Nitrogen and Sulfur Co-Doped Graphene as Efficient Electrode Material for L-Cysteine Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of N and S Co-doped Graphene Samples
2.3. Characterization Methods
2.4. Electrochemical Studies
3. Results and Discussions
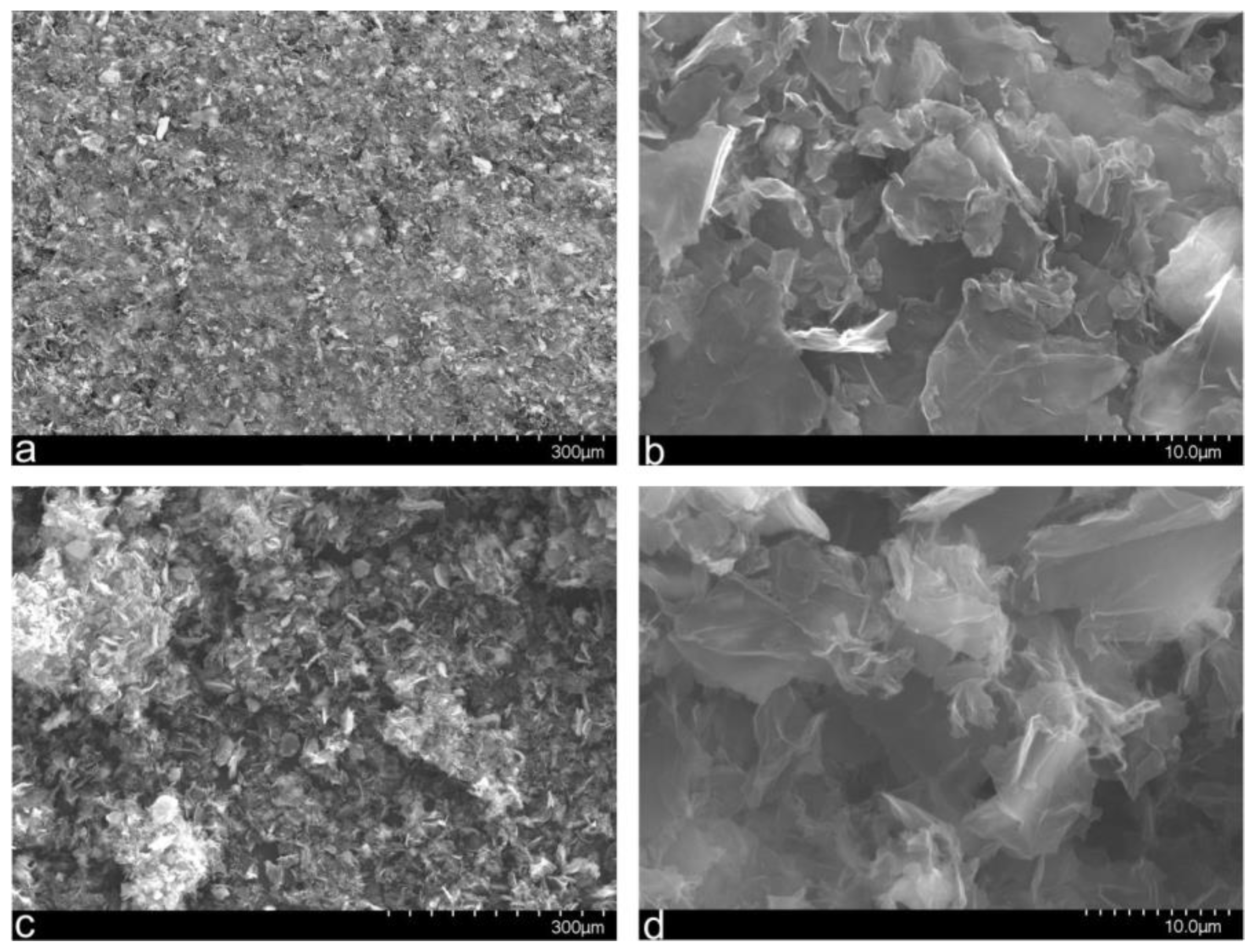
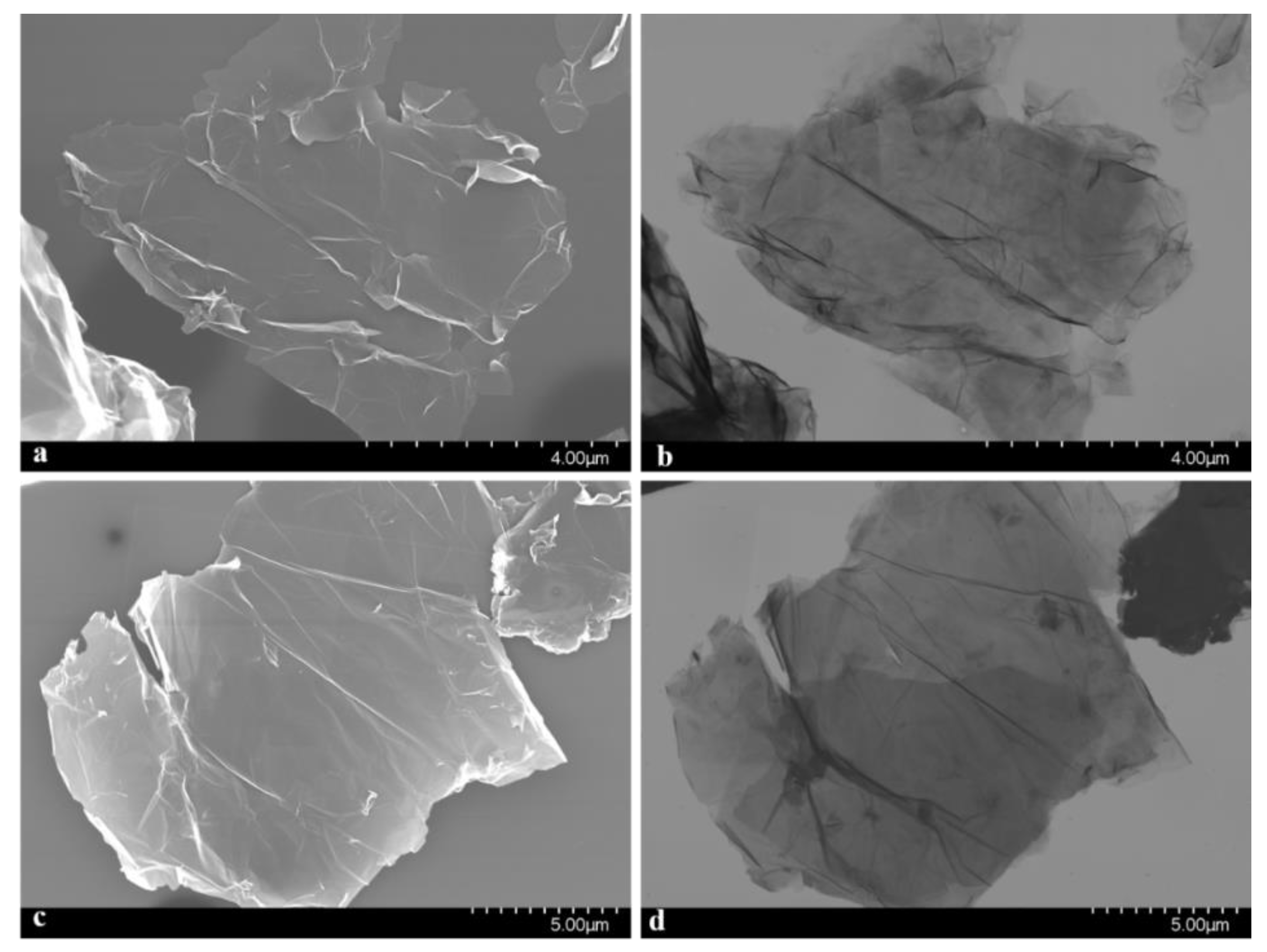
3.1. Synthesis and Characterization of the NSGr Samples
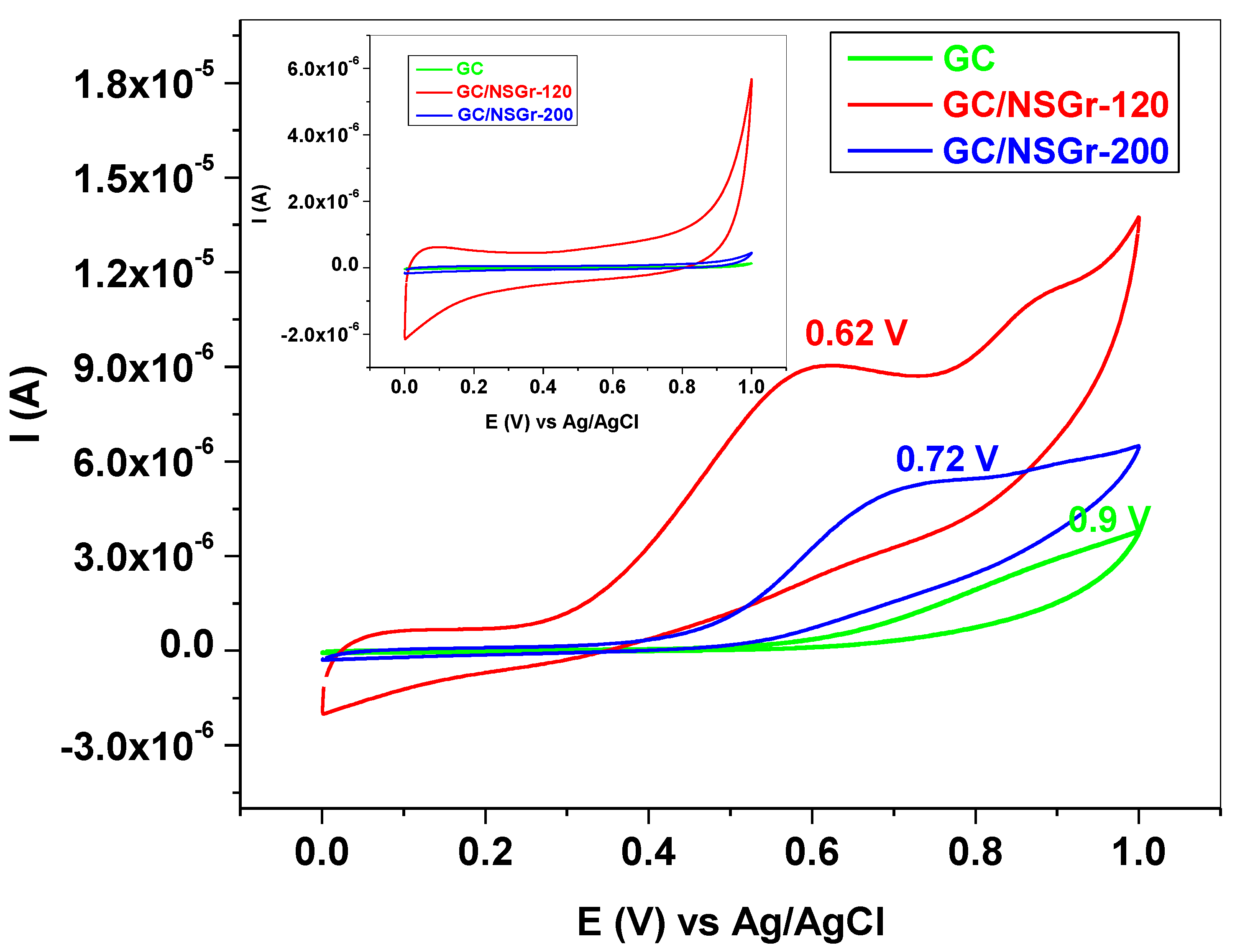
3.2. Electrochemical Characterization of Bare and Graphene-Modified Electrodes
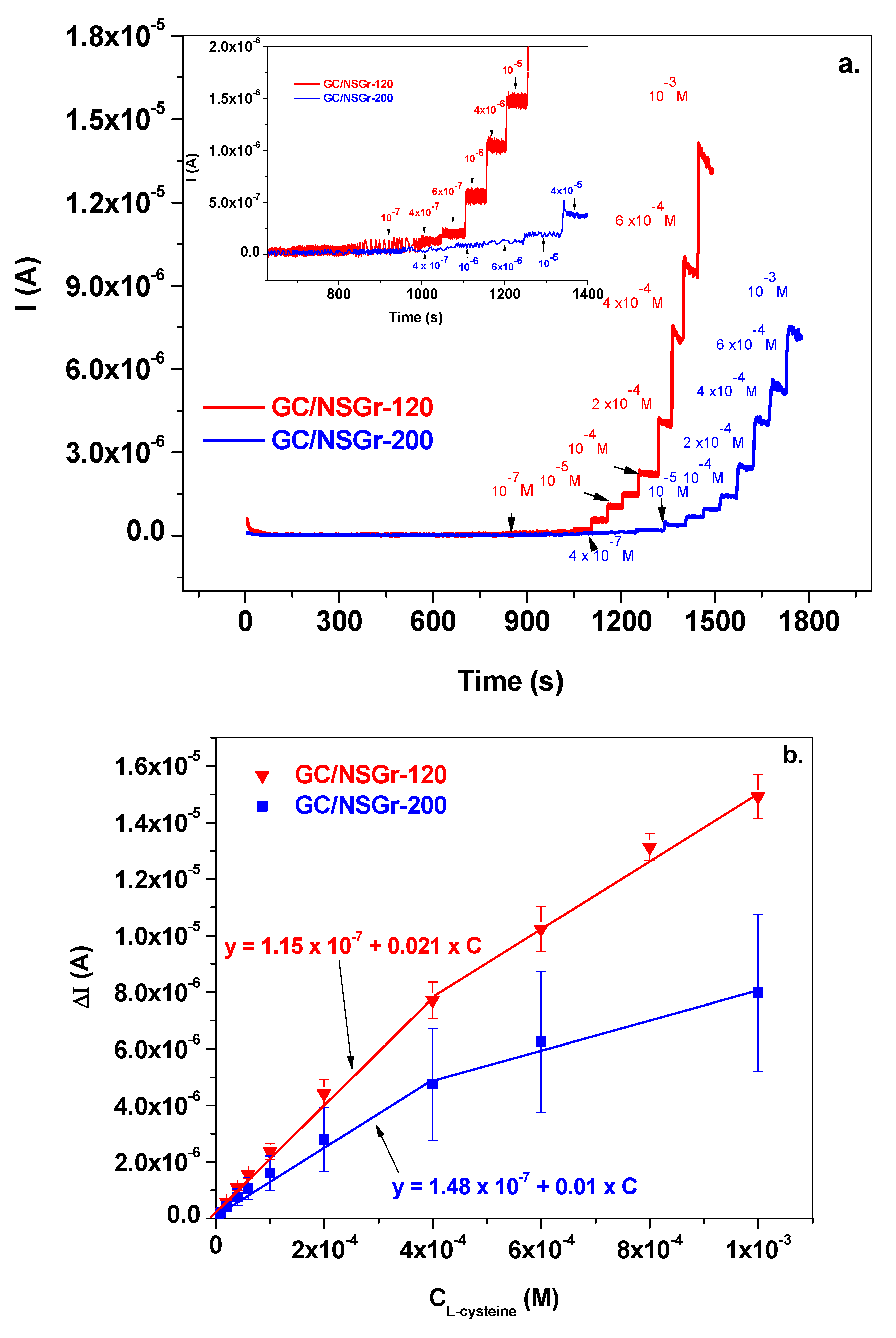
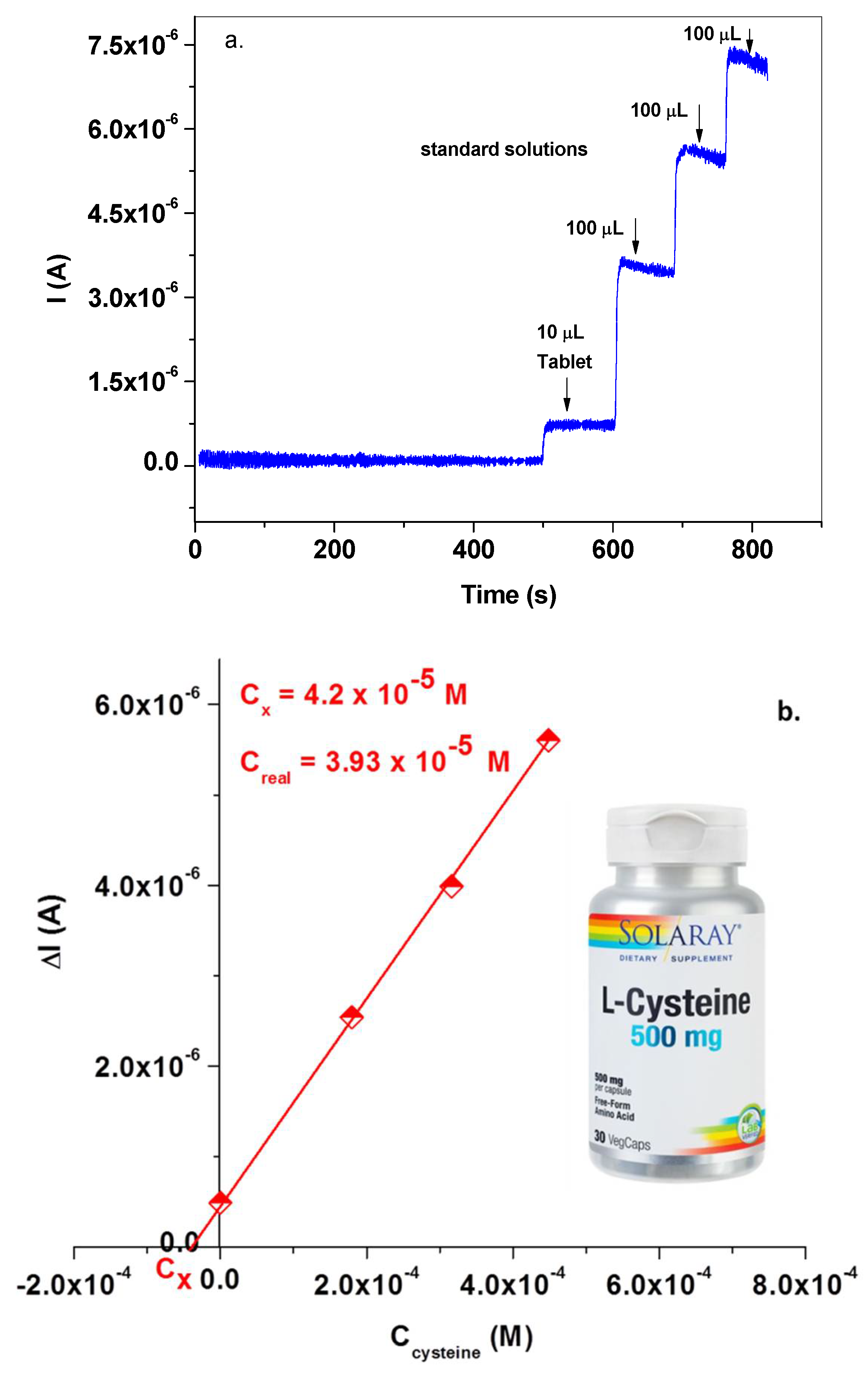
3.3. Electrochemical Detection of L-Cysteine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine metabolic circuitries: Druggable targets in cancer. Br. J. Cancer 2021, 124, 862–879. [Google Scholar] [CrossRef]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef]
- Cieslak, S.G.; Hutchinson, B.; Adhikari, R.; Steed, K.S.; Staudte, R.S.; Cox, P.; Rasch, A.; Black, E.; Araujo, A.; Wisco, J.J. The effects of L-Cysteine on Alzheimer’s disease pathology in APOE2, APOE3, and APOE4 homozygous mice. Brain Nerves 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Rehman, T.; Asim, M.; Muhammad, S.; Faisal, M.; Nazir, M.; Liu, A.Z.; Ahmad, H.; Siddeeg, A.; Abid, M.; Muhammad, R. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef]
- Głowacki, R.; Piechocka, J.; Borowczyk, K.; Information, R. A simple HPLC—UV method for simultaneous determination of cysteine and cysteinylglycine in biological fluids. Acta Chromatogr. 2016, 28, 333–346. [Google Scholar] [CrossRef]
- Tůma, P. Determination of amino acids by capillary and microchip electrophoresis with contactless conductivity detection—Theory, instrumentation and applications. Talanta 2021, 224, 121922. [Google Scholar] [CrossRef]
- Piechocka, J.; Wieczorek, M.; Głowacki, R. Gas Chromatography–Mass Spectrometry Based Approach for the Determination of Methionine-Related Sulfur-Containing Compounds in Human Saliva. Int. J. Mol. Sci. 2020, 21, 9252. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, J.; Guo, L.P.; Shang, Q.K. Electrochemical behavior of L-cysteine and its detection at ordered mesoporous carbon-modified glassy carbon electrode. Anal. Chem. 2007, 79, 5328–5335. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Su, W.Y.; Cheng, J.R.; Cheng, S.H. Electrochemical determination of cysteine based on conducting polymers/gold nanoparticles hybrid nanocomposites. Electrochim. Acta 2011, 56, 6887–6895. [Google Scholar] [CrossRef]
- Heidari, M.; Ghaffarinejad, A. Electrochemical sensor for L-cysteine by using a cobalt(II)/aluminum(III) layered double hydroxide as a nanocatalyst. Microchim. Acta 2019, 186, 365. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Ponraj, J.; Mahmoud, K.A. Sensitive electrochemical detection of l-cysteine based on a highly stable Pd@Ti3C2T:X(MXene) nanocomposite modified glassy carbon electrode. Anal. Methods 2019, 11, 3851–3856. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Alizadeh, Z. A certain electrochemical nanosensor based on functionalized multi-walled carbon nanotube for determination of cysteine in the presence of paracetamo. J. Nanostruct. 2020, 10, 258–267. [Google Scholar]
- Tajik, S.; Dourandish, Z.; Jahani, P.M.; Sheikhshoaie, I.; Beitollahi, H.; Asl, M.S.; Jang, H.W.; Shokouhimehr, M. Recent developments in voltammetric and amperometric sensors for cysteine detection. RSC Adv. 2021, 11, 5411–5425. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Q.; Yang, N. Recent Advances of Porous Graphene: Synthesis, Functionalization, and Electrochemical Applications. Small 2019, 15, e1903780. [Google Scholar] [CrossRef]
- Kaushal, S.; Kaur, M.; Kaur, N.; Kumari, V.; Singh, P.P. Heteroatom-doped graphene as sensing materials: A mini review. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, Y.; Wang, Q.; Xiao, J.; Chi, K.; Duan, X.; Chen, J.; Tang, C.; Sun, Y.; Xiao, F.; et al. Multi-element doping design of high-efficient carbocatalyst for electrochemical sensing of cancer cells. Sens. Actuators B Chem. 2018, 273, 108–117. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. [Google Scholar] [CrossRef]
- Sibul, R.; Kibena-põldsepp, E.; Mäeorg, U.; Merisalu, M.; Kikas, A. Electrochemistry Communications Sulphur and nitrogen co-doped graphene-based electrocatalysts for oxygen reduction reaction in alkaline medium. Electrochem. Commun. 2019, 109, 106603. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zheng, X.; Wang, Y. Synergistic effect of nitrogen and sulfur co-doped graphene as efficient metal-free counter electrode for dye-sensitized solar cells: A first-principle study. Comput. Mater. Sci. 2017, 136, 44–51. [Google Scholar] [CrossRef]
- Luo, Q.; Hao, F.; Wang, S.; Shen, H.; Zhao, L.; Li, J.; Grätzel, M.; Lin, H. Highly Efficient Metal-Free Sulfur-Doped and Nitrogen and Sulfur Dual-Doped Reduced Graphene Oxide Counter Electrodes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 17010–17018. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Z.; Sun, Y.; Zhang, G.; Guan, D.; Nan, J. Electrochimica Acta The kinetics investigation of nitrogen/sulfur co-doped reduced anode for lithium-ion batteries and its application in full cells. Electrochim. Acta 2021, 375, 138026. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, Y.; Liu, H.; Zhang, X.; Peng, W. One-step and rapid synthesis of nitrogen and sulfur co-doped graphene for hydrogen peroxide and glucose sensing. J. Electroanal. Chem. 2015, 742, 8–14. [Google Scholar] [CrossRef]
- Kumar, S.; Patra, S.; Madhuri, R.; Sharma, P.K. Dual doped graphene oxide for electrochemical sensing of europium ion. AIP Conf. Proc. 2017, 1832, 50068. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J.; Song, H.; Lin, X.; Zhang, S. Nature of the Synergistic Effect of N and S Co-Doped Graphene for the Enhanced Simultaneous Determination of Toxic Pollutants. ACS Appl. Mater. Interfaces 2019, 11, 44545–44555. [Google Scholar] [CrossRef]
- Xiao, L.; Yin, J.; Li, Y.; Yuan, Q.; Shen, H.; Hu, G.; Gan, W. Facile one-pot synthesis and application of nitrogen and sulfur-doped activated graphene in simultaneous electrochemical determination of hydroquinone and catechol. Analyst 2016, 141, 5555–5562. [Google Scholar] [CrossRef]
- Pogacean, F.; Socaci, C.; Pruneanu, S.M.; Biris, A.R.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide—A comparison study of their intrinsic peroxidase catalytic behavior. Sensors Actuators B Chem. 2015, 213, 474–483. [Google Scholar] [CrossRef]
- Gu, W.; Sevilla, M.; Magasinski, A.; Fuertes, A.B.; Yushin, G. Sulfur-containing activated carbons with greatly reduced content of bottle neck pores for double-layer capacitors: A case study for pseudocapacitance detection. Energy Environ. Sci. 2013, 6, 2465–2476. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the identification of sulfides and polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; Macdonald, B.J.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Sharma, R.; Chadha, N.; Saini, P. Determination of defect density, crystallite size and number of graphene layers in graphene analogues using X-ray diffraction and Raman spectroscopy. Indian J. Pure Appl. Phys. 2017, 55, 625–629. [Google Scholar]
- Coroş, M.; Pogăcean, F.; Roşu, M.-C.; Socaci, C.; Borodi, G.; Mageruşan, L.; Biriş, A.R.; Pruneanu, S. Simple and cost-effective synthesis of graphene by electrochemical exfoliation of graphite rods. RSC Adv. 2015, 6, 2651–2661. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, C.; Zheng, F.; Li, X.; Sun, C. Nitrogen and sulfur co-doped graphene nanoribbons: A novel metal- free catalyst for high performance electrochemical detection of 2, 4, 6-trinitrotoluene (TNT). Carbon 2018, 126, 328–337. [Google Scholar] [CrossRef]
- Chen, F.; Ma, L.; Ren, J.; Luo, X.; Liu, B.; Zhou, X. Sandwich-Type Nitrogen and Sulfur Co-doped Graphene-Backboned Porous Carbon Coated Separator for High Performance Lithium-Sulfur Batteries. Nanomaterials 2018, 8, 191. [Google Scholar] [CrossRef]
- Mannan, A.; Hirano, Y.; Quitain, A.T.; Koinuma, M.; Kida, T. Nitrogen, Sulfur Co-Doped Reduced Graphene Oxide: Synthesis and Characterization. Micro Nanosyst. 2020, 12, 129–134. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, M.; Jin, T.; Cheng, C.; Zheng, J.; Qian, Y. One-step synthesis of nitrogen, sulphur-codoped graphene as electrode material for supercapacitor with excellent cycling stability. Int. J. Electrochem. Sci. 2020, 15, 16–25. [Google Scholar] [CrossRef]
- Liang, S.; Han, C.; Meng, Q.; Tian, G. Nitrogen and sulfur co-doped NaTi2(PO4)3/hole graphene composite as high-performance electrosorption electrodes for hybrid capacitive deionization. J. Mater. Sci. 2020, 55, 6017–6029. [Google Scholar] [CrossRef]
- Prathish, K.P.; Barsan, M.M.; Geng, D.; Sun, X.; Brett, C.M. Chemically modified graphene and nitrogen-doped graphene: Electrochemical characterisation and sensing applications. Electrochim. Acta 2013, 114, 533–542. [Google Scholar] [CrossRef]
- Nkosi, D.; Pillay, J.; Ozoemena, K.I.; Nouneh, K.; Oyama, M. Heterogeneous electron transfer kinetics and electrocatalytic behaviour of mixed self-assembled ferrocenes and SWCNT layers. Phys. Chem. Chem. Phys. 2010, 12, 604–613. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Liu, Y.; Tian, Y.; Zhang, X. Nitrogen and sulfur dual-doped graphene for glucose biosensor application. J. Electroanal. Chem. 2015, 738, 100–107. [Google Scholar] [CrossRef]
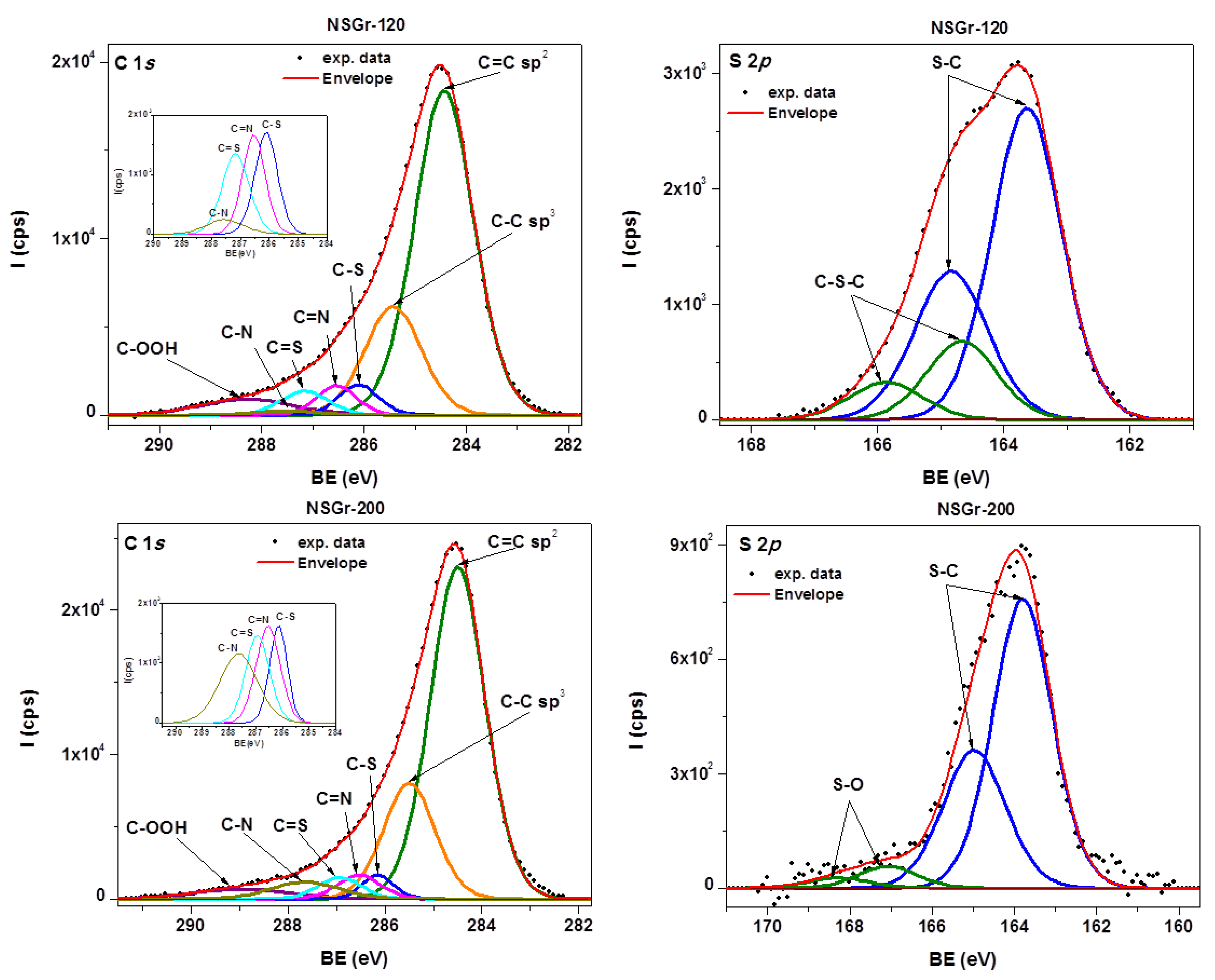
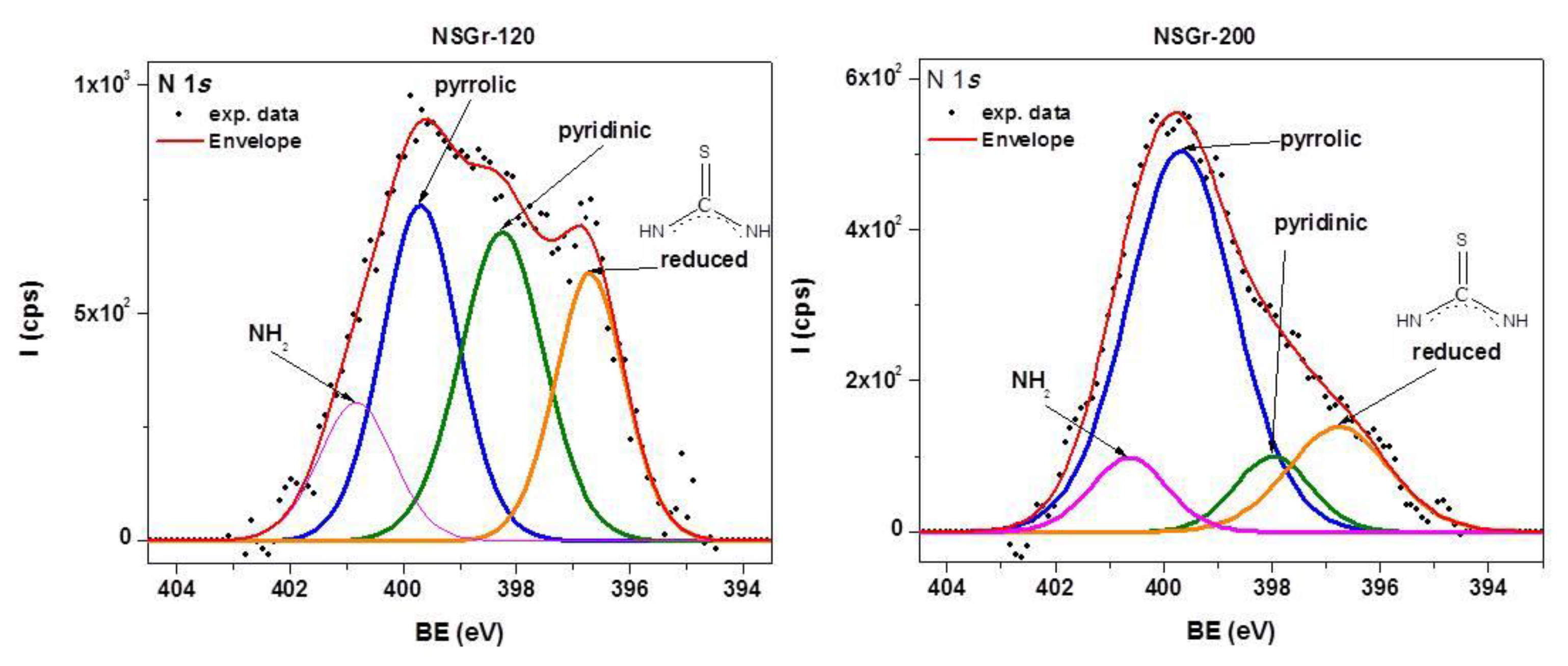


| Sample | Csp2/C(sp2 + sp3) (at. %) | Npyrr. (at.%) | Npyrid. (at.%) | –N–H2 (at.%) | Reduced –N–H2 (at. %) | S–C (at.%) | S–O (at.%) | C–S–C (at.%) |
|---|---|---|---|---|---|---|---|---|
| NSGr-120 | 75.91 | 2 | 2 | 0.8 | 1.47 | 9.35 | - | 2.35 |
| NSGr-200 | 75.49 | 1.64 | 0.23 | 0.43 | 0.22 | 2.8 | 0.2 | - |
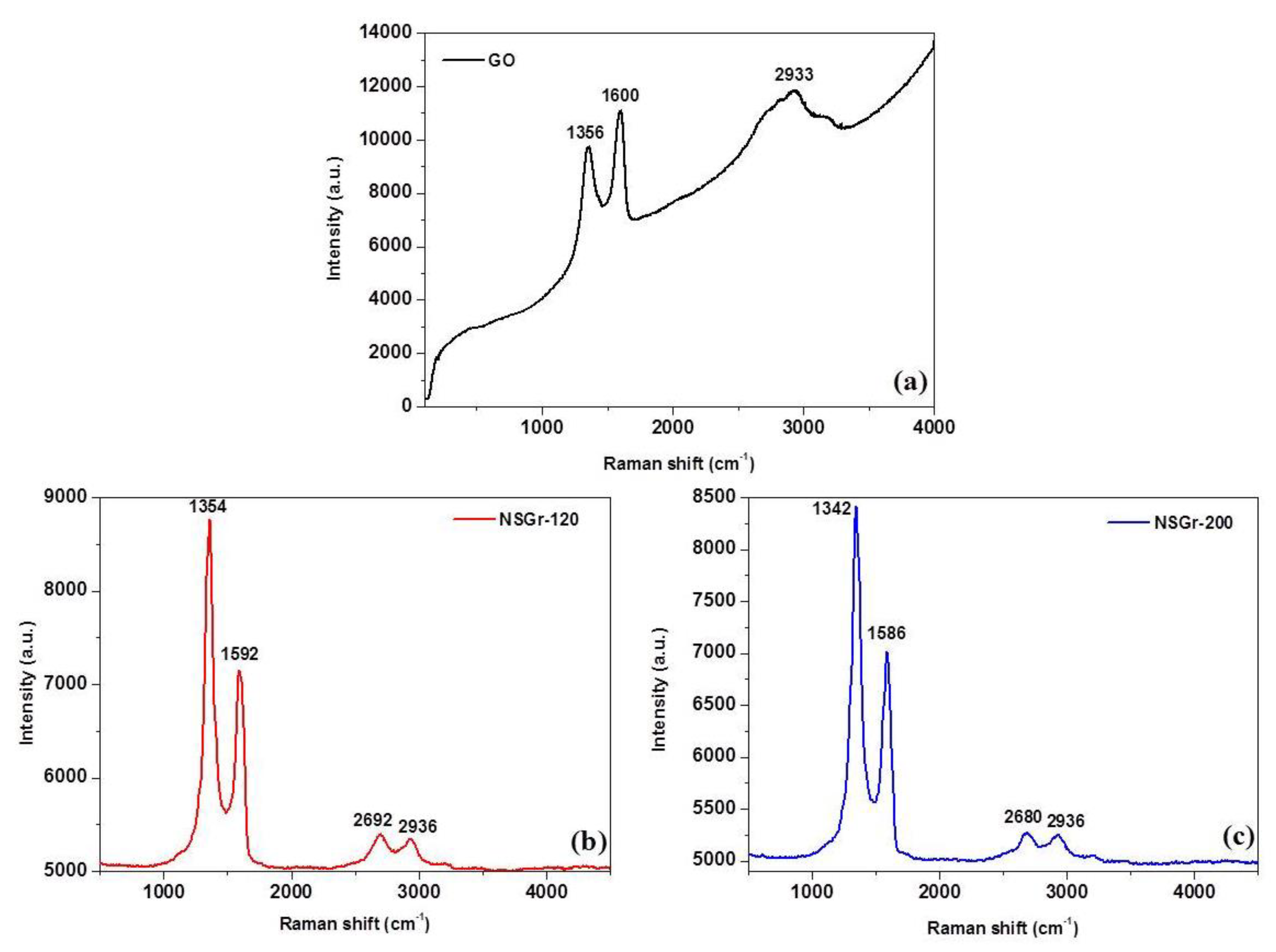
| Sample | G (cm−1) | D (cm−1) | 2D (cm−1) | D + D’ (cm−1) | ID/IG | La (nm) |
|---|---|---|---|---|---|---|
| GO | 1600 | 1356 | - | 2933 | 0.877 | 21.66 |
| NSGr-120 | 1592 | 1354 | 2692 | 2936 | 1.227 | 15.48 |
| NSGr-200 | 1586 | 1342 | 2680 | 2936 | 1.196 | 15.88 |
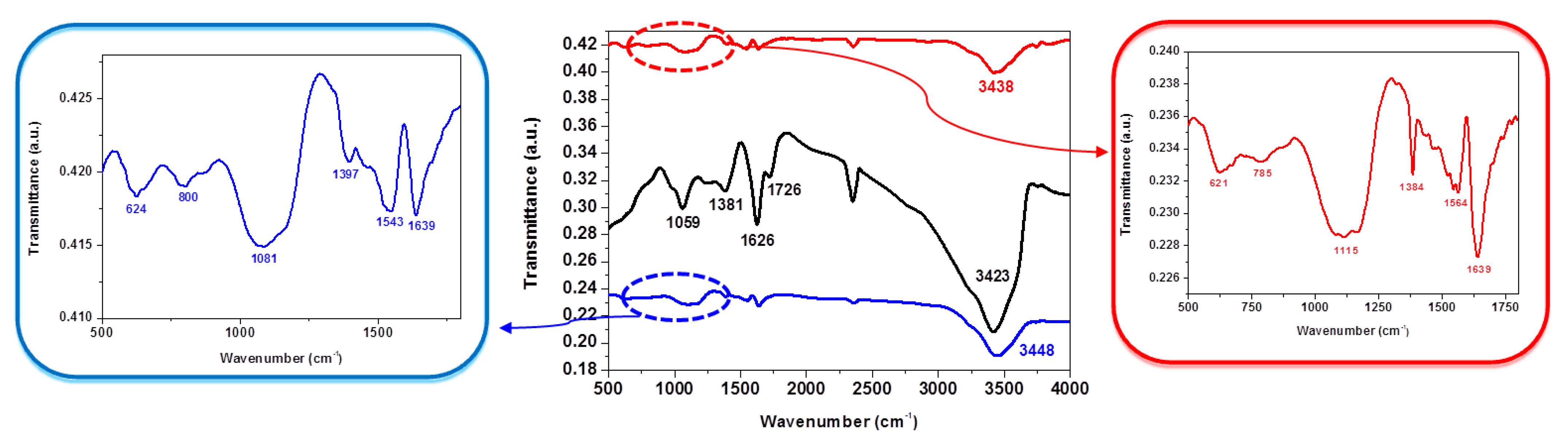
| Group Frequency (cm−1) | Peak Position (cm−1) GO | Peak Position (cm−1) NSGr-120 | Peak Position (cm−1) NSGr-200 |
|---|---|---|---|
| O–H stretching, 3550–3200 (broad, s) | 3423 | 3448 | 3438 |
| C=O stretching, 1750–1680 (s) | 1726 | - | - |
| C–O stretching, 1150–1085 (s) | 1059 | 1081 | 1115 |
| C=N, 1690–1640 (m), or C=C stretching, 1680–1600 (m) | 1626 | 1639 | 1639 |
| C–S stretching, 710–570 (w) | - | 624 | 621 |
| GO: Dopant (Weight) | Temperature (°C) | Reaction Time (h) | N, S at% | Ref. |
|---|---|---|---|---|
| 1:4 | 180 | 12 | 1.99 N 0.48 S | [34] |
| 1:1.75 | 180 | 12 | 1.86 N 5.26 S | [35] |
| 1:1 | 250 | 2 | 3.52 N 7.59 S | [36] |
| 1:10 | 150 | overnight | - | [37] |
| 1:1, 1:3, 1:5 | 180 | 12 | - | [38] |
| 1:1 | 120 | 3 | 4.8 N 9.35 S | This work |
| 200 | 2.3 N 2.8 S |
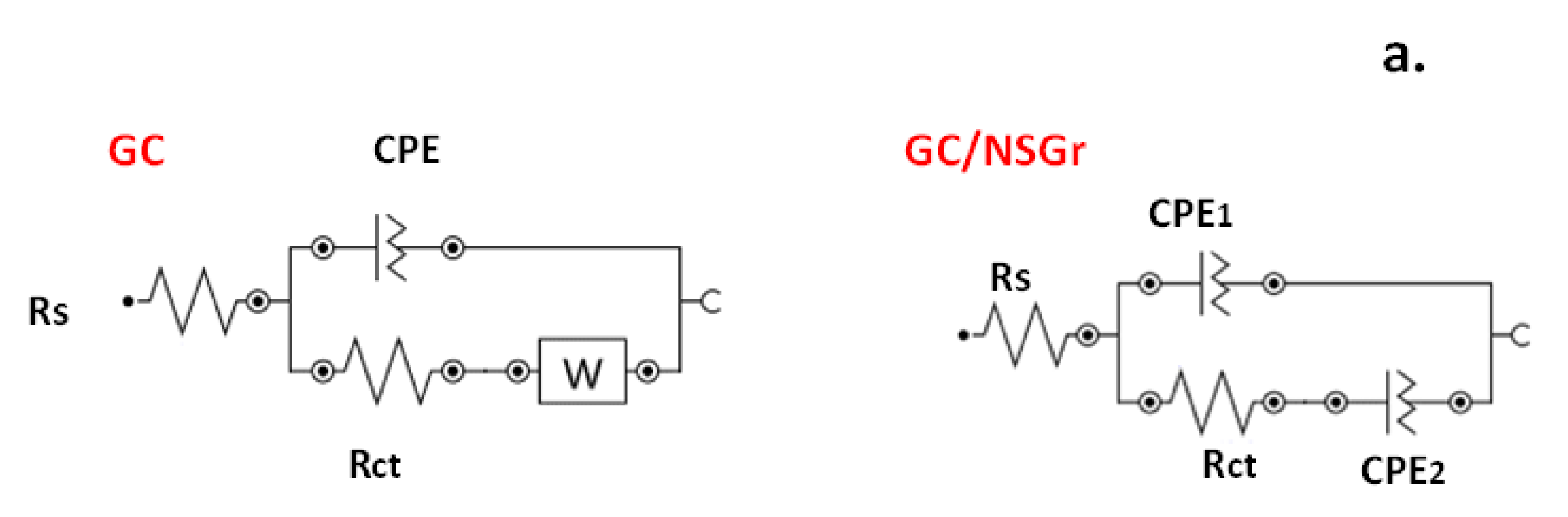
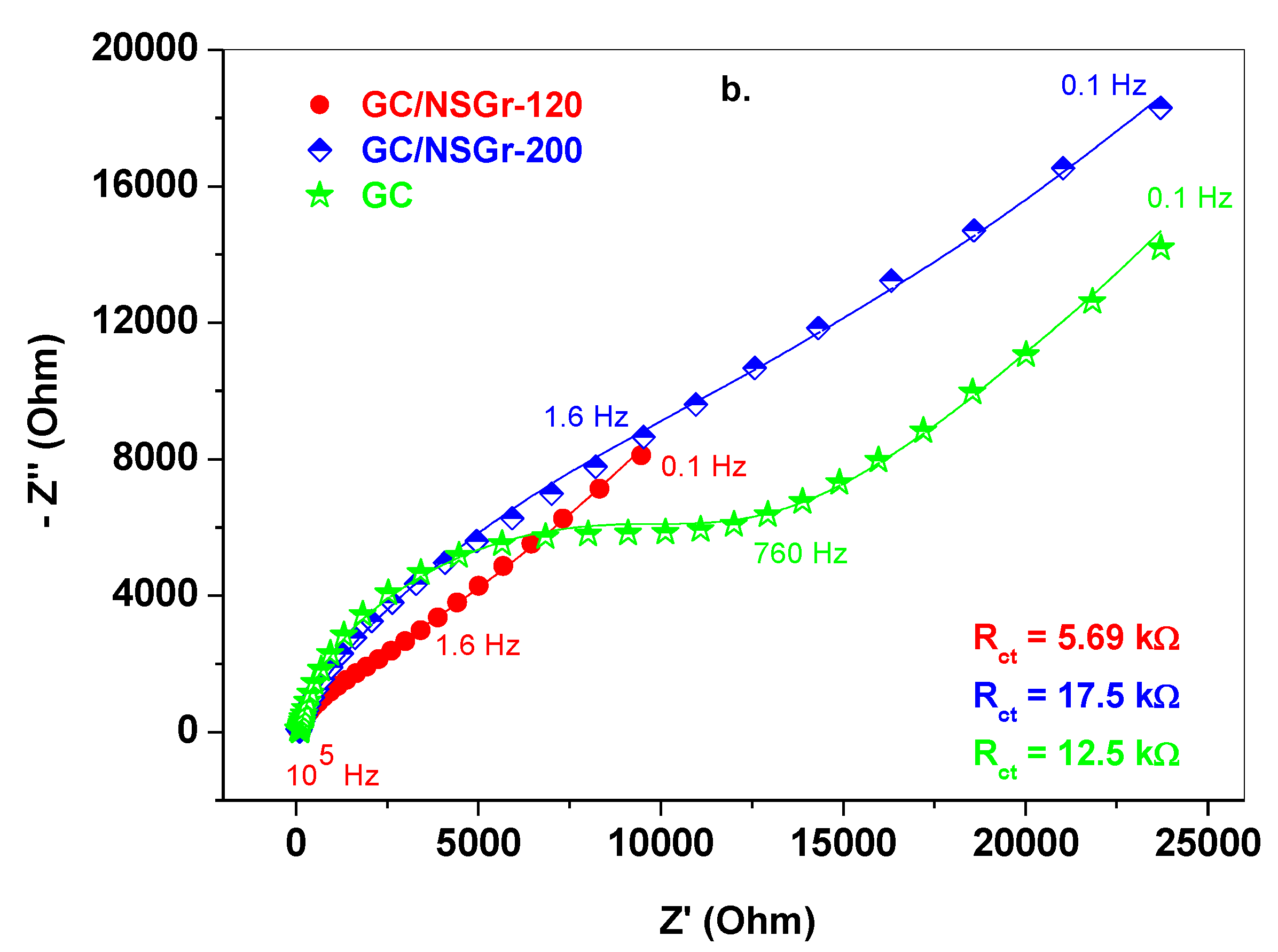
| Electrode | ΔEp (mV) | Ipa/Ipc | A (cm2) | E0′ (V) | Rct (kΩ) | Kapp (cm/s) |
|---|---|---|---|---|---|---|
| GC | 130 | 1.06 | 0.025 | 0.245 | 12.5 | 7.6 × 10−4 |
| GC/NSGr-120 | 80 | 1.0 | 0.055 | 0.24 | 5.2 | 9.3 × 10−4 |
| GC/NSGr-200 | 80 | 0.97 | 0.037 | 0.24 | 17.5 | 4.1 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varodi, C.; Pogăcean, F.; Cioriță, A.; Pană, O.; Leoștean, C.; Cozar, B.; Radu, T.; Coroș, M.; Ștefan-van Staden, R.I.; Pruneanu, S.-M. Nitrogen and Sulfur Co-Doped Graphene as Efficient Electrode Material for L-Cysteine Detection. Chemosensors 2021, 9, 146. https://doi.org/10.3390/chemosensors9060146
Varodi C, Pogăcean F, Cioriță A, Pană O, Leoștean C, Cozar B, Radu T, Coroș M, Ștefan-van Staden RI, Pruneanu S-M. Nitrogen and Sulfur Co-Doped Graphene as Efficient Electrode Material for L-Cysteine Detection. Chemosensors. 2021; 9(6):146. https://doi.org/10.3390/chemosensors9060146
Chicago/Turabian StyleVarodi, Codruța, Florina Pogăcean, Alexandra Cioriță, Ovidiu Pană, Cristian Leoștean, Bogdan Cozar, Teodora Radu, Maria Coroș, Raluca Ioana Ștefan-van Staden, and Stela-Maria Pruneanu. 2021. "Nitrogen and Sulfur Co-Doped Graphene as Efficient Electrode Material for L-Cysteine Detection" Chemosensors 9, no. 6: 146. https://doi.org/10.3390/chemosensors9060146
APA StyleVarodi, C., Pogăcean, F., Cioriță, A., Pană, O., Leoștean, C., Cozar, B., Radu, T., Coroș, M., Ștefan-van Staden, R. I., & Pruneanu, S.-M. (2021). Nitrogen and Sulfur Co-Doped Graphene as Efficient Electrode Material for L-Cysteine Detection. Chemosensors, 9(6), 146. https://doi.org/10.3390/chemosensors9060146








