Chlorin Endogenous to the North Pacific Brittle Star Ophiura sarsii for Photodynamic Therapy Applications in Breast Cancer and Glioblastoma Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Collection and Food Deprivation
2.2. Homogenization and Extraction
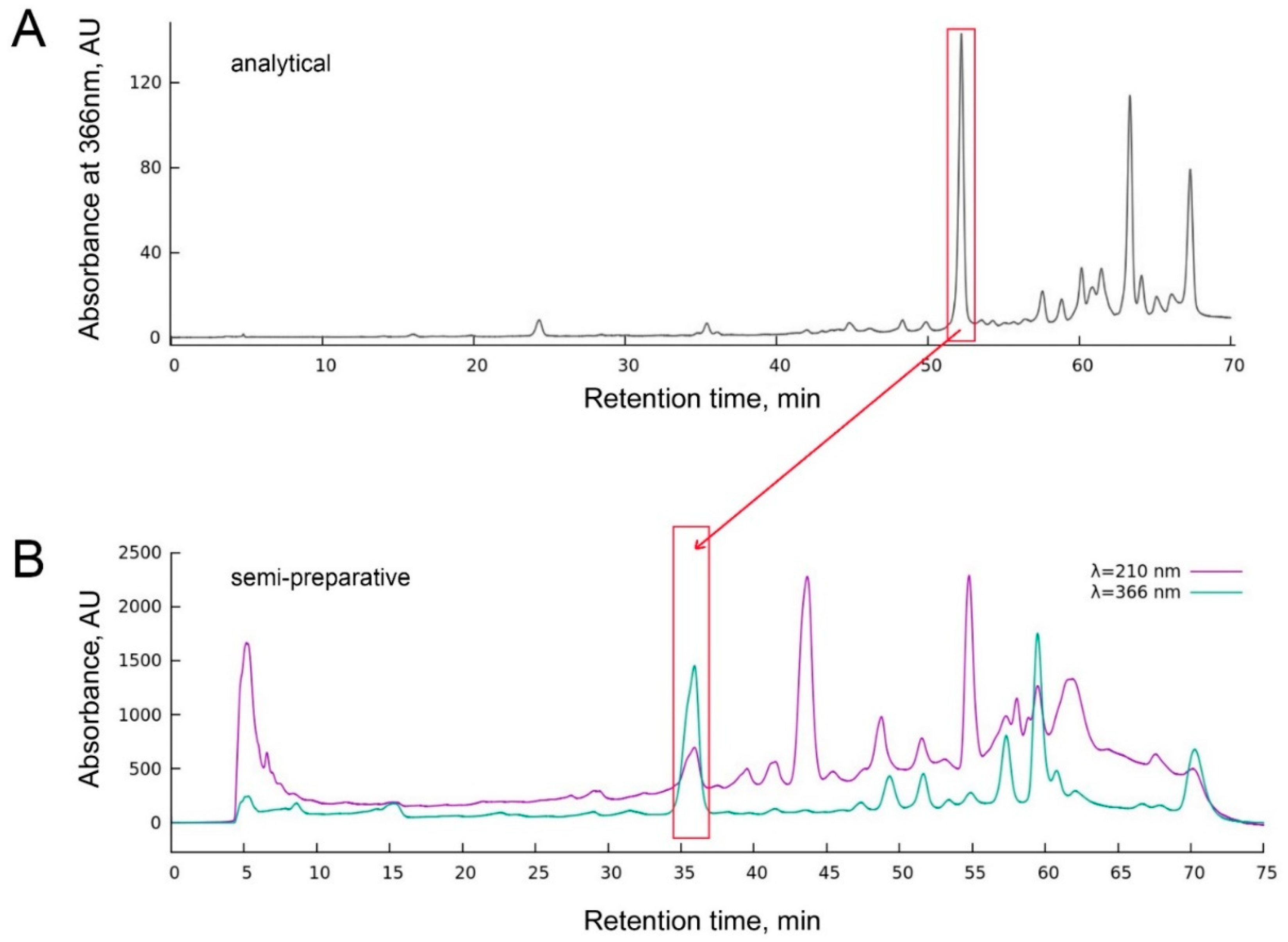
2.3. Analytical and Semi-Preparative HPLC
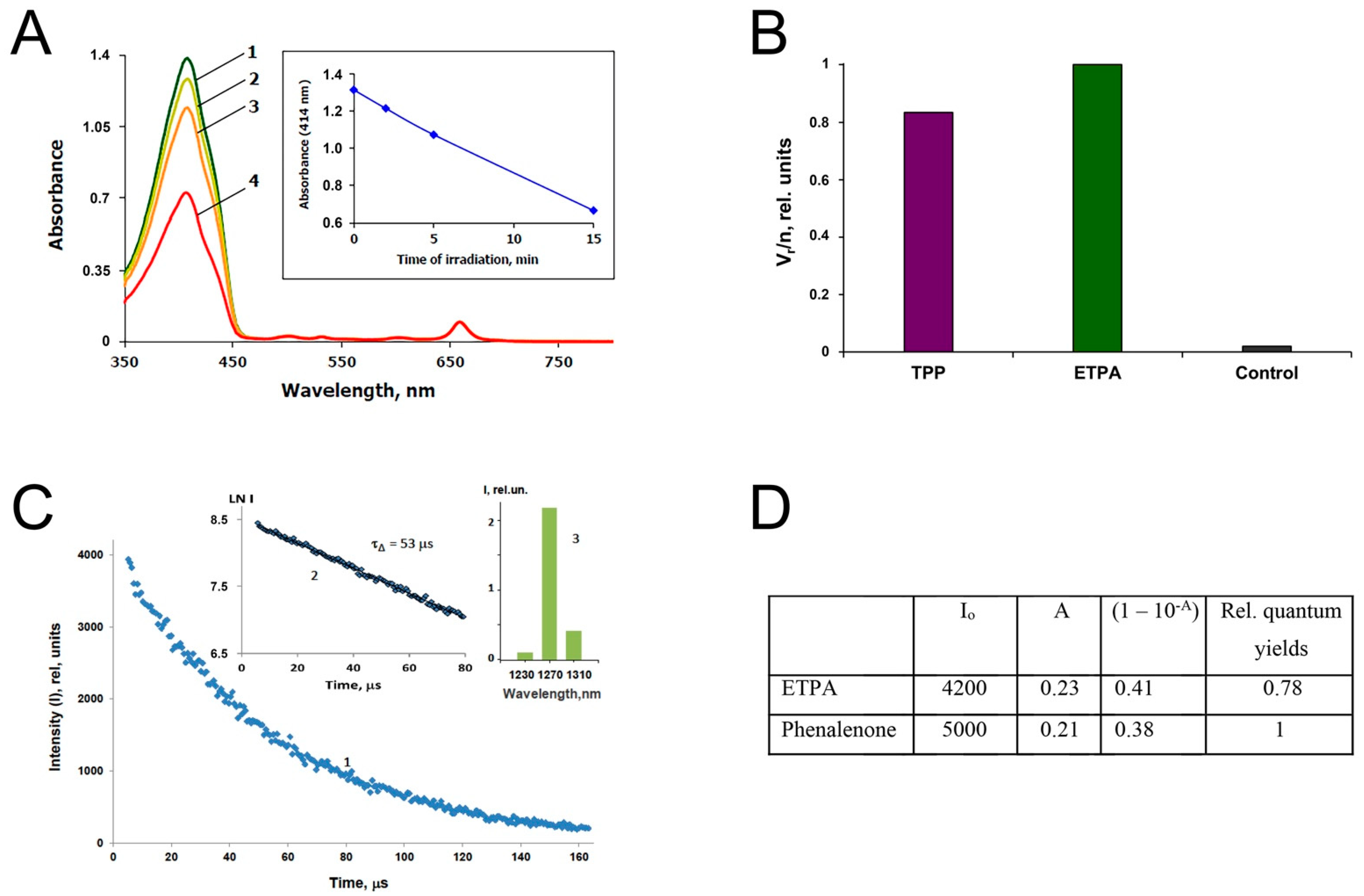
2.4. High-Resolution Spectrophotometry
2.5. Cells and Medium
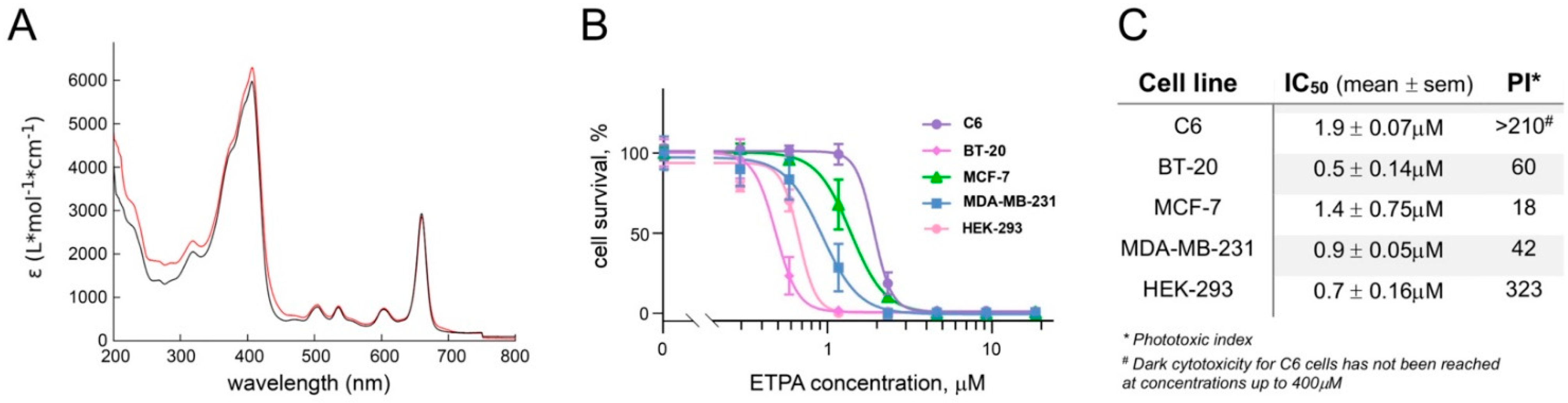
2.6. Photoxicity Assays
2.7. Determination of the Singlet Oxygen Quantum Yield of ETPA
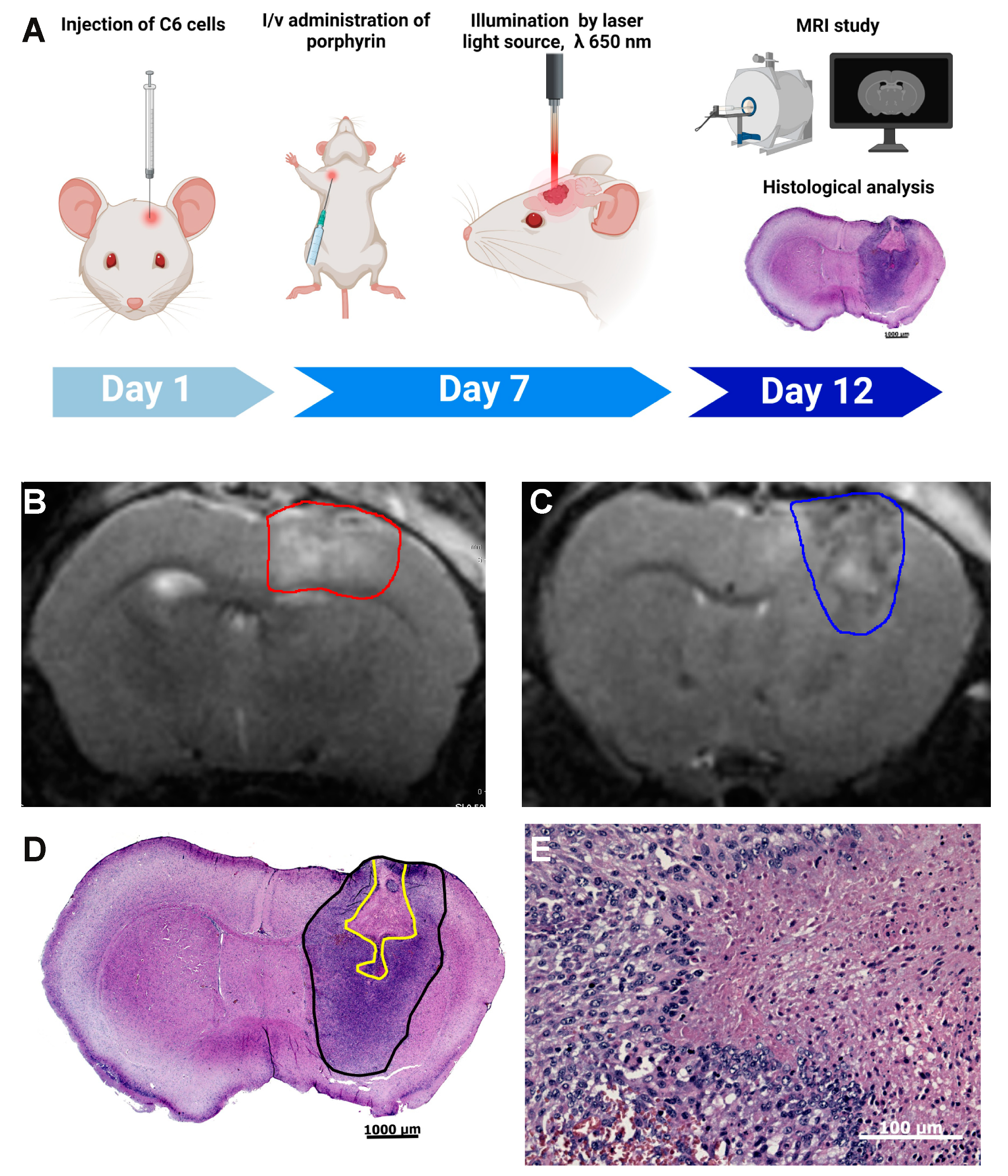
2.8. Mouse Experimentation
2.9. Cell Culture and Intracranial Tumor Implantation
2.10. Glioma Photodynamic Therapy (PDT)
2.11. Magnetic Resonance Imaging and Histological Studies of the Tumor Injury
3. Results
3.1. Chlorin Is Endogenous to O. sarsii
3.2. Phototoxicity of ETPA against a Panel of Cancer Lines
3.3. Singlet Oxygen Production by ETPA
3.4. Photodynamic Therapy with ETPA in a Mouse Model of Brain Tumor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dougherty, T.J. Introduction. In Methods in Molecular Biology; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2010; Volume 635, pp. 1–6. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Goswami, L.N.; Chen, Y.; Gryshuk, A.; Missert, J.R.; Oseroff, A.; Dougherty, T.J. Nature: A rich source for developing multifunctional agents. Tumor-imaging and photodynamic therapy. Lasers Surg. Med. 2006, 38, 445–467. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Nature: A vital source of leads for anticancer drug development. Phytochem. Rev. 2009, 8, 313–331. [Google Scholar] [CrossRef]
- Katanaev, V.L.; Blagodatski, A.; Xu, J.; Khotimchenko, Y.; Koval, A. Mining Natural Compounds to Target WNT Signaling: Land and Sea Tales. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021; Volume 269, pp. 215–248. [Google Scholar] [CrossRef]
- Katanaev, V.L.; Di Falco, S.; Khotimchenko, Y. The Anticancer Drug Discovery Potential of Marine Invertebrates from Russian Pacific. Mar. Drugs 2019, 17, 474. [Google Scholar] [CrossRef] [Green Version]
- Calestani, C.; Wessel, G.M. These Colors Don’t Run: Regulation of Pigment—Biosynthesis in Echinoderms. In Results and Problems in Cell Differentiation; Springer: Cham, Switzerland, 2018; Volume 65, pp. 515–525. [Google Scholar] [CrossRef]
- Blagodatski, A.; Cherepanov, V.; Koval, A.; Kharlamenko, V.I.; Khotimchenko, Y.S.; Katanaev, V.L. High-throughput targeted screening in triple-negative breast cancer cells identifies Wnt-inhibiting activities in Pacific brittle stars. Sci. Rep. 2017, 7, 11964. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, A.; Huber, R.; Marcourt, L.; Chardonnens, E.; Koval, A.; Khotimchenko, Y.S.; Ferreira Queiroz, E.; Wolfender, J.L.; Katanaev, V.L. A Cytotoxic Porphyrin from North Pacific Brittle Star Ophiura sarsii. Mar. Drugs 2021, 19, 11. [Google Scholar] [CrossRef]
- Kennedy, G.Y. Porphyrins in invertebrates. Ann. N. Y. Acad. Sci. 1975, 244, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Krasnovsky, A.A., Jr.; Kozlov, A.S.; Roumbal, Y.V. Photochemical investigation of the IR absorption bands of molecular oxygen in organic and aqueous environment. Photochem. Photobiol. Sci. 2012, 11, 988–997. [Google Scholar] [CrossRef]
- Krasnovsky, A.A.; Kozlov, A.S. Photonics of dissolved oxygen molecules. Comparison of the rates of direct and photosensitized excitation of oxygen and reevaluation of the oxygen absorption coefficients. J. Photochem. Photobiol. A Chem. 2016, 329, 167–174. [Google Scholar] [CrossRef]
- Krasnovsky, A.A.; Benditkis, A.S.; Kozlov, A.S. Kinetic Measurements of Singlet Oxygen Phosphorescence in Hydrogen-Free Solvents by Time-Resolved Photon Counting. Biochemistry 2019, 84, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Irtenkauf, S.M.; Sobiechowski, S.; Hasselbach, L.A.; Nelson, K.K.; Transou, A.D.; Carlton, E.T.; Mikkelsen, T.; deCarvalho, A.C. Optimization of Glioblastoma Mouse Orthotopic Xenograft Models for Translational Research. Comp. Med. 2017, 67, 300–314. [Google Scholar]
- Hill, J.S.; Kahl, S.B.; Stylli, S.S.; Nakamura, Y.; Koo, M.S.; Kaye, A.H. Selective tumor kill of cerebral glioma by photodynamic therapy using a boronated porphyrin photosensitizer. Proc. Natl. Acad. Sci. USA 1995, 92, 12126–12130. [Google Scholar] [CrossRef] [Green Version]
- Padrutt, R.; Babu, V.; Klingler, S.; Kalt, M.; Schumer, F.; Anania, M.I.; Schneider, L.; Spingler, B. Highly Phototoxic Transplatin-Modified Distyryl-BODIPY Photosensitizers for Photodynamic Therapy. ChemMedChem 2021, 16, 694–701. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.N.; Del Maestro, R.F.; Anderson, R. A simple and reproducible experimental in vivo glioma model. Can. J. Neurol. Sci. 1981, 8, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.S.; Kahl, S.B.; Kaye, A.H.; Stylli, S.S.; Koo, M.S.; Gonzales, M.F.; Vardaxis, N.J.; Johnson, C.I. Selective tumor uptake of a boronated porphyrin in an animal model of cerebral glioma. Proc. Natl. Acad. Sci. USA 1992, 89, 1785–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef]
- Grobben, B.; De Deyn, P.P.; Slegers, H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002, 310, 257–270. [Google Scholar] [CrossRef]
- Romanova, G.A.; Silachev, D.N.; Shakova, F.M.; Kvashennikova, Y.N.; Viktorov, I.V.; Shram, S.I.; Myasoedov, N.F. Neuroprotective and antiamnesic effects of Semax during experimental ischemic infarction of the cerebral cortex. Bull. Exp. Biol. Med. 2006, 142, 663–666. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, F.; Kalkanis, S.N.; Yang, H.; Zhang, Z.; Katakowski, M.; Hong, X.; Zheng, X.; Chopp, M. Combination of surgical resection and photodynamic therapy of 9L gliosarcoma in the nude rat. Photochem. Photobiol. 2006, 82, 1704–1711. [Google Scholar] [CrossRef]
- Aziz, F.; Telara, S.; Moseley, H.; Goodman, C.; Manthri, P.; Eljamel, M.S. Photodynamic therapy adjuvant to surgery in metastatic carcinoma in brain. Photodiagnosis Photodyn. Ther. 2009, 6, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Tzerkovsky, D.A.; Osharin, V.V.; Istomin, Y.P.; Alexandrova, E.N.; Vozmitel, M.A. Fluorescent diagnosis and photodynamic therapy for C6 glioma in combination with antiangiogenic therapy in subcutaneous and intracranial tumor models. Exp. Oncol. 2014, 36, 85–89. [Google Scholar] [PubMed]
- Akimoto, J.; Haraoka, J.; Aizawa, K. Preliminary clinical report on safety and efficacy of photodynamic therapy using talaporfin sodium for malignant gliomas. Photodiagnosis Photodyn. Ther. 2012, 9, 91–99. [Google Scholar] [CrossRef]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic therapy (PDT) for malignant brain tumors—where do we stand? Photodiagnosis Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, A.H.; Morstyn, G. Photoradiation therapy causing selective tumor kill in a rat glioma model. Neurosurgery 1987, 20, 408–415. [Google Scholar] [CrossRef]
- Olzowy, B.; Hundt, C.S.; Stocker, S.; Bise, K.; Reulen, H.J.; Stummer, W. Photoirradiation therapy of experimental malignant glioma with 5-aminolevulinic acid. J. Neurosurg. 2002, 97, 970–976. [Google Scholar] [CrossRef]
- Cameron, R.A.; Samanta, M.; Yuan, A.; He, D.; Davidson, E. SpBase: The sea urchin genome database and web site. Nucleic Acids Res. 2009, 37, D750–D754. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Zhang, Q.; Yuan, C.; Zhang, W.; Hu, L.; Wang, X.; Liu, M.; Han, B.; Ding, J.; Chang, Y. Genome-wide identification, characterisation and expression analysis of the ALAS gene in the Yesso scallop (Patinopecten yessoensis) with different shell colours. Gene 2020, 757, 144925. [Google Scholar] [CrossRef]
- Cho, J.; Kwon, D.H.; Kim, R.G.; Song, H.; Rosa-Neto, P.; Lee, M.C.; Kim, H.I. Remodeling of Neuronal Circuits After Reach Training in Chronic Capsular Stroke. Neurorehabilit. Neural Repair 2016, 30, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Lütken, C.F. Contribution to knowledge about the brittle stars. II. Overview of the West Indian ophiuras (title in Danish). Vidensk. Medd. Dan. Nat. Förening Kjøbenhavn 1856, 7, 1–19. [Google Scholar]
- Harris, J.L.; MacIsaac, K.; Gilkinson, K.D.; Kenchington, E.L. Feeding biology of Ophiura sarsii Lutken, 1855 on Banquereau bank and the effects of fishing. Mar. Biol. 2009, 156, 1891–1902. [Google Scholar] [CrossRef]
- Gavrilova, G.S.; Kucheryavenko, A.V. Commercial rearing of the sea cucumber Apostichopus japonicus in Peter the great bay: Methodical peculiarities and results of the work of a mariculture farm in Sukhodol Bight. Russ. J. Mar. Biol. 2010, 36, 539–547. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimenko, A.; Rodina, E.E.; Silachev, D.; Begun, M.; Babenko, V.A.; Benditkis, A.S.; Kozlov, A.S.; Krasnovsky, A.A.; Khotimchenko, Y.S.; Katanaev, V.L. Chlorin Endogenous to the North Pacific Brittle Star Ophiura sarsii for Photodynamic Therapy Applications in Breast Cancer and Glioblastoma Models. Biomedicines 2022, 10, 134. https://doi.org/10.3390/biomedicines10010134
Klimenko A, Rodina EE, Silachev D, Begun M, Babenko VA, Benditkis AS, Kozlov AS, Krasnovsky AA, Khotimchenko YS, Katanaev VL. Chlorin Endogenous to the North Pacific Brittle Star Ophiura sarsii for Photodynamic Therapy Applications in Breast Cancer and Glioblastoma Models. Biomedicines. 2022; 10(1):134. https://doi.org/10.3390/biomedicines10010134
Chicago/Turabian StyleKlimenko, Antonina, Elvira E. Rodina, Denis Silachev, Maria Begun, Valentina A. Babenko, Anton S. Benditkis, Anton S. Kozlov, Alexander A. Krasnovsky, Yuri S. Khotimchenko, and Vladimir L. Katanaev. 2022. "Chlorin Endogenous to the North Pacific Brittle Star Ophiura sarsii for Photodynamic Therapy Applications in Breast Cancer and Glioblastoma Models" Biomedicines 10, no. 1: 134. https://doi.org/10.3390/biomedicines10010134
APA StyleKlimenko, A., Rodina, E. E., Silachev, D., Begun, M., Babenko, V. A., Benditkis, A. S., Kozlov, A. S., Krasnovsky, A. A., Khotimchenko, Y. S., & Katanaev, V. L. (2022). Chlorin Endogenous to the North Pacific Brittle Star Ophiura sarsii for Photodynamic Therapy Applications in Breast Cancer and Glioblastoma Models. Biomedicines, 10(1), 134. https://doi.org/10.3390/biomedicines10010134







