Sensitive Electrochemical Detection of Phosphorylated-Tau Threonine 231 in Human Serum Using Interdigitated Wave-Shaped Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing of the IWE
2.3. Construction of the Biosensor IWE/SAM/EDC-NHS/anti–p-tau231
2.4. Investigation of the Modification of SAM on the IWE Surface
2.5. EIS Measurement
2.6. Cyclic Voltammetry (CV) Measurements
3. Results
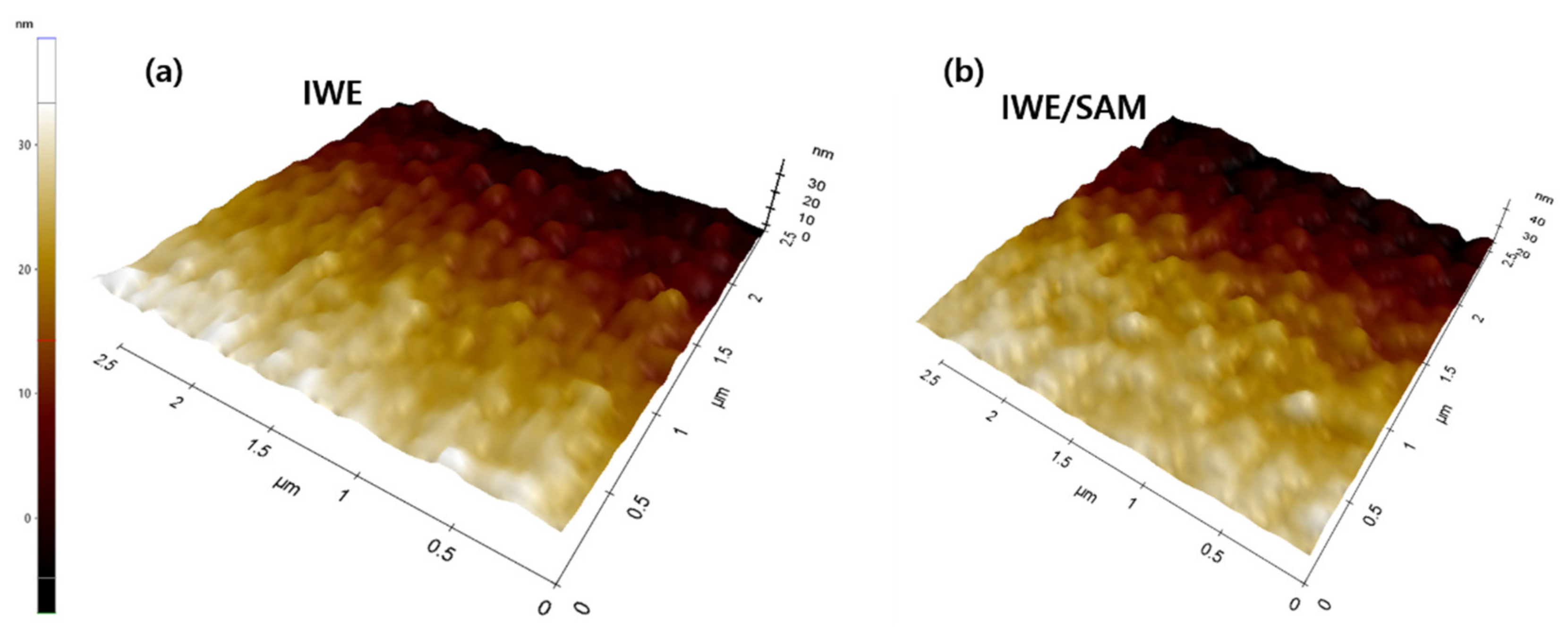
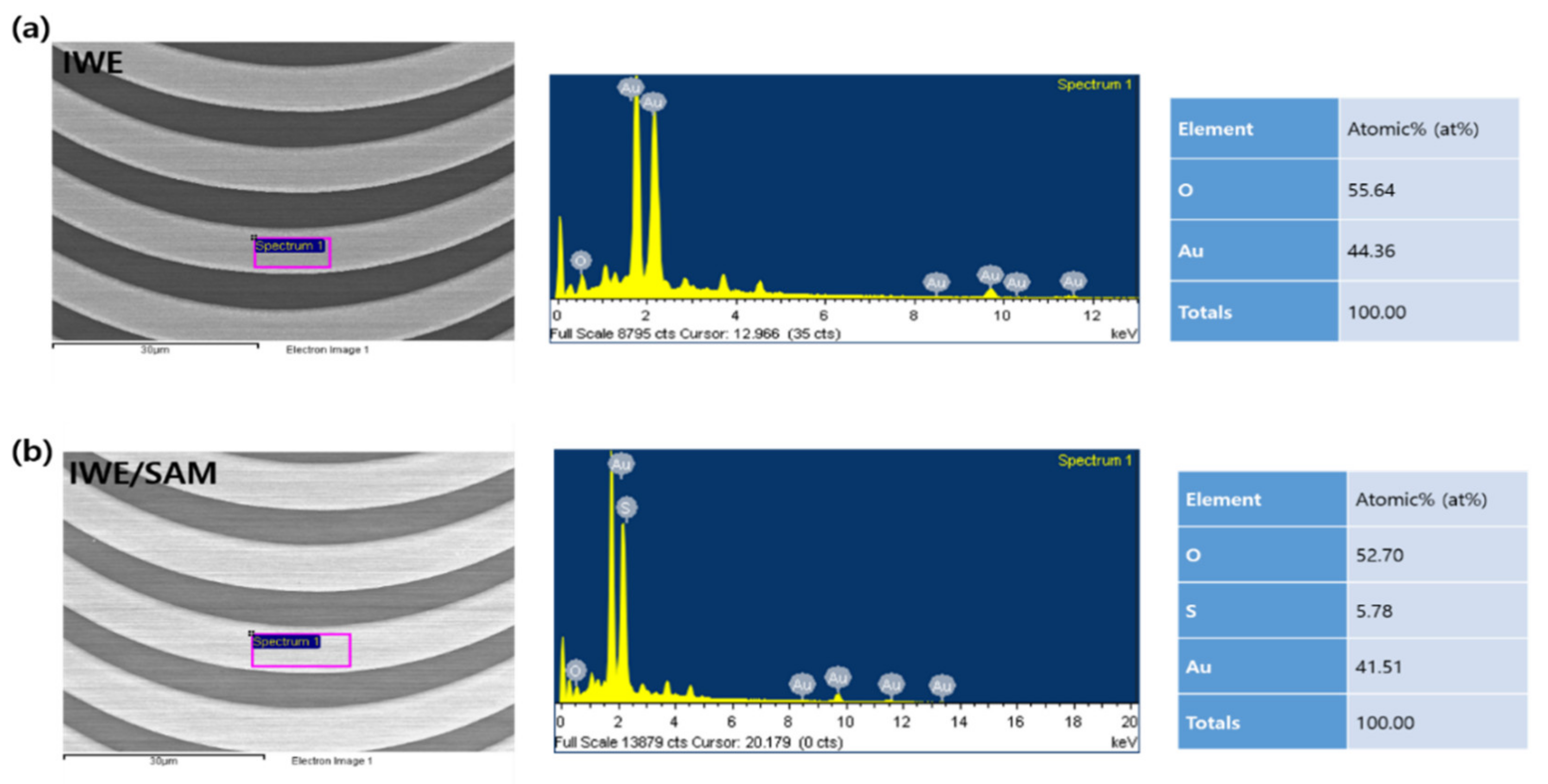
3.1. Characterization the Modification of SAM on the IWE
3.2. Electrochemical Characterization of the Biosensor (IWE/SAM/EDC-NHS/anti–p-tau231)
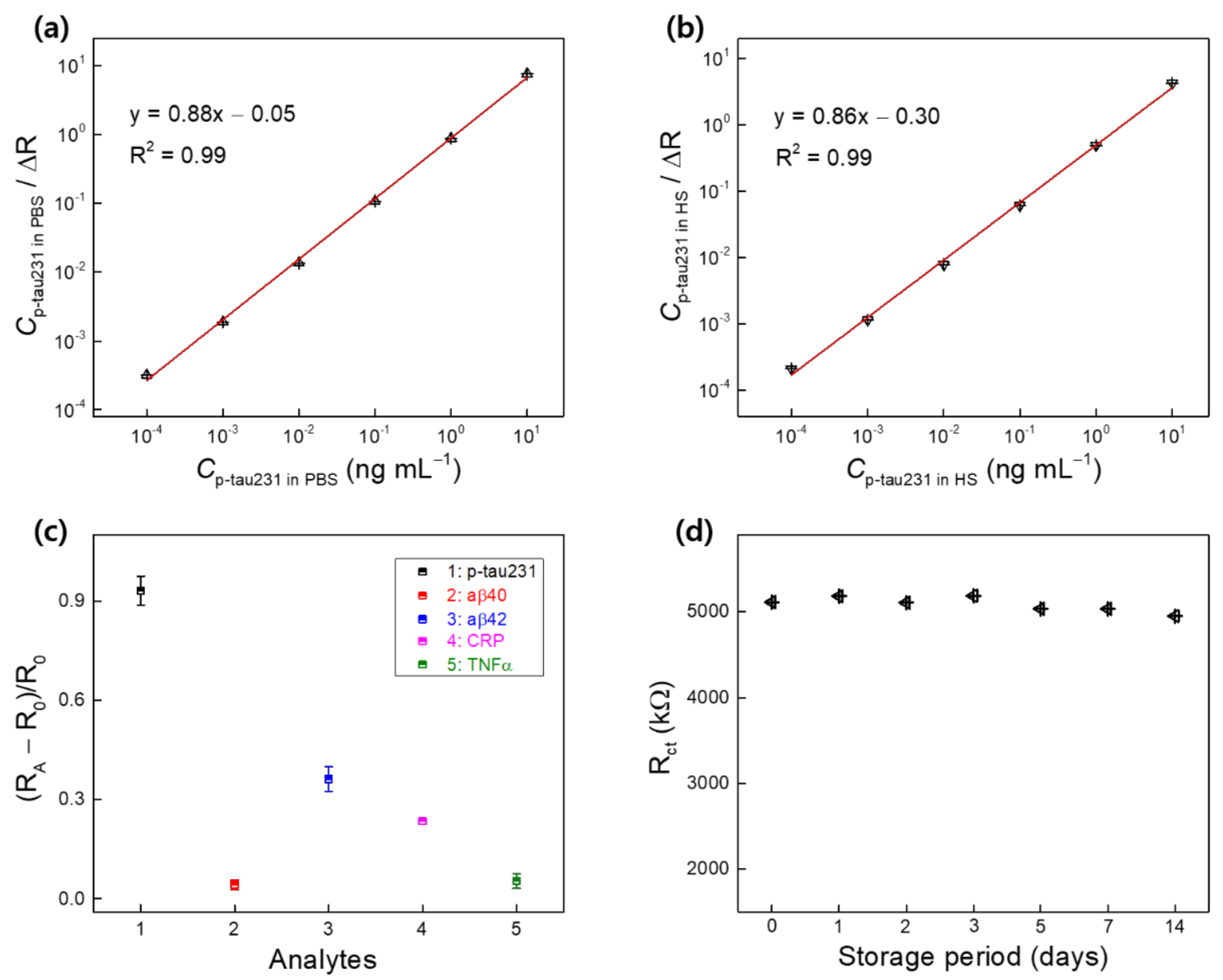
3.3. Detection of p-tau231 in PBS and HS using the Biosensor
3.4. Binding Affinity, Dissociation Constant (Kd), and Selectivity and Stability of the Biosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbaciauskaite, M.; Lei, Y.; Cho, Y.K. High-specificity antibodies and detection methods for quantifying phosphorylated tau from clinical samples. Antib. Ther. 2021, 4, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Karikari, T.K.; Ashton, N.J.; Rodríguez, J.L.; Milà-Alomà, M.; Gispert, J.D.; Salvadó, G.; Minguillon, C.; Fauria, K.; Shekari, M.; et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Aβ pathology are detected. EMBO Mol. Med. 2020, 12, e12921. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H.; Buerger, K.; Zinkowski, R.; Teipel, S.J.; Goernitz, A.; Andreasen, N.; Sjoegren, M.; DeBernardis, J.; Kerkman, D.; Ishiguro, K.; et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: A comparative cerebrospinal fluid study. Arch. Gen. Psychiatry 2004, 61, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Molinuevo, J.L.; Ayton, S.; Batrla, R.; Bednar, M.M.; Bittner, T.; Cummings, J.; Fagan, A.M.; Hampel, H.; Mielke, M.M.; Mikulskis, A.; et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018, 136, 821–853. [Google Scholar] [CrossRef] [Green Version]
- Shui, B.Q.; Tao, D.; Florea, A.; Cheng, J.; Zhao, Q.; Gu, Y.; Li, W.; Jaffrezic-Renault, N.; Mei, Y.; Guo, Z. Biosensors for Alz-heimer’s disease biomarker detection: A review. Biochimie 2018, 147, 13–24. [Google Scholar] [CrossRef]
- Chen, F.L.; Fan, Y.J.; Lin, J.D.; Hsiao, Y.C. Label-free, color-indicating, and sensitive biosensors of cholesteric liquid crystals on a single vertically aligned substrate. Biomed. Opt. Express 2019, 10, 4636–4642. [Google Scholar] [CrossRef]
- Fan, Y.J.; Chen, F.L.; Liou, J.C.; Huang, Y.W.; Chen, C.H.; Hong, Z.Y.; Lin, J.D.; Hsiao, Y.C. Label-Free Multi-Microfluidic Immunoassays with Liquid Crystals on Polydimethylsiloxane Biosensing Chips. Polymers 2020, 12, 395. [Google Scholar] [CrossRef] [Green Version]
- Chuang, E.Y.; Lin, P.Y.; Wang, P.F.; Kuo, T.R.; Chen, C.H.; Manga, Y.B.; Hsiao, Y.C. Label-Free, Smartphone-Based, and Sensitive Nano-Structural Liquid Crystal Aligned by Ceramic Silicon Compound-Constructed DMOAP-Based Biosensor for the Detection of Urine Albumin. Int. J. Nanomed. 2021, 16, 763–773. [Google Scholar] [CrossRef]
- Deng, J.; Han, D.; Yang, J. Applications of Microfluidics in Liquid Crystal-Based Biosensors. Biosensors 2021, 11, 385. [Google Scholar] [CrossRef]
- Le, H.T.N.; Cho, S.B. Deciphering the Disaggregation Mechanism of Amyloid Beta Aggregate by 4-(2-Hydroxyethyl)-1-Piperazinepropanesulfonic Acid Using Electrochemical Impedance Spectroscopy. Sensors 2021, 21, 788. [Google Scholar] [CrossRef]
- Le, H.T.N.; Park, J.S.; Cho, S.B. A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode. Micromachines 2020, 11, 791. [Google Scholar] [CrossRef]
- Le, H.T.N.; Jeong, H.K. Enhanced supramolecular recognition capability of gamma cyclodextrin-graphite oxide-carbon nanotube composite. Electrochim. Acta 2017, 250, 259–266. [Google Scholar] [CrossRef]
- Le, H.T.N.; Jeong, H.K. Cyclodextrin-Graphite Oxide-Carbon Nanotube Composites for Electrochemical Supramolecular Recognition. Electrochim. Acta 2017, 232, 7–12. [Google Scholar] [CrossRef]
- Le, H.N.; Jeong, H.K. β-Cyclodextrin–Graphite Oxide–Carbon Nanotube Composite for Enhanced Electrochemical Supramolecular Recognition. J. Phys. Chem. C 2015, 119, 18671–18677. [Google Scholar] [CrossRef]
- Esteves-Villanueva, J.O.; Trzeciakiewicz, H.; Martic, S. A protein-based electrochemical biosensor for detection of tau protein, a neurodegenerative disease biomarker. Analyst 2014, 139, 2823–2831. [Google Scholar] [CrossRef]
- Dai, Y.; Molazemhosseini, A.; Liu, C.C. A Single-Use, In Vitro Biosensor for the Detection of T-Tau Protein, A Biomarker of Neuro-Degenerative Disorders, in PBS and Human Serum Using Differential Pulse Voltammetry (DPV). Biosensors 2017, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Carlin, N.; Martic-Milne, S. Anti-Tau Antibodies Based Electrochemical Sensor for Detection of Tau Protein Biomarkers. J. Electrochem. Soc. 2018, 165, G3018–G3025. [Google Scholar] [CrossRef]
- Chikae, M.; Fukuda, T.; Kerman, K.; Idegami, K.; Miura, Y.; Tamiya, E. Amyloid-β detection with saccharide immobilized gold nanoparticle on carbon electrode. Bioelectrochemistry 2008, 74, 118–123. [Google Scholar] [CrossRef]
- Vestergaard, M.; Kerman, K.; Saito, M.; Nagatani, N.; Takamura, Y.; Tamiya, E. A Rapid Label-Free Electrochemical Detection and Kinetic Study of Alzheimer’s Amyloid Beta Aggregation. J. Am. Chem. Soc. 2005, 127, 11892–11893. [Google Scholar] [CrossRef]
- Lien, T.T.N.; Takamura, Y.; Tamiya, E.; Vestergaard, M.C. Modified screen printed electrode for development of a highly sensitive label-free impedimetric immunosensor to detect amyloid beta peptides. Anal. Chim. Acta 2015, 892, 69–76. [Google Scholar] [CrossRef]
- Wang, L.; Veselinovic, M.; Yang, L.; Geiss, B.J.; Dandy, D.S.; Chen, T.A. Sensitive DNA capacitive biosensor using interdigitated electrodes. Biosens. Bioelectron. 2017, 87, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.Y.; Chou, C.M.; Chen, T.H.; Chiou, P.C.; Hsiao, V.K.S.; Ching, C.T.S.; Sun, T.P. Enhanced Sensitivity Using Microfluidic, Interdigitated Microelectrode Based Capacitance Glucose Sensor Measured at 4 MHz. J. Electrochem. Soc. 2014, 161, B102–B105. [Google Scholar] [CrossRef]
- Buerger, K.; Teipel, S.J.; Zinkowski, R.; Blennow, K.; Arai, H.; Engel, R.; Hofmann–Kiefer, K.; McCulloch, C.; Ptok, U.; Heun, R.; et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology 2002, 59, 627–629. [Google Scholar] [CrossRef]
- Newton, L.; Slater, T.; Clark, N.; Vijayaraghavan, A. Self assembled monolayers (SAMs) on metallic surfaces (gold and graphene) for electronic applications. J. Mater. Chem. C. 2013, 1, 376–393. [Google Scholar] [CrossRef]
- Li, Z.; Chen, G.Y. Current Conjugation Methods for Immunosensors. Nanomaterials 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Yin, H.; Melissa, C.B.; Ian, I.S. Impedance Biosensor for Peanut Protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar]
- Le, H.T.N.; Kim, D.S.; Phan, L.M.T.; Cho, S.B. Ultrasensitive capacitance sensor to detect amyloid-beta 1-40 in human serum using supramolecular recognition of β-CD/RGO/ITO micro-disk electrode. Talanta 2022, 237, 122907. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, L.Q.; Hong, L.; Bush, W.D.; Carducci, T.; Simon, J.D. Quantification of the Binding Constant of Copper(II) to the Amyloid-Beta Peptide. J. Phys. Chem. B 2008, 112, 8160–8164. [Google Scholar] [CrossRef] [PubMed]
- Amniai, L.; Lippens, G.; Landrieu, I. Characterization of the AT180 epitope of phosphorylated Tau protein by a combined nuclear magnetic resonance and fluorescence spectroscopy approach. Biochem. Biophys. Res. Commun. 2011, 412, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.H.; Tu, C.; Cao, W.; Klein, A.; Ramsey, R.; Fennell, B.J.; Lambert, M.; Ni Shuilleabhain, D.; Autin, B.; Kouranova, E.; et al. An Ultra-specific Avian Antibody to Phosphorylated Tau Protein Reveals a Unique Mechanism for Phosphoepitope Recognition. J. Biol. Chem. 2012, 287, 44425–44434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Wang, L.; Maziuk, B.F.; Yao, X.; Wolozin, B.; Cho, Y.K. Directed evolution of a picomolar-affinity, high-specificity antibody targeting phosphorylated tau. J. Biol. Chem. 2018, 293, 12081–12094. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, H.T.N.; Cho, S. Sensitive Electrochemical Detection of Phosphorylated-Tau Threonine 231 in Human Serum Using Interdigitated Wave-Shaped Electrode. Biomedicines 2022, 10, 10. https://doi.org/10.3390/biomedicines10010010
Le HTN, Cho S. Sensitive Electrochemical Detection of Phosphorylated-Tau Threonine 231 in Human Serum Using Interdigitated Wave-Shaped Electrode. Biomedicines. 2022; 10(1):10. https://doi.org/10.3390/biomedicines10010010
Chicago/Turabian StyleLe, Hien T. Ngoc, and Sungbo Cho. 2022. "Sensitive Electrochemical Detection of Phosphorylated-Tau Threonine 231 in Human Serum Using Interdigitated Wave-Shaped Electrode" Biomedicines 10, no. 1: 10. https://doi.org/10.3390/biomedicines10010010
APA StyleLe, H. T. N., & Cho, S. (2022). Sensitive Electrochemical Detection of Phosphorylated-Tau Threonine 231 in Human Serum Using Interdigitated Wave-Shaped Electrode. Biomedicines, 10(1), 10. https://doi.org/10.3390/biomedicines10010010







