Beyond Gene Delivery: Strategies to Engineer the Surfaces of Viral Vectors
Abstract
:1. Introduction
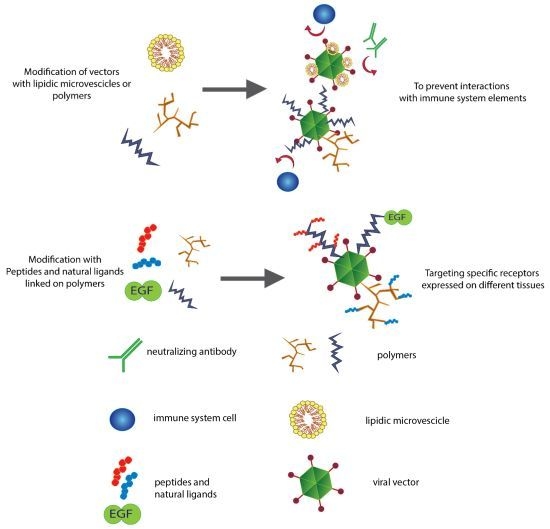
2. Fighting the Immune Response
2.1. Polyethylene Glycol and a New Generation of Polymers
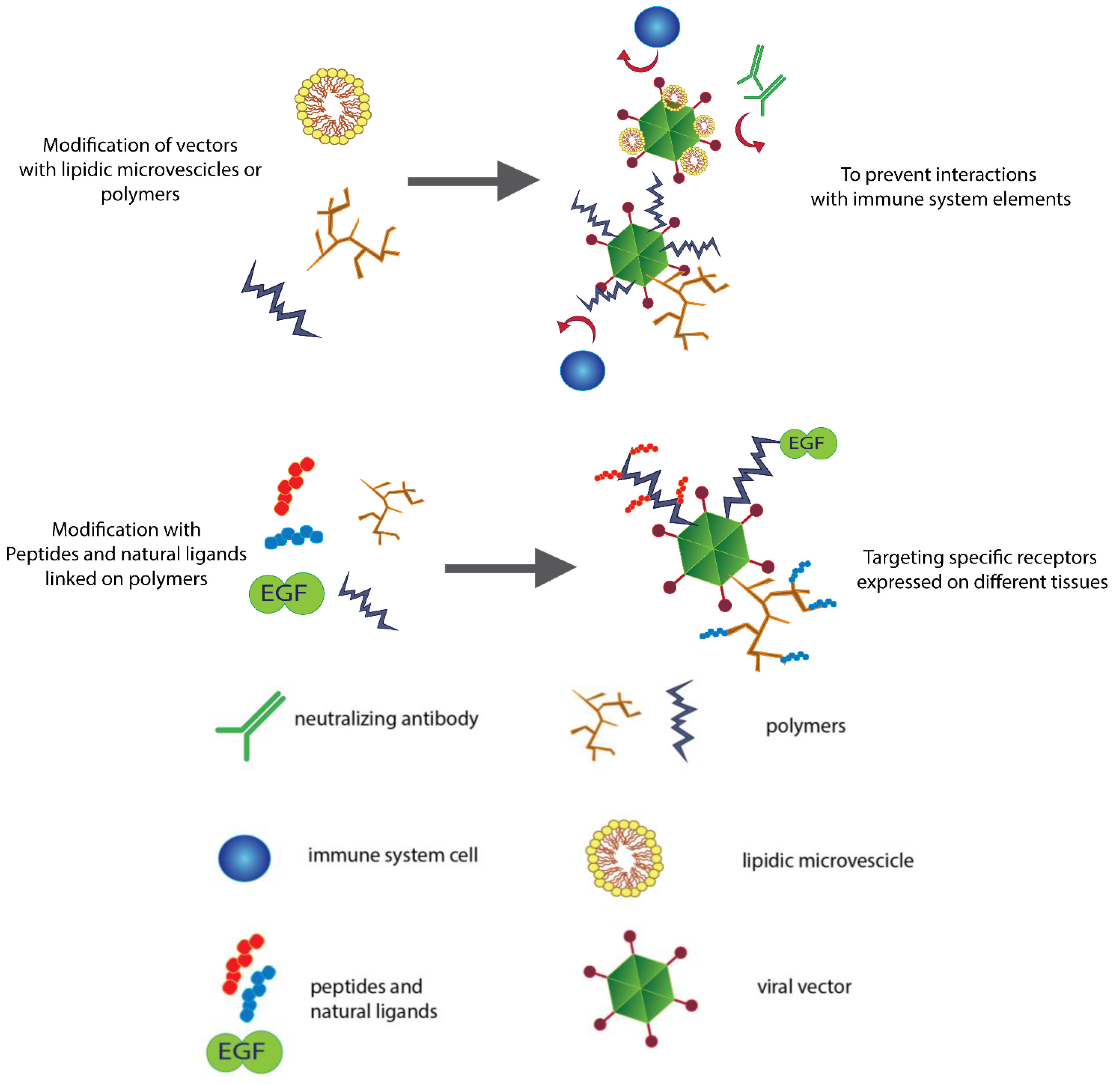
2.2. Lipids Can Hide Vectors
3. Controlling the Target
3.1. Passive Re-Targeting with Lipids
3.2. Passive Re-Targeting with Polymers and Magnetic Particles
3.3. Active Re-Targeting with Peptide Motifs
3.4. Active Re-Targeting with Natural Ligands
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kay, A.M.; Glorioso, G.; Naldini, L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001, 7, 33–40. [Google Scholar] [CrossRef]
- Young, L.S.; Searle, P.F.; Onion, D.; Mautner, V. Viral gene therapy strategies: From basic science to clinical application. J. Pathol. 2006, 208, 299–318. [Google Scholar] [CrossRef]
- Escors, D.; Breckpot, K. Lentiviral vectors in gene therapy: Their current status and future potential. Arch. Immunol. Ther. Exp. 2010, 58, 107–119. [Google Scholar] [CrossRef]
- Palmer, D.J.; Ng, P. Characterization of helper-dependent adenoviral vectors. Cold Spring Harb. Protoc. 2011, 2011, 867–870. [Google Scholar]
- Brunetti-Pierri, N.; Ng, P. Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum. Mol. Genet. 2011, 20, R7–R13. [Google Scholar] [CrossRef]
- Cooray, S.; Howe, S.J.; Thrasher, A.J. Retrovirus and lentivirus vector design and methods of cell conditioning. Methods Enzymol. 2012, 507, 29–57. [Google Scholar]
- Miyoshi, H.; Blomer, U.; Takahashi, M.; Gage, F.H.; Verma, I.M. Development of a self-inactivating lentivirus vector. J. Virol. 1998, 72, 8150–8157. [Google Scholar]
- Brunetti-Pierri, N.; Palmer, D.J.; Beaudet, A.L.; Carey, K.D.; Finegold, M.; Ng, P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004, 15, 35–46. [Google Scholar] [CrossRef]
- Muruve, D.A.; Cotter, M.J.; Zaiss, A.K.; White, L.R.; Liu, Q.; Chan, T.; Clark, S.A.; Ross, P.J.; Meulenbroek, R.A.; Maelandsmo, G.M.; et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 2004, 78, 5966–5972. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawabata, K.; Koizumi, N.; Sakurai, F.; Nakashima, K.; Sakurai, H.; Sasaki, T.; Okada, N.; Yamanishi, K.; Mizuguchi, H. Role of Myd88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 2007, 18, 753–762. [Google Scholar] [CrossRef]
- Martinez, J.; Huang, X.; Yang, Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc. Natl. Acad. Sci. USA 2010, 107, 6442–6447. [Google Scholar] [CrossRef]
- Cerullo, V.; Seiler, M.P.; Mane, V.; Brunetti-Pierri, N.; Clarke, C.; Bertin, T.K.; Rodgers, J.R.; Lee, B. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 2007, 15, 378–385. [Google Scholar] [CrossRef]
- Seiler, M.P.; Cerullo, V.; Lee, B. Immune response to helper dependent adenoviral mediated liver gene therapy: Challenges and prospects. Curr. Gene Ther. 2007, 7, 297–305. [Google Scholar] [CrossRef]
- Breckpot, K.; Escors, D.; Arce, F.; Lopes, L.; Karwacz, K.; van Lint, S.; Keyaerts, M.; Collins, M. HIV-1 lentiviral vector immunogenicity is mediated by toll-like receptor 3 (TLR3) and TLR7. J. Virol. 2010, 84, 5627–5636. [Google Scholar]
- Suzuki, M.; Cerullo, V.; Bertin, T.K.; Cela, R.; Clarke, C.; Guenther, M.; Brunetti-Pierri, N.; Lee, B. Myd88-dependent silencing of transgene expression during the innate and adaptive immune response to helper-dependent adenovirus. Hum. Gene Ther. 2010, 21, 325–336. [Google Scholar] [CrossRef]
- Rhee, E.G.; Blattman, J.N.; Kasturi, S.P.; Kelley, R.P.; Kaufman, D.R.; Lynch, D.M.; La Porte, A.; Simmons, N.L.; Clark, S.L.; Pulendran, B.; et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J. Virol. 2011, 85, 315–323. [Google Scholar] [CrossRef]
- Pradere, J.P.; Dapito, D.H.; Schwabe, R.F. The yin and yang of toll-like receptors in cancer. Oncogene 2013. [Google Scholar] [CrossRef]
- Alba, R.; Bradshaw, A.C.; Coughlan, L.; Denby, L.; McDonald, R.A.; Waddington, S.N.; Buckley, S.M.; Greig, J.A.; Parker, A.L.; Miller, A.M.; et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood 2010, 116, 2656–2664. [Google Scholar] [CrossRef]
- Bayo-Puxan, N.; Gimenez-Alejandre, M.; Lavilla-Alonso, S.; Gros, A.; Cascallo, M.; Hemminki, A.; Alemany, R. Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum. Gene Ther. 2009, 20, 1214–1221. [Google Scholar] [CrossRef]
- Edelstein, M.L.; Abedi, M.R.; Wixon, J.; Edelstein, R.M. Gene therapy clinical trials worldwide 1989–2004—An overview. J. Gene Med. 2004, 6, 597–602. [Google Scholar] [CrossRef]
- Raper, S.E.; Chirmule, N.; Lee, F.S.; Wivel, N.A.; Bagg, A.; Gao, G.-P.; Wilson, J.M.; Batshaw, M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003, 80, 148–158. [Google Scholar] [CrossRef]
- Schulte, M.; Sorkin, M.; Al-Benna, S.; Stupka, J.; Hirsch, T.; Daigeler, A.; Kesting, M.R.; Steinau, H.U.; Jacobsen, F.; Steinstraesser, L. Innate immune response after adenoviral gene delivery into skin is mediated by AIM2, NALP3, DAI and MDA5. SpringerPlus 2013, 2, 234. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of pegylation on biological therapies. Biodrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Croyle, M.A.; Le, H.T.; Linse, K.D.; Cerullo, V.; Toietta, G.; Beaudet, A.; Pastore, L. Pegylated helper-dependent adenoviral vectors: Highly efficient vectors with an enhanced safety profile. Gene Ther. 2005, 12, 579–587. [Google Scholar] [CrossRef]
- Wonganan, P.; Croyle, M.A. Pegylated adenoviruses: From mice to monkeys. Viruses 2010, 2, 468–502. [Google Scholar] [CrossRef]
- Tesfay, M.Z.; Kirk, A.C.; Hadac, E.M.; Griesmann, G.E.; Federspiel, M.J.; Barber, G.N.; Henry, S.M.; Peng, K.W.; Russell, S.J. Pegylation of vesicular stomatitis virus extends virus persistence in blood circulation of passively immunized mice. J. Virol. 2013, 87, 3752–3759. [Google Scholar] [CrossRef]
- Alemany, R.; Suzuki, K.; Curiel, D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000, 81, 2605–2609. [Google Scholar]
- Metzner, C.; Kochan, F.; Dangerfield, J.A. Postexit surface engineering of retroviral/lentiviral vectors. BioMed Res. Int. 2013, 2013, 253521:1–253521:8. [Google Scholar]
- Leggiero, E.; Astone, D.; Cerullo, V.; Lombardo, B.; Mazzaccara, C.; Labruna, G.; Sacchetti, L.; Salvatore, F.; Croyle, M.; Pastore, L. Pegylated helper-dependent adenoviral vector expressing human Apo A-I for gene therapy in ldlr-deficient mice. Gene Ther. 2013. [Google Scholar] [CrossRef] [Green Version]
- Kreppel, F.; Kochanek, S. Modification of adenovirus gene transfer vectors with synthetic polymers: A scientific review and technical guide. Mol. Ther. 2008, 16, 16–29. [Google Scholar] [CrossRef]
- Eto, Y.; Yoshioka, Y.; Ishida, T.; Yao, X.; Morishige, T.; Narimatsu, S.; Mizuguchi, H.; Mukai, Y.; Okada, N.; Kiwada, H.; et al. Optimized pegylated adenovirus vector reduces the anti-vector humoral immune response against adenovirus and induces a therapeutic effect against metastatic lung cancer. Biol. Pharm. Bull. 2010, 33, 1540–1544. [Google Scholar] [CrossRef]
- Lee, G.K.; Maheshri, N.; Kaspar, B.; Schaffer, D.V. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol. Bioeng. 2005, 92, 24–34. [Google Scholar] [CrossRef]
- Croyle, M.A.; Callahan, S.M.; Auricchio, A.; Schumer, G.; Linse, K.D.; Wilson, J.M.; Brunner, L.J.; Kobinger, G.P. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 2003, 78, 912–921. [Google Scholar]
- Ahn, C.H.; Chae, S.Y.; Bae, Y.H.; Kim, S.W. Synthesis of biodegradable multi-block copolymers of poly(l-lysine) and poly(ethylene glycol) as a non-viral gene carrier. J. Control. Release 2004, 97, 567–574. [Google Scholar]
- Zeng, Q.; Han, J.; Zhao, D.; Gong, T.; Zhang, Z.; Sun, X. Protection of adenovirus from neutralizing antibody by cationic PEG derivative ionically linked to adenovirus. Int. J. Nanomed. 2012, 7, 985–997. [Google Scholar]
- Fisher, K.D.; Seymour, L.W. HPMA copolymers for masking and retargeting of therapeutic viruses. Adv. Drug Deliv. Rev. 2010, 62, 240–245. [Google Scholar] [CrossRef]
- Wang, C.H.; Chan, L.W.; Johnson, R.N.; Chu, D.S.; Shi, J.; Schellinger, J.G.; Lieber, A.; Pun, S.H. The transduction of coxsackie and adenovirus receptor-negative cells and protection against neutralizing antibodies by HPMA-co-oligolysine copolymer-coated adenovirus. Biomaterials 2011, 32, 9536–9545. [Google Scholar] [CrossRef]
- Kim, P.H.; Kim, J.; Kim, T.I.; Nam, H.Y.; Yockman, J.W.; Kim, M.; Kim, S.W.; Yun, C.O. Bioreducible polymer-conjugated oncolytic adenovirus for hepatoma-specific therapy via systemic administration. Biomaterials 2011, 32, 9328–9342. [Google Scholar] [CrossRef]
- Singh, R.; Al-Jamal, K.T.; Lacerda, L.; Kostarelos, K. Nanoengineering artificial lipid envelopes around adenovirus by self-assembly. ACS Nano 2008, 2, 1040–1050. [Google Scholar] [CrossRef]
- Yilmazer, A.; Al-Jamal, W.T.; van den Bossche, J.; Kostarelos, K. The effect of artificial lipid envelopment of adenovirus 5 (ad5) on liver de-targeting and hepatotoxicity. Biomaterials 2013, 34, 1354–1363. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for coxsackie b viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Hastie, E.; Cataldi, M.; Marriott, I.; Grdzelishvili, V.Z. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013, 176, 16–32. [Google Scholar] [CrossRef]
- Finkelshtein, D.; Werman, A.; Novick, D.; Barak, S.; Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 2013, 110, 7306–7311. [Google Scholar]
- Glazkova, D.V.; Vetchinova, A.S.; Bogoslovskaia, E.V.; Zhogina, Y.A.; Markelov, M.L.; Shipulin, G.A. Downregulation of human CCR5 receptor gene expression using artificial microRNAs. Mol. Biol. 2013, 47, 419–428. [Google Scholar] [CrossRef]
- Martin, K.; Brie, A.; Saulnier, P.; Perricaudet, M.; Yeh, P.; Vigne, E. Simultaneous car- and alpha v integrin-binding ablation fails to reduce ad5 liver tropism. Mol. Ther. 2003, 8, 485–494. [Google Scholar] [CrossRef]
- Waddington, S.N.; McVey, J.H.; Bhella, D.; Parker, A.L.; Barker, K.; Atoda, H.; Pink, R.; Buckley, S.M.; Greig, J.A.; Denby, L.; et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 2008, 132, 397–409. [Google Scholar] [CrossRef]
- Wonganan, P.; Clemens, C.C.; Brasky, K.; Pastore, L.; Croyle, M.A. Species differences in the pharmacology and toxicology of PEGylated helper-dependent adenovirus. Mol. Pharm. 2011, 8, 78–92. [Google Scholar] [CrossRef]
- Matsui, H.; Sakurai, F.; Katayama, K.; Yamaguchi, T.; Okamoto, S.; Takahira, K.; Tachibana, M.; Nakagawa, S.; Mizuguchi, H. A hexon-specific PEGylated adenovirus vector utilizing blood coagulation factor x. Biomaterials 2012, 33, 3743–3755. [Google Scholar] [CrossRef]
- Doronin, K.; Shashkova, E.V.; May, S.M.; Hofherr, S.E.; Barry, M.A. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum. Gene Ther. 2009, 20, 975–988. [Google Scholar] [CrossRef]
- Mok, H.; Palmer, D.J.; Ng, P.; Barry, M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005, 11, 66–79. [Google Scholar]
- Hofherr, S.E.; Shashkova, E.V.; Weaver, E.A.; Khare, R.; Barry, M.A. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008, 16, 1276–1282. [Google Scholar] [CrossRef]
- Green, N.K.; Herbert, C.W.; Hale, S.J.; Hale, A.B.; Mautner, V.; Harkins, R.; Hermiston, T.; Ulbrich, K.; Fisher, K.D.; Seymour, L.W. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004, 11, 1256–1263. [Google Scholar] [CrossRef]
- Wang, I.J.; Jhuang, M.C.; Chen, Y.H.; Yeh, L.K.; Liu, C.Y.; Young, T.H. Chitosan modification of adenovirus to modify transfection efficiency in bovine corneal epithelial cells. PLoS One 2010, 5, e12085. [Google Scholar]
- Kang, S.H.; Zirbes, E.L.; Kole, R. Delivery of antisense oligonucleotides and plasmid DNA with various carrier agents. Antisense Nucleic Acid Drug Dev. 1999, 9, 497–505. [Google Scholar] [CrossRef]
- Vetter, A.; Virdi, K.S.; Espenlaub, S.; Rodl, W.; Wagner, E.; Holm, P.S.; Scheu, C.; Kreppel, F.; Spitzweg, C.; Ogris, M. Adenoviral vectors coated with PAMAM dendrimer conjugates allow car independent virus uptake and targeting to the egf receptor. Mol. Pharm. 2013, 10, 606–618. [Google Scholar] [CrossRef]
- Pandori, M.W.; Hobson, D.A.; Sano, T. Adenovirus-microbead conjugates possess enhanced infectivity: A new strategy for localized gene delivery. Virology 2002, 299, 204–212. [Google Scholar] [CrossRef]
- Mah, C.; Fraites, T.J., Jr.; Zolotukhin, I.; Song, S.; Flotte, T.R.; Dobson, J.; Batich, C.; Byrne, B.J. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther. 2002, 6, 106–112. [Google Scholar] [CrossRef]
- Sapet, C.; Pellegrino, C.; Laurent, N.; Sicard, F.; Zelphati, O. Magnetic nanoparticles enhance adenovirus transduction in vivo and in vivo. Pharm. Res. 2012, 29, 1203–1218. [Google Scholar] [CrossRef]
- Hoffman, J.A.; Giraudo, E.; Singh, M.; Zhang, L.; Inoue, M.; Porkka, K.; Hanahan, D.; Ruoslahti, E. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell 2003, 4, 383–391. [Google Scholar] [CrossRef]
- Yao, X.L.; Yoshioka, Y.; Ruan, G.X.; Chen, Y.Z.; Mizuguchi, H.; Mukai, Y.; Okada, N.; Gao, J.Q.; Nakagawa, S. Optimization and internalization mechanisms of PEGylated adenovirus vector with targeting peptide for cancer gene therapy. Biomacromolecules 2012, 13, 2402–2409. [Google Scholar] [CrossRef]
- Yao, X.; Yoshioka, Y.; Morishige, T.; Eto, Y.; Narimatsu, S.; Kawai, Y.; Mizuguchi, H.; Gao, J.Q.; Mukai, Y.; Okada, N.; et al. Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Mol. Ther. 2011, 19, 1619–1625. [Google Scholar] [CrossRef]
- Xiong, Z.; Cheng, Z.; Zhang, X.; Patel, M.; Wu, J.C.; Gambhir, S.S.; Chen, X. Imaging chemically modified adenovirus for targeting tumors expressing integrin alphavbeta3 in living mice with mutant herpes simplex virus type 1 thymidine kinase pet reporter gene. J. Nucl. Med. 2006, 47, 130–139. [Google Scholar]
- King, W.J.; Krebsbach, P.H. Cyclic-RGD peptides increase the adenoviral transduction of human mesenchymal stem cells. Stem Cells Dev. 2013, 22, 679–686. [Google Scholar] [CrossRef]
- Black, P.C.; Agarwal, P.K.; Dinney, C.P. Targeted therapies in bladder cancer–An update. Urol. Oncol. 2007, 25, 433–438. [Google Scholar]
- Bonsted, A.; Engesaeter, B.O.; Hogset, A.; Maelandsmo, G.M.; Prasmickaite, L.; D’Oliveira, C.; Hennink, W.E.; van Steenis, J.H.; Berg, K. Photochemically enhanced transduction of polymer-complexed adenovirus targeted to the epidermal growth factor receptor. J. Gene Med. 2006, 8, 286–297. [Google Scholar] [CrossRef]
- Morrison, J.; Briggs, S.S.; Green, N.; Fisher, K.; Subr, V.; Ulbrich, K.; Kehoe, S.; Seymour, L.W. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol. Ther. 2008, 16, 244–251. [Google Scholar] [CrossRef]
- Paul, A.; Shao, W.; Abbasi, S.; Shum-Tim, D.; Prakash, S. Pamam dendrimer-baculovirus nanocomplex for microencapsulated adipose stem cell-gene therapy: In vitro and in vivo functional assessment. Mol. Pharm. 2012, 9, 2479–2488. [Google Scholar] [CrossRef]
- Grunwald, G.K.; Vetter, A.; Klutz, K.; Willhauck, M.J.; Schwenk, N.; Senekowitsch-Schmidtke, R.; Schwaiger, M.; Zach, C.; Wagner, E.; Goke, B.; et al. Systemic image-guided liver cancer radiovirotherapy using dendrimer-coated adenovirus encoding the sodium iodide symporter as theranostic gene. J. Nucl. Med. 2013, 54, 1450–1457. [Google Scholar] [CrossRef]
- Walters, C.L.; Arend, R.C.; Armstrong, D.K.; Naumann, R.W.; Alvarez, R.D. Folate and folate receptor alpha antagonists mechanism of action in ovarian cancer. Gynecol. Oncol. 2013, 131, 493–498. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, H.; Xu, Y.; Liu, T.; Chen, S.; Wang, J.; Zhang, T. Expression of folate receptors in nasopharyngeal and laryngeal carcinoma and folate receptor-mediated endocytosis by molecular targeted nanomedicine. Int. J. Nanomed. 2013, 8, 2443–2451. [Google Scholar]
- Reddy, J.A.; Clapp, D.W.; Low, P.S. Retargeting of viral vectors to the folate receptor endocytic pathway. J. Control. Release 2001, 74, 77–82. [Google Scholar] [CrossRef]
- Kwon, O.J.; Kang, E.; Choi, J.W.; Kim, S.W.; Yun, C.O. Therapeutic targeting of chitosan-peg-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J. Control. Release 2013, 169, 257–265. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhu, N.L.; Chua, J.; Swenson, S.; Costa, F.K.; Schmitmeier, S.; Sosnowski, B.A.; Shichinohe, T.; Kasahara, N.; Chen, T.C. Retargeting of adenoviral vector using basic fibroblast growth factor ligand for malignant glioma gene therapy. J. Neurosurg. 2005, 103, 1058–1066. [Google Scholar] [CrossRef]
- Lanciotti, J.; Antonius, S.; Doukas, J.; Sosnowski, B.; Pierce, G.; Gregory, R.; Wadsworth, S.; O’Riordan, C. Targeting adenoviral vectors using heterofunctional polyethylene glycol fgf2 conjugates. Mol. Ther. 2003, 8, 99–107. [Google Scholar] [CrossRef]
- Green, N.K.; Morrison, J.; Hale, S.; Briggs, S.S.; Stevenson, M.; Subr, V.; Ulbrich, K.; Chandler, L.; Mautner, V.; Seymour, L.W.; et al. Retargeting polymer-coated adenovirus to the FGF receptor allows productive infection and mediates efficacy in a peritoneal model of human ovarian cancer. J. Gene Med. 2008, 10, 280–289. [Google Scholar] [CrossRef]
© 2013 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Capasso, C.; Hirvinen, M.; Cerullo, V. Beyond Gene Delivery: Strategies to Engineer the Surfaces of Viral Vectors. Biomedicines 2013, 1, 3-16. https://doi.org/10.3390/biomedicines1010003
Capasso C, Hirvinen M, Cerullo V. Beyond Gene Delivery: Strategies to Engineer the Surfaces of Viral Vectors. Biomedicines. 2013; 1(1):3-16. https://doi.org/10.3390/biomedicines1010003
Chicago/Turabian StyleCapasso, Cristian, Mari Hirvinen, and Vincenzo Cerullo. 2013. "Beyond Gene Delivery: Strategies to Engineer the Surfaces of Viral Vectors" Biomedicines 1, no. 1: 3-16. https://doi.org/10.3390/biomedicines1010003
APA StyleCapasso, C., Hirvinen, M., & Cerullo, V. (2013). Beyond Gene Delivery: Strategies to Engineer the Surfaces of Viral Vectors. Biomedicines, 1(1), 3-16. https://doi.org/10.3390/biomedicines1010003