Pretherapeutic Serum Albumin as an Outcome Prognosticator in Head and Neck Adenoid-Cystic Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistics
2.2. Ethical Statement
3. Results
3.1. Patients
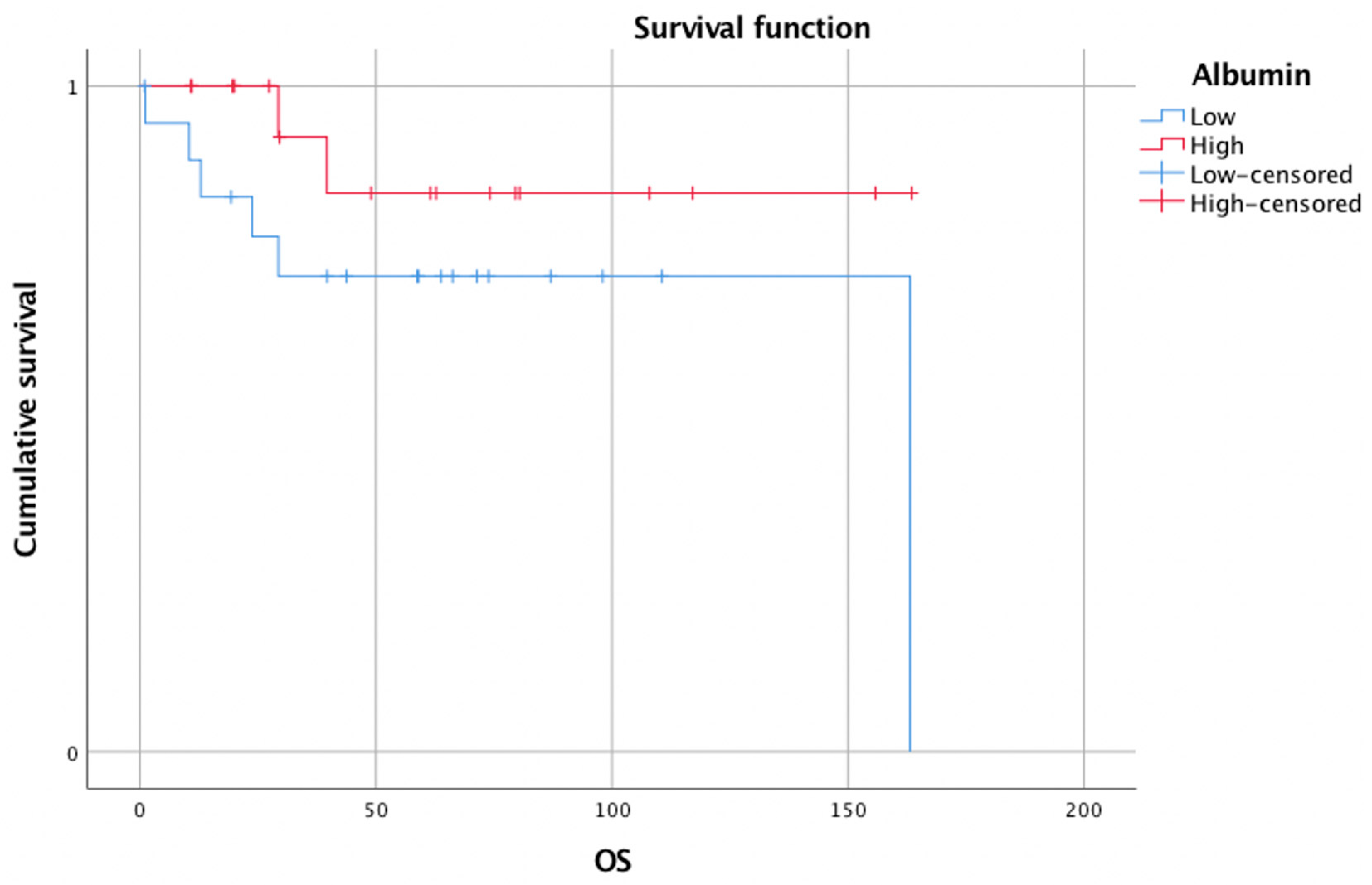
3.2. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spiro, R.H. Salivary neoplasms: Overview of a 35-year experience with 2807 patients. Head Neck Surg. 1986, 8, 177–184. [Google Scholar] [CrossRef]
- Mannelli, G.; Cecconi, L.; Fasolati, M.; Santoro, R.; Franchi, A.; Gallo, O. Parotid adenoid cystic carcinoma: Retrospective single institute analysis. Am. J. Otolaryngol. 2017, 38, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.J.; Roh, J.-L.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Risk factors and survival associated with distant metastasis in patients with carcinoma of the salivary gland. Ann. Surg. Oncol. 2016, 23, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Bjørndal, K.; Agander, T.K.; Wessel, I.; Homøe, P. Tumors of the sublingual gland: A national clinicopathologic study of 29 cases. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3847–3856. [Google Scholar] [CrossRef]
- Yamahara, K.; Mizukoshi, A.; Lee, K.; Ikegami, S. Pretherapeutic nutritional/inflammatory factors as predictors for survival of both early and advanced staged head and neck cancer patients. Auris Nasus Larynx 2021, 48, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Roh, J.-L.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Pretreatment albumin level predicts survival in head and neck squamous cell carcinoma. Laryngoscope 2017, 127, E437–E442. [Google Scholar] [CrossRef]
- Wu, N.; Chen, G.; Hu, H.; Pang, L.; Chen, Z. Low pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomas. Nutr. Cancer 2015, 67, 481–485. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, D.; Lao, X.; Liang, Y. The value of MYB as a prognostic marker for adenoid cystic carcinoma: Meta-analysis. Head Neck 2019, 41, 1517–1524. [Google Scholar] [CrossRef]
- Bazarsad, S.; Kim, J.Y.; Zhang, X.; Kim, K.-Y.; Lee, D.Y.; Ryu, M.H.; Kim, J. Ataxia-telangiectasia-mutated protein expression as a prognostic marker in adenoid cystic carcinoma of the salivary glands. Yonsei Med. J. 2018, 59, 717–726. [Google Scholar] [CrossRef]
- Kadletz, L.C.; Brkic, F.F.; Jank, B.J.; Schneider, S.; Cede, J.; Seemann, R.; Gruber, E.S.; Gurnhofer, E.; Heiduschka, G.; Kenner, L. AF1q expression associates with CD44 and STAT3 and impairs overall survival in adenoid cystic carcinoma of the head and neck. Pathol. Oncol. Res. 2020, 26, 1287–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brkic, F.F.; Kadletz, L.; Jank, B.; Mayer, C.; Heiduschka, G.; Brunner, M. Impact of pretherapeutic neutrophil-to-lymphocyte ratio, serum albumin, body-mass index, and advanced lung cancer inflammation index on clinical outcome in sinonasal squamous cell carcinoma. J. Cranio-Maxillofac. Surg. 2020, 48, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lis, C.G.; Grutsch, J.F.; Vashi, P.G.; Lammersfeld, C.A. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J. Parenter. Enteral. Nutr. 2003, 27, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Maltoni, M.; Amadori, D. Prognosis in advanced cancer. Hematol. Oncol. Clin. N. Am. 2002, 16, 715–729. [Google Scholar] [CrossRef]
- Mantzorou, M.; Koutelidakis, A.; Theocharis, S.; Giaginis, C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr. Cancer 2017, 69, 1151–1176. [Google Scholar] [CrossRef]
- Mattox, T.W. Cancer cachexia: Cause, diagnosis, and treatment. Nutr. Clin. Pr. 2017, 32, 599–606. [Google Scholar] [CrossRef]
- Nazha, B.; Moussaly, E.; Zaarour, M.; Weerasinghe, C.; Azab, B. Hypoalbuminemia in colorectal cancer prognosis: Nutritional marker or inflammatory surrogate? World J. Gastrointest. Surg. 2015, 7, 370–377. [Google Scholar] [CrossRef]
- Kennelly, P.J.; Murray, R.K.; Jacob, M.; Varghese, J. Plasma proteins & immunoglobulins. In Harper’s Illustrated Biochemistry; Rodwell, V.W., Bender, D.A., Botham, K.M., Kennelly, P.J., Weil, P.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2016; Available online: Accessmedicine.mhmedical.com/content.aspx?aid=1106060547 (accessed on 21 October 2021).
- Don, B.R.; Kaysen, G. Poor nutritional status and inflammation: Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Fleck, A.; Hawker, F.; Wallace, P.; Raines, G.; Trotter, J.; Ledingham, I.; Calman, K. Increased vascular permeability: A major cause of hypoalbuminaemia in disease and injury. Lancet 1985, 325, 781–784. [Google Scholar] [CrossRef]
- Kowalski-Saunders, P.W.J.; Winwood, P.J.; Arthur, M.J.P.; Wright, R. Reversible inhibition of albumin production by rat hepatocytes maintained on a laminin-rich gel (Engelbreth-Holm-Swarm) in response to secretory products of Kupffer cells and cytokines. Hepatology 1992, 16, 733–741. [Google Scholar] [CrossRef]
- Babson, A.L.; Winnick, T. Protein transfer in tumor-bearing rats. Cancer Res. 1954, 14, 606–611. [Google Scholar] [PubMed]
- Asher, V.; Lee, J.; Bali, A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med. Oncol. 2012, 29, 2005–2009. [Google Scholar] [CrossRef]
- Danan, D.; Shonka, D.C., Jr.; Selman, Y.; Chow, Z.; Smolkin, M.E.; Jameson, M.J. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope 2016, 126, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, A.; Saliba, J.; Castano, R.; Hier, M.; Zeitouni, A.G. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 2015, 37, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Brkic, F.F.; Kadletz, L.; Jank, B.; Cede, J.; Seemann, R.; Schneider, S.; Haymerle, G.; Parzefall, T.; Kenner, L.; Heiduschka, G. Pretreatment assessment of hematologic and inflammatory markers in adenoid cystic carcinoma: Neutrophil/lymphocyte ratio is associated with multiple recurrences. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 408–416. [Google Scholar] [CrossRef]
- Cordesmeyer, R.; Schliephake, H.; Kauffmann, P.; Tröltzsch, M.; Laskawi, R.; Ströbel, P.; Bremmer, F. Clinical prognostic factors of salivary adenoid cystic carcinoma: A single-center analysis of 61 patients. J. Cranio-Maxillofac. Surg. 2017, 45, 1784–1787. [Google Scholar] [CrossRef] [PubMed]

| Age | Years | |
|---|---|---|
| Mean | 57.2 | |
| Standard deviation | 14.2 | |
| Gender | n | % |
| Male | 16 | 43.3 |
| Female | 21 | 56.7 |
| T classification | n | % |
| T1 | 4 | 10.8 |
| T2 | 11 | 29.7 |
| T3 | 8 | 21.6 |
| T4 | 14 | 37.8 |
| N classification | n | % |
| N0 | 31 | 83.8 |
| N1 | 6 | 16.2 |
| N2 | 0 | 0 |
| N3 | 0 | 0 |
| M classification | n | % |
| M0 | 36 | 97.3 |
| M1 | 1 | 2.7 |
| Primary therapy | n | % |
| Surgery alone | 13 | 35.1 |
| Surgery with postoperative radiotherapy | 14 | 37.9 |
| Surgery with postoperative chemoradiotherapy | 1 | 2.7 |
| Radiotherapy | 6 | 16.2 |
| Chemoradiotherapy | 2 | 5.4 |
| Palliative chemotherapy | 1 | 2.7 |
| Localization | n | % |
| Minor salivary glands | 25 | 67.6 |
| Sublingual gland | 1 | 2.7 |
| Submandibular gland | 6 | 16.2 |
| Parotid gland | 5 | 13.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedl, M.; Stoiber, S.; Brkic, F.F.; Kadletz-Wanke, L. Pretherapeutic Serum Albumin as an Outcome Prognosticator in Head and Neck Adenoid-Cystic Carcinoma. Biomedicines 2022, 10, 191. https://doi.org/10.3390/biomedicines10010191
Friedl M, Stoiber S, Brkic FF, Kadletz-Wanke L. Pretherapeutic Serum Albumin as an Outcome Prognosticator in Head and Neck Adenoid-Cystic Carcinoma. Biomedicines. 2022; 10(1):191. https://doi.org/10.3390/biomedicines10010191
Chicago/Turabian StyleFriedl, Marlene, Stefan Stoiber, Faris F. Brkic, and Lorenz Kadletz-Wanke. 2022. "Pretherapeutic Serum Albumin as an Outcome Prognosticator in Head and Neck Adenoid-Cystic Carcinoma" Biomedicines 10, no. 1: 191. https://doi.org/10.3390/biomedicines10010191
APA StyleFriedl, M., Stoiber, S., Brkic, F. F., & Kadletz-Wanke, L. (2022). Pretherapeutic Serum Albumin as an Outcome Prognosticator in Head and Neck Adenoid-Cystic Carcinoma. Biomedicines, 10(1), 191. https://doi.org/10.3390/biomedicines10010191







