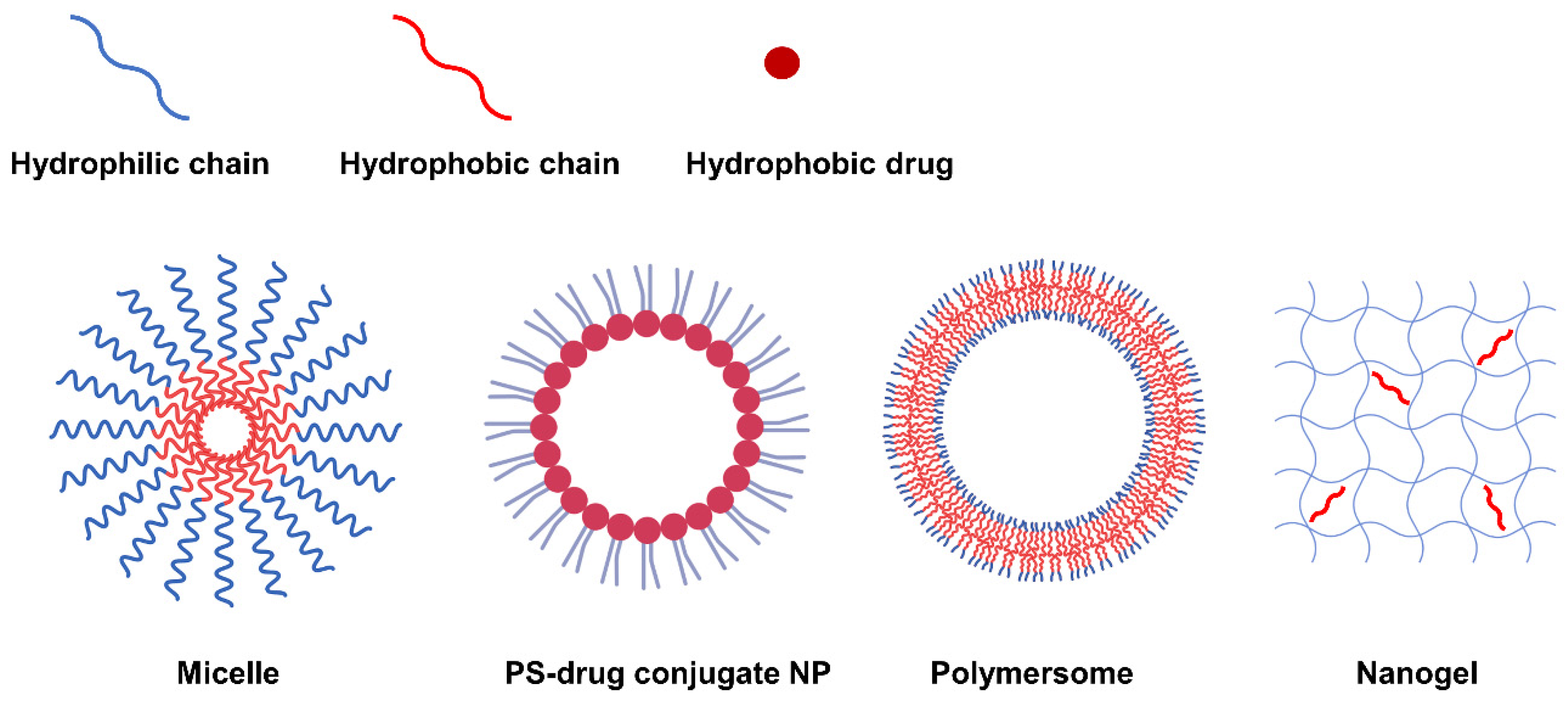
5.2. Self-Assembled NP via Transformation into Amphiphilic PS-Derivatives
5.2.1. Self-Assembly Methods for Amphiphilic PS Derivatives
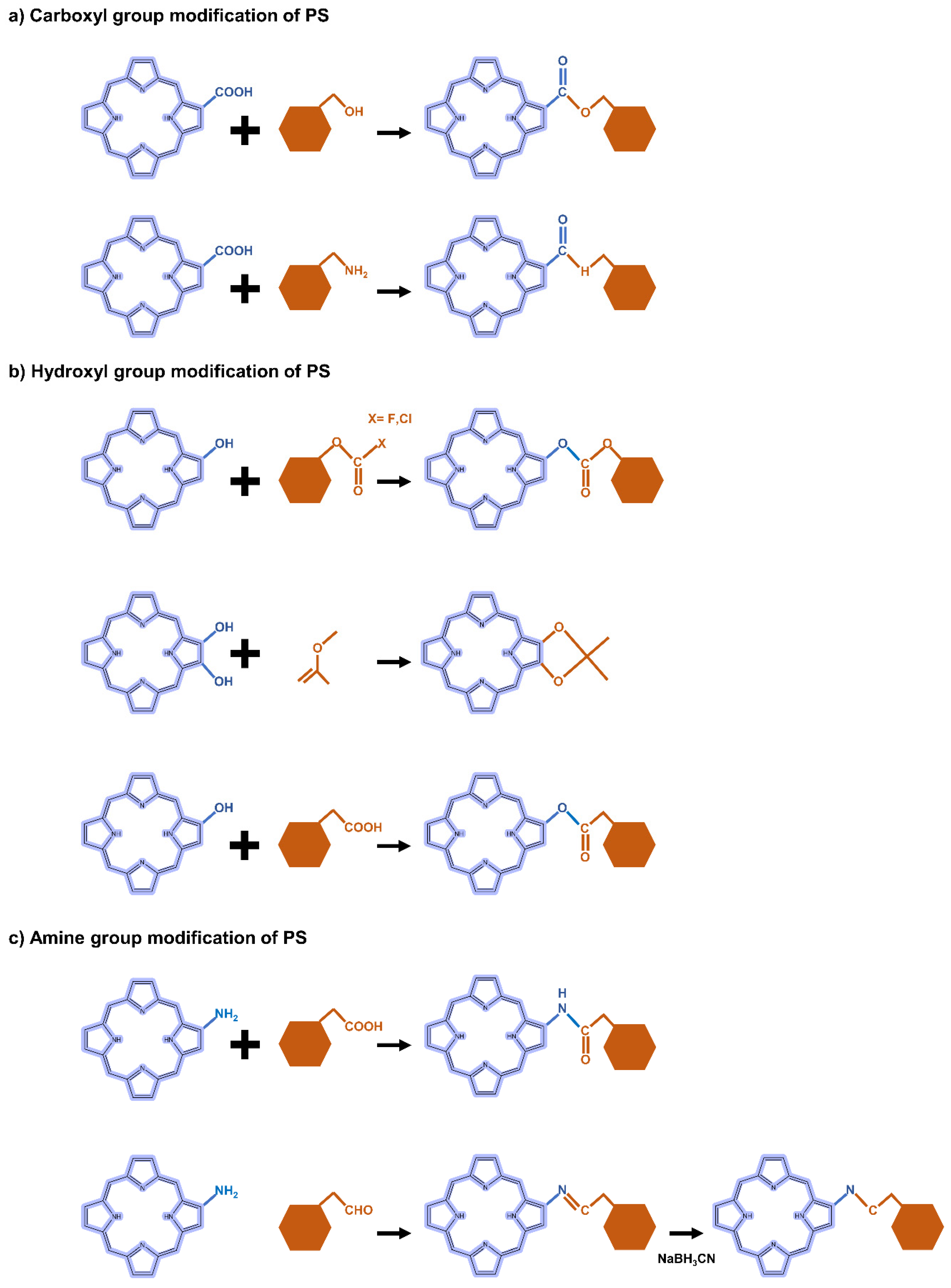
5.2.2. Carboxyl Group Modification of PS-Derivatives
5.2.3. Hydroxyl Group Modification of PS-Derivatives
5.2.4. Amine Group Modification of PS-Derivatives
5.2.5. Hyaluronic Acid-Modified NPs for PDT
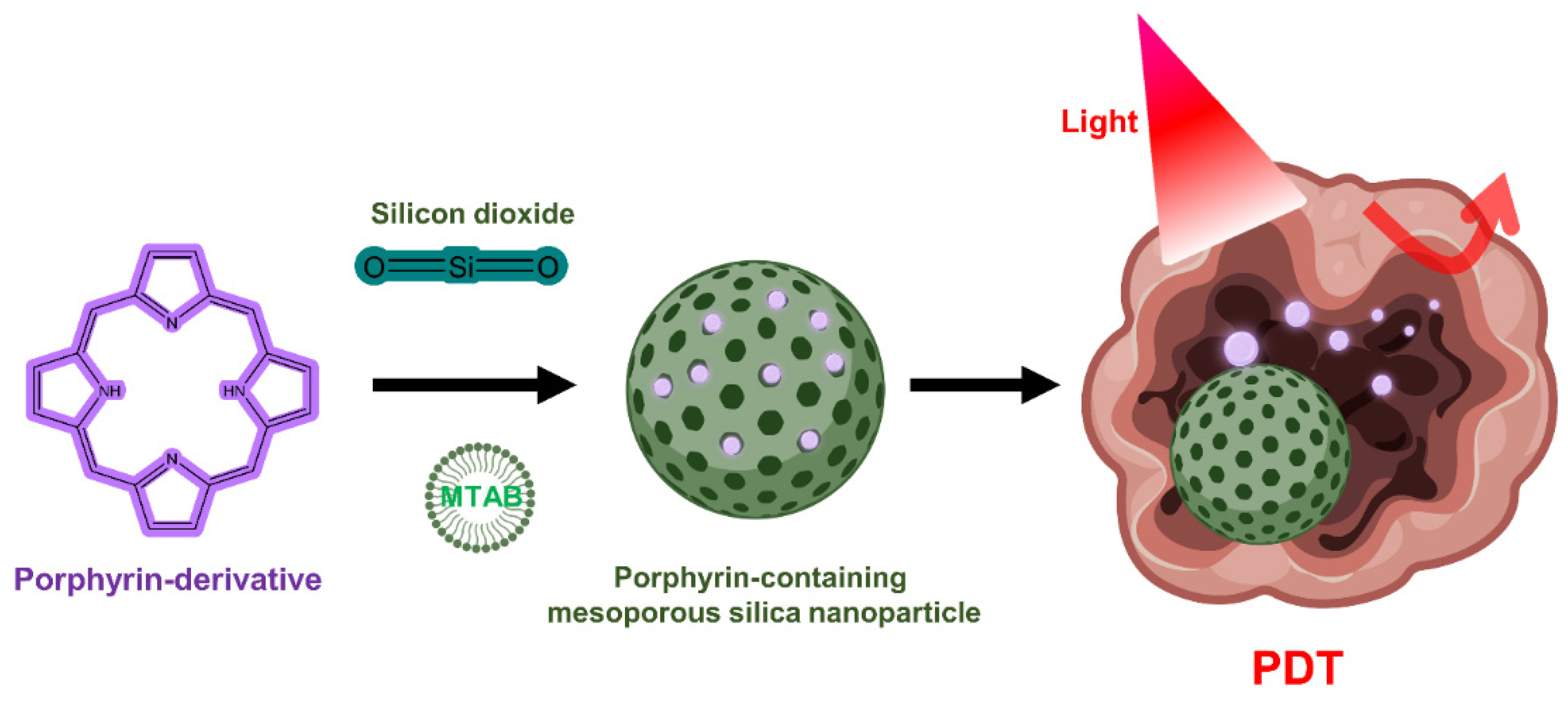
5.3. Application of Inorganic Nanomaterials in PDT
5.3.1. Silica Nanoparticles
5.3.2. Gold Nanoparticles
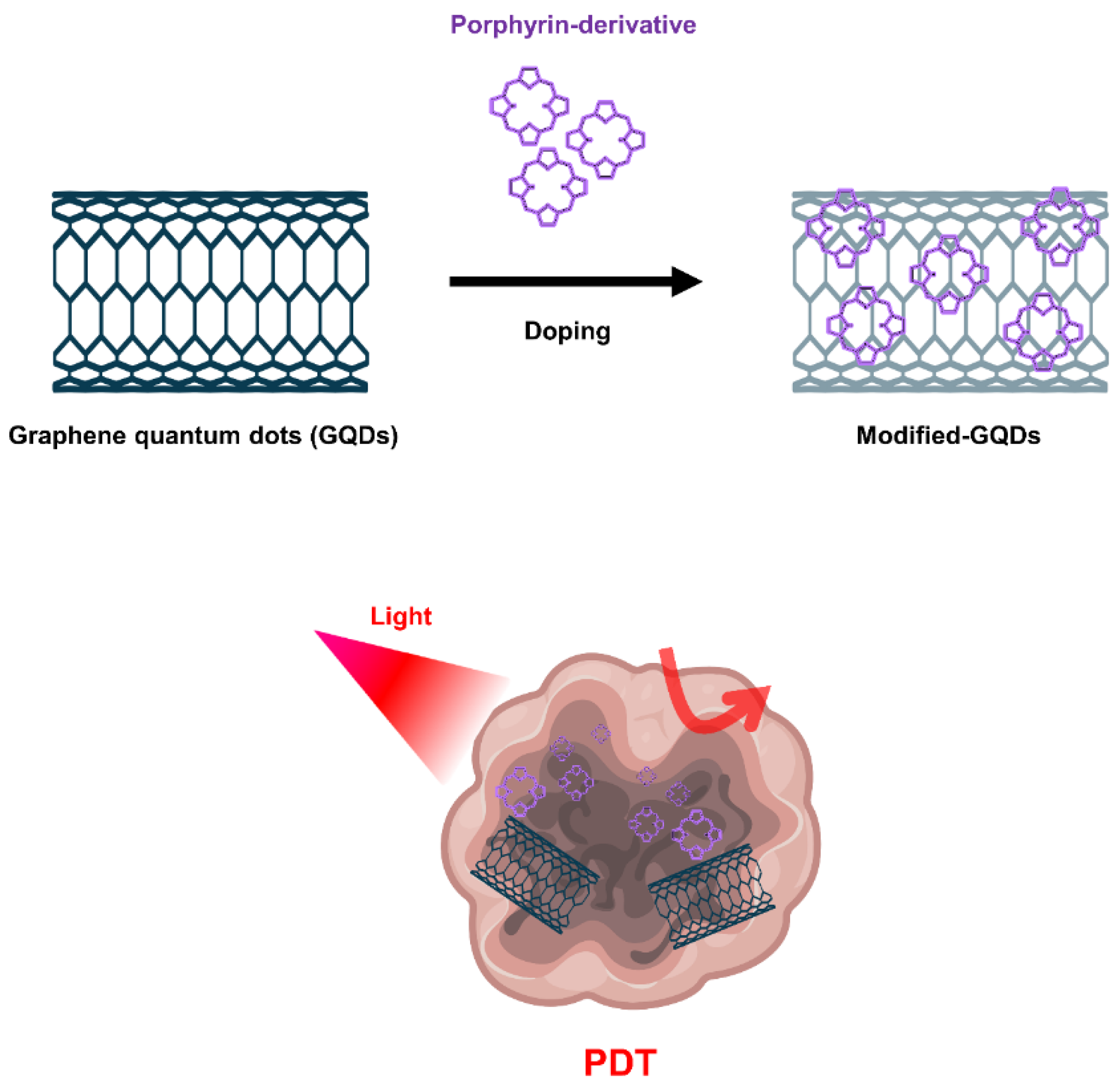
5.3.3. Graphene Nanomaterials
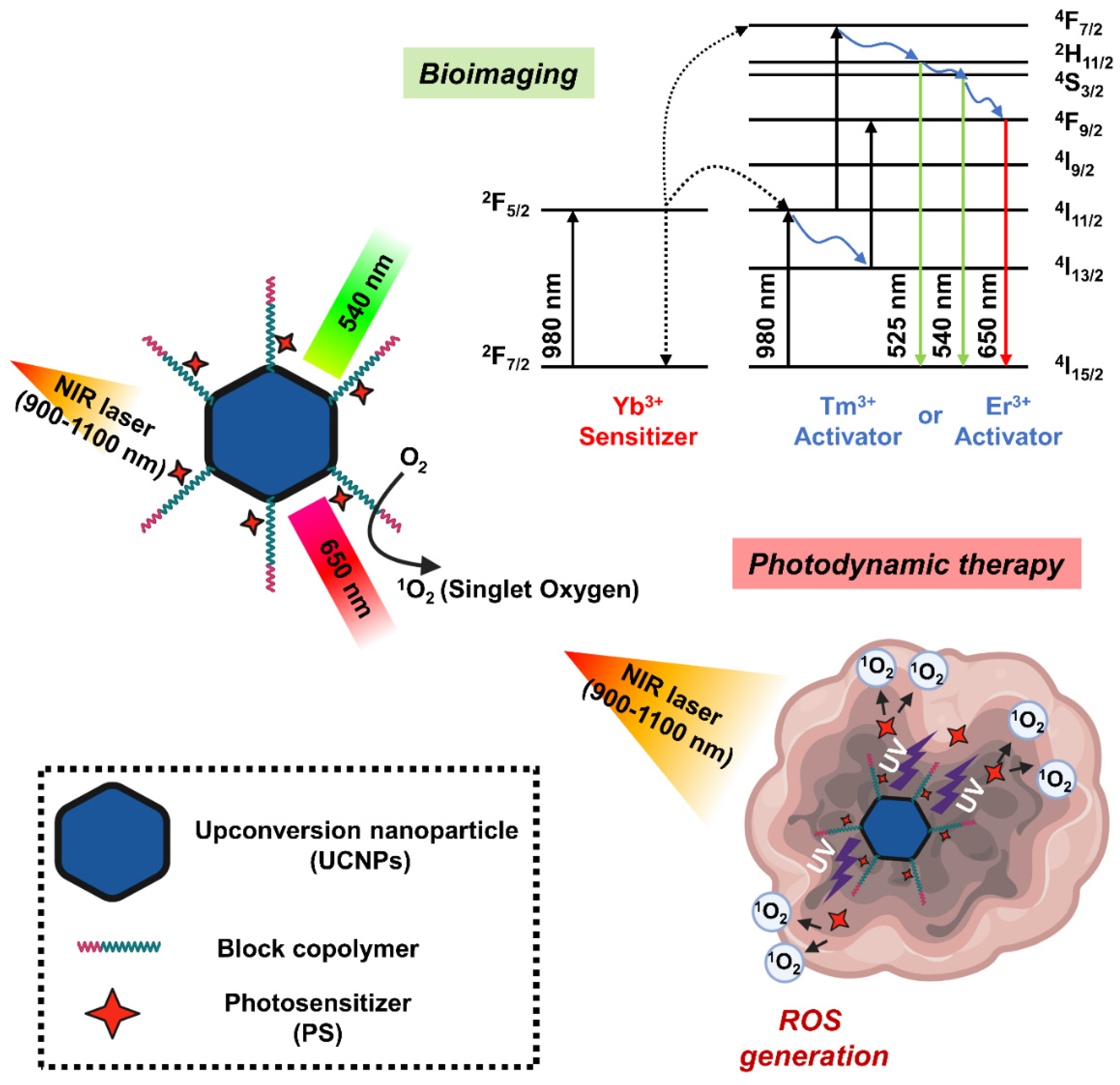
5.3.4. Upconversion Nanoparticles
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abramova, O.B.; Kaplan, M.A.; Grin, M.A.; Yuzhakov, V.V.; Suvorov, N.V.; Mironov, A.F.; Drozhzhina, V.V.; Churikova, T.P.; Kozlovtseva, E.A.; Bandurko, L.N.; et al. Photodynamic Therapy of Melanoma B16 with Chlorin E6 Conjugated with a PSMA-Ligand. Bull. Exp. Biol. Med. 2021, 171, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhao, S.; Shen, M.; Su, J.; Chen, X. Laser-assisted photodynamic therapy vs. conventional photodynamic therapy in non-melanoma skin cancers: Systematic review and meta-analysis of randomized controlled trials. Photodermatol. Photoimmunol. Photomed. 2021, 37, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Ishihara, R. Photodynamic Therapy for Esophageal Cancer. Clin. Endosc. 2021, 54, 494–498. [Google Scholar] [CrossRef]
- Yano, T.; Minamide, T.; Takashima, K.; Nakajo, K.; Kadota, T.; Yoda, Y. Clinical Practice of Photodynamic Therapy Using Talaporfin Sodium for Esophageal Cancer. J. Clin. Med. 2021, 10, 2785. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Huang, H.; Zhou, X.; Zhang, Z.; Ma, H.; Yao, Q.; Shao, K.; Sun, W.; Du, J.; Fan, J.; et al. Reversing Multidrug Resistance by Inducing Mitochondrial Dysfunction for Enhanced Chemo-Photodynamic Therapy in Tumor. ACS Appl. Mater. Interfaces 2021, 13, 45259–45268. [Google Scholar] [CrossRef]
- Shi, X.; Yang, X.; Liu, M.; Wang, R.; Qiu, N.; Liu, Y.; Yang, H.; Ji, J.; Zhai, G. Chondroitin sulfate-based nanoparticles for enhanced chemo-photodynamic therapy overcoming multidrug resistance and lung metastasis of breast cancer. Carbohydr. Polym. 2021, 254, 117459. [Google Scholar] [CrossRef]
- Akimoto, J. Photodynamic Therapy for Malignant Brain Tumors. Neurol. Med. Chir. (Tokyo) 2016, 56, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Novell, A.; Kamimura, H.A.S.; Cafarelli, A.; Gerstenmayer, M.; Flament, J.; Valette, J.; Agou, P.; Conti, A.; Selingue, E.; Badin, R.A.; et al. A new safety index based on intrapulse monitoring of ultra-harmonic cavitation during ultrasound-induced blood-brain barrier opening procedures. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Mulvihill, J.J.E.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug delivery across the blood-brain barrier: Recent advances in the use of nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Villasenor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell. Mol. Life Sci. 2019, 76, 1081–1092. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, B.; Zhao, R.; Zhang, Q.; Kong, X. Multifunctional nanoparticles as photosensitizer delivery carriers for enhanced photodynamic cancer therapy. Mater. Sci. Eng. C 2020, 115, 111099. [Google Scholar] [CrossRef] [PubMed]
- Biratu, E.S.; Schwenker, F.; Ayano, Y.M.; Debelee, T.G. A Survey of Brain Tumor Segmentation and Classification Algorithms. J. Imaging 2021, 7, 179. [Google Scholar] [CrossRef]
- Khan, M.A.; Lali, I.U.; Rehman, A.; Ishaq, M.; Sharif, M.; Saba, T.; Zahoor, S.; Akram, T. Brain tumor detection and classification: A framework of marker-based watershed algorithm and multilevel priority features selection. Microsc. Res. Tech. 2019, 82, 909–922. [Google Scholar] [CrossRef]
- Asano, K.; Hasegawa, S.; Matsuzaka, M.; Ohkuma, H. Brain tumor-related epilepsy and risk factors for metastatic brain tumors: Analysis of 601 consecutive cases providing real-world data. J. Neurosurg. 2021, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Weller, M.; Wen, P.Y.; Kros, J.M.; Aldape, K.; Chang, S. A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro-Oncology 2017, 19, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Tauziede-Espariat, A.; Burel-Vandenbos, F.; Pedeutour, F.; Gareton, A.; Saffroy, R.; Andreiuolo, F.; Blauwblomme, T.; Dangouloff-Ros, V.; Boddaert, N.; Lechapt, E.; et al. Intracranial chondromas: A histopathologic and molecular study of three cases. Clin. Neuropathol. 2020, 39, 171–178. [Google Scholar] [CrossRef]
- Robles, L.A.; Mundis, G.M. Chondromas of the Lumbar Spine: A Systematic Review. Glob. Spine. J. 2021, 11, 232–239. [Google Scholar] [CrossRef] [Green Version]
- DeLaney, T.F.; Liebsch, N.J.; Pedlow, F.X.; Adams, J.; Weyman, E.A.; Yeap, B.Y.; Depauw, N.; Nielsen, G.P.; Harmon, D.C.; Yoon, S.S.; et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J. Surg. Oncol. 2014, 110, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Villanueva-Meyer, J.E.; Goode, B.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Bastian, B.C.; Chamyan, G.; Maher, O.M.; Khatib, Z.; et al. The genetic landscape of ganglioglioma. Acta Neuropathol. Commun. 2018, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Sauer, A.; Blavin, J.; Lhermitte, B.; Speeg-Schatz, C. Conjunctival ganglioglioma as a feature of basal cell nevus syndrome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2011, 15, 387–388. [Google Scholar] [CrossRef]
- Erickson, N.J.; Schmalz, P.G.R.; Agee, B.S.; Fort, M.; Walters, B.C.; McGrew, B.M.; Fisher, W.S. Koos Classification of Vestibular Schwannomas: A Reliability Study. Neurosurgery 2019, 85, 409–414. [Google Scholar] [CrossRef]
- Younes, E.; Montava, M.; Bachelard-Serra, M.; Jaloux, L.; Salburgo, F.; Lavieille, J.P. Intracanalicular Vestibular Schwannomas: Initial Clinical Manifestation, Imaging Classification, and Risk Stratification for Management Proposal. Otol. Neurotol. 2017, 38, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, A.R.; Chakraborty, S. Aggressive pituitary adenomas: Is pathology the only feature of aggressiveness? Acta Neurochir. 2018, 160, 57–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.A.; Awad, A.W.; Brachman, D.; Coons, S.W.; McBride, H.; Youssef, E.; Nakaji, P.; Shetter, A.G.; Smith, K.A.; Spetzler, R.F.; et al. Long-term radiosurgical control of subtotally resected adult pineocytomas. J. Neurosurg. 2012, 117, 212–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, J.F.; Lamborn, K.R.; Chang, S.M.; Gilbert, M.R.; Cloughesy, T.F.; Aldape, K.; Yao, J.; Jackson, E.F.; Lieberman, F.; Robins, H.I.; et al. Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium study. J. Clin. Oncol. 2011, 29, 2689–2695. [Google Scholar] [CrossRef]
- Quesada, A.; Prada, F.A.; Aguilera, Y.; Espinar, A.; Carmona, A.; Prada, C. Peripapillary glial cells in the chick retina: A special glial cell type expressing astrocyte, radial glia, neuron, and oligodendrocyte markers throughout development. Glia 2004, 46, 346–355. [Google Scholar] [CrossRef]
- She, D.; Liu, J.; Xing, Z.; Zhang, Y.; Cao, D.; Zhang, Z. MR Imaging Features of Anaplastic Pleomorphic Xanthoastrocytoma Mimicking High-Grade Astrocytoma. AJNR Am. J. Neuroradiol. 2018, 39, 1446–1452. [Google Scholar] [CrossRef]
- Jungk, C.; Reinhardt, A.; Warta, R.; Capper, D.; Deimling, A.V.; Herold-Mende, C.; Unterberg, A. Extent of Resection, MGMT Promoter Methylation Status and Tumor Location Independently Predict Progression-Free Survival in Adult Sporadic Pilocytic Astrocytoma. Cancers 2019, 11, 1072. [Google Scholar] [CrossRef] [Green Version]
- Tjahjadi, M.; Arifin, M.Z.; Sobana, M.; Avianti, A.; Caropeboka, M.S.; Eka, P.A.; Agustina, H. Cystic pilomyxoid astrocytoma on suprasellar region in 7-year-old girl: Treatment and strategy. Asian J. Neurosurg. 2015, 10, 154–157. [Google Scholar] [CrossRef] [Green Version]
- Figarella-Branger, D.; Mokhtari, K.; Dehais, C.; Jouvet, A.; Uro-Coste, E.; Colin, C.; Carpentier, C.; Forest, F.; Maurage, C.A.; Vignaud, J.M.; et al. Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro-Oncology 2014, 16, 1244–1254. [Google Scholar] [CrossRef] [Green Version]
- Achey, R.L.; Khanna, V.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S. Incidence and survival trends in oligodendrogliomas and anaplastic oligodendrogliomas in the United States from 2000 to 2013: A CBTRUS Report. J. Neurooncol. 2017, 133, 17–25. [Google Scholar] [CrossRef]
- Ye, Z.; Price, R.L.; Liu, X.; Lin, J.; Yang, Q.; Sun, P.; Wu, A.T.; Wang, L.; Han, R.H.; Song, C.; et al. Diffusion Histology Imaging Combining Diffusion Basis Spectrum Imaging (DBSI) and Machine Learning Improves Detection and Classification of Glioblastoma Pathology. Clin. Cancer Res. 2020, 26, 5388–5399. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA. Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Pinheiro, L.; Brem, H. Delivery of local therapeutics to the brain: Working toward advancing treatment for malignant gliomas. Ther. Deliv. 2015, 6, 353–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Abbasi, J. Guided Ultrasound Opens Blood-Brain Barrier to Cancer Drugs. JAMA 2021, 326, 1785. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Tureli, A.E.; Gunday Tureli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdeel, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, L.; Cano, A.; Ettcheto, M.; Souto, E.B.; Espina, M.; Camins, A.; Garcia, M.L.; Sanchez-Lopez, E. Surface Functionalization of PLGA Nanoparticles to Increase Transport across the BBB for Alzheimer’s Disease. Appl. Sci. 2021, 11, 4305. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Berg, K.; Peng, Q. Photodynamic therapy mediated immune therapy of brain tumors. Neuroimmunol. Neuroinflamm. 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Fujimoto, S.; Yamaguchi, H.; Yamauchi, T.; Yoshimoto, T.; Tokuda, K. Photodynamic Therapy of Malignant Gliomas. Prog. Neurol. Surg. 2018, 32, 1–13. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Sampson, J.H. Temozolomide treatment outcomes and immunotherapy efficacy in brain tumor. J. Neurooncol. 2021, 151, 55–62. [Google Scholar] [CrossRef]
- Borah, B.M.; Cacaccio, J.; Durrani, F.A.; Bshara, W.; Turowski, S.G.; Spernyak, J.A.; Pandey, R.K. Sonodynamic therapy in combination with photodynamic therapy shows enhanced long-term cure of brain tumor. Sci. Rep. 2020, 10, 21791. [Google Scholar] [CrossRef]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef]
- Akimoto, J.; Haraoka, J.; Aizawa, K. Preliminary clinical report on safety and efficacy of photodynamic therapy using talaporfin sodium for malignant gliomas. Photodiagn. Photodyn. Ther. 2012, 9, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin derivative: A possible aid in the diagnosis and therapy of carcinoma of the bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef]
- Bay, C.; Vissing, A.C.; Thaysen-Petersen, D.; Lerche, C.M.; Togsverd-Bo, K.; Heydenreich, J.; Haedersdal, M. Skin reactions after photodynamic therapy are unaffected by 839 nm photobiomodulation therapy: A randomized, double-blind, placebo-controlled, clinical trial. Lasers Surg. Med. 2017, 49, 810–818. [Google Scholar] [CrossRef]
- Dixon, A.J.; Anderson, S.J.; Mazzurco, J.D.; Steinman, H.K. Novel photodynamic therapy does not prevent new skin cancers--randomized controlled trial. Dermatol. Surg. 2014, 40, 412–419. [Google Scholar] [CrossRef]
- Hendel, K.; Mogensen, M.; Wenande, E.; Dierickx, C.; Haedersdal, M.; Togsverd-Bo, K. Fractional 1,927 nm Thulium Laser Plus Photodynamic Therapy Compared and Combined for Photodamaged Decollete Skin: A Side-by-Side Randomized Controlled Trial. Lasers Surg. Med. 2020, 52, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Miola, A.C.; Ferreira, E.R.; Abbade, L.P.F.; Schmitt, J.V.; Miot, H.A. Randomized clinical trial testing the efficacy and safety of 0.5% colchicine cream versus photodynamic therapy with methyl aminolevulinate in the treatment of skin field cancerization: Study protocol. BMC Cancer 2018, 18, 340. [Google Scholar] [CrossRef] [Green Version]
- Miola, A.C.; Ferreira, E.R.; Lima, T.R.R.; Schmitt, J.V.; Abbade, L.P.F.; Miot, H.A. Effectiveness and safety of 0.5% colchicine cream vs. photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratosis and skin field cancerization of the forearms: A randomized controlled trial. Br. J. Dermatol. 2018, 179, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Togsverd-Bo, K.; Omland, S.H.; Wulf, H.C.; Sorensen, S.S.; Haedersdal, M. Primary prevention of skin dysplasia in renal transplant recipients with photodynamic therapy: A randomized controlled trial. Am. J. Transplant. 2015, 15, 2986–2990. [Google Scholar] [CrossRef] [Green Version]
- Hendren, S.K.; Hahn, S.M.; Spitz, F.R.; Bauer, T.W.; Rubin, S.C.; Zhu, T.; Glatstein, E.; Fraker, D.L. Phase II trial of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Ann. Surg. Oncol. 2001, 8, 65–71. [Google Scholar] [CrossRef]
- Hahn, S.M.; Fraker, D.L.; Mick, R.; Metz, J.; Busch, T.M.; Smith, D.; Zhu, T.; Rodriguez, C.; Dimofte, A.; Spitz, F.; et al. A phase II trial of intraperitoneal photodynamic therapy for patients with peritoneal carcinomatosis and sarcomatosis. Clin. Cancer Res. 2006, 12, 2517–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, H. Photodynamic therapy in gastrointestinal cancer: A realistic option? Drugs Aging 2000, 16, 81–86. [Google Scholar] [CrossRef]
- Dougherty, T.J. Photodynamic therapy in gastrointestinal cancer. Lasers Surg. Med. 1992, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Gossner, L.; Sroka, R.; Hahn, E.G.; Ell, C. Photodynamic therapy: Successful destruction of gastrointestinal cancer after oral administration of aminolevulinic acid. Gastrointest. Endosc. 1995, 41, 55–58. [Google Scholar] [CrossRef]
- Hayata, Y.; Kato, H.; Okitsu, H.; Kawaguchi, M.; Konaka, C. Photodynamic therapy with hematoporphyrin derivative in cancer of the upper gastrointestinal tract. Semin. Surg. Oncol. 1985, 1, 1–11. [Google Scholar] [CrossRef]
- Jin, M.L.; Yang, B.Q.; Zhang, W.; Ren, P. Evaluation of photodynamic therapy in advanced gastrointestinal cancer. J. Clin. Laser Med. Surg. 1991, 9, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Karanov, S.; Shopova, M.; Getov, H. Photodynamic therapy in gastrointestinal cancer. Lasers Surg. Med. 1991, 11, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kubba, A.K. Role of photodynamic therapy in the management of gastrointestinal cancer. Digestion 1999, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hayata, Y.; Kato, H.; Konaka, C.; Hayashi, N.; Tahara, M.; Saito, T.; Ono, J. Fiberoptic bronchoscopic photoradiation in experimentally induced canine lung cancer. Cancer 1983, 51, 50–56. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef]
- Fayter, D.; Corbett, M.; Heirs, M.; Fox, D.; Eastwood, A. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett’s oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol. Assess. 2010, 14, 1–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, P.J.; Wilson, B.C. Photodynamic therapy of malignant brain tumours. Can. J. Neurol. Sci. 1990, 17, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perria, C.; Casu, G.; Sgaramella, E. Proposal of a protocol for the photodynamic therapy of malignant brain tumours. J. Photochem. Photobiol. B 1990, 6, 443–449. [Google Scholar] [CrossRef]
- Muller, P.J.; Wilson, B.C. Photodynamic therapy for malignant newly diagnosed supratentorial gliomas. J. Clin. Laser Med. Surg. 1996, 14, 263–270. [Google Scholar] [CrossRef]
- Muller, P.J.; Wilson, B.C. Photodynamic therapy for recurrent supratentorial gliomas. Semin. Surg. Oncol. 1995, 11, 346–354. [Google Scholar] [CrossRef]
- Kaye, A.H.; Morstyn, G.; Brownbill, D. Adjuvant high-dose photoradiation therapy in the treatment of cerebral glioma: A phase 1-2 study. J. Neurosurg. 1987, 67, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Stylli, S.S.; Kaye, A.H.; MacGregor, L.; Howes, M.; Rajendra, P. Photodynamic therapy of high grade glioma-long term survival. J. Clin. Neurosci. 2005, 12, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Jesu Raj, J.G.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neurooncol. 2019, 141, 595–607. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Powers, S.K.; Witmer, P.; Brown, T. Optimal light dose for interstitial photodynamic therapy in treatment for malignant brain tumors. Lasers Surg. Med. 2000, 27, 224–234. [Google Scholar] [CrossRef]
- Calixto, G.M.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Garg, T.; Jain, N.K.; Rath, G.; Goyal, A.K. Nanotechnology-Based Photodynamic Therapy: Concepts, Advances, and Perspectives. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 389–439. [Google Scholar] [CrossRef]
- Feng, J.J.; Wang, S.Y.; Wang, Y.M.; Wang, L.P. Stem cell membrane-camouflaged bioinspired nanoparticles for targeted photodynamic therapy of lung cancer. J. Nanopart. Res. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Yang, C.; Fu, Y.; Huang, C.; Hu, D.; Zhou, K.; Hao, Y.; Chu, B.; Yang, Y.; Qian, Z. Chlorin e6 and CRISPR-Cas9 dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy. Biomaterials 2020, 255, 120194. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.I.; Kim, J.K.; Kim, C.H.; Kim, Y.J. Peptide 18-4/chlorin e6-conjugated polyhedral oligomeric silsesquioxane nanoparticles for targeted photodynamic therapy of breast cancer. Colloids Surf. B Biointerfaces 2020, 189, 110829. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Liu, F.; Lin, L.; Yan, N.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J. Control. Release 2020, 328, 631–639. [Google Scholar] [CrossRef]
- Cho, M.H.; Li, Y.; Lo, P.C.; Lee, H.; Choi, Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nano-Micro Lett. 2020, 12, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.Y.; Hu, X.L.; Xia, R.; Liu, S.; Pei, Q.; Chen, G.; Xie, Z.G.; Jing, X.B. A Paclitaxel Prodrug Activatable by Irradiation in a Hypoxic Microenvironment. Angew. Chem. Int. Ed. 2020, 59, 23198–23205. [Google Scholar] [CrossRef]
- Um, W.; Park, J.; Ko, H.; Lim, S.; Yoon, H.Y.; Shim, M.K.; Lee, S.; Ko, Y.J.; Kim, M.J.; Park, J.H.; et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials 2019, 224, 119494. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.F.; Qin, J.J.; Li, Z.; Ge, Q.; Zeng, W.H. Enhanced anti-tumor efficacy of 5-aminolevulinic acid-gold nanoparticles-mediated photodynamic therapy in cutaneous squamous cell carcinoma cells. Braz. J. Med. Biol. Res. 2020, 53, e8457. [Google Scholar] [CrossRef]
- Huang, C.; Chen, F.; Zhang, L.; Yang, Y.; Yang, X.; Pan, W. (99m)Tc Radiolabeled HA/TPGS-Based Curcumin-Loaded Nanoparticle for Breast Cancer Synergistic Theranostics: Design, in vitro and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 2987–2998. [Google Scholar] [CrossRef]
- Uthaman, S.; Pillarisetti, S.; Mathew, A.P.; Kim, Y.; Bae, W.K.; Huh, K.M.; Park, I.K. Long circulating photoactivable nanomicelles with tumor localized activation and ROS triggered self-accelerating drug release for enhanced locoregional chemo-photodynamic therapy. Biomaterials 2020, 232, 119702. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, J.; Liu, X.; Song, H.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Cascade of reactive oxygen species generation by polyprodrug for combinational photodynamic therapy. Biomaterials 2020, 255, 120210. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Uthaman, S.; Pillarisetti, S.; Noh, K.; Huh, K.M.; Park, I.K. Bioactivatable reactive oxygen species-sensitive nanoparticulate system for chemo-photodynamic therapy. Acta Biomater. 2020, 108, 273–284. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, X.J.; Fu, T.W.; Li, K.; He, Y.; Luo, Z.; Dai, L.L.; Zeng, R.; Cai, K.Y. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.Q.; Tian, J.J.; Zhu, H.H.; Hong, L.J.; Mao, Z.W.; Oliveira, J.M.; Reis, R.L.; Li, X. Tumor-Targeting Polycaprolactone Nanoparticles with Codelivery of Paclitaxel and IR780 for Combinational Therapy of Drug-Resistant Ovarian Cancer. ACS Biomater. Sci. Eng. 2020, 6, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Gao, Q.; Dong, X.; Yin, W.; Gu, Z.; Gan, Z.; Zhao, Y.; Yin, M. A Size-Reducible Nanodrug with an Aggregation-Enhanced Photodynamic Effect for Deep Chemo-Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 11384–11388. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. A pH-sensitive micelle composed of heparin, phospholipids, and histidine as the carrier of photosensitizers: Application to enhance photodynamic therapy of cancer. Int. J. Biol. Macromol. 2017, 98, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hwang, H.S.; Na, K. Photoresponsive Micelle-Incorporated Doxorubicin for Chemo-Photodynamic Therapy to Achieve Synergistic Antitumor Effects. Biomacromolecules 2018, 19, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shan, L.; Yao, Y.; Peng, F.; Jiang, S.; Yang, D.; Ling, G.; Zhang, P. Black phosphorus nanosheets and docetaxel micelles co-incorporated thermoreversible hydrogel for combination chemo-photodynamic therapy. Drug Deliv. Transl. Res. 2021, 11, 1133–1143. [Google Scholar] [CrossRef]
- Wu, J.; Xia, L.; Liu, Z.; Xu, Z.; Cao, H.; Zhang, W. Fabrication of a Dual-Stimuli-Responsive Supramolecular Micelle from a Pillar[5]arene-Based Supramolecular Diblock Copolymer for Photodynamic Therapy. Macromol. Rapid Commun. 2019, 40, e1900240. [Google Scholar] [CrossRef]
- Li, L.; Cho, H.; Kim, S.; Kang, H.C.; Huh, K.M. Polyelectrolyte nanocomplex formation of heparin-photosensitizer conjugate with polymeric scavenger for photodynamic therapy. Carbohydr. Polym. 2015, 121, 122–131. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Zhang, X.; Li, Z.; Zhang, Q.; Wang, W.; Pan, D.; Zheng, X.; Gu, Z.; Zhang, H.; et al. Tumor microenvironment-responsive PEGylated heparin-pyropheophorbide-a nanoconjugates for photodynamic therapy. Carbohydr. Polym. 2021, 255, 117490. [Google Scholar] [CrossRef]
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int. J. Nanomed. 2017, 12, 4117–4127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.T.; Ding, Y.F.; Han, Z.H.; Yuwen, L.; Ye, Z.; Mok, G.S.P.; Li, S.; Wang, L.H. Hyaluronic acid-based nanogels derived from multicomponent self-assembly for imaging-guided chemo-photodynamic cancer therapy. Carbohydr. Polym. 2021, 268, 118257. [Google Scholar] [CrossRef]
- Chang, E.; Bu, J.; Ding, L.; Lou, J.W.H.; Valic, M.S.; Cheng, M.H.Y.; Rosilio, V.; Chen, J.; Zheng, G. Porphyrin-lipid stabilized paclitaxel nanoemulsion for combined photodynamic therapy and chemotherapy. J. Nanobiotechnol. 2021, 19, 154. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, C.; Liu, Z.; Zhao, Q.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hyaluronic Acid-Functionalized Hollow Mesoporous Silica Nanoparticles as pH-Sensitive Nanocarriers for Cancer Chemo-Photodynamic Therapy. Langmuir 2021, 37, 2619–2628. [Google Scholar] [CrossRef]
- Potara, M.; Nagy-Simon, T.; Focsan, M.; Licarete, E.; Soritau, O.; Vulpoi, A.; Astilean, S. Folate-targeted Pluronic-chitosan nanocapsules loaded with IR780 for near-infrared fluorescence imaging and photothermal-photodynamic therapy of ovarian cancer. Colloids Surf. B Biointerfaces 2021, 203, 111755. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, H. Co-delivery of chitosan nanoparticles of 5-aminolevulinic acid and shGBAS for improving photodynamic therapy efficacy in oral squamous cell carcinomas. Photodiagn. Photodyn. Ther. 2021, 34, 102218. [Google Scholar] [CrossRef]
- Zhu, T.; Shi, L.; Ma, C.; Xu, L.; Yang, J.; Zhou, G.; Zhu, X.; Shen, L. Fluorinated chitosan-mediated intracellular catalase delivery for enhanced photodynamic therapy of oral cancer. Biomater. Sci. 2021, 9, 658–662. [Google Scholar] [CrossRef]
- Gaio, E.; Conte, C.; Esposito, D.; Miotto, G.; Quaglia, F.; Moret, F.; Reddi, E. Co-delivery of Docetaxel and Disulfonate Tetraphenyl Chlorin in One Nanoparticle Produces Strong Synergism between Chemo- and Photodynamic Therapy in Drug-Sensitive and -Resistant Cancer Cells. Mol. Pharm. 2018, 15, 4599–4611. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Peng, J.; Tan, L.; Wu, J.; Shi, K.; Qu, Y.; Wei, X.; Qian, Z. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified Docetaxel/IR820 Co-loaded micelles. Biomaterials 2016, 106, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, S.; Zhang, T.; Wan, G.; Chen, B.; Xiong, Q.; Zhang, J.; Zhang, W.; Wang, Y. Pullulan-coated phospholipid and Pluronic F68 complex nanoparticles for carrying IR780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int. J. Nanomed. 2017, 12, 8649–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Shi, X.; Zhang, Y.; Xu, J.; Ji, J.; Ye, L.; Yi, F.; Zhai, G. Photo-triggered self-destructive ROS-responsive nanoparticles of high paclitaxel/chlorin e6 co-loading capacity for synergetic chemo-photodynamic therapy. J. Control. Release 2020, 323, 333–349. [Google Scholar] [CrossRef] [PubMed]
- de Paula Rodrigues, R.; Tini, I.R.; Soares, C.P.; da Silva, N.S. Effect of photodynamic therapy supplemented with quercetin in HEp-2 cells. Cell Biol. Int. 2014, 38, 716–722. [Google Scholar] [CrossRef]
- Thakur, N.S.; Mandal, N.; Patel, G.; Kirar, S.; Reddy, Y.N.; Kushwah, V.; Jain, S.; Kalia, Y.N.; Bhaumik, J.; Banerjee, U.C. Co-administration of zinc phthalocyanine and quercetin via hybrid nanoparticles for augmented photodynamic therapy. Nanomedicine 2021, 33, 102368. [Google Scholar] [CrossRef]
- He, J.; Huang, X.; Li, Y.C.; Liu, Y.; Babu, T.; Aronova, M.A.; Wang, S.; Lu, Z.; Chen, X.; Nie, Z. Self-assembly of amphiphilic plasmonic micelle-like nanoparticles in selective solvents. J. Am. Chem. Soc. 2013, 135, 7974–7984. [Google Scholar] [CrossRef]
- Huntosova, V.; Datta, S.; Lenkavska, L.; Macajova, M.; Bilcik, B.; Kundekova, B.; Cavarga, I.; Kronek, J.; Jutkova, A.; Miskovsky, P.; et al. Alkyl Chain Length in Poly(2-oxazoline)-Based Amphiphilic Gradient Copolymers Regulates the Delivery of Hydrophobic Molecules: A Case of the Biodistribution and the Photodynamic Activity of the Photosensitizer Hypericin. Biomacromolecules 2021, 22, 4199–4216. [Google Scholar] [CrossRef]
- Li, H.; Yu, Z.; Wang, S.; Long, X.; Zhang, L.M.; Zhu, Z.; Yang, L. Photosensitizer-encapsulated amphiphilic chitosan derivative micelles: Photoactivity and enhancement of phototoxicity against human pancreatic cancer cells. J. Photochem. Photobiol. B Biol. 2015, 142, 212–219. [Google Scholar] [CrossRef]
- Bazylinska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405. [Google Scholar] [CrossRef] [Green Version]
- Malacarne, M.C.; Banfi, S.; Rugiero, M.; Caruso, E. Drug delivery systems for the photodynamic application of two photosensitizers belonging to the porphyrin family. Photochem. Photobiol. Sci. 2021, 20, 1011–1025. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Cai, H.; Liu, F.; Wang, Y.; Gao, Y.; Zhang, W. Photocontrollable release and enhancement of photodynamic therapy based on host-guest supramolecular amphiphiles. J. Mater. Chem. B 2015, 3, 7417–7426. [Google Scholar] [CrossRef] [PubMed]
- Elschner, T.; Wondraczek, H.; Heinze, T. Syntheses and detailed structure characterization of dextran carbonates. Carbohydr. Polym. 2013, 93, 216–223. [Google Scholar] [CrossRef]
- Yang, X.; Cai, X.; Yu, A.; Xi, Y.; Zhai, G. Redox-sensitive self-assembled nanoparticles based on alpha-tocopherol succinate-modified heparin for intracellular delivery of paclitaxel. J. Colloid Interface Sci. 2017, 496, 311–326. [Google Scholar] [CrossRef]
- Jena, S.K.; Sangamwar, A.T. Polymeric micelles of amphiphilic graft copolymer of alpha-tocopherol succinate-g-carboxymethyl chitosan for tamoxifen delivery: Synthesis, characterization and in vivo pharmacokinetic study. Carbohydr. Polym. 2016, 151, 1162–1174. [Google Scholar] [CrossRef]
- Wang, Y.H.; Song, S.Y.; Zhang, S.T.; Zhang, H.J. Stimuli-responsive nanotheranostics based on lanthanide-doped upconversion nanoparticles for cancer imaging and therapy: Current advances and future challenges. Nano Today 2019, 25, 38–67. [Google Scholar] [CrossRef]
- Pandya, A.D.; Overbye, A.; Sahariah, P.; Gaware, V.S.; Hogset, H.; Masson, M.; Hogset, A.; Maelandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-Loaded Photosensitizer-Chitosan Nanoparticles for Combinatorial Chemo- and Photodynamic-Therapy of Cancer. Biomacromolecules 2020, 21, 1489–1498. [Google Scholar] [CrossRef]
- Xu, X.; Chong, Y.; Liu, X.; Fu, H.; Yu, C.; Huang, J.; Zhang, Z. Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta Biomater. 2019, 84, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019, 83, 400–413. [Google Scholar] [CrossRef]
- Krajczewski, J.; Rucinska, K.; Townley, H.E.; Kudelski, A. Role of various nanoparticles in photodynamic therapy and detection methods of singlet oxygen. Photodiagn. Photodyn. Ther. 2019, 26, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Ghosh, S.; Chatterjee, S.; Das, J.; Brahmachari, G.; Sil, P.C. In vivo therapeutic evaluation of a novel bis-lawsone derivative against tumor following delivery using mesoporous silica nanoparticle based redox-responsive drug delivery system. Mater. Sci. Eng. C 2021, 126. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kuang, Y.; Liu, R.; Chen, Z.Y.; Jiang, B.B.; Sun, Z.G.; Chen, X.Q.; Li, C. Dual-pH-sensitive mesoporous silica nanoparticle-based drug delivery system for tumor-triggered intracellular drug release. J. Mater. Sci. 2018, 53, 10653–10665. [Google Scholar] [CrossRef]
- Han, R.L.; Wu, S.; Yan, Y.Y.; Chen, W.; Tang, K.Q. Construction of ferrocene modified and indocyanine green loaded multifunctional mesoporous silica nanoparticle for simultaneous chemodynamic/photothermal/photodynamic therapy. Mater. Today Commun. 2021, 26, 101842. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.R.; Jeon, H.; Kim, D.; Song, C.; Lee, N.; Choi, S.H.; Hyeon, T. Continuous O-2-Evolving MnFe2O4 Nanoparticle-Anchored Mesoporous Silica Nanoparticles for Efficient Photodynamic Therapy in Hypoxic Cancer. J. Am. Chem. Soc. 2017, 139, 10992–10995. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Y.; Zhang, P.; Zhang, X.; Zhou, Q.; Zhao, J.; Ren, L.Q. Self-enriched mesoporous silica nanoparticle composite membrane with remarkable photodynamic antimicrobial performances. J. Colloid Interfaces Sci. 2020, 559, 197–205. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Moorthy, M.S.; Manivasagan, P.; Seo, H.; Lee, K.D.; Oh, J. Chlorin e6 conjugated silica nanoparticles for targeted and effective photodynamic therapy. Photodiagn. Photodyn. Ther. 2017, 19, 212–220. [Google Scholar] [CrossRef]
- Huang, C.L.; Zhang, Z.M.; Guo, Q.; Zhang, L.; Fan, F.; Qin, Y.; Wang, H.; Zhou, S.; Ou, W.B.Y.; Sun, H.F.; et al. A Dual-Model Imaging Theragnostic System Based on Mesoporous Silica Nanoparticles for Enhanced Cancer Phototherapy. Adv. Healthc. Mater. 2019, 8, e1900840. [Google Scholar] [CrossRef]
- Dey, P.; Blakey, I.; Stone, N. Diagnostic prospects and preclinical development of optical technologies using gold nanostructure contrast agents to boost endogenous tissue contrast. Chem. Sci. 2020, 11, 8671–8685. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Huang, Y.K.; Kalluru, P.; Chiang, C.S.; Hwang, K.C. First Demonstration of Gold Nanorods-Mediated Photodynamic Therapeutic Destruction of Tumors via Near Infra-Red Light Activation. Small 2014, 10, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Lin, C.C.; Kalluru, P.; Chiang, C.S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.L.; Zhang, X.; Li, N.N.; Wang, B.J.; He, S.L. Absorption-dependent generation of singlet oxygen from gold bipyramids excited under low power density. RSC Adv. 2015, 5, 81897–81904. [Google Scholar] [CrossRef]
- Vankayala, R.; Sagadevan, A.; Vijayaraghavan, P.; Kuo, C.L.; Hwang, K.C. Metal Nanoparticles Sensitize the Formation of Singlet Oxygen. Angew. Chem. Int. Ed. 2011, 50, 10640–10644. [Google Scholar] [CrossRef]
- Pasparakis, G. Light-Induced Generation of Singlet Oxygen by Naked Gold Nanoparticles and its Implications to Cancer Cell Phototherapy. Small 2013, 9, 4130–4134. [Google Scholar] [CrossRef]
- Jiang, C.F.; Zhao, T.T.; Yuan, P.Y.; Gao, N.Y.; Pan, Y.L.; Guan, Z.P.; Zhou, N.; Xu, Q.H. Two-Photon Induced Photoluminescence and Singlet Oxygen Generation from Aggregated Gold Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4972–4977. [Google Scholar] [CrossRef]
- Pakravan, A.; Salehi, R.; Mahkam, M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si -Au and Fe -Au core-shell on photothermal cancer treatment. Photodiagn. Photodyn. Ther. 2021, 33, 102144. [Google Scholar] [CrossRef]
- Shih, C.Y.; Huang, W.L.; Chiang, I.T.; Su, W.C.; Teng, H.S. Biocompatible hole scavenger-assisted graphene oxide dots for photodynamic cancer therapy. Nanoscale 2021, 13, 8431–8441. [Google Scholar] [CrossRef]
- Mangalath, S.; Babu, P.S.S.; Nair, R.R.; Manu, P.M.; Krishna, S.; Nair, S.A.; Joseph, J. Graphene Quantum Dots Decorated with Boron Dipyrromethene Dye Derivatives for Photodynamic Therapy. ACS Appl. Nano Mater. 2021, 4, 4162–4171. [Google Scholar] [CrossRef]
- Roeinfard, M.; Zahedifar, M.; Darroudi, M.; Zak, A.K.; Sadeghi, E. Preparation and characterization of selenium-decorated graphene quantum dots with high afterglow for application in photodynamic therapy. Luminescence 2020, 35, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.Y.; Zhang, Y.N.; Shi, X.Q.; Du, X.Y.; Wang, X.Y.; Yu, A.H.; Zhai, G.X. Recent progress of functionalised graphene oxide in cancer therapy. J. Drug Target. 2019, 27, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Rong, P.F.; Yang, K.; Srivastan, A.; Kiesewetter, D.O.; Yue, X.Y.; Wang, F.; Nie, L.M.; Bhirde, A.; Wang, Z.; Liu, Z.; et al. Photosensitizer Loaded Nano-Graphene for Multimodality Imaging Guided Tumor Photodynamic Therapy. Theranostics 2014, 4, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Zhou, L.; Chen, X.; Wu, Q.; Song, Z.Y.; Wei, S.H.; Zhou, J.H.; Shen, J. Mutual sensitization mechanism and self-degradation property of drug delivery system for in vitro photodynamic therapy. Int. J. Pharmaceut. 2016, 498, 335–346. [Google Scholar] [CrossRef]
- Cho, Y.; Choi, Y. Graphene oxide-photosensitizer conjugate as a redox-responsive theranostic agent. Chem. Commun. 2012, 48, 9912–9914. [Google Scholar] [CrossRef]
- Ge, J.C.; Lan, M.H.; Zhou, B.J.; Liu, W.M.; Guo, L.; Wang, H.; Jia, Q.Y.; Niu, G.L.; Huang, X.; Zhou, H.Y.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.S.; Shao, Y.T.; Huang, K.S.; Chou, T.M.; Yang, C.H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.K.; Wei, Y.C. Upconversion nanoparticle as a theranostic agent for tumor imaging and therapy. J. Innov. Opt. Health Sci. 2016, 9, 1630006. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef]
- Zhang, P.; Steelant, W.; Kumar, M.; Scholfield, M. Versatile photosensitizers for photodynamic therapy at infrared excitation. J. Am. Chem. Soc. 2007, 129, 4526–4527. [Google Scholar] [CrossRef] [Green Version]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, S.J.; Bu, W.B.; Chen, Y.; Xiao, Q.F.; Liu, J.A.; Xing, H.Y.; Zhou, L.P.; Peng, W.J.; Shi, J.L. A Uniform Sub-50 nm-Sized Magnetic/Upconversion Fluorescent Bimodal Imaging Agent Capable of Generating Singlet Oxygen by Using a 980 nm Laser. Chem. Eur. J. 2012, 18, 7082–7090. [Google Scholar] [CrossRef]
- Wang, C.; Tao, H.Q.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef]
- Dou, Q.Q.; Teng, C.P.; Ye, E.Y.; Loh, X.J. Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int. J. Nanomed. 2015, 10, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Rodriguez, E.M.; Naccache, R.; Forgione, P.; Lamoureux, G.; Sanz-Rodriguez, F.; Scheglmann, D.; Capobianco, J.A. Chemical modification of temoporfin—A second generation photosensitizer activated using upconverting nanoparticles for singlet oxygen generation. Chem. Commun. 2014, 50, 12150–12153. [Google Scholar] [CrossRef] [Green Version]
- Li, K.M.; Hong, E.L.; Wang, B.; Wang, Z.Y.; Zhang, L.W.; Hu, R.X.; Wang, B.Q. Advances in the application of upconversion nanoparticles for detecting and treating cancers. Photodiagn. Photodyn. Ther. 2019, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Tu, L.; Li, Q.; Feng, Y.; Que, I.; Zhang, Y.; Liu, X.; Xue, B.; Cruz, L.J.; Chang, Y.; et al. Near Infrared Light Sensitive Ultraviolet-Blue Nanophotoswitch for Imaging-Guided "Off-On" Therapy. ACS Nano 2018, 12, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
| Grade | Tumor Types | Characteristics | |
|---|---|---|---|
| Low Grade | Grade I |
|
|
| Grade II |
|
| |
| High Grade | Grade III |
|
|
| Grade IV |
|
|
| Photosensitizer (PS) | Type of Nanomaterials | Tumor Type Treated | Results and Highlights | Year | Ref. |
|---|---|---|---|---|---|
| Chlorin e6 (Ce6) | Stem cell membrane-camouflaged bioinspired nanoparticles | Lung cancer | The enhanced antitumor effect of Ng/Ce6@SCV after NIR irradiation significantly inhibits primary tumor growth with fewer side effects. | 2020 | [80] |
| Hyaluronic acid (HA)-based nanomaterials | Primary tumor and melanoma | Multifunctional nanosystem (HPR@CCP) exerted combined photodynamic and immunotherapeutic activity to amplify the therapeutic effect on primary tumors and distant metastases. | 2020 | [81] | |
| Peptide p 18-4/chlorin e6 (Ce6)-conjugated polyhedral oligomeric silsesquioxane (PPC) nanoparticles | Breast cancer cells | Cancer-targeting peptide p 18-4/chlorin e6 (Ce6)-conjugated polyhedral oligomeric silsesquioxane (PPC) nanoparticles improved the targeting ability of Ce6 to breast cancer cells to enhance PDT efficacy. | 2020 | [82] | |
| Ce6 loaded to the peroxidase-mimic metal-organic framework (MOF) MIL-100 (Ce6@MIL-100) | Breast cancer cell (4T1 cell line) | Peroxidase mimic metal-organic framework efficiently ablated tumors in microenvironment. | 2020 | [83] | |
| A fucoidan-based theranostic nanogel consisting of a fucoidan backbone, redox-responsive cleavable linker and Ce6 | Human fibrosarcoma cell line (HT1080) | Fucoidan, the polymer backbone of the nanogel platform, enabled cancer targeting by P-selectin binding and enhanced the antitumor effect by inhibiting the binding of vascular endothelial growth factor. | 2020 | [84] | |
| Ligation of an anticancer cabazitaxel (CTX) drug via reactive oxygen species-activated thioketal linkage produces a dimeric TKdC prodrug, followed by co-assembly with a photosensitizer, Ce6 | Human melanoma patient-derived xenograft (PDX) | Administration of psTKdC NAs followed by laser irradiation produced durable tumor regression, with tumors completely eradicated in three of six PDXs. | 2020 | [81] | |
| Light-enhanced PTX nanoparticles (Ce6/PTX2-Azo NPs) were prepared by synthesizing a hypoxia-activated self-sacrificing prodrug of paclitaxel (PTX2-Azo) and encapsulating it with a peptide copolymer decorated with the photosensitizer Ce6 | The innately hypoxic microenvironment of most solid tumors | PTX2-Azo prevented premature drug leakage and realized specific release in a hypoxic tumor microenvironment, and the photosensitizer Ce6 efficiently generated singlet oxygen under light irradiation and acted as a positive amplifier to promote the release of PTX | 2020 | [85] | |
| Ce6-caspase 3 cleavable peptide (Asp-Glu-Val-Asp, DEVD)-anticancer drug monomethyl auristatin E (MMAE) conjugate, resulting in Ce6-DEVD-MMAE nanoparticles | Squamous cell carcinoma 7 (SCC7) | Light-induced therapeutic strategy based on apoptotic activation of Ce6-DEVD-MMAE nanoparticles can be used to treat solid tumors inaccessible to conventional PDT. | 2019 | [86] | |
| 5-aminolevulinic acid (5-ALA) | Gold nanoparticles (GNP) conjugated to 5-ALA | Nonmelanoma skin cancer Subcutaneous squamous cell carcinoma (cSCC) | GNP conjugated to 5-ALA significantly enhanced the antitumor efficacy of PDT in HaCat and A431 cells | 2020 | [87] |
| Gefitinib PLGA nanoparticles | Lung cancer | Synergistic therapeutic effects were identified by the combination of chemotherapy and photodynamic therapy | 2020 | [88] | |
| Pheophorbide A (PhA) | Photoactivatable nanomicelles, which are constructed by self-assembly of poly (ethylene glycol) (PEG)-stearamine (C18) conjugate (PTS) with a ROS-sensitive thioketal linker (TL) and co-loaded with doxorubicin (DOX) and photosensitizer pheophorbide A (PhA) | Colon cancer cell line (CT-26) | The gradual elevation of local ROS levels generated by photoactivated PhA synergistically inhibited tumor growth and enhanced anti-tumor immunity by ROS-induced release of DOX. | 2020 | [89] |
| Acid-responsive polygalactose-co-polycinnamaldehyde polyprodrug (PGGA) self-assembled with PhA | Hepatocarcinoma (HepG2) | Intravenous injection of PGCA@PA NPs strongly inhibited tumor growth of hepatocellular carcinoma with negligible side effects. | 2020 | [90] | |
| PEG-doxorubicin conjugate | Colon cancer (CT-26) | Synergistically maximized the efficacy of the combination of chemotherapy and photodynamic therapy. | 2020 | [91] | |
| IR780 | IR780 loaded on the prodrug micelle that consisted of camptothecin (CPT) andpolyethylene glycol (PEG) with further modification of iRGD peptide. | Glioma | The targeted prodrug system could effectively cross various barriers to reach the glioma site and greatly enhanced the antitumor effect with laser irradiation. | 2020 | [92] |
| Poly-ε-caprolactone nanoparticles (PCL NPs) modified with LHRH peptide and loaded with IR780 and paclitaxel (PTX) | Ovarian cancer | LHRH peptide modified PCL (PCL-LHRH) NPs demonstrated increased internalization in ovarian tumor cells in vitro and selective targeting in tumor xenografts in vivo | 2020 | [93] | |
| Indocyanine | Graphene oxide nanoparticle | Osteosarcoma | Nanoparticle consisting of polyethylene glycol (PEG), folic acid (FA), PS indocyanine green (ICG), and doxorubicin inhibited the proliferation and migration of osteosarcoma cells. | 2020 | [90] |
| Self-assembled nanoparticle with indocyanine, camptothecin, RGD peptide | Human cervical carcinoma cell lines (HeLa); Human hepatoma (BEL-7402) | This facile and effective self-assembly strategy to construct nanodrugs demonstrated enhanced performance for cancer theranostics. | 2018 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Lee, D.Y. Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors. Biomedicines 2022, 10, 96. https://doi.org/10.3390/biomedicines10010096
Kim HS, Lee DY. Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors. Biomedicines. 2022; 10(1):96. https://doi.org/10.3390/biomedicines10010096
Chicago/Turabian StyleKim, Hyung Shik, and Dong Yun Lee. 2022. "Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors" Biomedicines 10, no. 1: 96. https://doi.org/10.3390/biomedicines10010096
APA StyleKim, H. S., & Lee, D. Y. (2022). Nanomedicine in Clinical Photodynamic Therapy for the Treatment of Brain Tumors. Biomedicines, 10(1), 96. https://doi.org/10.3390/biomedicines10010096







