Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model undergoing Open-Heart Surgery with Cardiopulmonary Bypass
Abstract
1. Background
2. Materials and Methods
2.1. Materials
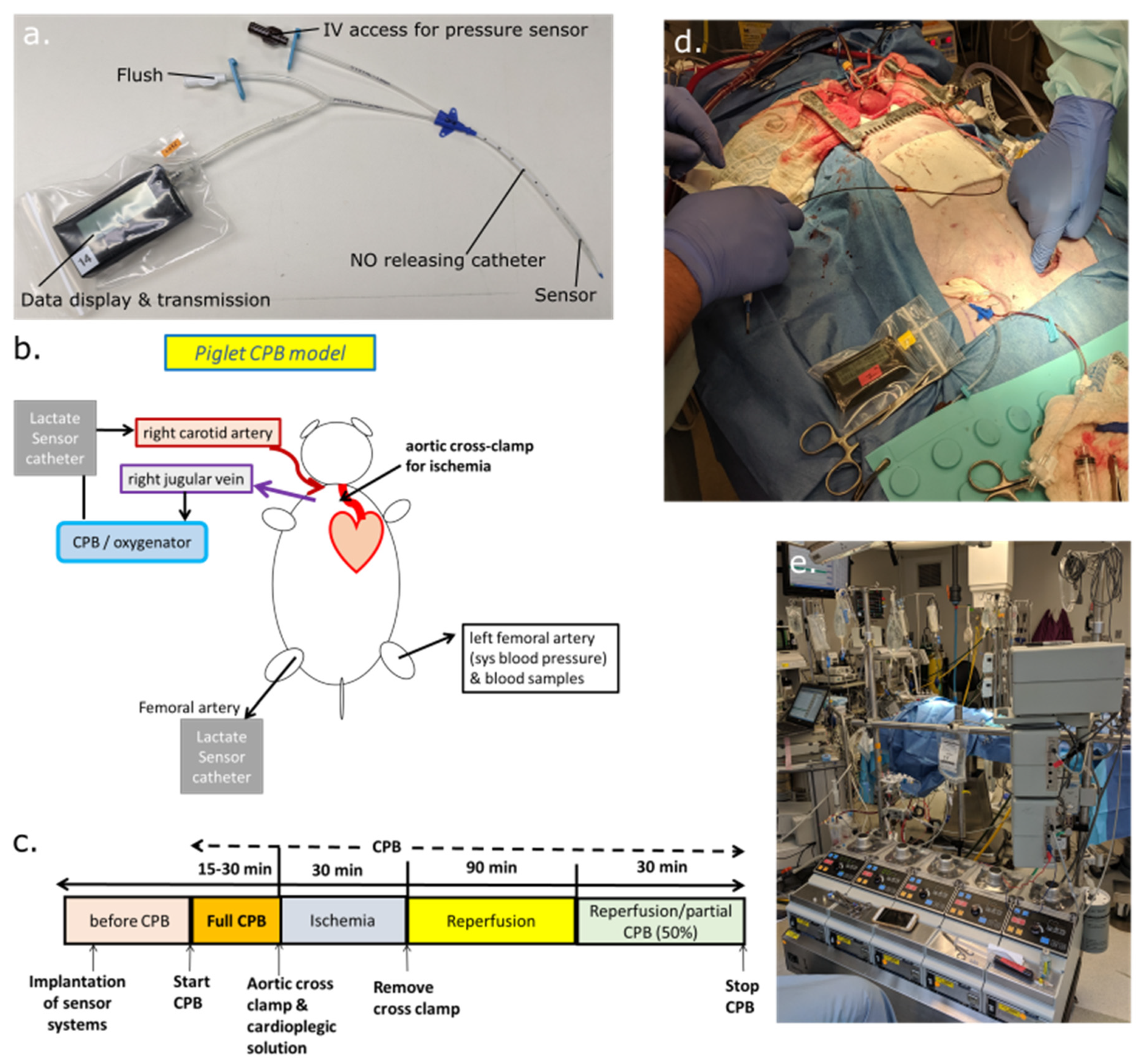
2.2. Sensor Fabrication and Catheter Assembly
2.3. Animal Preparation for Cardiopulmonary Bypass, Induced Cardioplegic Ischemia, and Reperfusion
3. Results
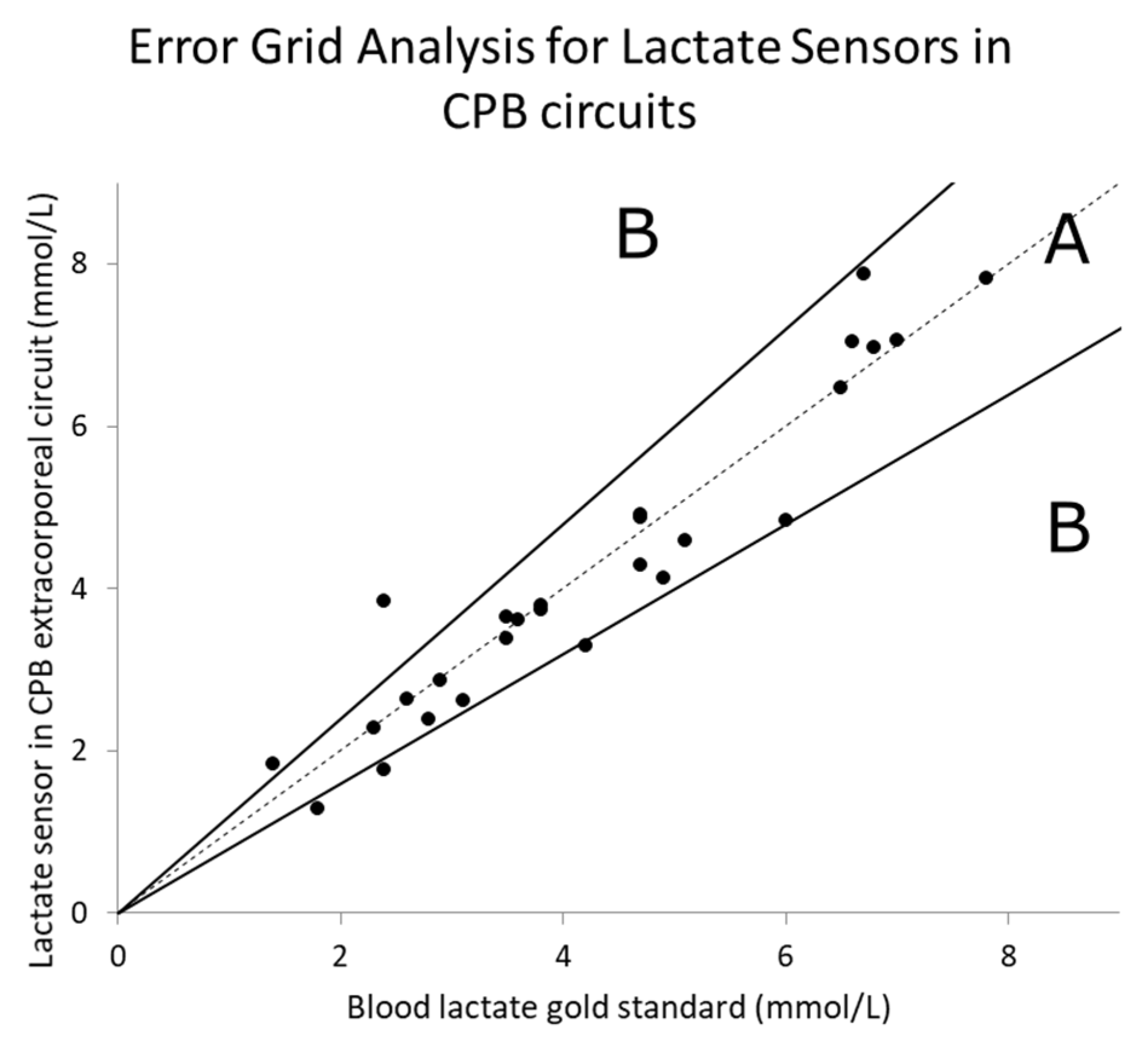
3.1. Lactate Measurement Results
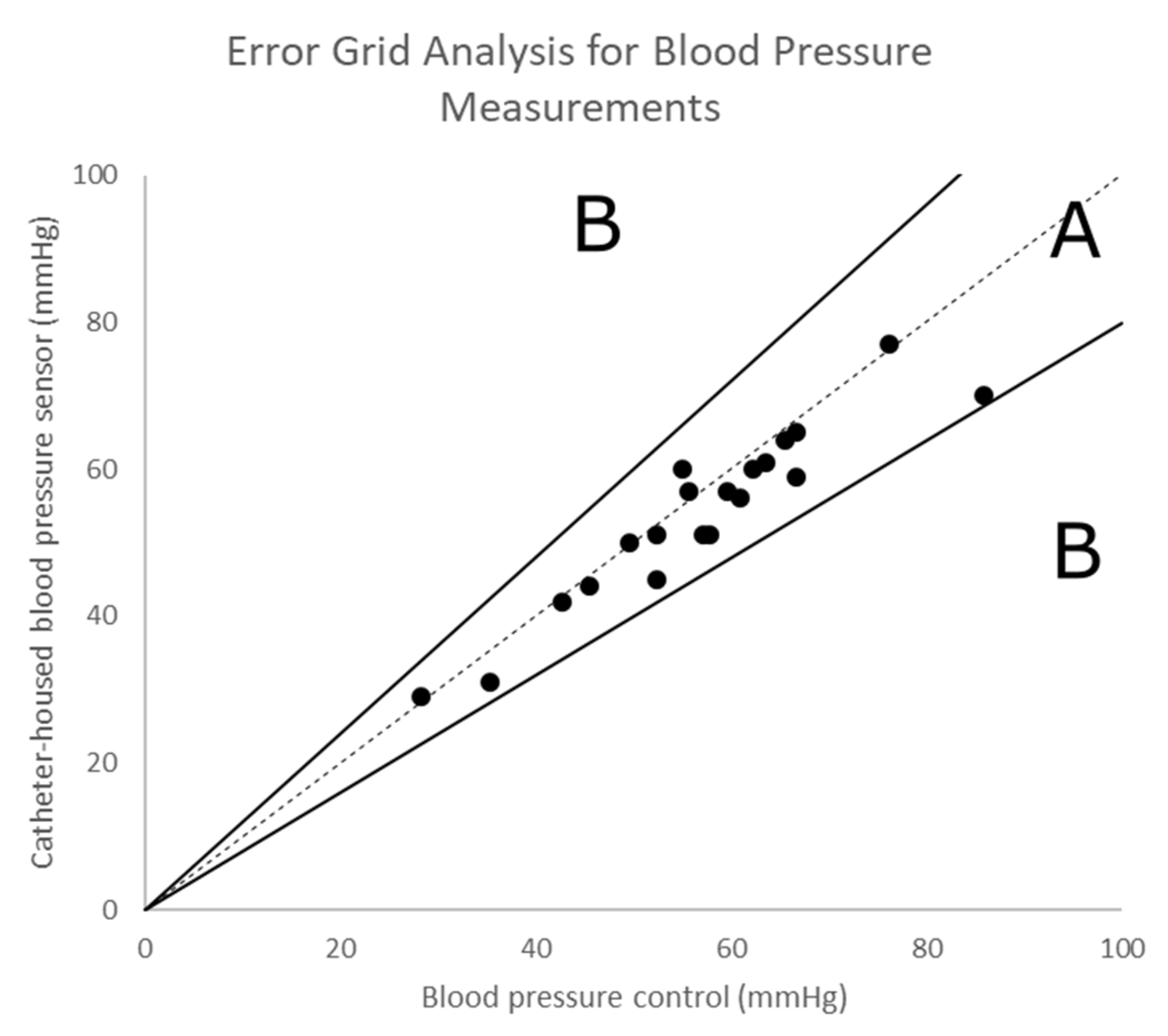
3.2. Continuous Monitoring of the Blood Pressure
4. Discussion
4.1. Accuracy and Performance of Lactate Sensors
4.2. Lower Body Hypoperfusion during CPB Interferes with Lactate Sensing
4.3. Recommendations for Future Uses and Potential Risks
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schumacher, K.R.; Reichel, R.A.; Vlasic, J.R.; Yu, S.; Donohue, J.; Gajarski, R.J.; Charpie, J.R. Rate of increase in serum lactate level risk-stratifies infants after surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Charpie, J.R.; Dekeon, M.K.; Goldberg, C.S.; Mosca, R.S.; Bove, E.L.; Kulik, T.J. Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. J. Thorac. Cardiovasc. Surg. 2000, 120, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J. The global burden of congenital heart disease. Cardiovasc J. Afr. 2013, 24, 141–145. [Google Scholar] [CrossRef]
- Jansen, T.C.; van Bommel, J.; Schoonderbeek, F.J.; Sleeswijk Visser, S.J.; van der Klooster, J.M.; Lima, A.P.; Willemsen, S.P.; Bakker, J. Early lactate-guided therapy in intensive care unit patients: A multicenter, open-label, randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, H.; Shahpori, R.; Stelfox, H.T.; Kirkpatrick, A.W.; Laupland, K.B. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit. Care. 2009, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vorwerk, C.; Loryman, B.; Coats, T.J.; Stephenson, J.A.; Gray, L.D.; Reddy, G.; Florence, L.; Butler, N. Prediction of mortality in adult emergency department patients with sepsis. Emerg. Med. J. 2009, 26, 254–258. [Google Scholar] [CrossRef] [PubMed]
- van Beest, P.; Kuiper, M.; Spronk, P.E. Lactate: An unusually sensitive parameter of ensuing organ failure? Crit. Care Med. 2010, 38, 337. [Google Scholar] [CrossRef]
- Manikis, P.; Jankowski, S.; Zhang, H.; Kahn, R.J.; Vincent, J.L. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am. J. Emerg. Med. 1995, 13, 619–622. [Google Scholar] [CrossRef]
- Roumen, R.M.; Redl, H.; Schlag, G.; Sandtner, W.; Koller, W.; Goris, R.J. Scoring systems and blood lactate concentrations in relation to the development of adult respiratory distress syndrome and multiple organ failure in severely traumatized patients. J. Trauma 1993, 35, 349–355. [Google Scholar] [CrossRef]
- Asgar, H.; Rishu, R.K.; Al-Dorzi, H.M.; Tamim, H.M.; Al-Qahtani, S.; Al-Ghamdi, G.; Arabi, Y.M. Even mild hyperlactatemia is associated with increased mortality in critically Ill patients. Crit. Care 2013, 17, R197. [Google Scholar]
- Abramson, D.; Scalea, T.M.; Hitchcock, R.; Trooskin, S.Z.; Henry, S.M.; Greenspan, J. Lactate clearance and survival following injury. J. Trauma Acute Care Surg. 1993, 35, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Gris, P.; Coffernils, M.; Kahn, R.J.; Vincent, J.L. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am. J. Surg. 1996, 171, 221–226. [Google Scholar] [CrossRef]
- Blow, O.; Magliore, L.; Claridge, J.A.; Butler, K.; Young, J.S. The golden hour and the silver day: Detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J. Trauma Acute Care Surg. 1999, 47, 964. [Google Scholar] [CrossRef] [PubMed]
- Kliegel, A.; Losert, H.; Sterz, F.; Holzer, M.; Zeiner, A.; Havel, C.; Laggner, A.N. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine 2004, 83, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; Dellinger, R.P.; Chansky, M.E.; Arnold, R.C.; Schorr, C.; Milcarek, B.; Hollenberg, S.M.; Parrillo, J.E. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007, 33, 970–977. [Google Scholar] [CrossRef]
- Jansen, T.C.; van Bommel, J.; Woodward, R.; Mulder, P.G.; Bakker, J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: A retrospective observational study *. Crit. Care Med. 2009, 37, 2369–2374. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef]
- Rusconi, A.M.; Bossi, I.; Lampard, J.G.; Szava-Kovats, M.; Bellone, A.; Lang, E. Early goal-directed therapy vs. usual care in the treatment of severe sepsis and septic shock: A systematic review and meta-analysis. Intern. Emerg. Med. 2015, 10, 731–743. [Google Scholar] [CrossRef]
- Rady, M.Y.; Rivers, E.P.; Nowak, R.M. Resuscitation of the critically III in the ED: Responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am. J. Emerg. Med. 1996, 14, 218–225. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Rhodes, A.; Perel, A.; Martin, G.S.; Della Rocca, G.; Vallet, B.; Pinsky, M.R.; Hofer, C.K.; Teboul, J.L.; de Boode, W.P.; et al. Clinical review: Update on hemodynamic monitoring—A consensus of 16. Crit. Care 2011, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Baram, M.; Vahid, B. Does central venous pressure predict fluid responsiveness?*: A Systematic review of the literature and the tale of seven mares. CHEST 2008, 134, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Coffernils, M.; Leon, M.; Gris, P.; Vincent, J.L. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. CHEST 1991, 99, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Donnino, M.; Clardy, P.; Talmor, D.; Shapiro, N.I. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007, 33, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.C.; van Bommel, J.; Mulder, P.G.; Rommes, J.H.; Schieveld, S.J.; Bakker, J. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: A pilot study. Crit. Care 2008, 12, R160. [Google Scholar] [CrossRef]
- Pölönen, P.; Ruokonen, E.; Hippeläinen, M.; Pöyhönen, M.; Takala, J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth. Analg. 2000, 90, 1052–1059. [Google Scholar] [CrossRef]
- Jones, A.E.; Shapiro, N.I.; Trzeciak, S.; Arnold, R.C.; Claremont, H.A.; Kline, J.A.; for the Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs. central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA 2010, 303, 739–746. [Google Scholar] [CrossRef]
- Jones, A.E. Point: Should lactate clearance be substituted for central venous oxygen saturation as goals of early severe sepsis and septic shock therapy? Yes. CHEST 2011, 140, 1406–1408. [Google Scholar] [CrossRef][Green Version]
- Bakker, J.; Nijsten, M.W.N.; Jansen, T.C. Clinical use of lactate monitoring in critically ill patients. Ann. Intensive Care 2013, 3, 12. [Google Scholar] [CrossRef]
- Frost, M.C.; Wolf, A.K.; Meyerhoff, M.E. In vivo sensors for continuous monitoring of blood gases, glucose and lactate: Biocompatibility challenges and potential solutions. In Detection Challenges in Clinical Diagnosis; Vadgama, P., Peteu, S., Eds.; RSC Publishing: London, UK, 2013. [Google Scholar]
- Gifford, R. Continuous glucose monitoring: 40 years, what we've learned and what’s next. ChemPhysChem 2013, 14, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.C.; Meyerhoff, M.E. Indwelling chemical sensors for real-time clinical monitoring: Progress and challenges. Curr. Opin. Chem. Biol. 2002, 6, 633–641. [Google Scholar] [CrossRef]
- Frost, M.; Meyerhoff, M.E. In vivo chemical sensors: Tackling biocompatibility. Anal. Chem. 2006, 78, 7370–7377. [Google Scholar] [CrossRef]
- Ganter, M.; Zollinger, A. Continuous intravascular blood gas monitoring: Development, current techniques, and clinical use of a commercial device. Br. J. Anaesth. 2003, 91, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Coule, L.W.; Truemper, E.J.; Steinhart, C.M.; Lutin, W.A. Accuracy and utility of a continuous intra-arterial blood gas monitoring system in pediatric patients. Crit. Care Med. 2001, 29, 420–426. [Google Scholar] [CrossRef]
- Shapiro, B.A. In-vivo monitoring of arterial blood gases and pH. Resp. Care 1992, 37, 165–169. [Google Scholar]
- Wahr, J.A.; Tremper, K.K. Continuous intravascular blood gas monitoring. J. Cardiothorac. Vasc. Anesth. 1994, 8, 342–353. [Google Scholar] [CrossRef]
- Meyerhoff, M.E. In vivo blood-gas and electrolyte sensors: Progress and challenges. Anal. Chem. 1993, 12, 257–266. [Google Scholar] [CrossRef]
- Mahutte, C.K.; Sassoon, C.S.; Muro, J.R.; Hansmann, D.R.; Maxwell, T.P.; Miller, W.W.; Yafuso, M. Progress in the development of a fluorescent intravascular blood gas system in man. J. Clin. Monit. 1990, 6, 147–157. [Google Scholar] [CrossRef]
- Bindra, D.S.; Zhang, Y.N.; Wilson, G.S.; Sternberg, R.; Thevenot, D.R.; Moatti, D.; Reach, G. Design and invitro studies of a needle-type glucose sensor for subcutaneous monitoring. Anal. Chem. 1991, 63, 1692–1696. [Google Scholar] [CrossRef]
- Ward, W.K.; Jansen, L.B.; Anderson, E.; Reach, G.; Klein, J.C.; Wilson, G.S. A new amperometric glucose microsensor: In vitro and short-term in vivo evaluation. Biosens. Bioelectron. 2002, 17, 181–189. [Google Scholar] [CrossRef]
- Wilson, G.S.; Gifford, R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 2005, 20, 2388–2403. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Y.; Wilson, G.S. A needle-type enzyme-based lactate sensor for in vivo monitoring. Anal. Chim. Acta 1993, 281, 503–511. [Google Scholar] [CrossRef]
- Baker, D.A.; Gough, D.A. A continuous, implantable lactate sensor. Anal. Chem. 1995, 67, 1536–1540. [Google Scholar] [CrossRef]
- Frost, M.C.; Rudich, S.M.; Zhang, H.; Maraschio, M.A.; Meyerhoff, M.E. In vivo biocompatibility and analytical performance of intravascular amperometric oxygen sensors prepared with improved nitric oxide-releasing silicone rubber coating. Anal. Chem. 2002, 74, 5942–5947. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.C.; Reynolds, M.M.; Meyerhoff, M.E. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials 2005, 26, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Major, T.C.; Bartlett, R.H.; Meyerhoff, M.E. Intravascular glucose/lactate sensors prepared with nitric oxide releasing poly(lactide-co-glycolide)-based coatings for enhanced biocompatibility. Biosens. Bioelectron. 2011, 26, 4276–4282. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Dellinger, E.P.; Gerberding, J.L.; Heard, S.O.; Maki, D.G.; Masur, H.; McCormick, R.D.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 2002, 51, 1–29. [Google Scholar]
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo. Clin. Proc. 2006, 81, 1159–1171. [Google Scholar] [CrossRef]
- Munoz, R.; Laussen, P.C.; Palacio, G.; Zienko, L.; Piercey, G.; Wessel, D.L. Changes in whole blood lactate levels during cardiopulmonary bypass for surgery for congenital cardiac disease: An early indicator of morbidity and mortality. J. Thorac. Cardiovasc. Surg. 2000, 119, 155–162. [Google Scholar] [CrossRef]
- Hajjar, L.A.; Almeida, J.P.; Fukushima, J.T.; Rhodes, A.; Vincent, J.L.; Osawa, E.A.; Galas, F.R.B.G. High lactate levels are predictors of major complications after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2013, 146, 455–460. [Google Scholar] [CrossRef]
- Ata, A.; Lee, J.; Bestle, S.L.; Desemone, J.; Stain, S.C. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch. Surg. 2010, 145, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Guvener, M.; Pasaoglu, I.; Demircin, M.; Oc, M. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr. J. 2002, 49, 531–537. [Google Scholar] [CrossRef]
- Ito, N.; Iwaya, T.; Ikeda, K.; Kimura, Y.; Akiyama, Y.; Konosu, M.; Ishida, K.; Fujiwara, H.; Otsuka, K.; Nitta, H.; et al. Hyperglycemia 3 days after esophageal cancer surgery is associated with an increased risk of postoperative infection. J. Gastrointest. Surg. 2014, 18, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Karidis, N.P.; Lekakos, L.; Dimitroulis, D. Stratification of patients who underwent colorectal surgery: Determining the risk of surgical site infection related to postoperative hyperglycemia. Arch. Surg. 2011, 146, 369. [Google Scholar] [CrossRef] [PubMed]
- Mraovic, B.; Suh, D.; Jacovides, C.; Parvizi, J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci. Technol. 2011, 5, 412–418. [Google Scholar] [CrossRef]
- Wolf, A.; Renehan, K.; Ho, K.K.Y.; Carr, B.D.; Chen, C.V.; Cornell, M.S.; Ye, M.; Rojas-Peña, A.; Chen, H. Evaluation of continuous lactate monitoring systems within a heparinized in vivo porcine model intravenously and subcutaneously. Biosensors 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.F.; Hibbs, J.B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991, 3, 65–70. [Google Scholar] [CrossRef]
- Mannick, J.B. Immunoregulatory and antimicrobial effects of nitrogen oxides. Proc. Am. Thorac. Soc. 2006, 3, 161–165. [Google Scholar] [CrossRef]
- Annich, G.M.; Meinhardt, J.P.; Mowery, K.A.; Ashton, B.A.; Merz, S.I.; Hirschl, R.B.; Meyerhoff, M.E.; Bartlett, R.H. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit. Care Med. 2000, 28, 915–920. [Google Scholar] [CrossRef]
- Fleser, P.S.; Nuthakki, V.K.; Malinzak, L.E.; Callahan, R.E.; Seymour, M.L.; Reynolds, M.M.; Merz, S.I.; Meyerhoff, M.E.; Bendick, P.J.; Zelenock, G.B.; et al. Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts. J. Vasc. Surg. 2004, 40, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.M.; Frost, M.C.; Meyerhoff, M.E. Nitric oxide-releasing hydrophobic polymers: Preparation, characterization, and potential biomedical applications. Free Radic. Biol. Med. 2004, 37, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 2, 1057–1058. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987, 148, 1482–1489. [Google Scholar] [CrossRef]
- Wolf, A.K.; Qin, Y.; Major, T.C.; Meyerhoff, M.E. Improved thromboresistance and analytical performance of intravascular amperometric glucose sensors using optimized nitric oxide release coatings. Chin. Chem. Lett. 2015, 26, 464–468. [Google Scholar] [CrossRef]
- Yan, Q.Y.; Peng, B.; Su, G.; Cohan, B.E.; Major, T.C.; Meyerhoff, M.E. Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration. Anal. Chem. 2011, 83, 8341–8346. [Google Scholar] [CrossRef]
- Carelli, I.; Chiarotto, I.; Curulli, A.; Palleschi, G. Electropolymerization of hydroxybenzene and aminobenzene isomers on platinum electrodes to assemble interference-free electrochemical biosensors. Electrochim. Acta 1996, 41, 1793–1800. [Google Scholar] [CrossRef]
- Geise, R.J.; Adams, J.M.; Barone, N.J.; Yacynych, A.M. Electropolymerized films to prevent interferences and electrode fouling in biosensors. Biosens. Bioelectron. 1991, 6, 151–160. [Google Scholar] [CrossRef]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef]
- Holst, K.A.; Said, S.M.; Nelson, T.J.; Cannon, B.C.; Dearani, J.A. Current interventional and surgical management of congenital heart disease: Specific focus on valvular disease and cardiac arrhythmias. Circ. Res. 2017, 120, 1027–1044. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, K.K.Y.; Peng, Y.-W.; Ye, M.; Tchouta, L.; Schneider, B.; Hayes, M.; Toomasian, J.; Cornell, M.; Rojas-Pena, A.; Charpie, J.; et al. Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model undergoing Open-Heart Surgery with Cardiopulmonary Bypass. Chemosensors 2020, 8, 56. https://doi.org/10.3390/chemosensors8030056
Ho KKY, Peng Y-W, Ye M, Tchouta L, Schneider B, Hayes M, Toomasian J, Cornell M, Rojas-Pena A, Charpie J, et al. Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model undergoing Open-Heart Surgery with Cardiopulmonary Bypass. Chemosensors. 2020; 8(3):56. https://doi.org/10.3390/chemosensors8030056
Chicago/Turabian StyleHo, Kenneth Kwun Yin, Yun-Wen Peng, Minyi Ye, Lise Tchouta, Bailey Schneider, McKenzie Hayes, John Toomasian, Marie Cornell, Alvaro Rojas-Pena, John Charpie, and et al. 2020. "Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model undergoing Open-Heart Surgery with Cardiopulmonary Bypass" Chemosensors 8, no. 3: 56. https://doi.org/10.3390/chemosensors8030056
APA StyleHo, K. K. Y., Peng, Y.-W., Ye, M., Tchouta, L., Schneider, B., Hayes, M., Toomasian, J., Cornell, M., Rojas-Pena, A., Charpie, J., & Chen, H. (2020). Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model undergoing Open-Heart Surgery with Cardiopulmonary Bypass. Chemosensors, 8(3), 56. https://doi.org/10.3390/chemosensors8030056




