Potentiometric Carboxylate Sensors Based on Carbazole-Derived Acyclic and Macrocyclic Ionophores
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Equipment
2.3. Preparation of the Membranes
2.4. Preparation of the Sensors
2.5. Electrochemical Impedance Spectroscopy
2.6. Potentiometry
3. Results and Discussion
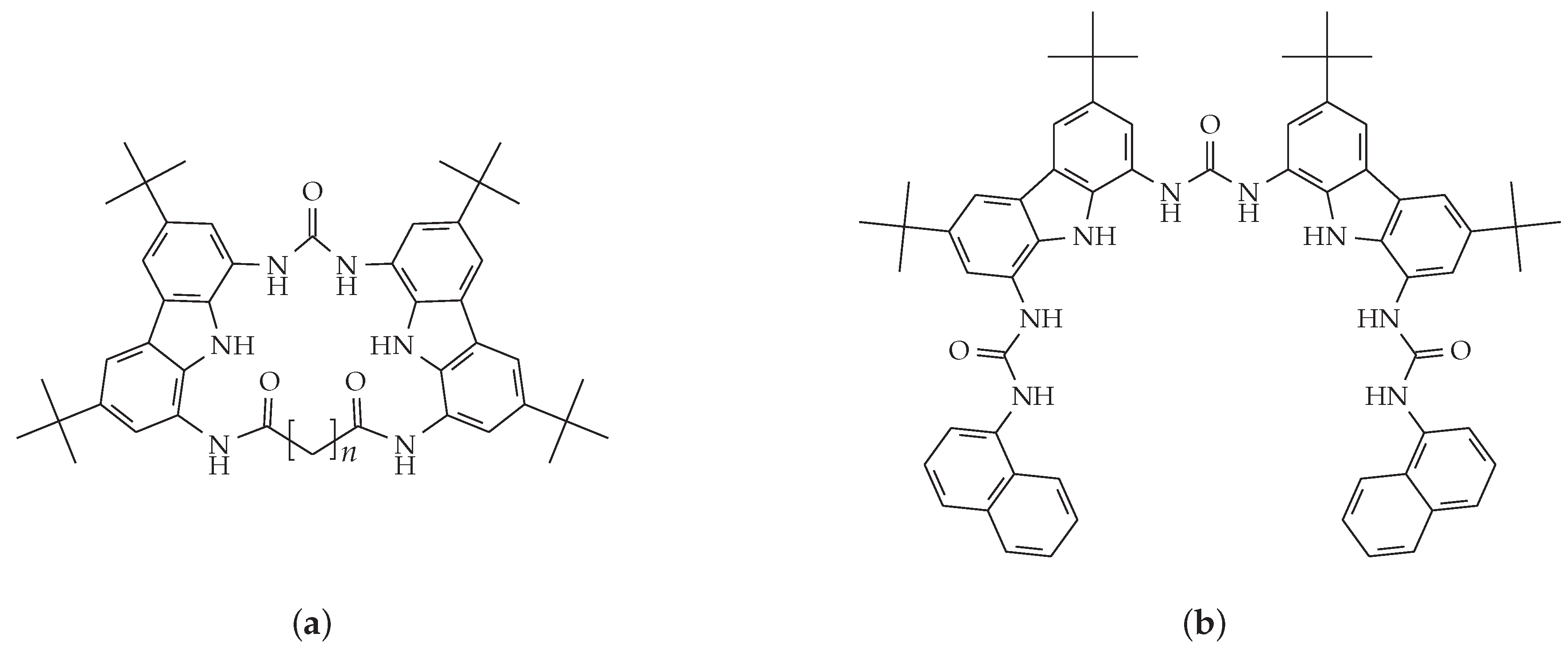
3.1. Receptors, Membranes, and Electrode Bodies
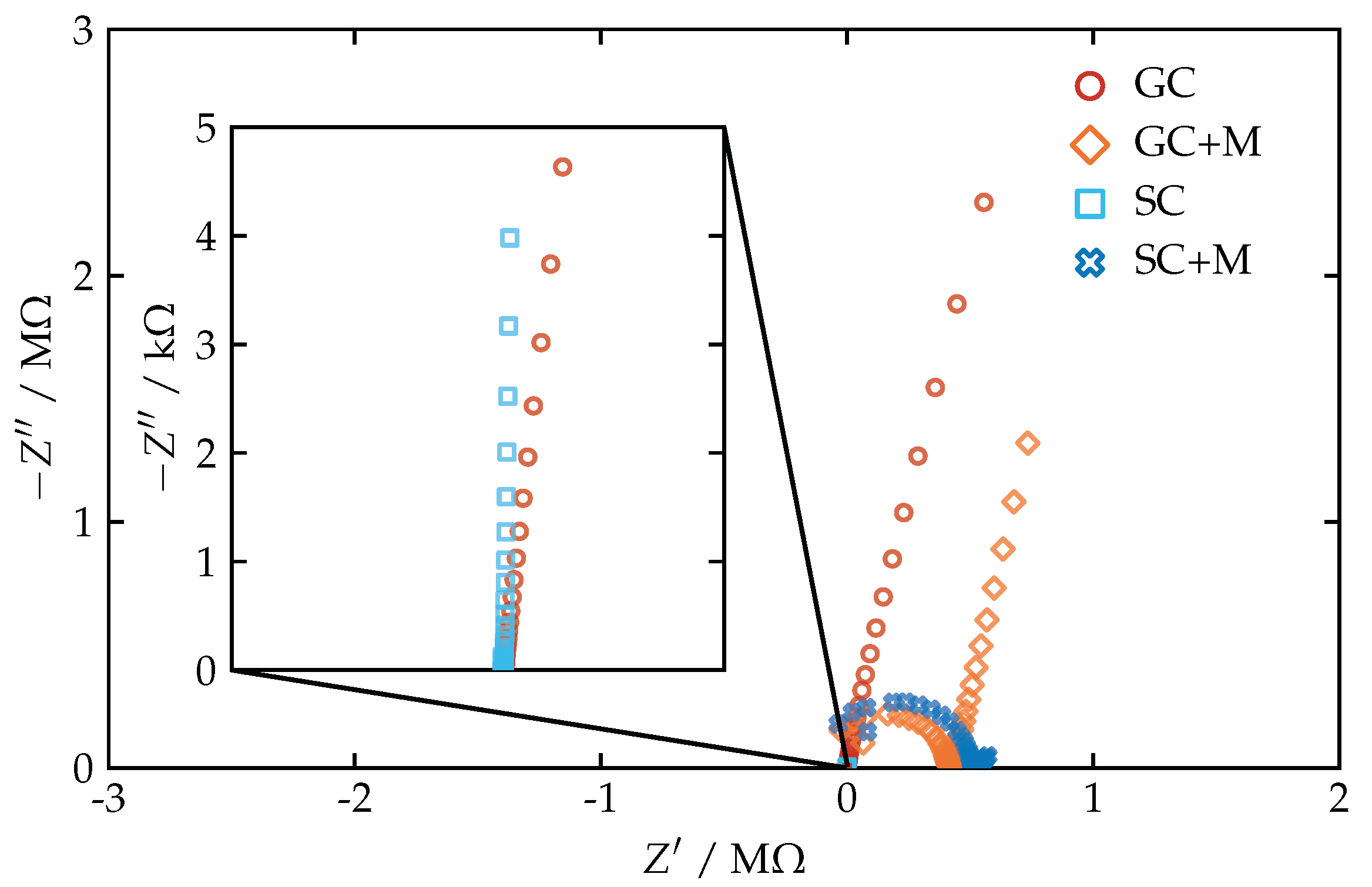
3.2. Minimizing the Effects of Dissolved CO2 and Chloride Leakage from the Reference Electrode
3.3. Electrochemical Impedance Spectroscopy
3.4. Potentiometric Calibration
3.5. Potentiometric pH Sensitivity
3.6. Potentiometric Selectivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Acyclic receptor |
| CWE | Coated wire electrode |
| DMSO | Dimethyl sulfoxide |
| DOS | Bis(2-ethylhexyl) sebacate |
| EDOT | 3,4-ethylenedioxythiophene |
| EIS | Electrochemical impedance spectroscopy |
| HMW | High molecular weight |
| GC | Glassy carbon |
| ISE | Ion-selective electrode |
| ISM | Ion-selective membrane |
| MC5 | Macrocyclic receptor with five methylene units in the linker |
| MC9 | Macrocyclic receptor with nine methylene units in the linker |
| MC12 | Macrocyclic receptor with twelve methylene units in the linker |
| OCP | Open-circuit potential |
| o-NPOE | 2-nitrophenyl octyl ether |
| PCTFE | Poly(chlorotrifluoroethylene) |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PVC | Poly(vinyl chloride) |
| RMS | Root mean square |
| SC-ISE | Solid-contact ion-selective electrode |
| TDMACl | Tridodecylmethylammonium chloride |
| THF | Tetrahydrofuran |
Appendix A
Appendix A.1. Receptor Binding Constants
| Ion | ||||
|---|---|---|---|---|
| AC | MC5 | MC9 | MC12 | |
| 5.39 | 4.93 | 5.82 | 5.40 | |
| 4.98 | 5.00 | 5.69 | 5.00 | |
| 4.20 | 4.17 | 4.95 | 4.41 | |
| 3.83 | 3.36 | 4.07 | 3.62 | |
| 3.63 | 4.06 | 4.59 | 3.86 | |
Appendix A.2. Electrochemical Impedance Spectroscopy

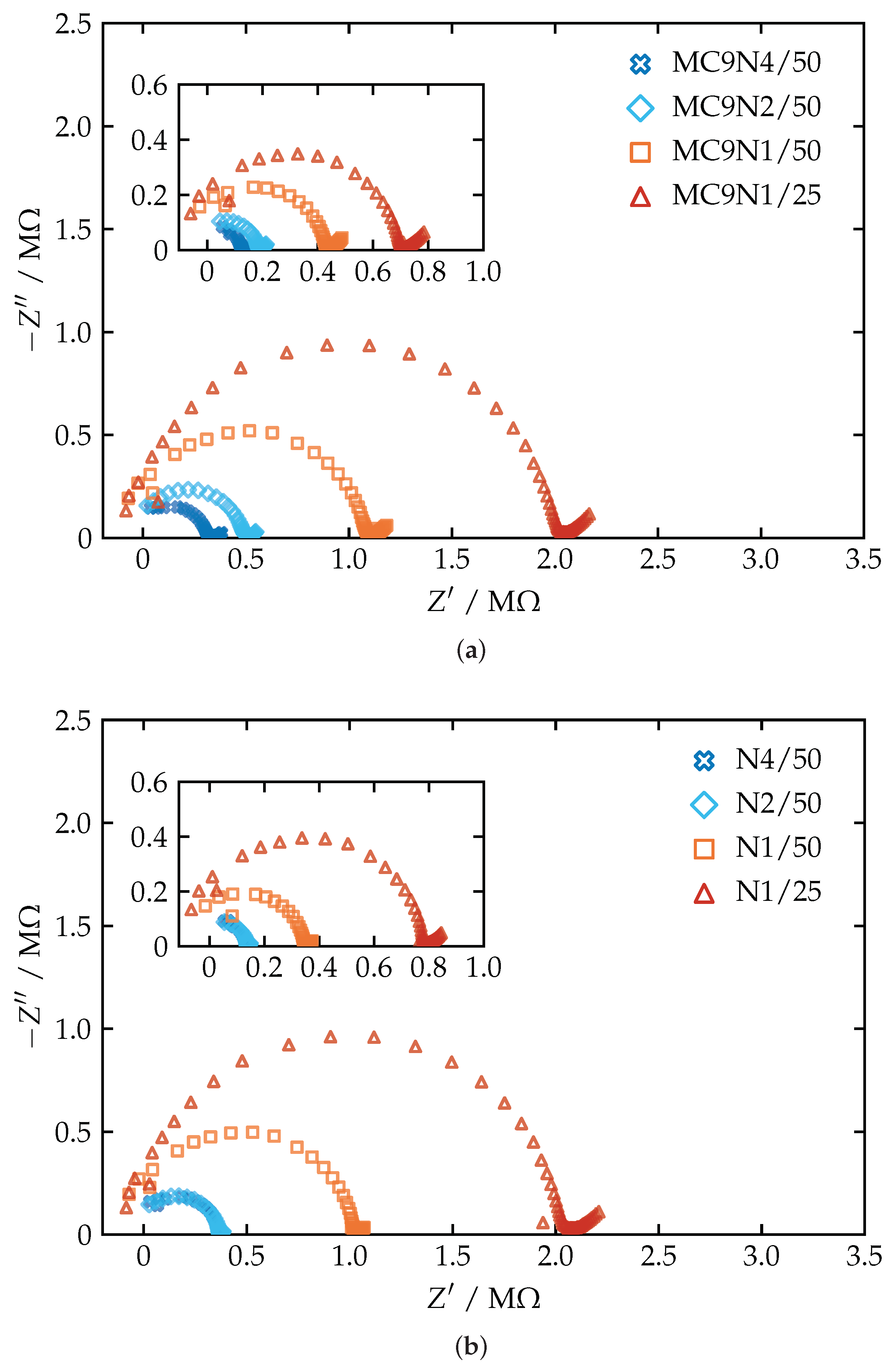
| Membrane | (M) | (pF) | (S m) | |||||
|---|---|---|---|---|---|---|---|---|
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| N1/25 | 2.0 | 0.8 | 11.1 | 11.3 | 7.4 | 12.6 | 18.9 | 12.8 |
| N1/50 | 1.0 | 0.3 | 9.5 | 12.7 | 14.9 | 29.5 | 16.1 | 14.4 |
| N2/50 | 0.4 | 0.1 | 11.3 | 14.2 | 39.5 | 72.7 | 19.1 | 16.1 |
| D2/50 | 6.8 | 4.8 | 5.9 | 6.0 | 2.2 | 2.1 | 9.9 | 6.8 |
| N4/50 | 0.4 | 0.1 | 11.4 | 14.8 | 42.2 | 78.0 | 19.3 | 16.7 |
| MC5N2/50 | 0.6 | 0.2 | 11.1 | 14.1 | 27.0 | 45.9 | 18.8 | 16.0 |
| MC5D2/50 | 8.1 | 4.8 | 6.2 | 6.4 | 1.9 | 2.1 | 10.5 | 7.2 |
| MC9N1/25 | 2.0 | 0.7 | 11.2 | 12.1 | 7.7 | 13.6 | 18.9 | 13.7 |
| MC9N1/50 | 1.0 | 0.4 | 9.6 | 12.2 | 15.0 | 24.0 | 16.2 | 13.8 |
| MC9N2/50 | 0.5 | 0.2 | 11.8 | 13.7 | 31.4 | 52.3 | 19.9 | 15.6 |
| MC9D2/50 | 5.5 | 3.1 | 6.1 | 6.4 | 2.7 | 3.2 | 10.3 | 7.3 |
| MC9N4/50 | 0.3 | 0.1 | 12.0 | 14.7 | 50.0 | 84.3 | 20.3 | 16.7 |
| MC12N2/50 | 0.4 | 0.2 | 11.6 | 14.5 | 34.9 | 66.4 | 19.6 | 16.4 |
| MC12D2/50 | 5.7 | 3.1 | 6.3 | 6.4 | 2.6 | 3.2 | 10.7 | 7.3 |
| ACN2/50 | 0.7 | 0.3 | 11.2 | 12.5 | 21.6 | 37.2 | 18.9 | 14.1 |
| ACD2/50 | 9.3 | 4.2 | 6.2 | 6.2 | 1.6 | 2.4 | 10.5 | 7.0 |
| Membrane | SD of (M) | SD of (pF) | SD of (S m) | SD of | ||||
|---|---|---|---|---|---|---|---|---|
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| N1/25 | 0.1 | <0.1 | 0.3 | 0.3 | 0.2 | 0.7 | 0.5 | 0.4 |
| N1/50 | <0.1 | <0.1 | 0.6 | 0.2 | 0.7 | 0.7 | 0.9 | 0.2 |
| N2/50 | <0.1 | <0.1 | 0.9 | 0.1 | 1.7 | 0.2 | 1.5 | 0.2 |
| D2/50 | 0.3 | 0.5 | 0.6 | 0.2 | 0.1 | 0.2 | 1.0 | 0.2 |
| N4/50 | <0.1 | <0.1 | 0.2 | 0.5 | 1.3 | 2.6 | 0.4 | 0.6 |
| MC5N2/50 | <0.1 | <0.1 | 0.1 | 0.4 | 0.2 | 1.1 | 0.1 | 0.4 |
| MC5D2/50 | 0.1 | 0.4 | 0.6 | 0.1 | <0.1 | 0.2 | 1.1 | 0.2 |
| MC9N1/25 | 0.2 | <0.1 | <0.1 | 0.1 | 0.9 | 0.8 | <0.1 | 0.1 |
| MC9N1/50 | 0.1 | <0.1 | 0.4 | 0.4 | 1.0 | 1.5 | 0.7 | 0.4 |
| MC9N2/50 | <0.1 | <0.1 | 0.1 | 0.4 | 1.3 | 2.2 | 0.2 | 0.5 |
| MC9D2/50 | 0.4 | 0.1 | 0.7 | <0.1 | 0.2 | 0.1 | 1.2 | <0.1 |
| MC9N4/50 | <0.1 | <0.1 | 0.3 | 0.2 | 2.0 | 3.7 | 0.4 | 0.3 |
| MC12N2/50 | <0.1 | <0.1 | 1.0 | <0.1 | 1.3 | 3.0 | 1.6 | <0.1 |
| MC12D2/50 | 0.2 | 0.2 | 0.5 | <0.1 | 0.1 | 0.2 | 0.8 | <0.1 |
| ACN2/50 | <0.1 | <0.1 | 0.7 | 0.1 | 0.6 | 0.4 | 1.2 | 0.1 |
| ACD2/50 | <0.1 | <0.1 | 0.3 | 0.2 | <0.1 | <0.1 | 0.5 | 0.2 |
| L () | (M) | (pF) | (S m) | |
|---|---|---|---|---|
| 100 | 0.6 0.1 | 17.0 2.0 | 12.1 0.9 | 12.7 0.2 |
| 150 | 0.8 0.0 | 11.4 0.2 | 13.1 0.9 | 13.5 0.3 |
| 240 | 1.2 0.1 | 8.8 0.2 | 12.9 0.8 | 15.8 0.7 |
| 270 | 1.3 0.0 | 7.9 0.2 | 13.6 0.4 | 16.1 0.5 |
| 300 | 2.1 0.2 | 11.2 1.2 | 7.2 0.4 | 19.0 1.4 |
Appendix A.3. Potentiometric Calibrations
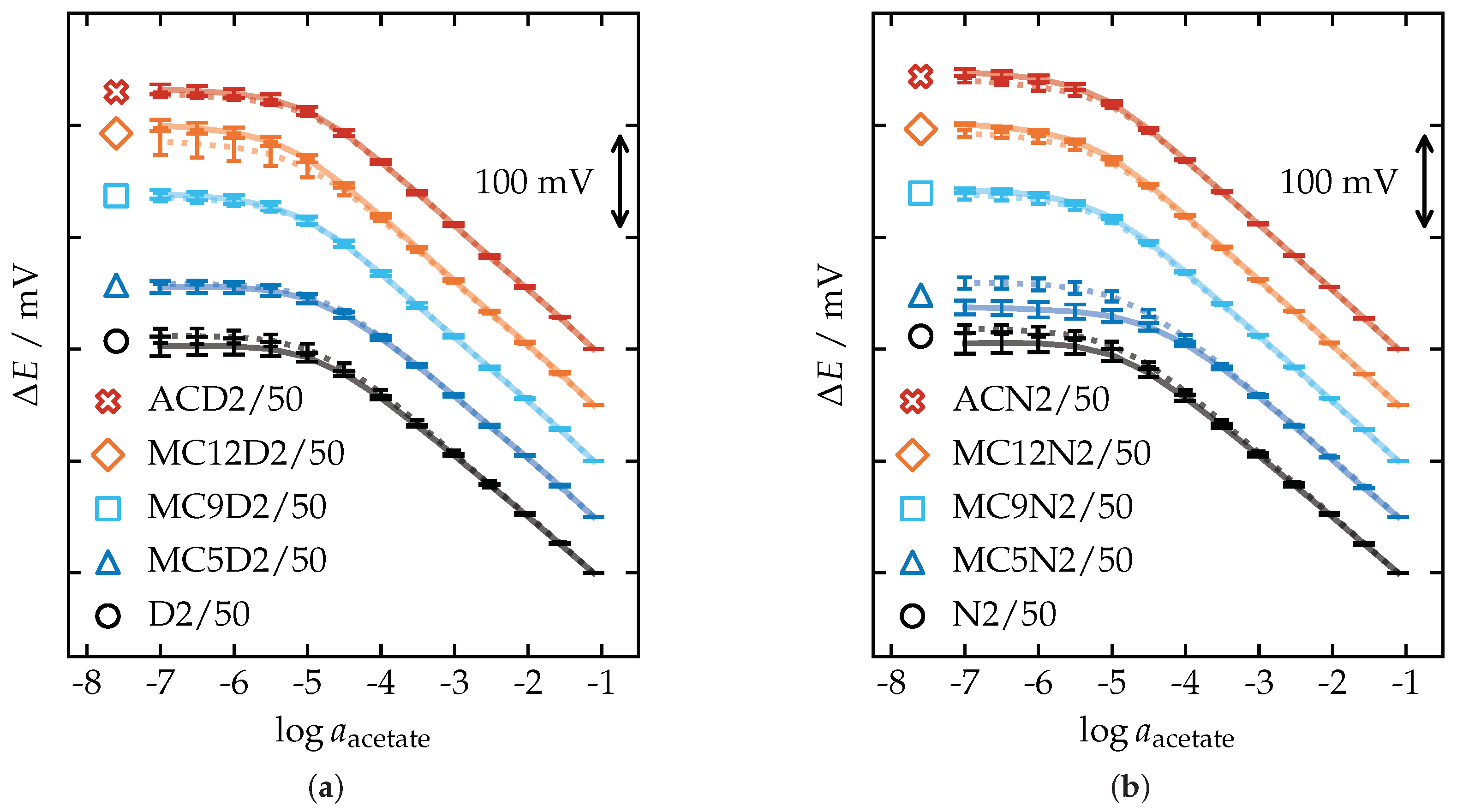
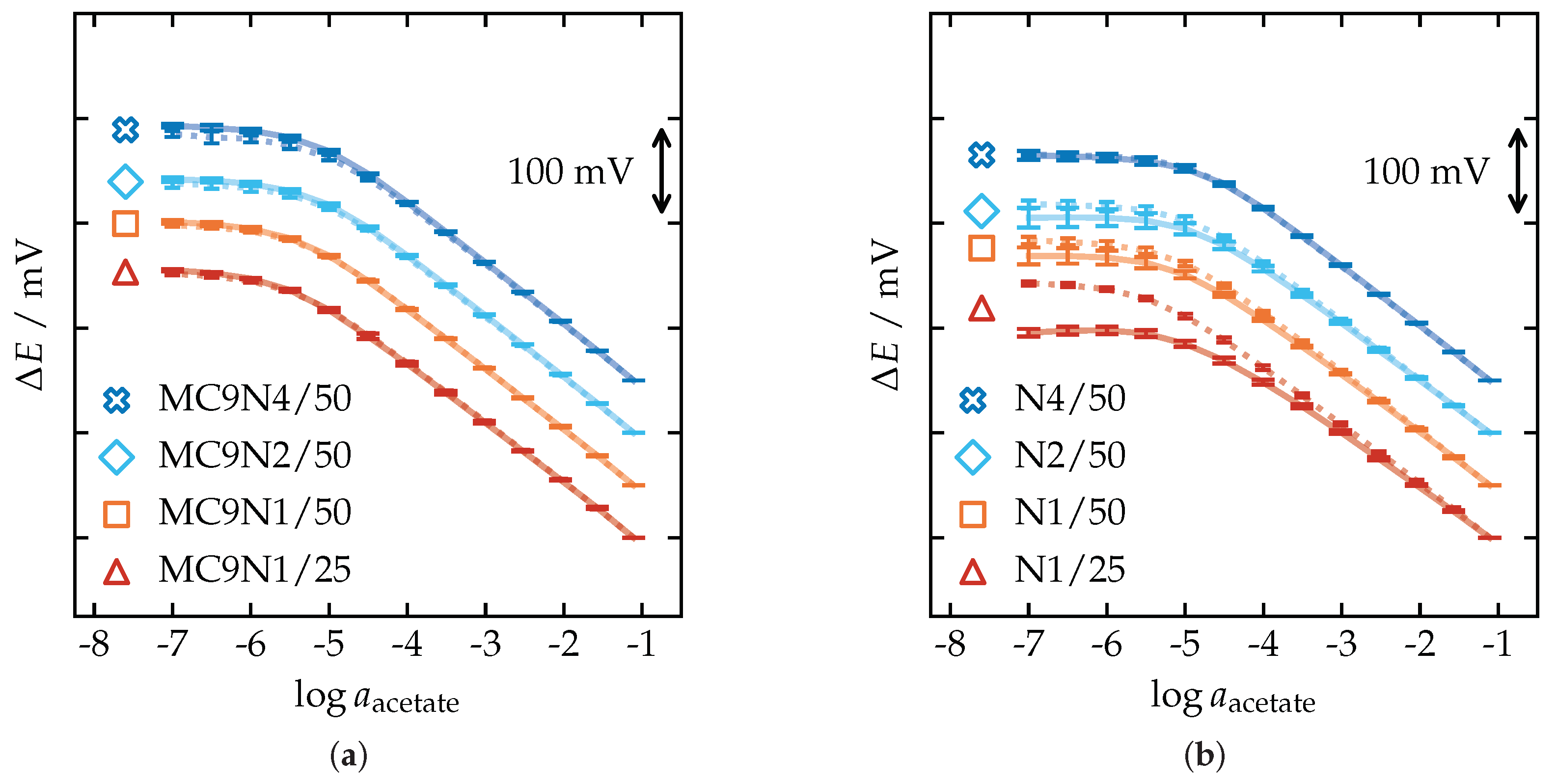
| Membrane | Slope (mV/dec) | |||||
|---|---|---|---|---|---|---|
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| N1/25 | −51.51 | −55.50 | −3.92 | −4.50 | −4.90 | −5.44 |
| N1/50 | −54.68 | −56.76 | −3.92 | −4.00 | −5.08 | −5.20 |
| N2/50 | −54.23 | −56.13 | −4.00 | −4.00 | −4.87 | −4.97 |
| D2/50 | −54.44 | −55.55 | −3.88 | −4.00 | −4.82 | −4.90 |
| N4/50 | −56.84 | −56.78 | −4.00 | −4.00 | −4.86 | −4.88 |
| MC5N2/50 | −55.98 | −55.82 | −3.51 | −3.88 | −4.44 | −4.83 |
| MC5D2/50 | −55.59 | −55.60 | −3.88 | −4.00 | −4.77 | −4.82 |
| MC9N1/25 | −56.58 | −56.99 | −4.50 | −4.50 | −5.56 | −5.48 |
| MC9N1/50 | −57.37 | −57.58 | −4.50 | −4.50 | −5.44 | −5.38 |
| MC9N2/50 | −58.37 | −57.81 | −4.00 | −4.00 | −5.22 | −5.20 |
| MC9D2/50 | −57.64 | −57.51 | −4.00 | −4.00 | −5.21 | −5.17 |
| MC9N4/50 | −58.66 | −57.84 | −4.00 | −4.00 | −5.22 | −5.15 |
| MC12N2/50 | −58.55 | −58.01 | −4.00 | −4.00 | −5.37 | −5.27 |
| MC12D2/50 | −58.15 | −57.55 | −4.00 | −3.88 | −5.38 | −5.19 |
| ACN2/50 | −58.43 | −58.30 | −4.00 | −4.00 | −5.32 | −5.21 |
| ACD2/50 | −57.57 | −57.56 | −4.00 | −4.00 | −5.11 | −5.03 |
| Membrane | SD of Slope (mV/dec) | SD of | SD of | |||
|---|---|---|---|---|---|---|
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| N1/25 | 0.69 | 0.81 | 0.20 | 0.07 | 0.08 | |
| N1/50 | 0.40 | 0.56 | 0.20 | 0.15 | 0.02 | |
| N2/50 | 0.82 | 0.43 | 0.10 | 0.04 | ||
| D2/50 | 0.21 | 1.15 e | 0.25 | 0.14 | 0.04 e | |
| N4/50 | 0.33 | 0.10 | 0.05 | 0.04 | ||
| MC5N2/50 | 0.49 | 0.35 | 0.25 | 0.09 | 0.07 | |
| MC5D2/50 | 0.55 | 0.77 | 0.25 | 0.10 | 0.04 | |
| MC9N1/25 | 0.91 | 0.62 | 0.07 | 0.06 | ||
| MC9N1/50 | 0.31 | 0.26 | 0.03 | 0.02 | ||
| MC9N2/50 | 0.19 | 0.20 | 0.02 | 0.05 | ||
| MC9D2/50 | 0.98 | 0.75 | 0.04 | 0.04 | ||
| MC9N4/50 | 0.05 | 0.10 | 0.03 | 0.04 | ||
| MC12N2/50 | 0.21 | 0.13 | 0.01 | 0.04 | ||
| MC12D2/50 | 0.60 | 0.67 | 0.25 | 0.07 | 0.20 | |
| ACN2/50 | 0.25 | 0.38 | 0.04 | 0.02 | ||
| ACD2/50 | 0.80 | 0.61 | 0.05 | 0.02 | ||
Appendix A.4. pH Sensitivity
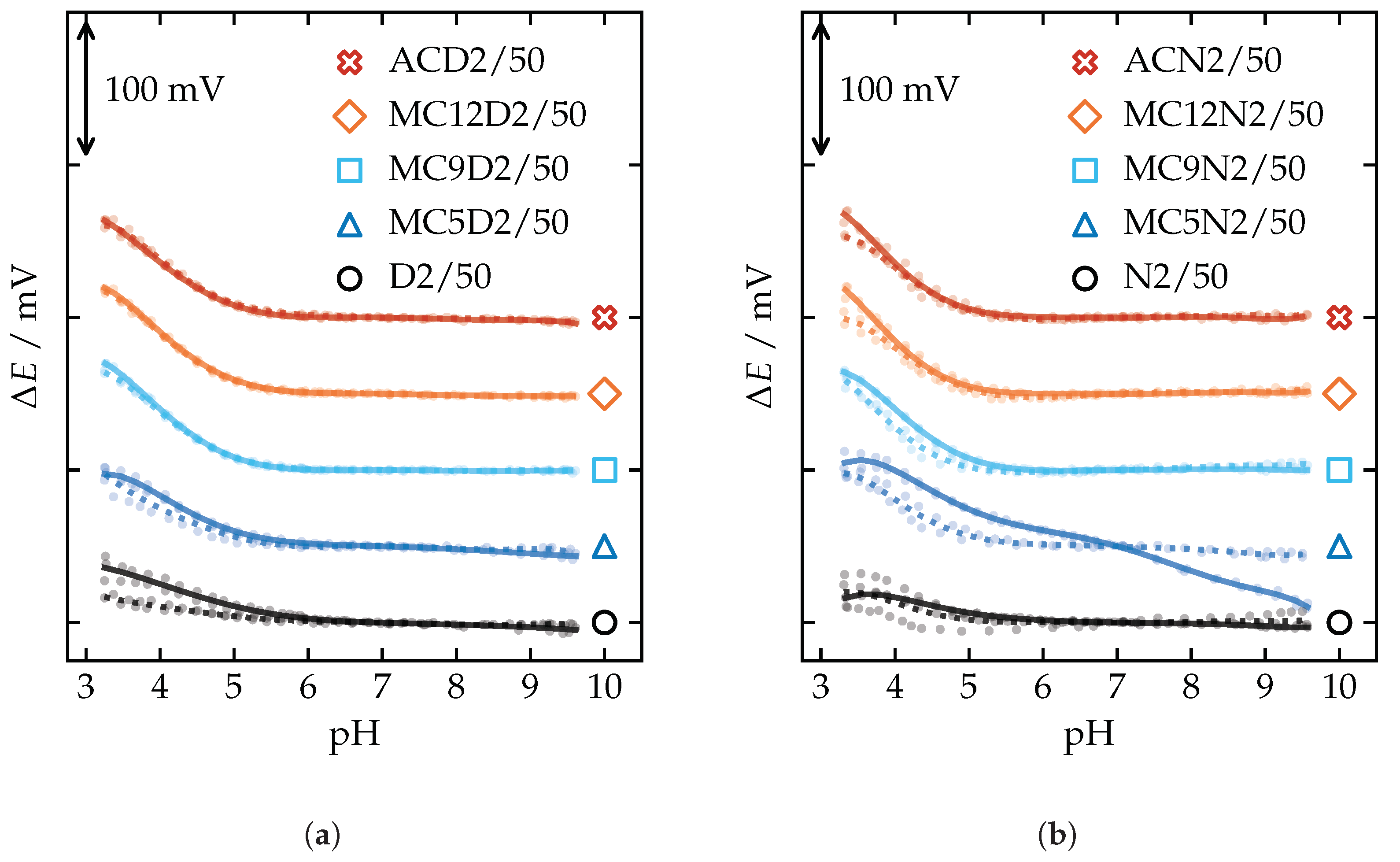
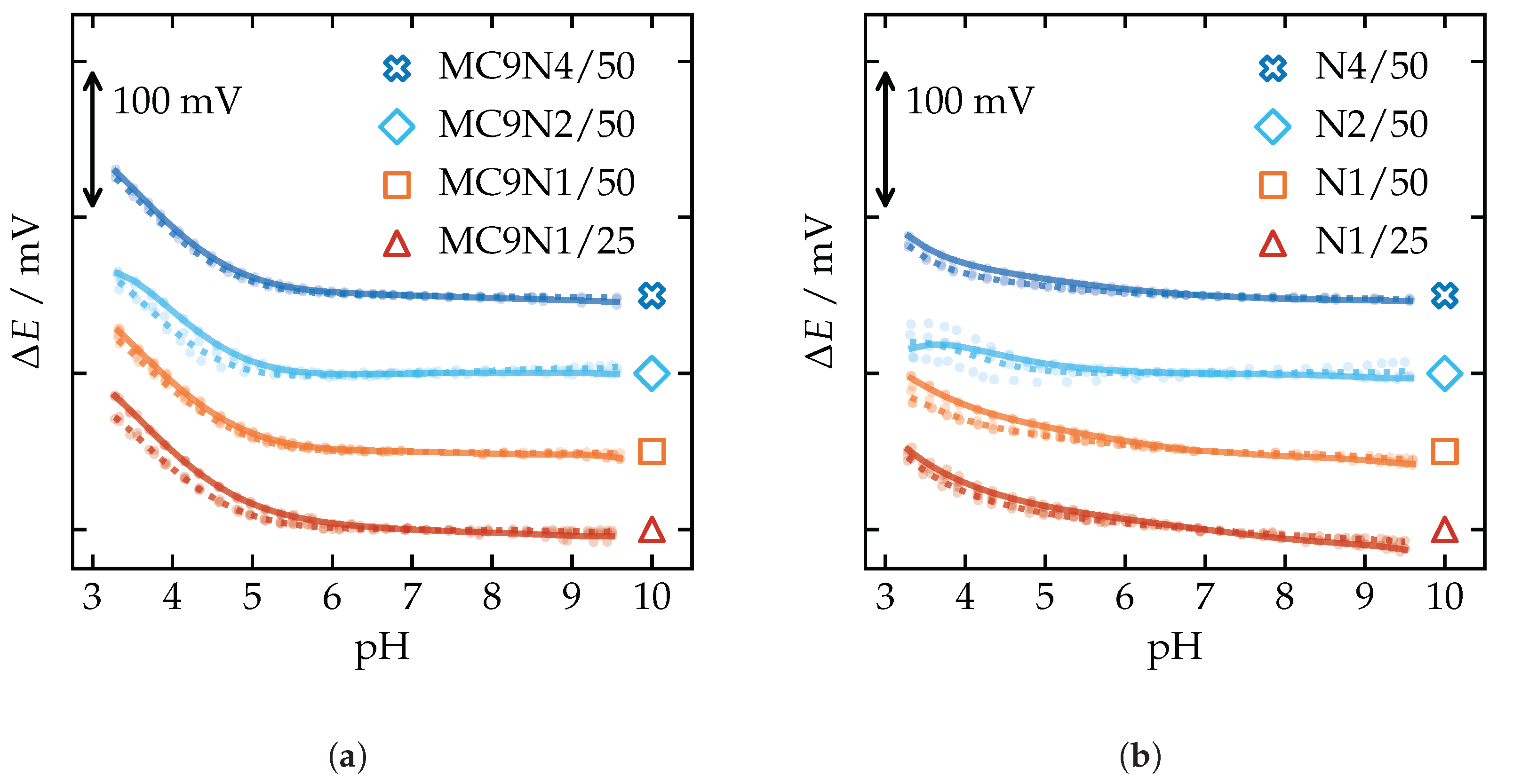
Appendix A.5. Selectivity Coefficients
| Ion, j | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D2/50 | MC5D2/50 | MC9D2/50 | MC12D2/50 | ACD2/50 | ||||||
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| −0.35 | −0.14 | −1.15 | −1.19 | −2.03 | −2.01 | −2.28 | −2.21 | −1.51 | −1.49 | |
| −0.14 | 0.00 | −1.49 | −1.18 | −1.11 | −1.02 | −1.63 | −1.67 | −0.67 | −0.61 | |
| 0.09 | 0.25 | −0.89 | −0.91 | −0.69 | −0.58 | −1.32 | −1.20 | −0.73 | −0.66 | |
| 0.17 | 0.32 | −0.60 | −0.58 | −0.29 | −0.16 | −0.81 | −0.74 | 0.29 | 0.35 | |
| 0.42 | 0.43 | 0.58 | 0.72 | 0.30 | 0.31 | −0.08 | −0.10 | 0.12 | 0.15 | |
| 0.43 | 0.47 | 0.19 | 0.13 | 0.14 | 0.19 | −0.20 | −0.23 | −0.19 | −0.21 | |
| 0.49 | 0.52 | −0.11 | −0.10 | −0.25 | −0.23 | −0.27 | −0.26 | −0.35 | −0.31 | |
| 1.44 | 1.44 | 1.64 | 1.72 | 0.98 | 1.00 | 1.10 | 1.11 | 1.48 | 1.50 | |
| 1.52 | 1.50 | 0.92 | 0.98 | −0.58 | −0.54 | −0.83 | −0.80 | −0.19 | −0.09 | |
| 2.86 | 2.80 | 2.60 | 2.77 | 2.16 | 2.24 | 2.06 | 2.12 | 2.17 | 2.22 | |
| 2.92 | 2.89 | 1.59 | 1.68 | 0.24 | 0.31 | −0.03 | 0.02 | 0.23 | 0.32 | |
| 3.63 | 3.62 | 1.10 | 1.04 | 0.66 | 0.77 | 0.18 | 0.25 | 0.20 | 0.24 | |
| 5.00 | 4.95 | 2.23 | 2.27 | 1.46 | 1.58 | 1.02 | 1.14 | 0.96 | 1.03 | |
| 5.64 | 5.58 | 2.26 | 2.32 | 1.27 | 1.40 | 1.11 | 1.26 | 1.20 | 1.28 | |
| Ion, j | SD of | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D2/50 | MC5D2/50 | MC9D2/50 | MC12D2/50 | ACD2/50 | ||||||
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| 0.01 | 0.04 | 0.09 | 0.07 | 0.09 | 0.08 | 0.06 | 0.15 | 0.03 | ||
| 0.01 | 0.05 | 0.05 | 0.03 | 0.03 | 0.03 | 0.12 | 0.02 | 0.02 | ||
| 0.03 | 0.02 | 0.03 | 0.04 | 0.02 | 0.05 | 0.02 | 0.07 | 0.02 | ||
| 0.06 | 0.10 | 0.18 | 0.03 | 0.05 | 0.02 | 0.07 | 0.03 | 0.02 | ||
| <0.01 | 0.07 | 0.07 | 0.01 | <0.01 | 0.02 | <0.01 | <0.01 | 0.01 | ||
| 0.01 | 0.08 | 0.01 | <0.01 | 0.04 | 0.04 | <0.01 | 0.04 | 0.01 | ||
| <0.01 | 0.03 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | ||
| 0.02 | 0.01 | 0.06 | 0.01 | 0.01 | <0.01 | <0.01 | 0.01 | <0.01 | ||
| 0.04 | 0.01 | 0.01 | 0.01 | 0.04 | 0.05 | <0.01 | 0.02 | 0.01 | ||
| 0.06 | 0.02 | 0.04 | 0.02 | 0.07 | 0.02 | 0.03 | 0.01 | 0.01 | ||
| 0.05 | 0.01 | 0.01 | 0.04 | 0.06 | 0.05 | 0.03 | 0.04 | 0.02 | ||
| 0.04 | 0.03 | 0.06 | 0.05 | 0.09 | 0.04 | 0.02 | 0.03 | 0.02 | ||
| 0.08 | <0.01 | 0.07 | 0.02 | 0.02 | 0.04 | 0.01 | <0.01 | 0.01 | ||
| 0.10 | 0.02 | 0.10 | 0.01 | 0.02 | 0.03 | 0.01 | <0.01 | <0.01 | ||
| Ion, j | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N2/50 | MC5N2/50 | MC9N2/50 | MC12N2/50 | ACN2/50 | ||||||
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| −0.84 | −0.61 | −0.82 | −1.24 | −2.14 | −2.23 | −2.35 | −2.42 | −1.86 | −1.97 | |
| −0.55 | −0.39 | −0.57 | −0.61 | −1.78 | −1.78 | −2.39 | −2.10 | −1.32 | −1.32 | |
| −0.36 | −0.14 | −0.73 | −1.13 | −1.32 | −1.24 | −2.17 | −1.88 | −1.42 | −1.38 | |
| −0.21 | −0.04 | −0.01 | 0.33 | −0.94 | −0.85 | −1.68 | −1.11 | −0.30 | −0.24 | |
| 0.18 | 0.28 | 0.71 | 0.60 | 0.22 | 0.23 | −0.06 | −0.02 | −0.33 | −0.27 | |
| 0.60 | 0.66 | 0.28 | 0.33 | 0.25 | 0.26 | −0.16 | −0.14 | −0.26 | −0.24 | |
| 0.64 | 0.70 | −0.18 | −0.15 | −0.31 | −0.30 | −0.33 | −0.32 | −0.48 | −0.41 | |
| 1.27 | 1.28 | 1.39 | 1.48 | 0.96 | 0.96 | 1.08 | 1.14 | 1.29 | 1.28 | |
| 1.71 | 1.69 | 1.04 | 1.14 | −0.80 | −0.83 | −1.03 | −1.04 | −0.40 | −0.46 | |
| 2.83 | 2.76 | 2.46 | 2.65 | 2.04 | 2.12 | 1.91 | 2.05 | 2.03 | 2.07 | |
| 3.25 | 3.30 | 1.79 | 1.91 | 0.09 | 0.14 | −0.17 | −0.00 | 0.01 | 0.03 | |
| 4.21 | 4.22 | 1.21 | 1.37 | 0.80 | 0.94 | 0.22 | 0.45 | 0.24 | 0.29 | |
| 5.61 | 5.57 | 2.49 | 2.64 | 1.48 | 1.68 | 1.04 | 1.44 | 0.84 | 0.89 | |
| 6.20 | 6.07 | 2.56 | 2.67 | 1.00 | 1.18 | 1.01 | 1.52 | 0.96 | 1.01 | |
| Ion, j | SD of | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N2/50 | MC5N2/50 | MC9N2/50 | MC12N2/50 | ACN2/50 | ||||||
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| <0.01 | 0.01 | 0.33 | <0.01 | 0.01 | 0.02 | <0.01 | 0.04 | 0.05 | 0.02 | |
| 0.02 | 0.02 | 0.22 | 0.05 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | |
| 0.04 | 0.02 | 0.09 | 0.06 | 0.02 | 0.03 | 0.02 | <0.01 | 0.03 | 0.01 | |
| 0.04 | 0.03 | 0.01 | 0.04 | 0.02 | 0.05 | 0.04 | <0.01 | <0.01 | 0.02 | |
| 0.06 | 0.03 | 0.03 | 0.04 | 0.04 | 0.02 | 0.04 | 0.01 | 0.01 | 0.03 | |
| 0.05 | 0.02 | 0.01 | 0.04 | 0.04 | 0.02 | 0.04 | 0.02 | <0.01 | 0.04 | |
| 0.06 | 0.01 | 0.03 | 0.01 | <0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.14 | |
| 0.02 | 0.06 | <0.01 | <0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | |
| 0.05 | 0.05 | 0.01 | <0.01 | <0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | |
| 0.03 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | |
| <0.01 | 0.01 | <0.01 | 0.03 | 0.01 | 0.02 | 0.03 | <0.01 | 0.03 | <0.01 | |
| 0.03 | 0.01 | 0.02 | 0.04 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | <0.01 | |
| 0.06 | 0.02 | 0.06 | 0.04 | 0.03 | 0.01 | 0.02 | 0.01 | 0.03 | 0.02 | |
| 0.08 | 0.03 | 0.02 | 0.05 | 0.04 | 0.01 | 0.01 | 0.04 | 0.01 | 0.02 | |
| Ion, j | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N4/50 | MC9N4/50 | N1/50 | MC9N1/50 | N1/25 | MC9N1/25 | |||||||
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| −0.44 | −0.44 | −2.22 | −2.29 | −1.21 | −1.09 | −2.33 | −2.37 | −0.56 | −1.16 | −2.05 | −2.26 | |
| −0.19 | −0.22 | −1.47 | −1.46 | −1.20 | −0.89 | −2.11 | −2.03 | −1.06 | −1.61 | −2.29 | −2.27 | |
| 0.11 | 0.12 | −0.97 | −0.88 | −0.94 | −0.65 | −1.68 | −1.55 | −1.52 | −1.25 | −1.95 | −1.72 | |
| 0.15 | 0.17 | −0.55 | −0.46 | −0.78 | −0.56 | −1.29 | −1.12 | −1.29 | −1.04 | −1.64 | −1.36 | |
| 0.43 | 0.44 | 0.28 | 0.38 | 0.20 | 0.08 | 0.17 | 0.18 | 0.52 | 0.15 | 0.31 | 0.19 | |
| 0.71 | 0.71 | 0.25 | 0.36 | 0.62 | 0.66 | 0.17 | 0.23 | 0.51 | 0.66 | 0.15 | 0.24 | |
| 0.73 | 0.77 | −0.28 | −0.18 | 0.69 | 0.74 | −0.29 | −0.30 | 0.52 | 0.71 | −0.31 | −0.31 | |
| 1.33 | 1.35 | 0.94 | 0.97 | 1.31 | 1.33 | 1.01 | 0.99 | 1.15 | 1.24 | 0.97 | 0.97 | |
| 1.75 | 1.78 | −0.79 | −0.80 | 1.72 | 1.78 | −0.85 | −0.85 | 1.52 | 1.74 | −0.94 | −0.86 | |
| 2.86 | 2.89 | 2.11 | 2.18 | 2.81 | 2.81 | 1.99 | 2.13 | 2.68 | 2.77 | 1.80 | 2.12 | |
| 3.21 | 3.29 | 0.18 | 0.22 | 3.22 | 3.33 | −0.02 | 0.12 | 3.17 | 3.35 | −0.22 | 0.12 | |
| 4.10 | 4.17 | 0.89 | 0.98 | 4.15 | 4.23 | 0.65 | 0.89 | 4.19 | 4.33 | 0.43 | 0.91 | |
| 5.42 | 5.52 | 1.61 | 1.70 | 5.51 | 5.54 | 1.32 | 1.61 | 5.65 | 5.71 | 1.11 | 1.62 | |
| 5.99 | 6.05 | 1.17 | 1.24 | 6.11 | 6.07 | 0.88 | 1.12 | 6.27 | 6.27 | 0.64 | 1.10 | |
| Ion, j | SD of | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N4/50 | MC9N4/50 | N1/50 | MC9N1/50 | N1/25 | MC9N1/25 | |||||||
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.10 | 0.16 | 0.07 | 0.02 | |
| 0.03 | 0.03 | 0.01 | 0.01 | 0.03 | <0.01 | 0.02 | 0.01 | 0.10 | 0.07 | 0.01 | 0.02 | |
| 0.04 | 0.03 | 0.01 | 0.02 | 0.07 | 0.02 | 0.01 | 0.02 | 0.10 | 0.06 | 0.03 | 0.01 | |
| 0.05 | 0.04 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | 0.02 | 0.04 | 0.06 | 0.04 | 0.02 | |
| 0.04 | 0.01 | 0.01 | 0.02 | 0.14 | 0.01 | 0.01 | 0.01 | 0.27 | 0.26 | 0.08 | 0.03 | |
| 0.03 | 0.02 | 0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | 0.02 | 0.03 | 0.04 | <0.01 | |
| 0.04 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.04 | 0.04 | 0.01 | |
| 0.03 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | <0.01 | 0.04 | 0.01 | 0.05 | 0.01 | |
| 0.03 | 0.04 | 0.03 | 0.03 | 0.01 | 0.02 | 0.03 | 0.01 | 0.05 | 0.04 | 0.03 | 0.01 | |
| 0.03 | 0.03 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.09 | 0.02 | 0.04 | 0.01 | |
| 0.03 | 0.04 | 0.01 | 0.02 | 0.01 | 0.01 | <0.01 | 0.01 | 0.12 | 0.02 | 0.05 | 0.01 | |
| 0.02 | 0.05 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.14 | 0.03 | 0.05 | 0.01 | |
| 0.02 | 0.04 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.17 | 0.03 | 0.05 | 0.01 | |
| 0.02 | 0.05 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.18 | 0.03 | 0.05 | 0.01 | |
References
- Scholz, F. From the Leiden jar to the discovery of the glass electrode by Max Cremer. J. Solid State Electrochem. 2011, 15, 5–14. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.a.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Muñoz, R.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Haber, F.; Klemensiewicz, Z. Über elektrische Phasengrenzkräfte. Z. Phys. Chem. 1909, 67U, 385–431. [Google Scholar] [CrossRef]
- Eisenman, G.; Rudin, D.O.; Casby, J.U. Glass electrode for measuring sodium ion. Science 1957, 126, 831–834. [Google Scholar] [CrossRef]
- Frant, M.S.; Ross, J.W. Electrode for sensing fluoride ion activity in solution. Science 1966, 154, 1553–1555. [Google Scholar] [CrossRef]
- Ross, J.W. Calcium-selective electrode with liquid ion exchanger. Science 1967, 156, 1378–1379. [Google Scholar] [CrossRef]
- Bloch, R.; Shatkay, A.; Saroff, H. Fabrication and evaluation of membranes as specific electrodes for calcium ions. Biophys. J. 1967, 7, 865–877. [Google Scholar] [CrossRef]
- Pechenkina, I.; Mikhelson, K.N. Materials for the ionophore-based membranes for ion-selective electrodes: Problems and achievements. Russ. J. Electrochem. 2015, 51, 93–102. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (technical report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Umezawa, Y.; Umezawa, K.; Bühlmann, P.; Hamada, N.; Aoki, H.; Nakanishi, J.; Sato, M.; Xiao, K.P.; Nishimura, Y. Potentiometric selectivity coefficients of ion-selective electrodes. Part II. Inorganic anions (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 923–994. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Hamada, N. Potentiometric selectivity coefficients of ion-selective electrodes. Part III. Organic ions (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 995–1099. [Google Scholar] [CrossRef]
- Gale, P.A. Anion receptor chemistry: Highlights from 1999. Coord. Chem. Rev. 2001, 213, 79–128. [Google Scholar] [CrossRef]
- Gale, P.A. Anion and ion-pair receptor chemistry: Highlights from 2000 and 2001. Coord. Chem. Rev. 2003, 240, 191–221. [Google Scholar] [CrossRef]
- Gale, P.A.; Quesada, R. Anion coordination and anion-templated assembly: Highlights from 2002 to 2004. Coord. Chem. Rev. 2006, 250, 3219–3244. [Google Scholar] [CrossRef]
- Gale, P.A.; García-Garrido, S.E.; Garric, J. Anion receptors based on organic frameworks: Highlights from 2005 and 2006. Chem. Soc. Rev. 2008, 37, 151–190. [Google Scholar] [CrossRef]
- Caltagirone, C.; Gale, P.A. Anion receptor chemistry: Highlights from 2007. Chem. Soc. Rev. 2009, 38, 520–563. [Google Scholar] [CrossRef]
- Gale, P.A. Anion receptor chemistry: Highlights from 2008 and 2009. Chem. Soc. Rev. 2010, 39, 3746–3771. [Google Scholar] [CrossRef]
- Wenzel, M.; Hiscock, J.R.; Gale, P.A. Anion receptor chemistry: Highlights from 2010. Chem. Soc. Rev. 2012, 41, 480–520. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Busschaert, N.; Haynes, C.J.; Karagiannidis, L.E.; Kirby, I.L. Anion receptor chemistry: Highlights from 2011 and 2012. Chem. Soc. Rev. 2014, 43, 205–241. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.A.; Martin, K.; Haav, K.; Toom, L.; Mayeux, C.; Pung, A.; Gale, P.A.; Hiscock, J.R.; Brooks, S.J.; Kirby, I.L.; et al. Towards the Discrimination of Carboxylates by Hydrogen-Bond Donor Anion Receptors. Chem.-Eur. J. 2015, 21, 5145–5160. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Howe, E.N.; Wu, X. Anion receptor chemistry. Chem 2016, 1, 351–422. [Google Scholar] [CrossRef]
- Gale, P.A.; Howe, E.N.; Wu, X.; Spooner, M.J. Anion receptor chemistry: Highlights from 2016. Coord. Chem. Rev. 2018, 375, 333–372. [Google Scholar] [CrossRef]
- Chen, L.; Berry, S.N.; Wu, X.; Howe, E.N.; Gale, P.A. Advances in Anion Receptor Chemistry. Chem 2020, 6, 61–141. [Google Scholar] [CrossRef]
- Kadam, S.A.; Haav, K.; Toom, L.; Pung, A.; Mayeux, C.; Leito, I. Multidentate Anion Receptors for Binding Glyphosate Dianion: Structure and Affinity. Eur. J. Org. Chem. 2017, 2017, 1396–1406. [Google Scholar] [CrossRef]
- Martin, K.; Nõges, J.; Haav, K.; Kadam, S.A.; Pung, A.; Leito, I. Exploring selectivity of 22 acyclic urea-, carbazole- and indolocarbazole-based receptors towards 11 monocarboxylates. Eur. J. Org. Chem. 2017, 2017, 5231–5237. [Google Scholar] [CrossRef]
- Tshepelevitsh, S.; Kadam, S.A.; Darnell, A.; Bobacka, J.; Rüütel, A.; Haljasorg, T.; Leito, I. LogP Determination for Highly Lipophilic Hydrogen Bonding Anion Receptor Molecules. Anal. Chim. Acta 2020, 1132, 123–133. [Google Scholar] [CrossRef]
- Rüütel, A.; Yrjänä, V.; Kadam, S.A.; Saar, I.; Ilisson, M.; Darnell, A.; Haav, K.; Haljasorg, T.; Toom, L.; Bobacka, J.; et al. Design, synthesis and application of carbazole macrocycles in anion sensors. Beilstein J. Org. Chem. 2020, 16, 1901–1914. [Google Scholar] [CrossRef]
- Martin, K.; Kadam, S.A.; Mattinen, U.; Bobacka, J.; Leito, I. Solid-contact Acetate-selective Electrode Based on a 1,3-bis(carbazolyl)urea-ionophore. Electroanalysis 2019, 31, 1061–1066. [Google Scholar] [CrossRef]
- MacInnes, D.A. The Principles of Electrochemistry; Dover: Mineola, NY, USA, 1961. [Google Scholar]
- Dahlgren, B. ChemPy: A package useful for chemistry written in Python. J. Open Source Softw. 2018, 3, 565. [Google Scholar] [CrossRef]
- Debye, P.; Hückel, E. The theory of electrolytes. I. Freezing point depression and related phenomena [Zur Theorie der Elektrolyte. I. Gefrierpunktserniedrigung und verwandte Erscheinungen]. Phys. Z. 1923, 24, 185–206, Translated and typeset by Michael J. Braus (2020). [Google Scholar]
- Koch, S.; Graves, C.; Vels Hansen, K.; DTU Energy. Elchemea Analytical. Available online: https://www.elchemea.com/ (accessed on 9 November 2020).
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Plasticizer-free all-solid-state potassium-selective electrode based on poly (3-octylthiophene) and valinomycin. Anal. Chim. Acta 1999, 385, 195–202. [Google Scholar] [CrossRef]
- Amemiya, S.; Bühlmann, P.; Umezawa, Y.; Jagessar, R.C.; Burns, D.H. An ion-selective electrode for acetate based on a urea-functionalized Porphyrin as a Hydrogen-Bonding Ionophore. Anal. Chem. 1999, 71, 1049–1054. [Google Scholar] [CrossRef]
- Dulic, N.; Horváth, L.; Horvai, G.; Tóth, K.; Pungor, E. Dielectric behavior of PVC membranes plasticized with dioctyl sebacate or o-nitrophenyl-octyl ether. Electroanalysis 1990, 2, 533–537. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, H.; Wang, J. Electrochemical Sensors, Biosensors and Their Biomedical Applications; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Mikhelson, K.N. Ion-Selective Electrodes; Springer: Berlin/Heidelberg, Germany, 2013; Volume 81. [Google Scholar]
- Szigeti, Z.; Vigassy, T.; Bakker, E.; Pretsch, E. Approaches to improving the lower detection limit of polymeric membrane ion-selective electrodes. Electroanalysis 2006, 18, 1254–1265. [Google Scholar] [CrossRef]
- Lindfors, T.; Sundfors, F.; Höfler, L.; Gyurcsányi, R.E. FTIR-ATR Study of Water Uptake and Diffusion Through Ion-Selective Membranes Based on Plasticized Poly(vinyl chloride). Electroanalysis 2009, 21, 1914–1922. [Google Scholar] [CrossRef]
- Hambly, B.; Guzinski, M.; Pendley, B.; Lindner, E. Evaluation, Pitfalls and Recommendations for the “Water Layer Test” for Solid Contact Ion-selective Electrodes. Electroanalysis 2020, 32, 781–791. [Google Scholar] [CrossRef]
- Janata, J. Principles of Chemical Sensors, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Horvai, G.; Graf, E.; Toth, K.; Pungor, E.; Buck, R.P. Plasticized poly (vinyl chloride) properties and characteristics of valinomycin electrodes. 1. High-frequency resistances and dielectric properties. Anal. Chem. 1986, 58, 2735–2740. [Google Scholar] [CrossRef]
- Antonisse, M.M.G.; Snellink-Ruël, B.H.M.; Ion, A.C.; Engbersen, J.F.J.; Reinhoudt, D.N. Synthesis of novel uranyl salophene derivatives and evaluation as sensing molecules in chemically modified field effect transistors (CHEMFETs). J. Chem. Soc. Perkin Trans. 2 1999, 6, 1211–1218. [Google Scholar] [CrossRef]
- Lee, H.K.; Song, K.; Seo, H.R.; Jeon, S. Polymeric Acetate-Selective Electrodes Based on meso-(α, α, α, α)-Tetrakis-[(2-arylphenylurea) phenyl] porphyrins: Electormic and pH Effects. Bull. Korean Chem. Soc. 2002, 23, 1409–1412. [Google Scholar]
- Heering, A.; Stoica, D.; Camões, F.; Anes, B.; Nagy, D.; Nagyné Szilágyi, Z.; Quendera, R.; Ribeiro, L.; Bastkowski, F.; Born, R.; et al. Symmetric Potentiometric Cells for the Measurement of Unified pH Values. Symmetry 2020, 12, 1150. [Google Scholar] [CrossRef]
- Górski, Ł.; Mroczkiewicz, M.; Pietrzak, M.; Malinowska, E. Metalloporphyrin-based acetate-selective electrodes as detectors for enzymatic acetylcholine determination in flow-injection analysis system. Anal. Chim. Acta 2009, 644, 30–35. [Google Scholar] [CrossRef]
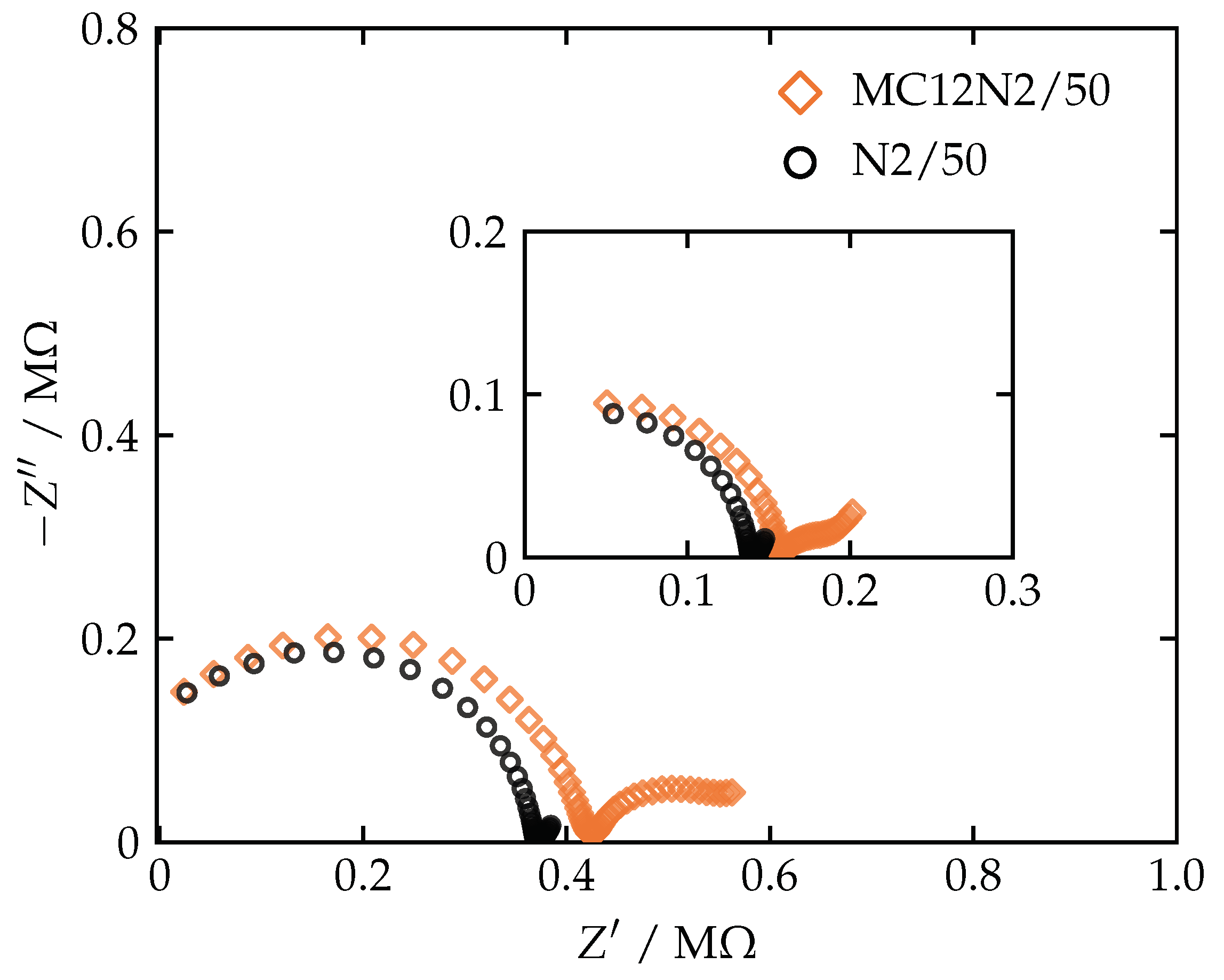
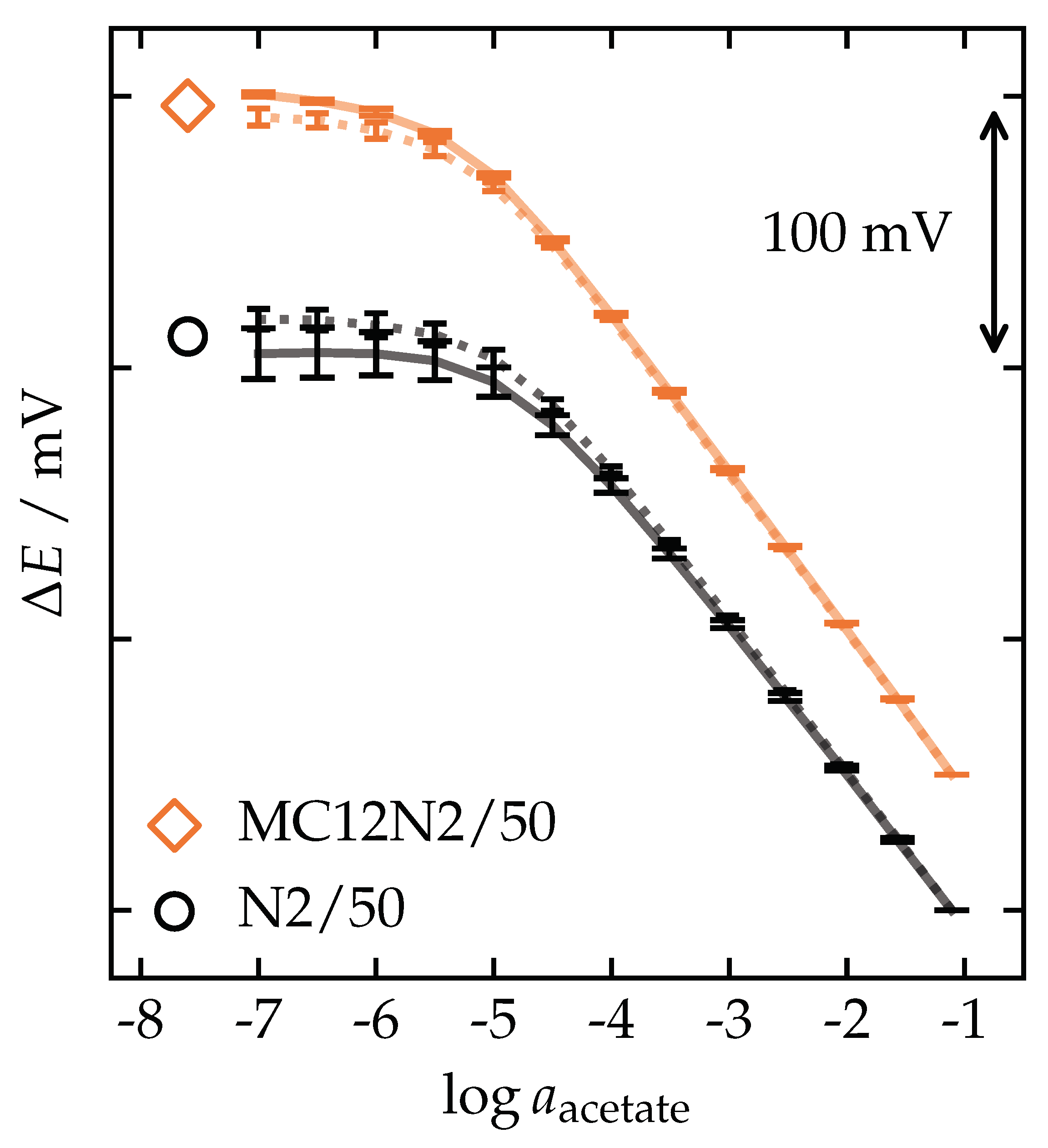

| Membrane | (Total Mass) | ||||||
|---|---|---|---|---|---|---|---|
| Ionophore | TDMACl | PVC | DOS | o-NPOE | THF | Anion Exchanger: Ionophore | |
| N1/25 | 0.03 | 5.67 | 11.31 | 82.99 | |||
| N1/50 | 0.06 | 5.66 | 11.24 | 83.04 | |||
| N2/50 | 0.11 | 5.48 | 11.39 | 83.02 | |||
| D2/50 | 0.12 | 5.61 | 11.27 | 83.00 | |||
| N4/50 | 0.23 | 5.59 | 11.19 | 82.98 | |||
| MC5N2/50 | 0.34 | 0.13 | 5.48 | 11.10 | 82.94 | 52.24 | |
| MC5D2/50 | 0.34 | 0.12 | 5.42 | 11.13 | 82.99 | 49.26 | |
| MC9N1/25 | 0.17 | 0.03 | 5.60 | 11.26 | 82.93 | 27.72 | |
| MC9N1/50 | 0.17 | 0.06 | 5.58 | 11.22 | 82.97 | 49.57 | |
| MC9N2/50 | 0.34 | 0.12 | 5.42 | 11.20 | 82.93 | 49.31 | |
| MC9D2/50 | 0.34 | 0.12 | 5.48 | 11.07 | 82.98 | 52.16 | |
| MC9N4/50 | 0.68 | 0.23 | 5.51 | 10.85 | 82.72 | 50.05 | |
| MC12N2/50 | 0.33 | 0.12 | 5.34 | 11.26 | 82.95 | 53.53 | |
| MC12D2/50 | 0.33 | 0.11 | 5.33 | 11.23 | 83.00 | 49.66 | |
| ACN2/50 | 0.34 | 0.10 | 5.56 | 11.04 | 82.96 | 49.62 | |
| ACD2/50 | 0.34 | 0.10 | 5.45 | 11.12 | 83.00 | 49.62 | |
| Membrane | Slope (mV/dec) | |||||
|---|---|---|---|---|---|---|
| PVC | PCTFE | PVC | PCTFE | PVC | PCTFE | |
| N2/50 | −54.23 | −56.13 | −4.00 | −4.00 | −4.87 | −4.97 |
| MC12N2/50 | −58.55 | −58.01 | −4.00 | −4.00 | −5.37 | −5.27 |
| Ion, j | ||||
|---|---|---|---|---|
| N2/50 | MC12N2/50 | |||
| PVC | PCTFE | PVC | PCTFE | |
| −0.84 | −0.61 | −2.35 | −2.42 | |
| −0.55 | −0.39 | −2.39 | −2.10 | |
| −0.36 | −0.14 | −2.17 | −1.88 | |
| −0.21 | −0.04 | −1.68 | −1.11 | |
| 0.18 | 0.28 | −0.06 | −0.02 | |
| 0.60 | 0.66 | −0.16 | −0.14 | |
| 0.64 | 0.70 | −0.33 | −0.32 | |
| 1.27 | 1.28 | 1.08 | 1.14 | |
| 1.71 | 1.69 | −1.03 | −1.04 | |
| 2.83 | 2.76 | 1.91 | 2.05 | |
| 3.25 | 3.30 | −0.17 | −0.00 | |
| 4.21 | 4.22 | 0.22 | 0.45 | |
| 5.61 | 5.57 | 1.04 | 1.44 | |
| 6.20 | 6.07 | 1.01 | 1.52 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yrjänä, V.; Saar, I.; Ilisson, M.; Kadam, S.A.; Leito, I.; Bobacka, J. Potentiometric Carboxylate Sensors Based on Carbazole-Derived Acyclic and Macrocyclic Ionophores. Chemosensors 2021, 9, 4. https://doi.org/10.3390/chemosensors9010004
Yrjänä V, Saar I, Ilisson M, Kadam SA, Leito I, Bobacka J. Potentiometric Carboxylate Sensors Based on Carbazole-Derived Acyclic and Macrocyclic Ionophores. Chemosensors. 2021; 9(1):4. https://doi.org/10.3390/chemosensors9010004
Chicago/Turabian StyleYrjänä, Ville, Indrek Saar, Mihkel Ilisson, Sandip A. Kadam, Ivo Leito, and Johan Bobacka. 2021. "Potentiometric Carboxylate Sensors Based on Carbazole-Derived Acyclic and Macrocyclic Ionophores" Chemosensors 9, no. 1: 4. https://doi.org/10.3390/chemosensors9010004
APA StyleYrjänä, V., Saar, I., Ilisson, M., Kadam, S. A., Leito, I., & Bobacka, J. (2021). Potentiometric Carboxylate Sensors Based on Carbazole-Derived Acyclic and Macrocyclic Ionophores. Chemosensors, 9(1), 4. https://doi.org/10.3390/chemosensors9010004






