The Current Role of Parathyroid Fine-Needle Biopsy (P-FNAB) with iPTH-Washout Concentration (iPTH-WC) in Primary Hyperparathyroidism: A Single Center Experience and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Inclusion Criteria
- Confirmed PHPT:
- -
- documented PTH-related hypercalcemia (laboratory assessment at the same time) with:
- -
- serum iPTH >69 pg/mL (laboratory reference ranges 11–69 pg/mL)
- -
- serum total calcium >10 mg% (laboratory reference ranges 8.9–10.0 mg/dL)
- -
- additionally, if serum total calcium was between 10.0 and 10.99 mg/dL—at least two independent assessments on different occasions had to be documented
- Age above 18 years old.
2.3. Exclusion Criteria
- Patients with suspicion of enlarged parathyroid gland(s) but without laboratory abnormalities typical of PHPT.
- Secondary or tertiary hyperparathyroidism.
- Age below 18 years old.
2.4. P-FNAB Procedure
- Disinfection of the operating field;
- Biopsy of suspected structure (freehand technique) with a standard needles 25 G (0.5 × 25 mm or 0.5 × 40 mm) or 23 G (0.6 × 25 mm or 0.6 × 40 mm);
- Visualization of the tip of the needle in PG according to authors’ own scale, QuOBo (Quality of Biopsy; see below for details);
- Aspiration of material—usually small amount in the tip of the syringe;
- Topping up with 0.9% saline up to approximately 1 mL (if necessary);
- Transfer of the material (iPTH washout) to a test tube;
- Immediate transfer to the laboratory and iPTH-WC assessment;
- Simultaneous compression of biopsied field;
- USG control directly after P-FNAB and 15–30 min later.
2.5. Laboratory Tests
2.6. Ultrasonography
2.7. Parathyroid Quality of Biopsy Scale (QuOBo)
2.8. Safety Protocol Scale and Compliance Scale
2.9. Statistical Methods
3. Results
3.1. Study Group
3.2. Study Group—Positive vs. Negative Results
4. Discussion
4.1. Embryology and Anatomy of PGs in Aspect of P-FNAB
4.2. P-FNAB
4.2.1. Technical Approach to P-FNAB
4.2.2. Cut-Off Values
4.2.3. Safety of P-FNAB
4.3. Other Imaging Techniques
4.3.1. Tc99-MIBI
4.3.2. P-FNAB with Cytological Examination
4.3.3. Multiphase CT
4.3.4. PET Imaging
4.3.5. Parathyroid Venous Sampling (PVS)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| P-FNAB | parathyroid fine needle biopsy |
| iPTH-WC | intact parathormone washout concentration |
| PHPT | primary hyperparathyroidism |
| PG(s) | parathyroid gland(s) |
| PA(s) | parathyroid adenoma(s) |
| MI ptx | minimally invasive parathyroidectomy |
| Ptx | parathyroidectomy |
| Tc99 m-MIBI | Technetium 99 m sestamibi scintigraphy |
| US | cervical ultrasound |
| CT | computed tomography |
| PET | positron emission tomography |
| MRI | magnetic resonance imaging |
| (i)PTH | (intact) parathyroid hormone |
References
- National Guideline Centre (UK). Hyperparathyroidism (Primary): Diagnosis, Assessment and Initial Diagnosis, Assessment and Initial Management; National Institute for Health and Care Excellence: London, UK, 2019. [Google Scholar]
- Rao, S.D. Epidemiology of parathyroid disorders. Best Pr. Res. Clin. Endocrinol. Metab. 2018, 32, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Silverberg, S.J. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2018, 14, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Udelsman, R. Primary hyperparathyroidism. Curr. Treat. Options Oncol. 2001, 2, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Cusano, N.E.; Cipriani, C.; Bilezikian, J.P. Management of normocalcemic primary hyperparathyroidism. Best Pr. Res. Clin. Endocrinol. Metab. 2018, 32, 837–845. [Google Scholar] [CrossRef]
- Golden, S.H.; Robinson, K.A.; Saldanha, I.; Anton, B.; Ladenson, P.W. Prevalence and Incidence of Endocrine and Metabolic Disorders in the United States: A Comprehensive Review. J. Clin. Endocrinol. Metab. 2009, 94, 1853–1878. [Google Scholar] [CrossRef]
- Khan, A.A.; Hanley, D.A.; Rizzoli, R.; Bollerslev, J.; Young, J.; Rejnmark, L.; Thakker, R.; D’Amour, P.; Paul, T.; Van Uum, S.; et al. Primary hyperparathyroidism: Review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos. Int. 2017, 28, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Urkan, M.; Peker, Y.S.; Ozturk, E. Minimally Invasive Parathyroidectomy for Primary Hyperparathyroidism. Acta Endocrinol. 2019, 15, 182–186. [Google Scholar] [CrossRef]
- Witteveen, J.E.; Kievit, J.; Stokkel, M.P.M.; Morreau, H.; Romijn, J.A.; Hamdy, N.A.T. Limitations of Tc99m-MIBI-SPECT Imaging Scans in Persistent Primary Hyperparathyroidism. World J. Surg. 2010, 35, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.; Wang, T.S.; Farrokhyar, F.; Roman, S.A.; Sosa, J.A. A Meta-analysis of Preoperative Localization Techniques for Patients with Primary Hyperparathyroidism. Ann. Surg. Oncol. 2011, 19, 577–583. [Google Scholar] [CrossRef]
- Treglia, G.; Trimboli, P.; Huellner, M.; Giovanella, L. GiovanellaImaging in primary hyperparathyroidism: Focus on the evidence-based diagnostic performance of different methods. Minerva Endocrinol. 2018, 43, 133–143. [Google Scholar] [CrossRef]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in Parathyroid Imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 151–166. [Google Scholar] [CrossRef]
- Aliyev, A.; Ismayilov, R.; Musayev, J.; Yusifov, S. Fine-needle aspiration washout in the diagnosis of cystic parathyroid adenoma. BMJ Case Rep. 2020, 13, e234084. [Google Scholar] [CrossRef]
- Bancos, I.; Grant, C.S.; Nadeem, S.; Stan, M.; Reading, C.C.; Sebo, T.J.; Algeciras-Schimnich, A.; Singh, R.J. Risks and Benefits of Parathyroid Fine-Needle Aspiration with Parathyroid Hormone Washout. Endocr. Pr. 2012, 18, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Dimashkieh, H.; Krishnamurthy, S. Ultrasound guided fine needle aspiration biopsy of parathyroid gland and lesions. Cytojournal 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Ketha, H.; Lasho, M.A.; Algeciras-Schimnich, A. Analytical and clinical validation of parathyroid hormone (PTH) measurement in fine-needle aspiration biopsy (FNAB) washings. Clin. Biochem. 2016, 49, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Caleo, A.; Vitale, M.; Valvano, L.; Siano, M.; Angrisani, B.; Forlenza, M.; Massari, A.; Puzziello, A.; Salzano, F.; Zeppa, P. Fine needle cytology pre-surgical differentiation of parathyroid neoplasms: Is it reliable? Cytopathol. 2017, 28, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Shi, L.; Zhu, G.; Zhu, L.; Bao, J.; Fan, J.; Hu, Y.; Zhou, B.; Lv, Z. Fine-needle aspiration with rapid parathyroid hormone assay to identify parathyroid gland in thyroidectomy. Medicine 2020, 99, e19840. [Google Scholar] [CrossRef]
- Kim, J.; Horowitz, G.; Hong, M.; Orsini, M.; Asa, S.; Higgins, K. The dangers of parathyroid biopsy. J. Otolaryngol. Head Neck Surg. 2017, 46, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Bliss, R.D.; Gauger, P.G.; Delbridge, L.W. Surgeon’s Approach to the Thyroid Gland: Surgical Anatomy and the Importance of Technique. World J. Surg. 2000, 24, 891–897. [Google Scholar] [CrossRef]
- Akerström, G.; Malmaeus, J.; Bergström, R. Surgical anatomy of human parathyroid glands. Surgery 1984, 95, 14–21. [Google Scholar]
- Maser, C.; Donovan, P.; Santos, F.; Donabedian, R.; Rinder, C.; Scoutt, L.; Udelsman, R. Sonographically Guided Fine Needle Aspiration with Rapid Parathyroid Hormone Assay. Ann. Surg. Oncol. 2006, 13, 1690–1695. [Google Scholar] [CrossRef]
- Boi, F.; Lombardo, C.; Cocco, M.C.; Piga, M.; Serra, A.; Lai, M.L.; Calò, P.G.; Nicolosi, A.; Mariotti, S. Thyroid diseases cause mismatch between MIBI scan and neck ultrasound in the diagnosis of hyperfunctioning parathyroids: Usefulness of FNA–PTH assay. Eur. J. Endocrinol. 2013, 168, 49–58. [Google Scholar] [CrossRef]
- Castellana, M.; Virili, C.; Palermo, A.; Giorgino, F.; Giovanella, L.; Trimboli, P. Primary hyperparathyroidism with surgical indication and negative or equivocal scintigraphy: Safety and reliability of PTH washout. A systematic review and meta-analysis. Eur. J. Endocrinol. 2019, 181, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Q.; Lai, X.; Sun, J.; Jiang, Y.; Ren, X.; Zhang, Q.; Meng, Z.; Li, J.; Dai, Q. Value of preoperative ultrasound-guided fine-needle aspiration for localization in Tc-99m MIBI-negative primary hyperparathyroidism patients. Medicine 2017, 96, e9051. [Google Scholar] [CrossRef]
- Marcocci, C.; Mazzeo, S.; Bruno-Bossio, G.; Picone, A.; Vignali, E.; Ciampi, M.; Viacava, P.; Naccarato, A.G.; Miccoli, P.; Iacconi, P.; et al. Preoperative localization of suspicious parathyroid adenomas by assay of parathyroid hormone in needle aspirates. Eur. J. Endocrinol. 1998, 139, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimboli, P.; D’Aurizio, F.; Tozzoli, R.; Giovanella, L. Measurement of thyroglobulin, calcitonin, and PTH in FNA washout fluids. Clin. Chem. Lab. Med. 2017, 55, 914–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydın, C.; Polat, S.B.; Dellal, F.D.; Kaya, C.; Dogan, H.T.; Turkolmez, S.; Kılıç, M.; Ersoy, R.; Çakır, B. The diagnostic value of parathyroid hormone washout in primary hyperparathyroidism patients with negative or equivocal 99 m Tc-MIBI results. Diagn. Cytopathol. 2019, 47, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ince, S.; Emer, O.; Deveci, S.; Okuyucu, K.; Alagoz, E.; San, H.; Ayan, A.; Karacalioglu, O.; Haymana, C.; Gunalp, B.; et al. Complementary role of parathormone washout test to 99m Tc-MIBI parathyroid scintigraphy and histopathologic analysis of cell types in parathyroid adenomas. Rev. Esp. Med. Nucl. Imagen Mol. 2018, 37, 205–210. [Google Scholar] [CrossRef]
- Canpolat, A.G.; Şahin, M.; Ediboğlu, E.; Erdoğan, M.F.; Güllü, S.; Demir, Özgür; Emral, R.; Çorapçıoğlu, D. Diagnostic accuracy of parathyroid hormone levels in washout samples of suspicious parathyroid adenomas: A single-centre retrospective cohort study. Clin. Endocrinol. 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Ozderya, A.; Temizkan, S.; Cetin, K.; Ozugur, S.; Gul, A.E.; Aydin, K. The Results of PArathyroid Hormone Assay in Parathyroid Aspirates in Pre-Operative Localization of Parathyroid Adenomas for Focused Parathyroidectomy in Patients with Negative or Suspicious Technetium-99M-Sestamibi Scans. Endocr. Pract. 2017, 23, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Pekkolay, Z.; Tuzcu, Ş.A. Importance of Parathyroid Hormone Needle Aspiration Washout in Adenoma Localization in Primary Hyperparathyroidism. Med. Sci. Monit. 2019, 25, 1694–1698. [Google Scholar] [CrossRef]
- Kendrick, M.L.; Charboneau, J.W.; Curlee, K.J.; Van Heerden, J.A.; Farley, D.R. Risk of parathyromatosis after fine-needle aspiration. Am. Surg. 2001, 67, 290–294. [Google Scholar]
- Yeh, R.; Tay, Y.-K.D.; Tabacco, G.; Dercle, L.; Kuo, J.H.; Bandeira, L.; McManus, C.; Leung, D.K.; Lee, J.A.; Bilezikian, J.P. Diagnostic Performance of 4D CT and Sestamibi SPECT/CT in Localizing Parathyroid Adenomas in Primary Hyperparathyroidism. Radiology 2019, 291, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Hehenwarter, L.; Paymani, Z.; Rendl, G.; Imamovic, L.; Rettenbacher, R.; Tsybrovskyy, O.; Langsteger, W.; Pirich, C. 18F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with 99mTc-MIBI or 99mTc-tetrofosmin SPECT/CT: A prospective dual-centre study in 100 patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1762–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, L.; Ravelli, I.; Magnani, F.; Iacobone, M.; Giraudo, C.; Camozzi, V.; Spimpolo, A.; Cecchin, D. 18F-choline PET/CT and PET/MRI in primary and recurrent hyperparathyroidism: A systematic review of the literature. Ann. Nucl. Med. 2020, 34, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Taslakian, B.; Trerotola, S.O.; Sacks, B.; Oklu, R.; Deipolyi, A. The Essentials of Parathyroid Hormone Venous Sampling. Cardiovasc. Interv. Radiol. 2017, 40, 9–21. [Google Scholar] [CrossRef] [PubMed]
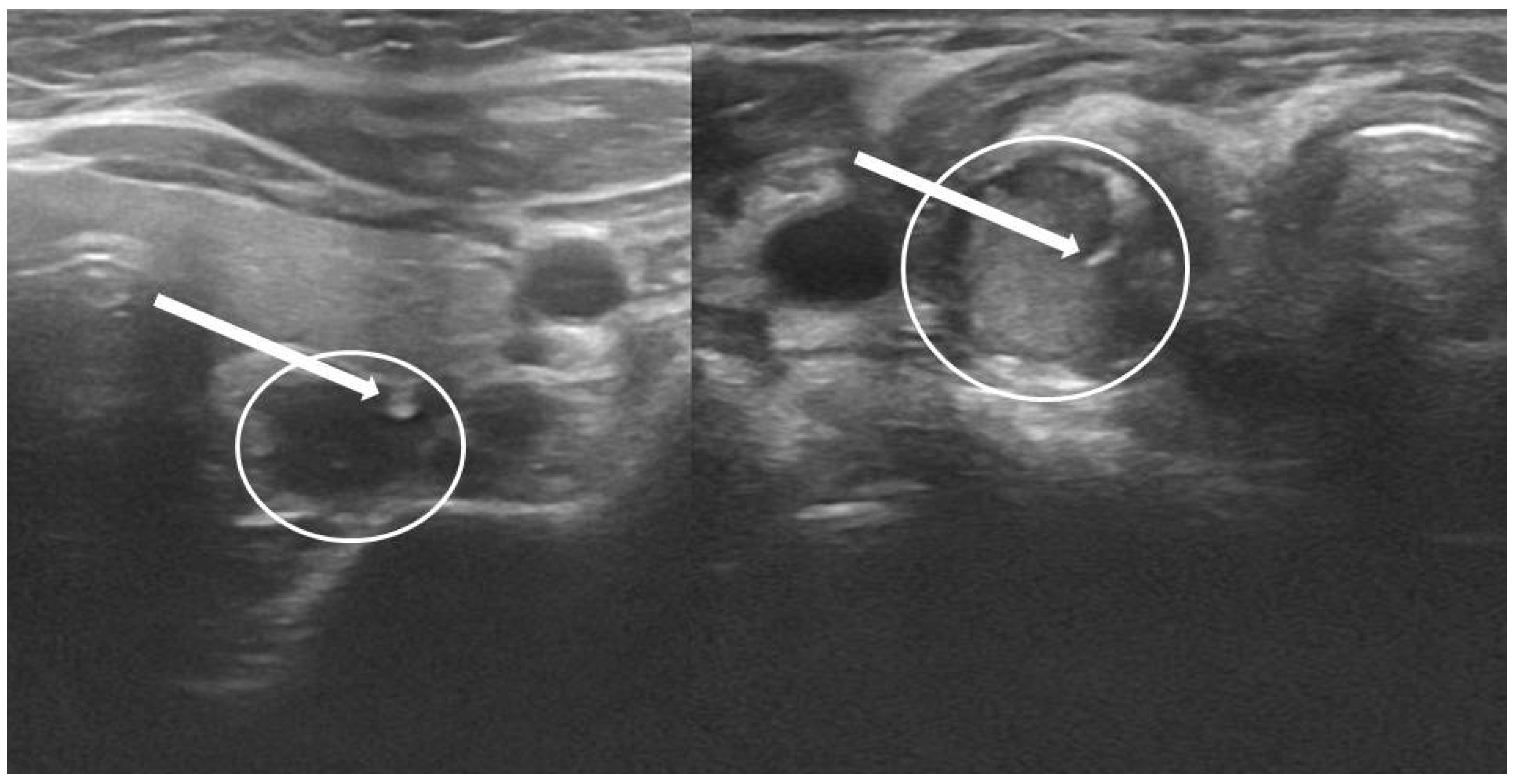
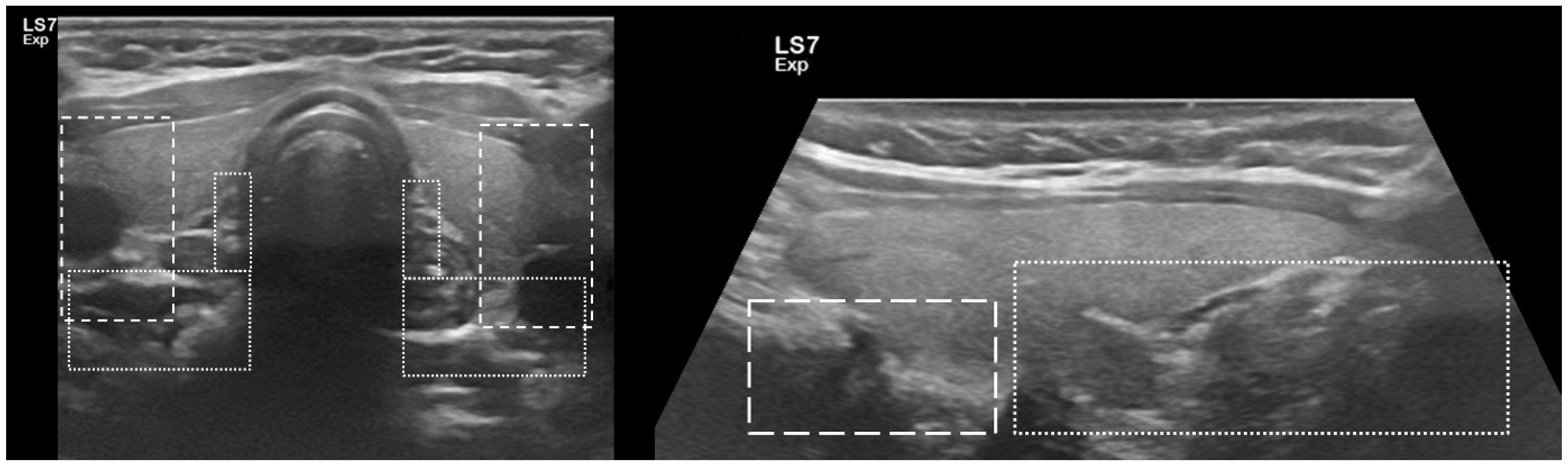
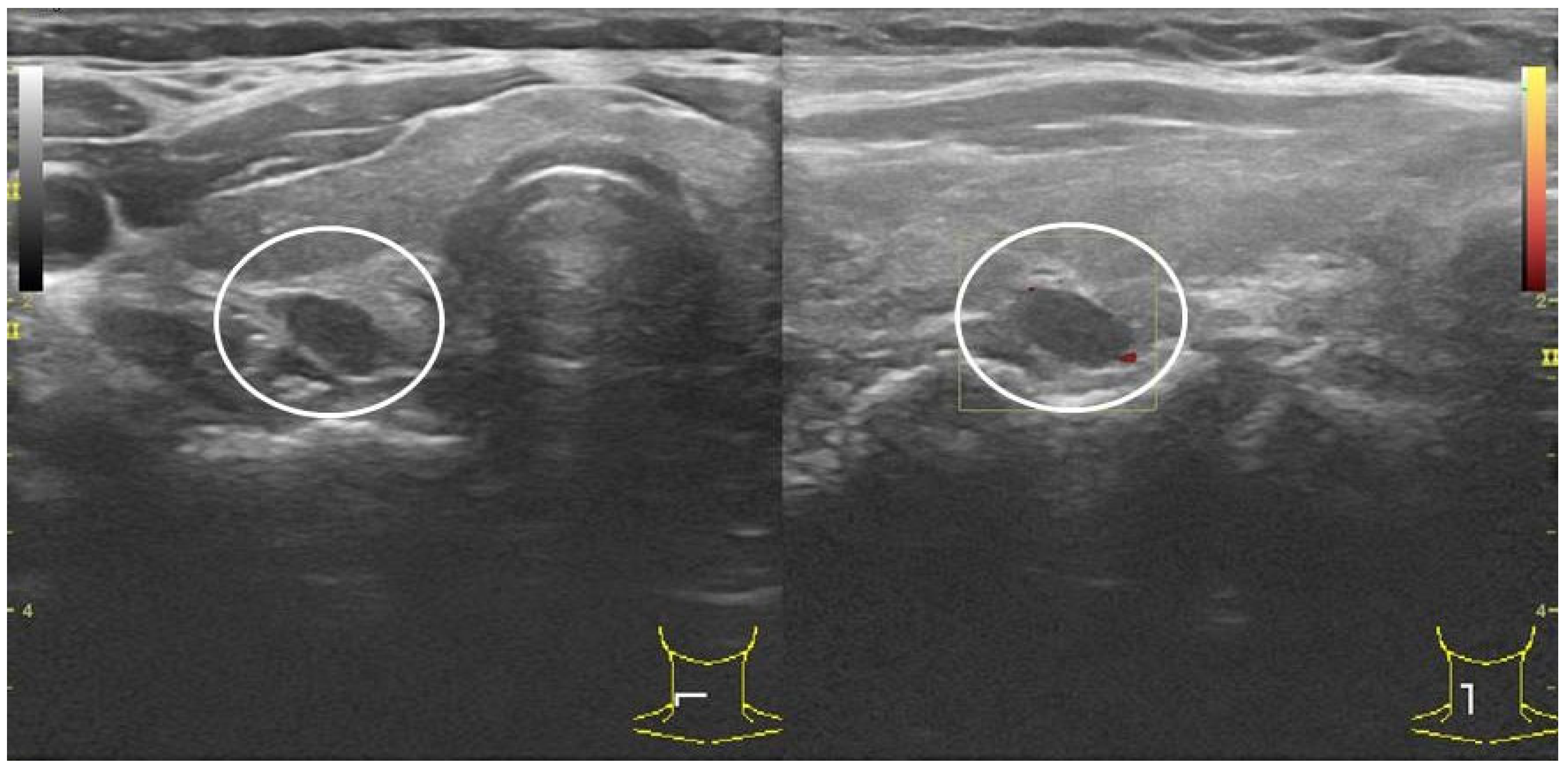
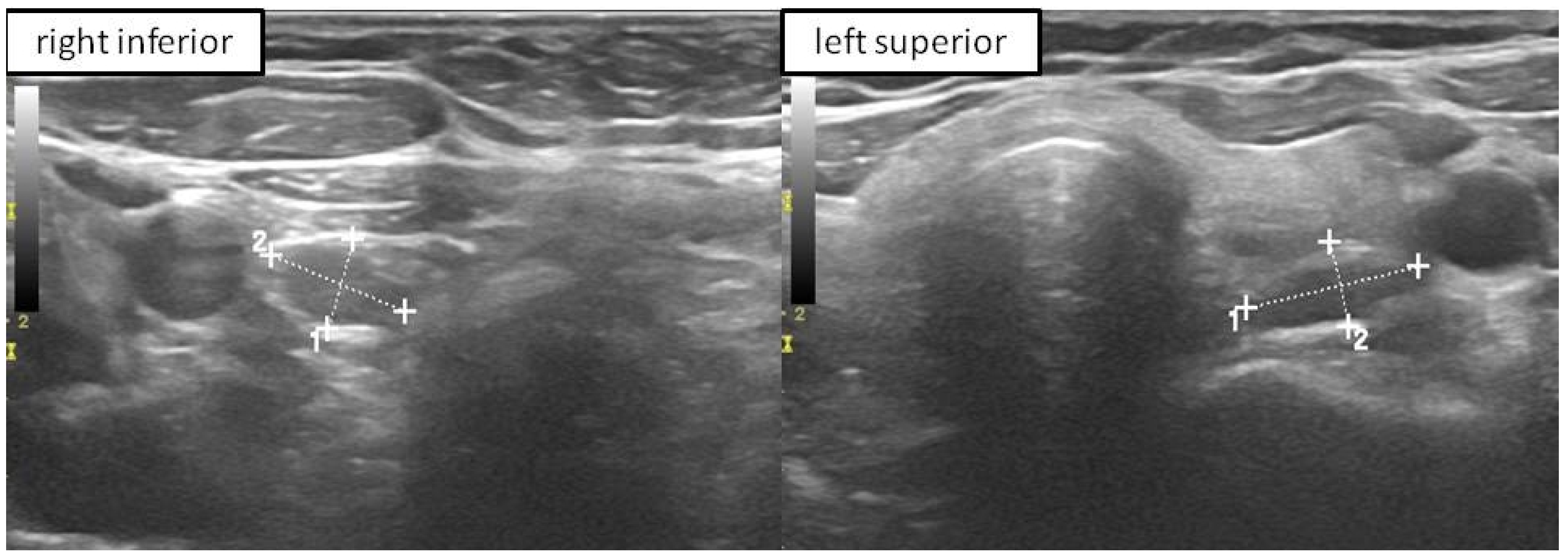
| QuOBo Scale | Description |
|---|---|
| 0 | needle tip and the shadow of the needle is not visible—iPTH-WC should not be assessed |
| 1 | needle tip visible outside the parathyroid, but close to its borders |
| 2 | needle tip visible inside the parathyroid close to its borders |
| 3 | needle tip clearly visible in the middle of the parathyroid |
| Compliance Scale | Description |
| 1 (weak) | e.g., strong hyperventilation, involuntary swallowing—weak visualization |
| 2 (medium) | e.g., mild hyperventilation—the parathyroid visible during US, but not visible all the time during P-FNAB |
| 3 (good) | perfect compliance—the parathyroid visible during whole procedure |
| Complications Scale | Description |
| 0 (no complication) | no complications |
| 1 (mild) | e.g., skin bruises of more 1 cm in diameter, pain in VAS scale 2–5 |
| 2 (moderate) | e.g., small hematoma visible in USG, pain in VAS > 5 |
| 3 (major) | e.g., necessary surgical intervention after FNAB, PG abscess, large hematoma visible in USG, hoarseness |
| iPTH-WC Diagnostic Category | |||
|---|---|---|---|
| Characteristic | Negative, N = 10 1 | Positive, N = 133 1 | p-Value 2 |
| Age | 62 (54; 72) | 60 (50; 69) | 0.4 |
| Male sex | 2 (20%) | 14 (11%) | 0.3 |
| Serum Ca [mg/dL] | 11.10 (10.85; 11.70) | 10.80 (10.40; 11.50) | 0.13 |
| Serum Pi [mg/dL] | 2.70 (2.50; 2.90) | 2.50 (2.20; 2.77) | 0.2 |
| Serum iPTH [pg/mL] | 114 (90; 133) | 116 (81; 168) | 0.9 |
| Largest dimension [cm] | 1.79 (1.40; 2.00) | 1.51 (1.13; 2.01) | 0.4 |
| Volume [mL] | 0.58 (0.45; 1.13) | 0.51 (0.29; 1.15) | 0.7 |
| Shape Index 3 | 0.50 (0.35; 0.59) | 0.49 (0.40; 0.60) | >0.9 |
| MIBI retention 4 | 6 (86%) | 38 (57%) | 0.2 |
| Washout iPTH [pg/mL] | 8 (7; 22) | 16856 (4030; 72,214) | <0.001 |
| Washout: serum iPTH ratio | 0 (0; 0) | 158 (38; 667) | <0.001 |
| Characteristic | Pts Assessed | n (%) |
|---|---|---|
| Compliance Scale | 40 | |
| 0 | 1 (2.5%) | |
| 1 | 2 (5.0%) | |
| 2 | 3 (7.5%) | |
| 3 | 34 (85%) | |
| Parathyroid Quality of Biopsy Scale | 92 | |
| 0 | 1 (1.1%) | |
| 1 | 5 (5.4%) | |
| 2 | 22 (24%) | |
| 3 | 64 (70%) | |
| Safety Protocol Scale | 40 | |
| None | 34 (85%) | |
| Mild | 3 (7.5%) | |
| Moderate | 3 (7.5%) | |
| Major | 0 (0%) |
| Patient | Doctor |
|---|---|
|
|
| Tips and tricks | |
| |
| Author | Numbers of Patients with iPTH-WC | Needle Gauge | Number of Passes | Buffer (0.9% NaCl) | Cut-Off |
|---|---|---|---|---|---|
| Maser; 2006 [22] | 6 | 25 | 2 to 7 | 1 mL | >40 pg/mL |
| Li; 2017 [25] | 11 | 21 | - | - | >57 pg/mL |
| Marocci; 1998 [26] | 12 | 22 | 2 | 0.5 mL of PTH free serum | >100 pg/mL |
| Kuzu; 2012 [27] | 12 | 22 | - | 1 mL | >1 × iPTH |
| Aydin; 2019 [28] | 20 | 25 | - | 0.5 mL | >1 × iPTH |
| Ince; 2018 [29] | 21 | 22 | - | 1 mL | >2 × iPTH |
| Boi; 2013 [23] | 27 | 22–25 | 1 or 2 | 0.5 mL | 103 pg/mL |
| Gokcay; 2018 [30] | 29 | 25 | - | 1 mL | >1 × iPTH |
| Ozderya; 2017 [31] | 44 | 27 | 1 or 2 | 1 mL | >1 × iPTH |
| Pekkolay; 2019 [32] | 49 | 22 | - | 1 mL | >1 × iPTH |
| Bancos; 2012 [14] | 67 (tertiary HPT included) | 25 | 6 | 0.5–1.5 mL | >1000 pg/mL or >1 × iPTH |
| Obołończyk; 2021 | 143 | 23 or 25 | 1 to 2 | 1 mL | 0.5–1 × iPTH PG possible >1 × iPTH PG certain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obołończyk, Ł.; Karwacka, I.; Wiśniewski, P.; Sworczak, K.; Osęka, T. The Current Role of Parathyroid Fine-Needle Biopsy (P-FNAB) with iPTH-Washout Concentration (iPTH-WC) in Primary Hyperparathyroidism: A Single Center Experience and Literature Review. Biomedicines 2022, 10, 123. https://doi.org/10.3390/biomedicines10010123
Obołończyk Ł, Karwacka I, Wiśniewski P, Sworczak K, Osęka T. The Current Role of Parathyroid Fine-Needle Biopsy (P-FNAB) with iPTH-Washout Concentration (iPTH-WC) in Primary Hyperparathyroidism: A Single Center Experience and Literature Review. Biomedicines. 2022; 10(1):123. https://doi.org/10.3390/biomedicines10010123
Chicago/Turabian StyleObołończyk, Łukasz, Izabela Karwacka, Piotr Wiśniewski, Krzysztof Sworczak, and Tomasz Osęka. 2022. "The Current Role of Parathyroid Fine-Needle Biopsy (P-FNAB) with iPTH-Washout Concentration (iPTH-WC) in Primary Hyperparathyroidism: A Single Center Experience and Literature Review" Biomedicines 10, no. 1: 123. https://doi.org/10.3390/biomedicines10010123
APA StyleObołończyk, Ł., Karwacka, I., Wiśniewski, P., Sworczak, K., & Osęka, T. (2022). The Current Role of Parathyroid Fine-Needle Biopsy (P-FNAB) with iPTH-Washout Concentration (iPTH-WC) in Primary Hyperparathyroidism: A Single Center Experience and Literature Review. Biomedicines, 10(1), 123. https://doi.org/10.3390/biomedicines10010123






