Canthaxanthin Biofabrication, Loading in Green Phospholipid Vesicles and Evaluation of In Vitro Protection of Cells and Promotion of Their Monolayer Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bioproduction and of Canthaxanthin
2.3. Canthaxanthin Analysis
2.4. Ability of Canthaxanthin to Scavenge Free Radicals
2.5. Vesicle Preparation
2.6. Vesicle Characterisation
2.7. Ability of Canthaxanthin Formulations to Inhibit the Generation of Nitric Oxide in Macrophages
2.8. In Vitro Cytotoxicity of Formulations
2.9. In Vitro Protective Effect of Formulations against Oxidative Damage in Fibroblasts
2.10. In Vitro Scratch Assay
2.11. Statistical Analysis of Data
3. Results
3.1. Canthaxanthin Bioproduction and Quantification
3.2. Vesicle Characterisation
3.3. Evaluation of the Antioxidant Activity of Formulations
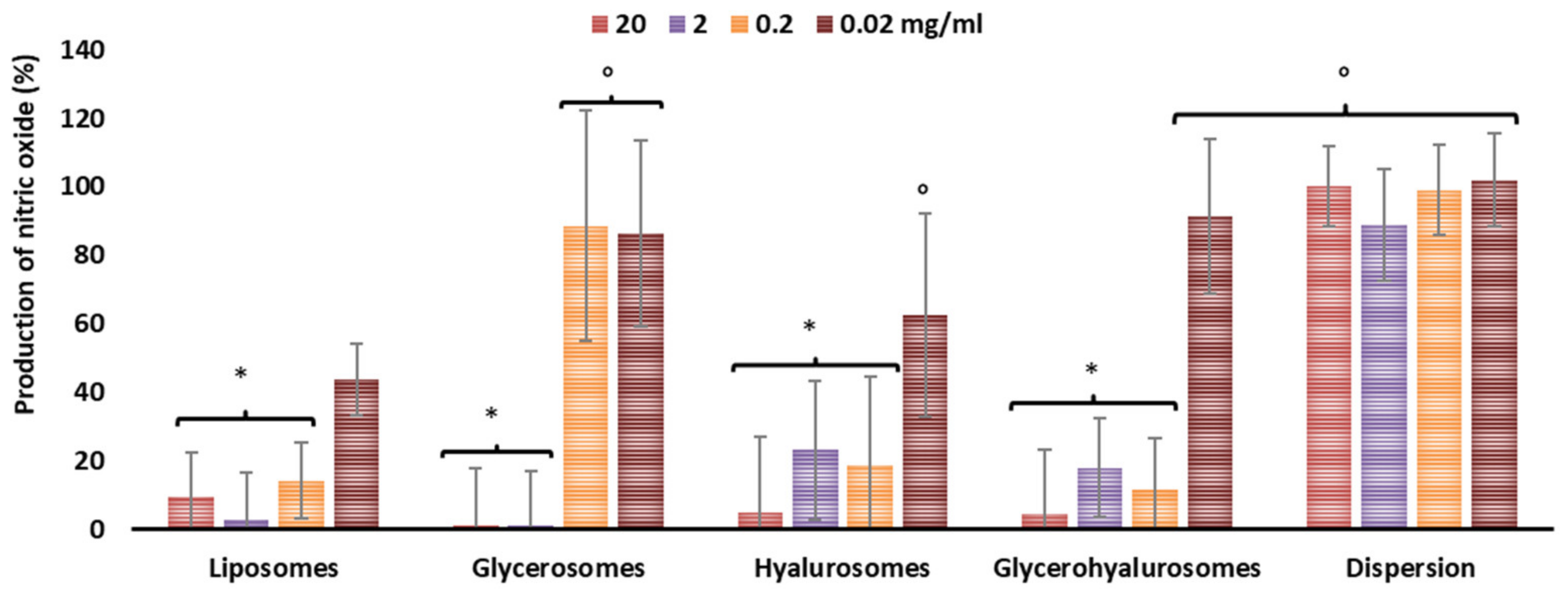
3.4. Inhibition of Nitric Oxide Generation in Cells
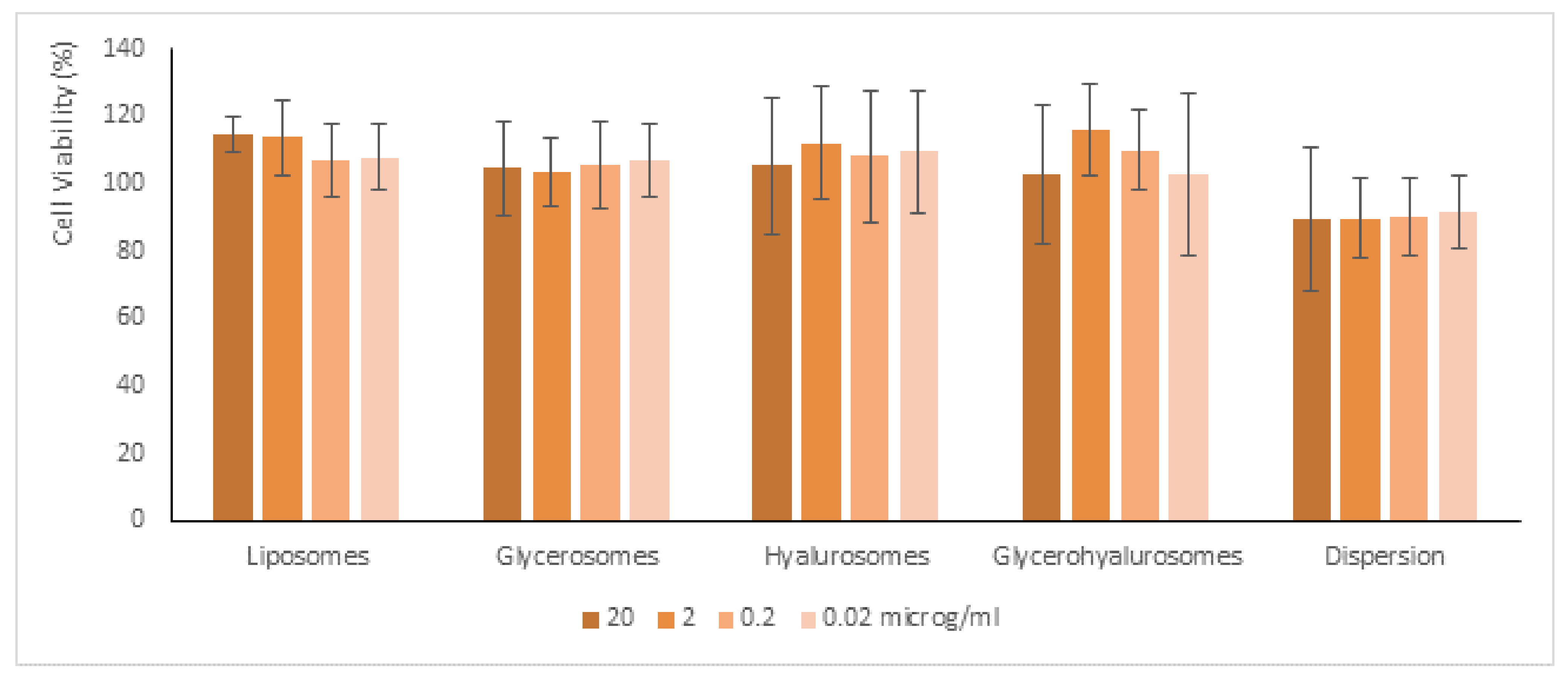
3.5. Biocompatibility of Formulations
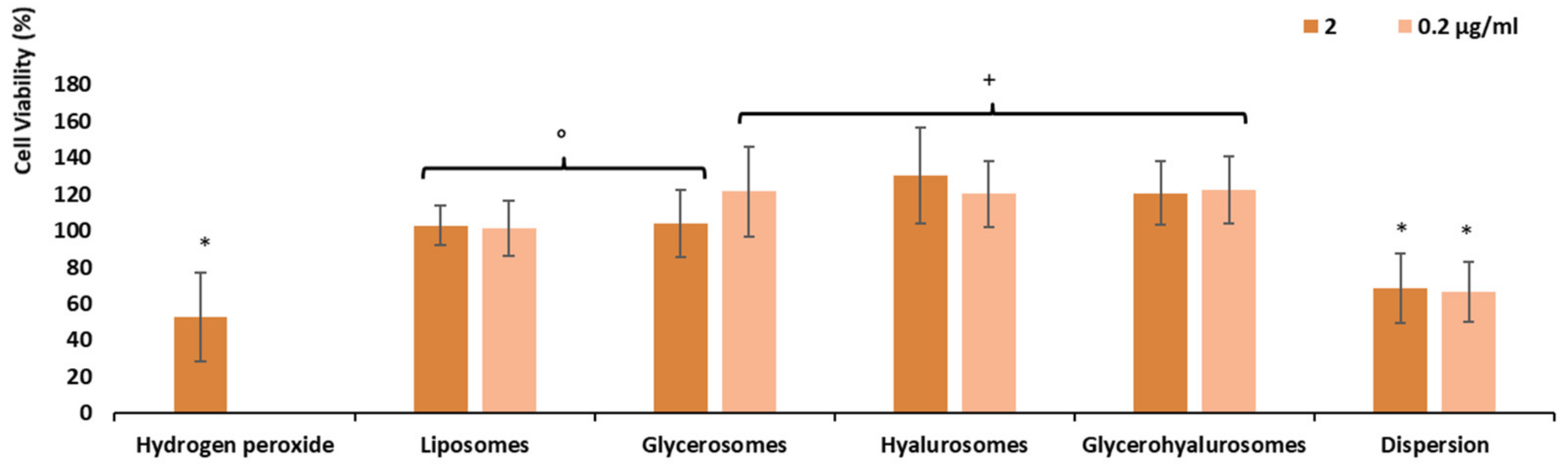
3.6. Protective Effect of the Formulations against Damages Induced by Hydrogen Peroxide
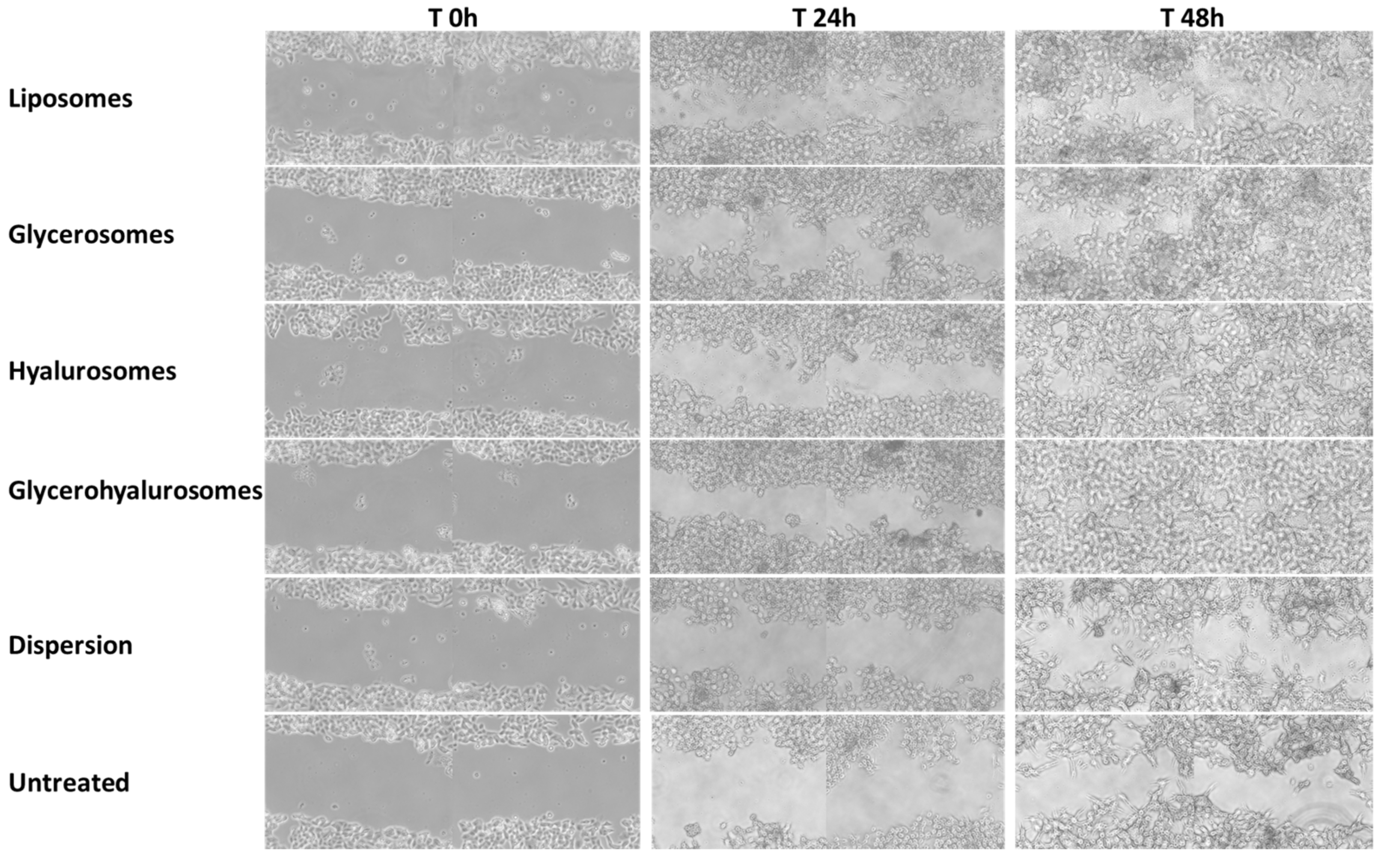
3.7. In Vitro Scratch Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Oliver, J.; Palou, A. Chromatographic Determination of Carotenoids in Foods. J. Chromatogr. A 2000, 881, 543–555. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Gilmore, A.; Iii, W.M.A. In Vivo Functions of Carotenoids in Higher Plants. Wiley Online Libr. 1996, 10, 403–412. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Rebelo, B.A.; Farrona, S.; Rita Ventura, M.; Abranches, R. Canthaxanthin, a Red-Hot Carotenoid: Applications, Synthesis, and Biosynthetic Evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, K.; Felix, N.; Prabu, E. A Review on the Application and Effect of Carotenoids with Respect to Canthaxanthin in the Culture of Fishes and Crustaceans. Int. J. Fish. Aquat. Stud. 2020, 8, 128–133. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From Molecule to Function. Mol. Nutr. Food Res. 2017, 61, 1600469. [Google Scholar] [CrossRef] [PubMed]
- Chandi, G.K.; Gill, B.S. Production and Characterization of Microbial Carotenoids as an Alternative to Synthetic Colors: A Review. Int. J. Food Prop. 2011, 14, 503–513. [Google Scholar] [CrossRef]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor Cultivation of Microalgae for Carotenoid Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Khodaiyan, K.; Razavi, S.H.; Emam-Djomeh, Z.; Mousavi, S.M.A.; Hejazi, M.A. Effect of Culture Conditions on Canthaxanthin Production by Dietzia Natronolimnaea HS-1. J. Microbiol. Biotechnol. 2007, 17, 195–201. Available online: https://www.researchgate.net/publication/5795841_Effect_of_culture_conditions_on_canthaxanthin_production_by_Dietzia_natronolimnaea_HS-1 (accessed on 26 November 2021).
- Ravaghi, M.; Razavi, S.H.; Mousavi, S.M.; Sinico, C.; Fadda, A.M. Stabilization of Natural Canthaxanthin Produced by Dietzia Natronolimnaea HS-1 by Encapsulation in Niosomes. LWT 2016, 73, 498–504. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, M. Characterizing the Natural Canthaxanthin/2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Carbohydr. Polym. 2014, 101, 1147–1153. [Google Scholar] [CrossRef]
- Allaw, M.; Castangia, I.; Manca, M.L.; Manconi, M. From Plants to Phospholipid Vesicle Products: A Comprehensive Review on the Incorporation of Plant- Derived Phytochemicals into Phospholipids Vesicles Designed for Skin Applications with Special Focus on Scalability and in Vitro and in Vivo Efficacy. J. Drug Deliv. Sci. Technol. 2021, in press.
- Manca, M.L.; Manconi, M.; Zaru, M.; Castangia, I.; Cabras, A.; Cappa, N.; Fadda, A.M. Hyalurosomes, Their Use in Topical Cosmetic or Pharmaceutical Composition and Their Preparation Process. U.S. Patent 14/950,696, 2 June 2016. [Google Scholar]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A New Tool for Effective Dermal and Transdermal Drug Delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Catalán-Latorre, A.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Phycocyanin-Encapsulating Hyalurosomes as Carrier for Skin Delivery and Protection from Oxidative Stress Damage. J. Mater. Sci. Mater. Med. 2016, 27, 75. [Google Scholar] [CrossRef]
- Allaw, M.; Pleguezuelos-Villa, M.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Nacher, A.; Diez-Sales, O.; Saurí, A.R.; Ferrer, E.E.; Fadda, A.M.; et al. Innovative Strategies to Treat Skin Wounds with Mangiferin: Fabrication of Transfersomes Modified with Glycols and Mucin. Nanomedicine 2020, 15, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Manconia, M.; Pendás, J.; Ledón, N.; Moreira, T.; Sinico, C.; Saso, L.; Fadda, A.M. Phycocyanin Liposomes for Topical Anti-Inflammatory Activity: In-Vitro in-Vivo Studies. J. Pharm. Pharmacol. 2009, 61, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Gharibzahedi, T.; Razavi, S.H.; Mousavi, M. Kinetic Analysis and Mathematical Modeling of Cell Growth and Canthaxanthin Biosynthesis by Dietzia Natronolimnaea HS-1 on Waste Molasses Hydrolysate. RSC Adv. 2013, 3, 23495–23502. [Google Scholar] [CrossRef]
- Razavi, S.; Blanchard, F.; Marc, I. UV-HPLC/APCI-MS Method for Separation and Identification of the Carotenoids Produced by Sporobolomyces Ruberrimus H110. Chem. Eng. 2006, 25, 1–10. [Google Scholar]
- Zaru, M.; Manca, M.L.; Fadda, A.M.; Orsini, G. Glycerosomes and Use Thereof in Pharmaceutical and Cosmetic Preparations for Topical Applications. U.S. Patent 8,778,367, 15 July 2014. [Google Scholar]
- Manconi, M.; Manca, M.; Caddeo, C.; Sarais, G.; Palmieri, A.; D’Hallewin, G.; Fadda, A. Citrus Limon Extract Loaded in Vesicular Systems for the Protection of Oral Cavity. Medicines 2018, 5, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Manconi, M.; Meloni, M.C.; Marongiu, F.; Allaw, M.; Usach, I.; Peris, J.E.; Escribano-Ferrer, E.; Tuberoso, C.I.G.; Gutierrez, G.; et al. Nanotechnology for Natural Medicine: Formulation of Neem Oil Loaded Phospholipid Vesicles Modified with Argan Oil as a Strategy to Protect the Skin from Oxidative Stress and Promote Wound Healing. Antioxidants 2021, 10, 670. [Google Scholar] [CrossRef]
- Olmos, E.; Mehmood, N.; Husein, L.H.; Goergen, J.L.; Fick, M.; Delaunay, S. Effects of Bioreactor Hydrodynamics on the Physiology of Streptomyces. Bioprocess Biosyst. Eng. 2013, 36, 259–272. [Google Scholar] [CrossRef]
- Reynders, M.B.; Rawlings, D.E.; Harrison, S.T.L. Demonstration of the Crabtree Effect in Phaffia Rhodozyma during Continuous and Fed-Batch Cultivation. Biotechnol. Lett. 2016, 19, 549–552. [Google Scholar] [CrossRef]
- Abruzzo, A.; Cappadone, C.; Farruggia, G.; Luppi, B.; Bigucci, F.; Cerchiara, T. Glycyrrhetinic Acid Liposomes and Hyalurosomes on Spanish Broom, Flax, and Hemp Dressings to Heal Skin Wounds. Molecules 2020, 25, 2558. [Google Scholar] [CrossRef]
- Paschke, A.; Zunker, K.; Wigotzki, M.; Steinhart, H. Determination of the IgE-Binding Activity of Soy Lecithin and Refined and Non-Refined Soybean Oils. J. Chromatogr. B: Biomed. Sci. Appl. 2001, 756, 249–254. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Identification and Quantification of Phenolic Compounds in Rapeseed Originated Lecithin and Antioxidant Activity Evaluation. LWT 2016, 73, 397–405. [Google Scholar] [CrossRef]
- Padwad, Y.; Ganju, L.; Jain, M.; Chanda, S.; Karan, D.; Banerjee, P.K.; Sawhney, R.C. Effect of Leaf Extract of Seabuckthorn on Lipopolysaccharide Induced Inflammatory Response in Murine Macrophages. Int. Immunopharmacol. 2006, 6, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Meikle, T.G.; Drummond, C.J.; Yang, Y.; Conn, C.E. Comparison of Cubosomes and Liposomes for the Encapsulation and Delivery of Curcumin. Soft Matter 2021, 17, 3306–3313. [Google Scholar] [CrossRef]
- Takahashi, M.; Kitamoto, D.; Asikin, Y.; Takara, K.; Wada, K. Liposomes Encapsulating Aloe Vera Leaf Gel Extract Significantly Enhance Proliferation and Collagen Synthesis in Human Skin Cell Lines. J. Oleo Sci. 2009, 58, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Protects Keratinocyte Cell Membranes Following Exposure to UVB and Hydrogen Peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef] [PubMed]
- Scano, A.; Ebau, F.; Manca, M.L.; Cabras, V.; Marincola, F.C.; Manconi, M.; Pilloni, M.; Fadda, A.M.; Ennas, G. Novel Drug Delivery Systems for Natural Extracts: The Case Study of Vitis Vinifera Extract-SiO2 Nanocomposites. Int. J. Pharm. 2018, 551, 84–96. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Hydrocolloids, S.X.-F. Biopolymer-Coated Liposomes by Electrostatic Adsorption of Chitosan (Chitosomes) as Novel Delivery Systems for Carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Ramakrishnan, B.; Ratha, S.K.; Bhaskar, S.; Chinnasamy, S. Valorisation of Molasses by Oleaginous Yeasts for Single Cell Oil (SCO) and Carotenoids Production. Environ. Technol. Innov. 2021, 21, 101281. [Google Scholar] [CrossRef]
- Robert, C.; Couëdelo, L.; Vaysse, C.; Michalski, M.C. Vegetable Lecithins: A Review of Their Compositional Diversity, Impact on Lipid Metabolism and Potential in Cardiometabolic Disease Prevention. Biochimie 2020, 169, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Matricardi, P.; Lampis, S.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Molecular Arrangements and Interconnected Bilayer Formation Induced by Alcohol or Polyalcohol in Phospholipid Vesicles. Colloids Surf. B Biointerfaces 2014, 117, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of Positively and Negatively Charged Liposomes on Skin Permeation of Drugs. J. Drug Target. 2001, 9, 49–59. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M. Microbial Canthaxanthin: Perspectives on Biochemistry and Biotechnological Production. Eng. Life Sci. 2013, 13, 408–417. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in Liposome Technology: Preparation Techniques and Applications in Food, Functional Foods, and Bioactive Delivery: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]



| Canthaxanthin | Lecithin | Hyaluronan | Water | Glycerol | |
|---|---|---|---|---|---|
| mg/mL | mg/mL | mg/mL | mL | mL | |
| Liposomes | 20 | 120 | - | 1 | - |
| Glycerosomes | 20 | 120 | - | 0.7 | 0.3 |
| Hyalurosomes | 20 | 120 | 1 | 0.7 | - |
| Glycerohyalurosomes | 20 | 120 | 1 | 0.7 | 0.3 |
| DM | (PI) | ZP (mV) | EE (%) | |
|---|---|---|---|---|
| Empty liposomes | * 66 ± 2 | 0.25 | −54 ± 3 | − |
| Empty glycerosomes | * 65 ± 3 | 0.23 | −49 ± 5 | − |
| Empty hyalurosomes | * 64 ± 2 | 0.24 | −57 ± 7 | − |
| Empty glycerohyalurosomes | * 59 ± 3 | 0.23 | −49 ± 3 | |
| Canthaxanthin liposomes | ° 72 ± 6 | 0.22 | −48 ± 6 | 57 ± 5 |
| Canthaxanthin glycerosomes | ° 71 ± 5 | 0.26 | −49 ± 2 | # 78 ± 3 |
| Canthaxanthin hyalurosomes | § 87 ± 11 | 0.25 | −54 ± 9 | # 76 ± 6 |
| Canthaxanthin glycerohyalurosomes | * 61 ± 4 | 0.22 | −49 ± 5 | # 81 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castangia, I.; Manca, M.L.; Razavi, S.H.; Nácher, A.; Díez-Sales, O.; Peris, J.E.; Allaw, M.; Terencio, M.C.; Usach, I.; Manconi, M. Canthaxanthin Biofabrication, Loading in Green Phospholipid Vesicles and Evaluation of In Vitro Protection of Cells and Promotion of Their Monolayer Regeneration. Biomedicines 2022, 10, 157. https://doi.org/10.3390/biomedicines10010157
Castangia I, Manca ML, Razavi SH, Nácher A, Díez-Sales O, Peris JE, Allaw M, Terencio MC, Usach I, Manconi M. Canthaxanthin Biofabrication, Loading in Green Phospholipid Vesicles and Evaluation of In Vitro Protection of Cells and Promotion of Their Monolayer Regeneration. Biomedicines. 2022; 10(1):157. https://doi.org/10.3390/biomedicines10010157
Chicago/Turabian StyleCastangia, Ines, Maria Letizia Manca, Seyed Hadi Razavi, Amparo Nácher, Octavio Díez-Sales, José Esteban Peris, Mohamad Allaw, Maria Carmen Terencio, Iris Usach, and Maria Manconi. 2022. "Canthaxanthin Biofabrication, Loading in Green Phospholipid Vesicles and Evaluation of In Vitro Protection of Cells and Promotion of Their Monolayer Regeneration" Biomedicines 10, no. 1: 157. https://doi.org/10.3390/biomedicines10010157
APA StyleCastangia, I., Manca, M. L., Razavi, S. H., Nácher, A., Díez-Sales, O., Peris, J. E., Allaw, M., Terencio, M. C., Usach, I., & Manconi, M. (2022). Canthaxanthin Biofabrication, Loading in Green Phospholipid Vesicles and Evaluation of In Vitro Protection of Cells and Promotion of Their Monolayer Regeneration. Biomedicines, 10(1), 157. https://doi.org/10.3390/biomedicines10010157








