Possibilities and Challenges for Quantitative Optical Sensing of Hydrogen Peroxide
Abstract
1. Introduction
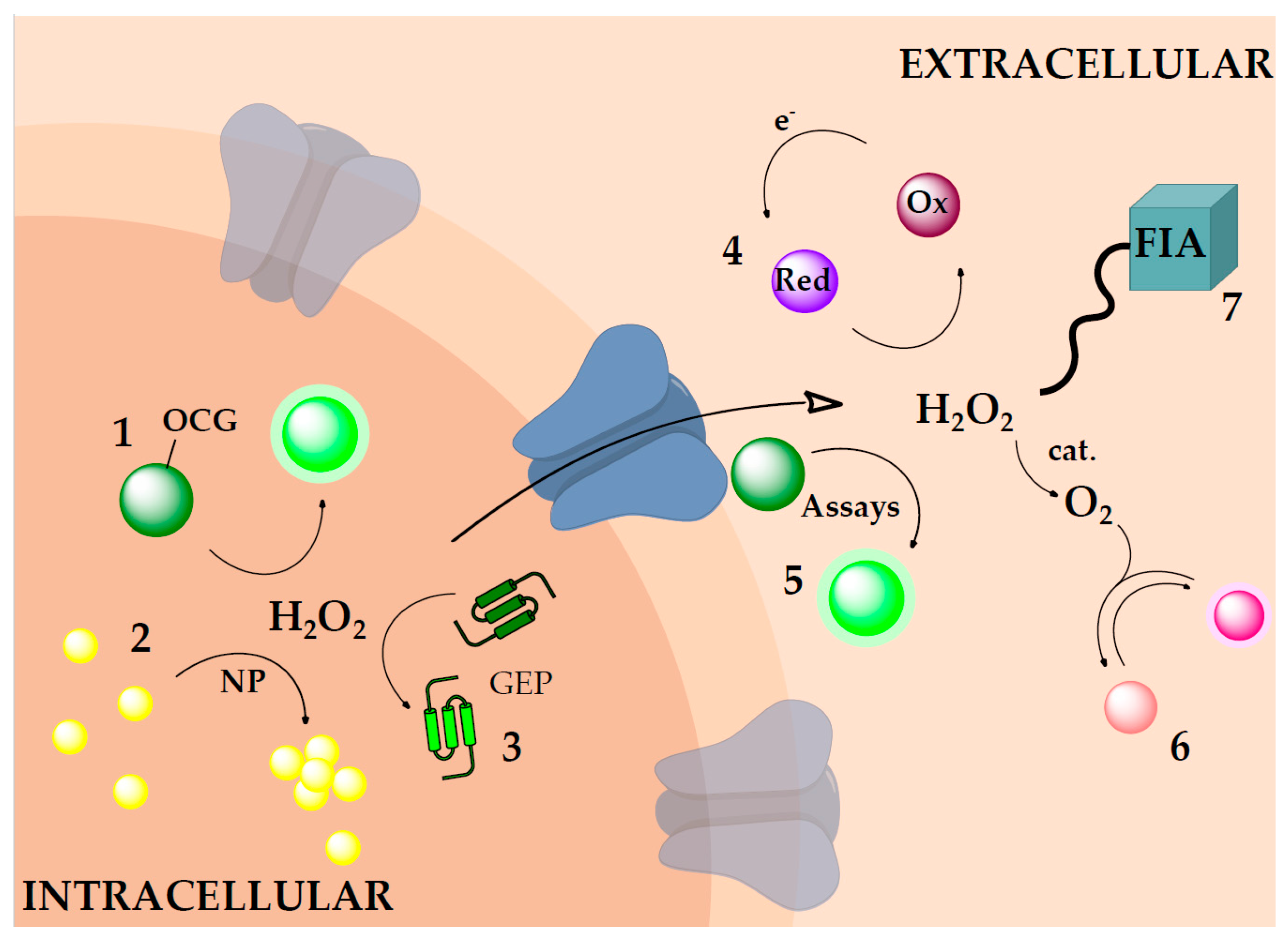
2. Intracellular Hydrogen Peroxide Measurement
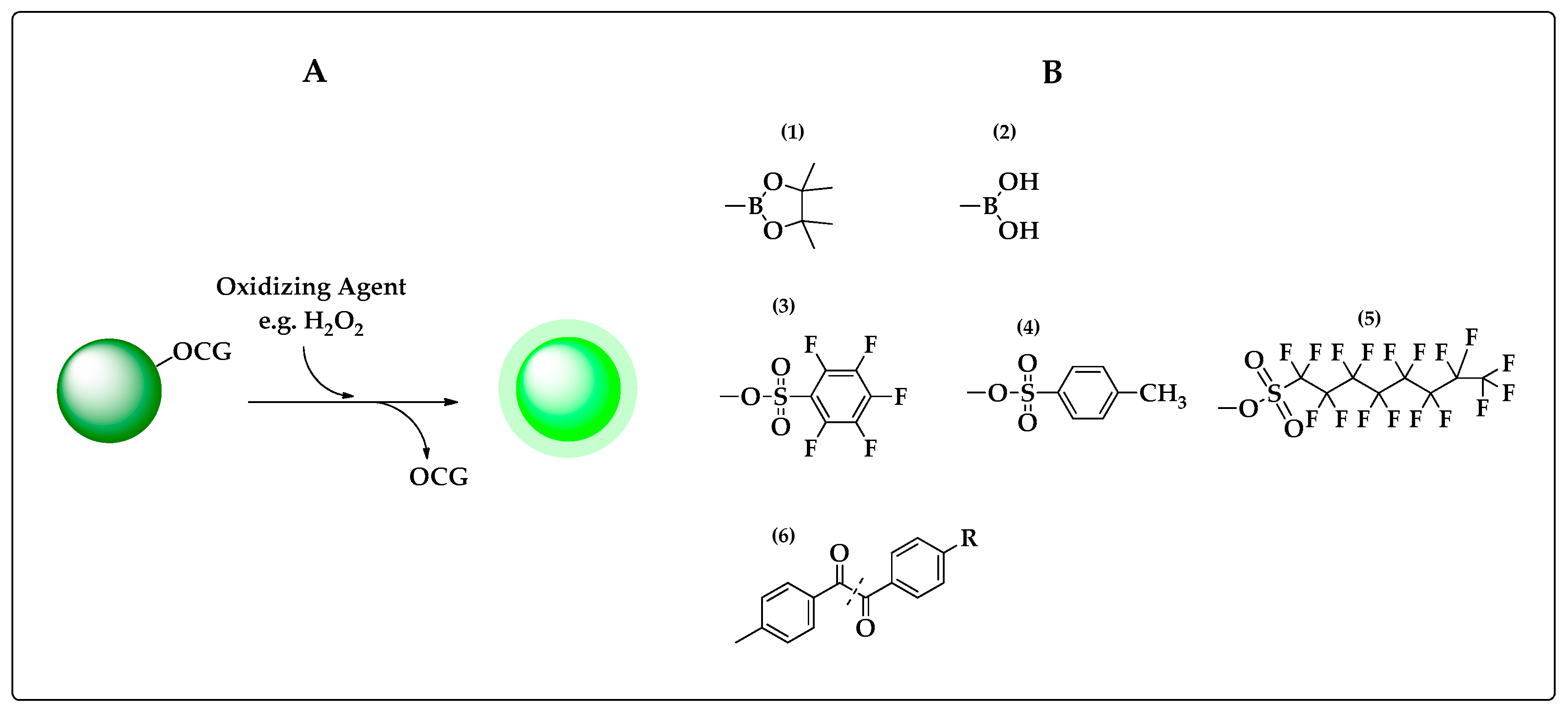
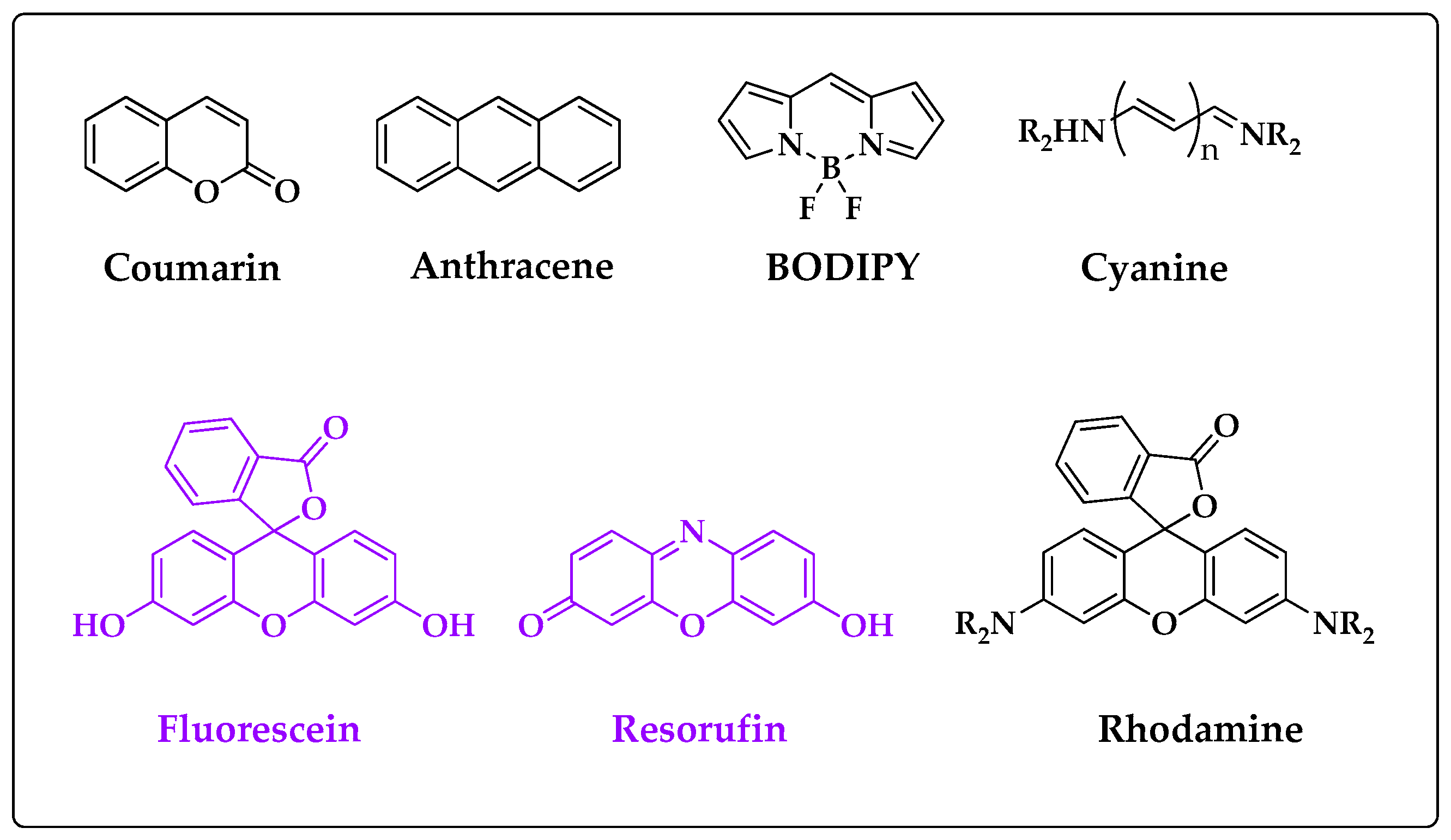
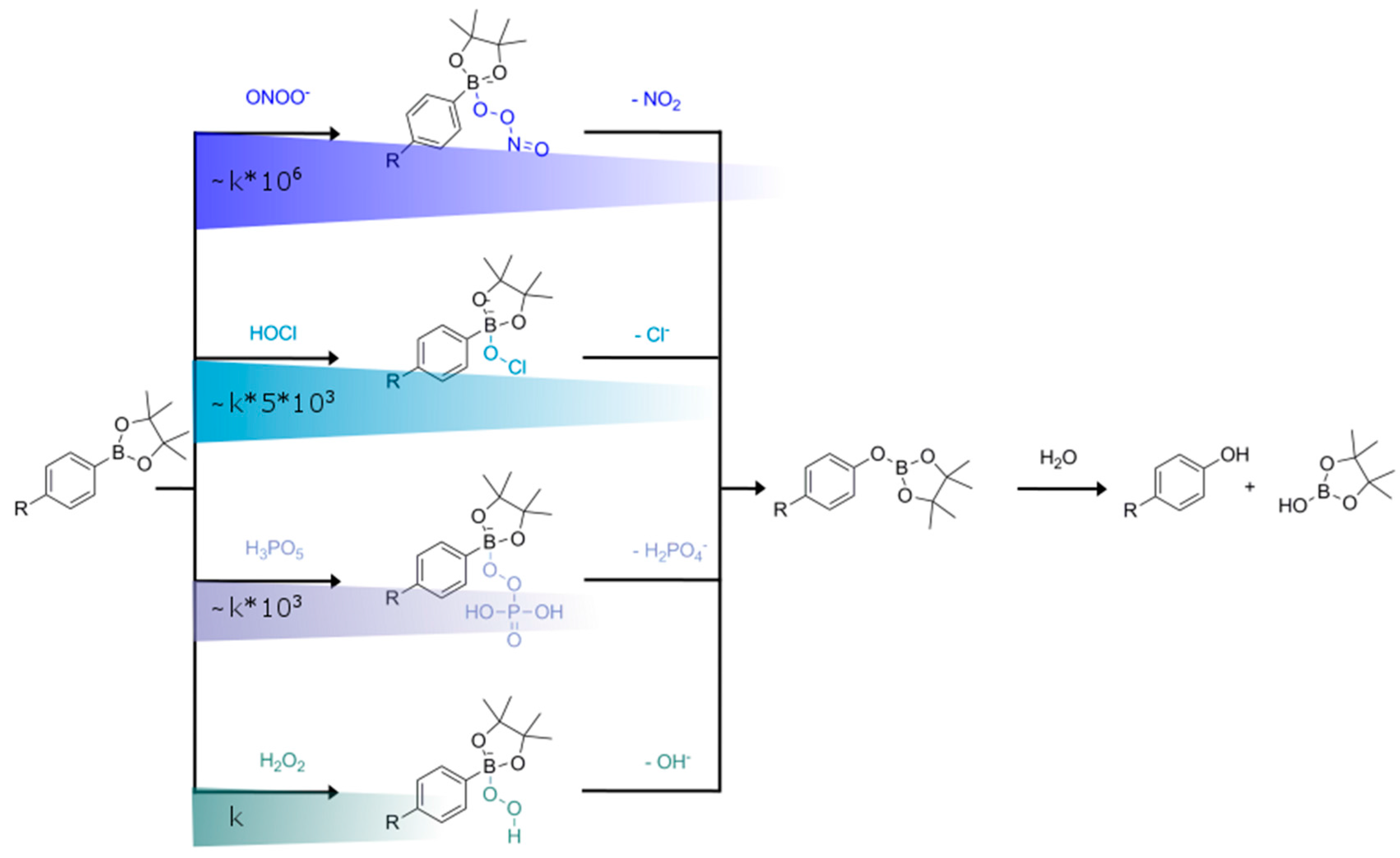
2.1. Oxidative Cleavage–Based Probes
2.2. Nanomaterials
2.3. Genetically Encoded Probes—Fluorescent Proteins
3. Extracellular Hydrogen Peroxide Measurements
3.1. Chemiluminescent Reactions
3.2. Kits for H2O2 Detection
4. Intermediate Sensing Systems
4.1. Flow Injection Analysis
4.2. Redox Systems
5. Fully Reversible Optical Chemical Sensors
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W. Lighting up H2O2: The molecule that is a “necessary evil” in the cell. Angew. Chem. Int. Ed. 2009, 48, 3022–3024. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Channon, K.M. Free radicals and redox signalling in cardiovascular disease. Heart 2004, 90, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, K.; Chen, D.; Mao, Y.; Gu, Y. A novel colorimetric and near-infrared fluorescent probe for hydrogen peroxide imaging in vitro and in vivo. RSC Adv. 2015, 5, 85957–85963. [Google Scholar] [CrossRef]
- Schäferling, M.; Grögel, D.B.M. Luminescent probes for detection and imaging of hydrogen peroxide. Microchim. Acta 2011, 174, 1–18. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.L. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Tabner, B.J.; El-Agnaf, O.M.A.; Turnbull, S.; German, M.J.; Paleologou, K.E.; Hayashi, Y.; Cooper, L.J.; Fullwood, N.J.; Allsop, D. Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia. J. Biol. Chem. 2005, 280, 35789–35792. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, J.; Liang, S.H.; Xu, Y.; Moore, A.; Ran, C. Imaging hydrogen peroxide in Alzheimer’s disease via cascade signal amplification. Sci. Rep. 2016, 6, 35613. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.V.; Garnett, M.; Chu, B. Spatiotemporal Oscillations in Biological Molecules: Hydrogen Peroxide and Parkinson’s Disease. Int. J. Electrochem. Sci. 2008, 3, 1364–1385. [Google Scholar]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Lisanti, M.P.; Martinez-Outschoorn, U.E.; Lin, Z.; Pavlides, S.; Whitaker-Menezes, D.; Pestell, R.G.; Howell, A.; Sotgia, F. Hydrogen peroxide fuels aging, inflammation, cancer metabolism and metastasis—The seed and soil also needs “fertilizer”. Cell Cycle 2011, 10, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.P.; Faccenda, J.; Innes, J.A.; Greening, A.P. Expired hydrogen peroxide in breath condensate of cystic fibrosis patients. Eur. Respir. J. 1999, 13, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Haskew-Layton, R.E.; Payappilly, J.B.; Smirnova, N.A.; Ma, T.C.; Chan, K.K.; Murphy, T.H.; Guo, H.; Langley, B.; Sultana, R.; Butterfield, D.A.; et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 17385–17390. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The Biological Chemistry of Hydrogen Peroxide. In Methods in Enzymology; Elsevier Inc.: Philadelphia, PA, USA, 2013; Volume 528, pp. 3–25. ISBN 9780124058811. [Google Scholar]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. Mutagenesis and Stress Responses Induced in Escherichia coli by Hydrogen Peroxide. J. Bacteriol. 1987, 169, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Orrenius, S. Dual regulation of caspase activity by hydrogen peroxide: Implications for apoptosis. FEBS Lett. 1997, 414, 552–556. [Google Scholar] [CrossRef]
- Gonza, B.; Demple, B. Homeostatic Regulation of Intracellular Hydrogen Peroxide Concentration in Aerobically Growing Escherichia coli. J. Bacteriol. 1997, 179, 382–388. [Google Scholar]
- Downs, C.A.; Fauth, J.E.; Halas, J.C.; Dustan, P.; Bemiss, J.; Woodley, C.M. Oxidative stress and seasonl coral bleaching. Free Radic. Biol. Med. 2002, 33, 533–543. [Google Scholar] [CrossRef]
- Febria, C.M.; Lesack, L.F.W.; Gareis, J.A.L.; Bothwell, M.L. Patterns of hydrogen peroxide among lakes of the Mackenzie Delta, western Canadian Arctic. Can. J. Fish. Aquat. Sci. 2006, 63, 2107–2118. [Google Scholar] [CrossRef]
- Reimer, H. The Daily Changing Pattern of Hydrogen Peroxide in New Zealand. Environ. Toxicol. Chem. 1996, 15, 652–662. [Google Scholar]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; DiMento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Dhawan, V. Reactive Oxygen and Nitrogen Species: Generation of ROS. In Studies on Respiratory Disorders; Ganguly, N., Jindal, S., Biswal, S., Barnes, P., Pawankar, R., Eds.; Humana Press: New York, NY, USA, 2014; pp. 27–47. ISBN 9781493904976. [Google Scholar]
- Bent, H.A.; Liebman, J.F. Paradigms and paradoxes: The weak bonds in elemental halogens, peroxides, disulfides, interhalogens, noble gas monohalide cations, and isoelectronic species. Struct. Chem. 2011, 22, 371–372. [Google Scholar] [CrossRef]
- Neftel, A.; Jacob, P.; Klockow, D. Long-term record of H2O2 in polar ice cores. Tellus 1986, 262–270. [Google Scholar] [CrossRef]
- Sigg, A.; Neftel, A. Seasonal variations in hydrogen peroxide in polar ice cores. Ann. Glaciol. 1988, 10, 157–162. [Google Scholar] [CrossRef]
- Guo, H.; Aleyasin, H.; Dickinson, B.C.; Haskew-layton, R.E.; Ratan, R.R. Recent advances in hydrogen peroxide imaging for biological applications. Cell Biosci. 2014, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S.; Resch-Genger, U. Standardization and Quality Assurance in Fluorescence Measurements I; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540752066. [Google Scholar]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical Sensors Definitions and Classifications. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Probes, Sensors, and Labels: Why is Real Progress Slow? Angew. Chem. Int. Ed. 2013, 52, 9864–9865. [Google Scholar] [CrossRef] [PubMed]
- Eror, N.G.; Coppersmith, S.N.; Dean, P.D.; Murray, R.W.; Peercy, P.S.; Rogers, C.A.; Sadoway, D.R.; Thome, J.R.; Wagner, J.W. Expanding the Vision of Sensor Materials; National Academy Press: Washington, DC, USA, 1995; ISBN 0309587433. [Google Scholar]
- Li, R.; Liu, X.; Qiu, W.; Zhang, M. In Vivo Monitoring of H2O2 with Polydopamine and Prussian Blue-coated Microelectrode. Anal. Chem. 2016, 88, 7769–7776. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. Prussian Blue-Based First-Generation Biosensor. A Sensitive Amperometric Electrode for Glucose. Anal. Chem. 1995, 67, 2419–2423. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.; Wen, W.; Zhao, Y. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Calas-Blanchard, C.; Catanante, G.; Noguer, T. Electrochemical Sensor and Biosensor Strategies for ROS/RNS Detection in Biological Systems. Electroanalysis 2014, 26, 1277–1286. [Google Scholar] [CrossRef]
- Zhao, Z.; Ou, Q.; Yin, X.; Liu, J. Nanomaterial-Based Electrochemical Hydrogen Peroxide Biosensor. Int. J. Biosens. Bioelectron. 2017, 2, 25–28. [Google Scholar] [CrossRef]
- Burmistrova, N.A.; Kolontaeva, O.A.; Duerkop, A. New Nanomaterials and Luminescent Optical Sensors for Detection of Hydrogen Peroxide. Chemosensors 2015, 3, 253–273. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Jacewicz, D.; Chmurzyński, L. Fluorescent Probes Used for Detection of Hydrogen Peroxide under Biological Conditions. Crit. Rev. Anal. Chem. 2016, 46, 171–200. [Google Scholar] [CrossRef] [PubMed]
- Soh, N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem. 2006, 386, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-E.K.; Kopelman, R. Nanoparticle PEBBLE Sensors in Live Cells. In Methods in Enzymology; Elsevier Inc.: Philadelphia, PA, USA, 2012; Volume 504, pp. 419–470. ISBN 9780123918574. [Google Scholar]
- Winterbourn, C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta 2014, 1840, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.B.; Agrawal, A.; Manchester, M.; Cohen, S.M. Readily Accessible Fluorescent Probes for Sensitive Biological Imaging of Hydrogen Peroxide. Chembiochem 2013, 14, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D.; Rutkoski, T.J.; Raines, R.T. Tuning the pKa of Fluorescein to Optimize Binding Assays. Anal. Chem. 2007, 79, 6775–6782. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.; Villegas, M.L.; Bertolotti, S.G.; Previtali, C.M.; Neumann, M.G.; Encinas, M.V. The Excited-State Interaction of Resazurin and Resorufin with Amines in Aqueous Solutions. Photophysics and Photochemical Reaction. Photochem. Photobiol. 2002, 76, 385–390. [Google Scholar] [CrossRef]
- Song, D.; Lim, M.; Cho, S.; Park, S.-J.; Cho, J.; Kang, D.; Rhee, S.G.; You, Y.; Nam, W. A fluorescence turn-on H2O2 probe exhibits lysosome-localized fluorescence signals. Chem. Commun. 2012, 48, 5449–5451. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chang, T.-S.; Jeong, W.; Kang, D. Methods for Detection and Measurement of Hydrogen Peroxide Inside and Outside of Cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Dickinson, B.C.; Chang, C.J. Boronate-based fluorescent probes: Imaging hydrogen peroxide in living systems. Methods Enzymol. 2013, 526, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Huynh, C.; Chang, C.J. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 2010, 132, 5906–5915. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Tulyathan, O.; Isacoff, E.Y.; Chang, C.J. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 2007, 3, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Zielonka, J.; Lopez, M.; Joseph, J.; Kalyanaraman, B. Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic. Biol. Med. 2009, 47, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Labutti, J.N.; Gates, K.S. Biologically Relevant Chemical Properties of Peroxymonophosphate (=O3POOH). Bioorg. Med. Chem. Lett. 2009, 19, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Sikora, A.; Hardy, M.; Joseph, J.; Dranka, B.P.; Kalyanaraman, B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem. Res. Toxicol. 2012, 25, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fukuyasu, Y.; Yoshida, S.; Fukuda, M.; Saeki, K.; Matsuno, H.; Yamauchi, Y.; Yoshida, K.; Hirata, K.; Miyamoto, K. Fluorescent Probes for Hydrogen Peroxide Based on a Non-Oxidative Mechanism. Angew. Chem. 2004, 43, 2389–2391. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Tang, B.; Huang, H.; Yang, G.; Chen, Z.; An, L. Strong red fluorescent probes suitable for detecting hydrogen peroxide generated by mice peritoneal macrophages. Chem. Commun. 2005, 5974–5976. [Google Scholar] [CrossRef] [PubMed]
- Mohr, G.J. New chromogenic and fluorogenic reagents and sensors for neutral and ionic analytes based on covalent bond formation—A review of recent developments. Anal. Bioanal. Chem. 2006, 386, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, F.; Wang, H.; Wang, S.; Wang, L.; Tang, B. Sulfonate-based fluorescent probes for imaging hydrogen peroxide in living cells. Sci. China Ser. B Chem. 2009, 52, 734–740. [Google Scholar] [CrossRef]
- Abo, M.; Urano, Y.; Hanaoka, K.; Terai, T.; Komatsu, T.; Nagano, T. Development of a Highly Sensitive Fluorescence Probe for Hydrogen Peroxide. J. Am. Chem. Soc. 2011, 133, 10629–10637. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-usmar, V.; Davies, K.J.A.; Dennery, P.A.; Jay, H.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2′,7′-Dichlorofluorescein as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 25, 227–231. [Google Scholar] [CrossRef]
- Koren, K.; Jensen, P.Ø.; Kühl, M. Development of a rechargeable optical hydrogen peroxide sensor—Sensor design and biological application. Analyst 2016, 141, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R.; Lenarczuk, T.; Głab, S. Optical sensing schemes for Prussian Blue/Prussian White film system. Anal. Chim. Acta 2000, 424, 27–35. [Google Scholar] [CrossRef]
- Miller, E.W.; Bian, S.X.; Chang, C.J. A Fluorescent Sensor for Imaging Reversible Redox Cycles in Living Cells. J. Am. Chem. Soc. 2007, 129, 3458–3459. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Gee, K.R. Redox-Dependent Trafficking of 2,3,4,5,6- Pentafluoreodihydrotetramethylrosamine, a Novel Fluorogenic Indicator of Cellular Oxidative Activity. Free Radic. Biol. Med. 2000, 28, 1266–1278. [Google Scholar] [CrossRef]
- Xu, K.; Qiang, M.; Gao, W.; Su, R.; Li, N.; Gao, Y.; Xie, Y.; Kong, F.; Tang, B. A near-infrared reversible fluorescent probe for real-time imaging of redox status changes in vivo. Chem. Sci. 2013, 4, 1079–1086. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Introduction to Nanoparticles. In Microwaves in Nanoparticle Synthesis: Fundamentals and Applications Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2013; pp. 1–24. [Google Scholar]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Santos, H.M.; Garcia-Pardo, J.; Diniz, M.; Lorenzo, J.; Rodríguez-González, B.; Capelo, J.L.; Lodeiro, C. Synthesis of functionalized fluorescent silver nanoparticles and their toxicological effect in aquatic environments (Goldfish) and HEPG2 cells. Front. Chem. 2013, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tagad, C.K.; Kim, H.U.; Aiyer, K.; More, P.; Kim, T.; Hyun Moh, S.; Kulkarni, A.; Sabharwal, S.G. A sensitive hydrogen peroxide optical sensor based on polysaccharide stabilized silver nanoparticles. RCS Adv. 2013, 3, 22940–22943. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S.; Jeon, H.-J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Peng, C.; Liu, C.; Xie, Z. Preparation of a fluorescent silver nanoprism–dye complex for detection of hydrogen peroxide in milk. Anal. Methods 2015, 7, 9749–9752. [Google Scholar] [CrossRef]
- Liu, C.; Ding, Y.; Li, Q.; Lin, Y. Photochemical synthesis of glutathione-stabilized silver nanoclusters for fluorometric determination of hydrogen peroxide. Microchim. Acta 2017, 184, 2497–2503. [Google Scholar] [CrossRef]
- Yuan, Z.; Hu, C.-C.; Chang, H.-T.; Lu, C. Gold nanoparticles as sensitive optical probes. Analyst 2016, 141, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Shiang, Y.-C.; Huang, C.-C.; Chang, H.-T. Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose. Chem. Commun. 2009, 3437–3439. [Google Scholar] [CrossRef] [PubMed]
- Molaabasi, F.; Hosseinkhani, S.; Moosavi-Movahedi, A.A.; Shamsipur, M. Hydrogen peroxide sensitive hemoglobin-capped gold nanoclusters as a fluorescence enhancing sensor for the label-free detection of glucose. RSC Adv. 2015, 5, 33123–33135. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Shen, J. Preparation of Stable Luminescent Poly(methyl methacrylate)–Europium Complex Nanospheres and Application in the Detection of Hydrogen Peroxide with the Biocatalytic Growth of Gold Nanoparticles. J. Appl. Polym. Sci. 2013, 845–850. [Google Scholar] [CrossRef]
- Chang, H.C.; Ho, J.A.A. Gold Nanocluster-Assisted Fluorescent Detection for Hydrogen Peroxide and Cholesterol Based on the Inner Filter Effect of Gold Nanoparticles. Anal. Chem. 2015, 87, 10362–10367. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, Z.-F.; Shi, M.-J.; Xu, Y.; Wu, Y.-L. Light Emission of Gold Nanoparticles Induced by the Reaction of Bis(2,4,6-trichlorophenyl) Oxalate and Hydrogen Peroxide. Anal. Chem. 2005, 77, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, O.; Khene, S.; Nyokong, T. Fluorescence “Switch on” of Conjugates of CdTe @ ZnS Quantum Dots with Al, Ni and Zn Tetraamino-Phthalocyanines by Hydrogen Peroxide: Characterization and Applications as Luminescent Nanosensors. J. Fluoresc. 2013, 23, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Zhang, J. H2O2- and pH-sensitive CdTe quantum dots as fluorescence probes for the detection of glucose. J. Biol. Chem. Lumin. 2013, 28, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Ibañez, E.; Goicoechea, J.; Urrutia, A.; Matias, I.R.; Arregui, F.J. A self-referenced optical colorimetric sensor based on silver and gold nanoparticles for quantitative determination of hydrogen peroxide. Sens. Actuators B Chem. 2017, 251, 624–631. [Google Scholar] [CrossRef]
- Garg, S.; Rong, H.; Miller, C.J.; Waite, T.D. Oxidative Dissolution of Silver Nanoparticles by Chlorine: Implications to Silver Nanoparticle Fate and Toxicity. Environ. Sci. Technol. 2016, 50, 3890–3896. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Kim, J.H.; Lee, J.S.; Ahn, J.; Kim, M.-G.; Park, C.B. Multi-layered stacks of fluorescent dye-doped silica nanoparticles decorated by gold nanoparticles for solid-phase optical biosensing. J. Mater. Chem. 2011, 21, 17623–17626. [Google Scholar] [CrossRef]
- Li, Z.; Guo, S.; Yuan, Z.; Lu, C. Carbon quantum dot-gold nanocluster nanosatellite for ratiometric fluorescence probe and imaging for hydrogen peroxide in living cells. Sens. Actuators B Chem. 2017, 241, 821–827. [Google Scholar] [CrossRef]
- Chu, C.-S.; Hsieh, M.-W.; Su, Z.-R. Hydrogen peroxide sensing based on carbon quantum dots. MATEC Web Conf. ICFST 2016, 59, 1–4. [Google Scholar] [CrossRef]
- Gan, Z.; Gui, Q.; Shan, Y.; Pan, P.; Zhang, N.; Zhang, L. Photoluminescence of MoS2 quantum dots quenched by hydrogen peroxide: A fluorescent sensor for hydrogen peroxide. J. Appl. Phys. 2016, 120, 104503. [Google Scholar] [CrossRef]
- Chon, C.H.; Li, D. Quantum Dot. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Berlin, Germany, 2008; pp. 1767–1769. ISBN 978-0-387-32468-5. [Google Scholar]
- Barroso, M.M. Quantum Dots in Cell Biology. J. Histochem. Cytochem. 2011, 59, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Lee, Y.-E.K.; Xu, H.; Philbert, M.A.; Kopelman, R. Nanoencapsulation Method for High Selectivity Sensing of Hydrogen Peroxide inside Live Cells. Lett. Anal. Chem. 2010, 82, 2165–2169. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Khaja, S.; Velasquez-castano, J.C.; Dasari, M.; Sun, C.; Petros, J.; Taylor, W.R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Hwang, O.; Yoo, D.; Khang, G.; Lee, D. Detection of Hydrogen Peroxide in vitro and in vivo Using Peroxalate Chemiluminescent Micelles. Bull. Korean Chem. Soc. 2011, 32, 2187–2192. [Google Scholar] [CrossRef]
- Banquy, X.; Suarez, F.; Argaw, A.; Rabanel, J.; Grutter, P.; Giasson, S. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. RCS Soft Matter 2009, 5, 3984–3991. [Google Scholar] [CrossRef]
- Barata, A.G.; Dick, T.P. In Vivo Imaging of H2O2 Production in Drosophila. In Methods in Enzymology; Elsevier Inc.: Berlin, Germany, 2013; Volume 526, pp. 61–82. ISBN 9780124058835. [Google Scholar]
- Mishina, N.M.; Markvicheva, K.N.; Bilan, D.S.; Matlashov, M.E.; Shirmanova, M.V.; Liebl, D.; Schultz, C.; Lukyanov, S.; Belousov, V.V. Visualization of Intracellular Hydrogen Peroxide with HyPer, a Genetically Encoded Fluorescent Probe. In Methods in Enzymology; Elsevier Inc.: Berlin, Germany, 2013; Volume 526, pp. 45–59. ISBN 9780124058835. [Google Scholar]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, Y.G.; Bilan, D.S.; Matlashov, M.E.; Mishina, N.M.; Markvicheva, K.N.; Subach, O.M.; Subach, F.V.; Bogeski, I.; Hoth, M.; Enikolopov, G.; et al. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Trush, M.A.; Li, Y.R. A Highly Sensitive Chemiluminometric Assay for Real-Time Detection of Biological Hydrogen Peroxide Formation. React. Oxyg. Species 2016, 1, 216–227. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G.D.; Stratis-Cullum, D.N. Biosensors. In Encyclopedia of Microbiology (Third Edition); Elsevier Inc.: Berlin, Germany, 2009; pp. 88–103. [Google Scholar]
- Chandross, E.A. A New Chemiluminescent System. Tetrahedron Lett. 1963, 761–765. [Google Scholar]
- Kuntzleman, T.S.; Rohrer, K.; Schultz, E. The Chemistry of Lightsticks: Demonstrations To Illustrate Chemical Processes. J. Chem. Educ. 2012, 89, 910–916. [Google Scholar] [CrossRef]
- Jacob, P.; Tavares, T.M.; Rocha, V.C.; Klockow, D. Atmospheric H2O2 field measurements in a tropical environment: Bahia, Brazil. Atmos. Environ. 1990, 24A, 377–382. [Google Scholar] [CrossRef]
- Malehorn, C.L.; Riehlt, T.E.; Hinze, W.L. Improved Determination of Hydrogen Peroxide or Lucigenin by Measurement of lucigenin Chemiluminescence in Organised Assemblies. Analyst 1986, 111, 941–947. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Kuppusamy, P.; Roubaud, V.; Zweier, J.L.; Trush, M.A. Validation of Lucigenin (Bis-N-methylacridinium) as a Chemilumigenic Probe for Detecting Superoxide Anion Radical Production by Enzymatic and Cellular Systems. J. Biol. Chem. 1998, 273, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, H.; Wang, Z.; Su, M.; Xiao, H.; Liang, S. Synthesis of a novel chemiluminescent reagent for the determination of hydrogen peroxide in snow waters. Talanta 2001, 53, 983–990. [Google Scholar] [CrossRef]
- Mátai, A.; Hideg, E. A comparison of colorimetric assays detecting hydrogen peroxide in leaf extracts. Anal. Methods 2017, 9, 2357–2360. [Google Scholar] [CrossRef]
- Hong, J.; Maguhn, J.; Freitag, D.; Kettrup, A. Determination of H2O2 and organic peroxides by high-performance liquid chromatography with post-column UV irradiation, derivatization and fluorescence detection. Fresenius J. Anal. Chem. 1998, 361, 124–128. [Google Scholar] [CrossRef]
- Zaitsu, K.; Ohkura, Y. New Fluorogenic Substrates for Horseradish Peroxidase: Rapid and Sensitive Assays for Hydrogen Peroxide and the Peroxidase. Anal. Biochem. 1980, 109, 109–113. [Google Scholar] [CrossRef]
- Perschke, H.; Broda, E. Determination of Very Small Amounts of Hydrogen Peroxide. Nature 1961, 190, 257–258. [Google Scholar] [CrossRef]
- Andreae, W.A. A Sensitive Method for the Estimation of Hydrogen Peroxide in Biological Materials. Nature 1955, 175, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.G.; Brignac, P.J.; Juneau, M. New Substrates for the Fluorometric Determination of Oxidative Enzymes. Anal. Chem. 1969, 40, 1256–1263. [Google Scholar] [CrossRef]
- Puch, W.; Cooper, P.H.; Baggiolini, M. Assay of H2O2 Production by Macrophages and Neutrophils with Homovanillic Acid and Horse-Radish Peroxidase. J. Immunol. Methods 1983, 63, 347–357. [Google Scholar]
- Zhou, M.; Diwu, Z.; Panchuk-Voloshina, N.; Haugland, R.P. A Stable Nonfluorescent Derivative of Resorufin for the Fluorometric Determination of Trace Hydrogen Peroxide: Applications in Detecting the Activity of Phagocyte NADPH Oxidase and Other Oxidases. Anal. Biochem. 1997, 253, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Jaffe, J.S.; Schulman, E.S.; Raible, D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 1997, 202, 133–141. [Google Scholar] [CrossRef]
- Votyakova, T.V.; Reynolds, I.J. Detection of hydrogen peroxide with Amplex Red: Interference by NADH and reduced glutathione auto-oxidation. Arch. Biochem. Biophys. 2004, 431, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Debski, D.; Smulik, R.; Zielonka, J.; Michałowski, B.; Jakubowska, M.; Debowska, K.; Adamus, J.; Marcinek, A.; Kalyanaraman, B.; Sikora, A. Mechanism of oxidative conversion of Amplex® Red to resorufin: Pulse radiolysis and enzymatic studies. Free Radic. Biol. Med. 2016, 95, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Treumann, A.; Bell, A.; Vistoli, G.; Nelson, G.; Hay, S.; Von Zglinicki, T. Carboxylesterase converts Amplex red to resorufin: Implications for mitochondrial H2O2 release assays. Free Radic. Biol. Med. 2016, 90, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Edman, L.; Rigler, R. Memory landscapes of single-enzyme molecules. Proc. Natl. Acad. Sci. USA 2000, 97, 8266–8271. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.J.; Moegling, J.K.; Kieber, R.J.; Kiddle, J.J. A chemiluminescence method for the analysis of H2O2 in natural waters. Mar. Chem. 2000, 70, 191–200. [Google Scholar] [CrossRef]
- King, D.W.; Cooper, W.J.; Rusak, S.A.; Peake, B.M.; Kiddle, J.J.; Sullivan, D.W.O.; Melamed, M.L.; Morgan, C.R.; Theberge, S.M. Flow Injection Analysis of H2O2 in Natural Waters Using Acridinium Ester Chemiluminescence: Method Development and Optimization Using a Kinetic Model. Anal. Chem. 2007, 79, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.W.; Morgan, C.A.; Kieber, D.J.; King, D.W.; Snow, J.A.; Heikes, B.G.; Mopper, K.; Kiddle, J.J. Hydrogen peroxide method intercomparison study in seawater. Mar. Chem. 2005, 97, 4–13. [Google Scholar] [CrossRef]
- Morris, J.J.; Johnson, Z.I.; Wilhelm, S.W.; Zinser, E.R. Diel regulation of hydrogen peroxide defenses by open ocean microbial communities. J. Plankton Res. 2016, 38, 1103–1114. [Google Scholar] [CrossRef]
- Schneider, R.J.; Roe, K.L.; Hansel, C.M.; Voelker, B.M. Species-Level Variability in Extracellular Production Rates of Reactive Oxygen Species by Diatoms. Front. Chem. 2016, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shiller, A.M. Determination of Subnanomolar Levels of Hydrogen Peroxide in Seawater by Reagent-Injection Chemiluminescence Detection. Anal. Chem. 1999, 71, 1975–1980. [Google Scholar] [CrossRef]
- Sullivan, D.W.O.; Hanson, A.K.; Kester, D.R. Stopped flow luminol chemiluminescence determination of Fe(II) and reducible iron in seawater at subnanomolar levels. Mar. Chem. 1995, 49, 65–77. [Google Scholar] [CrossRef]
- Price, D.; Mantoura, R.F.C.; Worsfold, P.J. Shipboard determination of hydrogen peroxide in the western Mediterranean sea using flow injection with chemiluminescence detection. Anal. Chim. Acta 1998, 371, 205–215. [Google Scholar] [CrossRef]
- Hopwood, M.J.; Rapp, I.; Schlosser, C.; Achterberg, E.P. Hydrogen peroxide in deep waters from the Mediterranean Sea, South Atlantic and South Pacific Oceans. Sci. Rep. 2017, 7, 43436. [Google Scholar] [CrossRef] [PubMed]
- Tüğsüz, T.; Gök, E.; Ateş, S. Determination of H2O2 Content of Various Water Samples Using a Chemiluminescence Technique. Turkish J. Chem. 2003, 27, 41–47. [Google Scholar]
- Gonçalves, C.; dos Santos, M.A.; Fornaro, A.; Pedrotti, J.J. Hydrogen Peroxide in the Rainwater of Sao Paulo Megacity: Measurements and Controlling Factors. J. Braz. Chem. Soc. 2010, 21, 331–339. [Google Scholar] [CrossRef]
- Akbari Khorami, H.; Wild, P.; Brolo, A.G.; Djilali, N. pH-Dependent response of a hydrogen peroxide sensing probe. Sens. Actuators B Chem. 2016, 237, 113–119. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Huo, F.; Ning, P.; Meng, X.; Yin, C. Reversible Ratiometric Fluorescent Probe for Sensing Bisulfate/H2O2 and Its Application in Zebrafish. Anal. Chem. 2017, 89, 8079–8083. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S.; Schäferling, M.; Dürkop, A. Reversible Optical Sensor Membrane for Hydrogen Peroxide Using an Immobilized Fluorescent Probe, and its Application to a Glucose Biosensor. Microchim. Acta 2003, 143, 221–227. [Google Scholar] [CrossRef]
- Rakicioglu, Y.; Perrin, J.H.; Schulman, S.G. Increased luminescence of the tetracycline–europium(III) system following oxidation by hydrogen peroxide. J. Pharm. Biomed. Anal. 1999, 20, 397–399. [Google Scholar] [CrossRef]
- Dehaen, G.; Absillis, G.; Driesen, K.; Binnemans, K.; Parac-Vogt, T.N. (Tetracycline) europium(III) Complex as Luminescent Probe for Hydrogen Peroxide Detection. Helv. Chim. Acta 2009, 92, 2387–2397. [Google Scholar] [CrossRef]
- Voraberger, H.S.; Trettnak, W.; Ribitsch, V. Optochemical hydrogen peroxide sensor based on oxygen detection. Sens. Actuators B 2003, 90, 324–331. [Google Scholar] [CrossRef]
- Posch, H.E.; Wolfbeis, O.S. Optical Sensor for Hydrogen Peroxide. Mikrochim. Acta 1989, 1, 41–50. [Google Scholar] [CrossRef]
- Rusak, S.A.; Richard, L.E.; Peake, B.M.; Cooper, W.J. Steady state hydrogen peroxide concentrations across the Subtropical Convergence east of New Zealand. In Proceedings of the ASLO 2007 Aquatic Sciences Meeting, Santa Fe, NM, USA, 4–9 February 2005; pp. 3–4. [Google Scholar]
- Yuan, J.; Shiller, A.M. Distribution of hydrogen peroxide in the northwest Pacific Ocean. Geochem. Geophys. Geosyst. 2005, 6, 1–13. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ushiroda, T.; Takeda, K.; Kumamoto, Y.-I.; Tsubota, H. Diurnal and seasonal dirstribution of hydrogen peroxide in seawater of the Seto Inland Sea. Geochem. J. 1993, 27, 103–115. [Google Scholar] [CrossRef]
- Zika, R.; Saltzman, E.; Chameides, W.L.; Davis, D.D. H2O2 Levels in Rainwater Collected in South Florida and the Bahama Islands. J. Geophys. Res. 1982, 87, 5015–5017. [Google Scholar] [CrossRef]
- Kelly, T.J.; Daum, P.H.; Schwartz, S.E. Measurements of Peroxides in Cloudwater and Rain. J. Geophys. Res. 1985, 90, 7861–7871. [Google Scholar] [CrossRef]
- Jacob, P.; Klockow, D. Measurements of hydrogen peroxide in Antarctic ambient air, snow and firn cores. Anal. Chem. 1993, 429–434. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.; Petasne, R.G.; Plane, J.M.C. Photochemical Formation of H2O2 in Natural Waters Exposed to Sunlight. Environ. Sci. Technol. 1988, 22, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Meslé, M.M.; Beam, J.P.; Jay, Z.J.; Bodle, B.; Bogenschutz, E.; Inskeep, W.P. Hydrogen Peroxide Cycling in High-Temperature Acidic Geothermal Springs and Potential Implications for Oxidative Stress Response. Front. Mar. Sci. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Ryan, C.S.; Kleinberg, I. Bacteria in Human Mouths Involved in the Production and Utilization of Hydrogen Peroxide. Arch. Oral Biol. 1995, 40, 753–763. [Google Scholar] [CrossRef]
- Long, L.H.; Evans, P.J.; Halliwell, B. Hydrogen Peroxide in Human Urine: Implications for Antioxidant Defense and Redox Regulation. Biochem. Biophys. Res. Commun. 1999, 262, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Madhusoodanan, U.K.; Nayak, S.; Jacob, J. Urinary hydrogen peroxide: A probable marker of oxidative stress in malignancy. Clin. Chim. Acta 2003, 334, 205–209. [Google Scholar] [CrossRef]
- Sznajder, I.J.; Fraiman, A.; Hall, J.B.; Sanders, W.; Schmidt, G.; Crawford, G.; Nahum, A.; Factor, P.; Wood, L.D.H. Increased Hydrogen Peroxide in the Expired Breath of Patients with Acute Hypoxemic Respiratory Failure. Clin. Investig. Crit. Care 1969, 96, 606–612. [Google Scholar] [CrossRef]
- Loukides, S.; Horvath, I.; Wodehouse, T.; Cole, P.J.; Barnes, P.J. Elevated Levels of Expired Breath Hydrogen Peroxide in Bronchiectasis. Am. J. Respir. Crit. Care Med. 1998, 158, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Jöbsis, R.Q.; Schellekens, S.L.; Fakkel-Kroesbergen, A.; Raatgeep, R.H.C.; de Jongste, J.C. Hydrogen peroxide in breath condensate during a common cold. Mediators Inflamm. 2001, 10, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Kronseder, A.; Karrasch, S.; Neff, P.A.; Haaks, M.; Koczulla, A.R.; Reinhold, P.; Nowak, D.; Jörres, R.A. Hydrogen peroxide in exhaled air: A source of error, a paradox and its resolution. EJR Open Res. 2016, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Yamamoto, Y.; Niclas, D.; Ames, B.N. Evaluation of an lsoluminol Chemiluminescence Assay for the Detection of Hydroperoxides in Human Blood Plasma. Anal. Biochem. 1988, 175, 120–130. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brodsky, M.H.; Baker, J.C.; Ames, B.N. Detection and Characterization of Lipid Hydroperoxides at Picomole Levels by High-Performance Liquid Chromatography. Anal. Biochem. 1987, 160, 7–13. [Google Scholar] [CrossRef]
- Varma, S.D.; Devamanoharan, P.S. Hydrogen Peroxide in Human Blood. Free Radic. Res. Commun. 1990, 14, 125–131. [Google Scholar] [CrossRef]
- Lacy, F.; Connor, D.T.O.; Schmid-Schönbein, G.W. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J. Hypertens. 1998, 16, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Deskur, E.; Dylewicz, P.; Rychlewski, T.; Wilk, M.; Wysocki, H. Exercise-induced increase in hydrogen peroxide plasma levels is diminished by endurance training after myocardial infarction. Int. J. Cardiol. 1998, 67, 219–224. [Google Scholar] [CrossRef]
- Lacy, F.; Kailasam, M.T.; Connor, D.T.O.; Schmid-scho, G.W.; Parmer, R.J. Plasma Hydrogen Peroxide Production in Human Essential Hypertension: Role of Heredity, Gender and Ethnicity. Hypertension 2000, 36, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Wierusz-Wysocka, B.; Wysocki, H.; Byks, H.; Zozulińska, D.; Wykretowicz, A.; Kaźmierczak, M. Metabolic control quality and free radical activity in diabetic patients. Diabetes Res. Clin. Pract. 1995, 27, 193–197. [Google Scholar] [CrossRef]
- Tsukimori, K.; Yoshitomi, T.; Morokuma, S.; Fukushima, K.; Wake, N. Serum Uric Acid Levels Correlate with Plasma Hydrogen Peroxide and Protein Carbonyl Levels in Preeclampsia. Am. J. Hypertens. 2008, 21, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Hochstein, P.; Davies, K.J.A. Hydrogen Peroxide Production by Red Blood Cells. Free Radic. Biol. Med. 1994, 16, 123–129. [Google Scholar] [CrossRef]
- Mapson, L.W. Influence of Halides on the Oxidation of Ascorbic Acid. Biochem. J. 1945, 39, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Pirie, A. Glutathione Peroxidase in Lens and a Source of Hydrogen Peroxide in Aqueous Humour. Biochem. J. 1965, 96, 244–253. [Google Scholar] [CrossRef] [PubMed]
- García-Castiñeiras, S.; Velázques, S.; Martínez, P.; Torres, N. Humor Hydrogen Peroxide Dichlorophenol-indophenol. Exp. Eye Res. 1992, 55, 9–19. [Google Scholar] [CrossRef]
- Spector, A.; Garner, W.H. Hydrogen Peroxide and Human Cataract. Exp. Eye Res. 1981, 33, 673–681. [Google Scholar] [CrossRef]
- Armoza-Zvuloni, R.; Schneider, A.; Sher, D.; Shaked, Y. Rapid Hydrogen Peroxide release from the coral Stylophora pistillata during feeding and in response to chemical and physical stimuli. Sci. Rep. 2016, 6, 21000. [Google Scholar] [CrossRef] [PubMed]
- Armoza-Zvuloni, R.; Schneider, A.; Shaked, Y. Rapid Hydrogen Peroxide Release during Coral-Bacteria Interactions. Front. Mar. Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Goyen, S.; Pernice, M.; Szabó, M.; Warner, M.E.; Ralph, P.J.; Suggett, D.J. A molecular physiology basis for functional diversity of hydrogen peroxide production amongst Symbiodinium spp. (Dinophyceae). Mar. Biol. 2017, 164, 1–12. [Google Scholar] [CrossRef]
- Abrahamsson, K.; Choo, K.; Pedersén, M.; Johansson, G.; Snoeijs, P. Effects of temperature on the production of hydrogen peroxide and volatile halocarbons by brackish-water algae. Phytochemistry 2003, 64, 725–734. [Google Scholar] [CrossRef]
- Collén, J.; Jiménez Del Río, M.; García-Reina, G.; Pedersén, M. Photosynthetic production of hydrogen peroxide by Ulva rigida C. Ag. (Chlorophyta). Planta 1995, 196, 225–230. [Google Scholar] [CrossRef]
- Patterson, P.C.O.; Myers, J. Photosynthetic Production of Hydrogen Peroxide by Anacystis nidulans. Plant Physiol. 1973, 51, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kalavathi, D.F.; Subramanian, G. Hydrogen Peroxide Photoproduction by A Marine Cyanobacterium Oscillatoria boryana BDU 92181 with Potential Use in Bioremediation. Biosci. Biotechnol. Res. Asia 2013, 10, 929–935. [Google Scholar] [CrossRef]
- Suggett, D.J.; Warner, M.E.; Smith, D.J.; Davey, P.; Hennige, S.; Baker, N.R. Photosynthesis and Production of Hydrogen Peroxide by Symbiodinium (Pyrrhophyta) Phylotypes with Different Thermal Tolerance. J. Phycol. 2008, 44, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Mishin, V.; Gray, J.P.; Heck, D.E.; Laskin, D.L.; Laskin, J.D. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic. Biol. Med. 2010, 48, 1485–1491. [Google Scholar] [CrossRef] [PubMed]

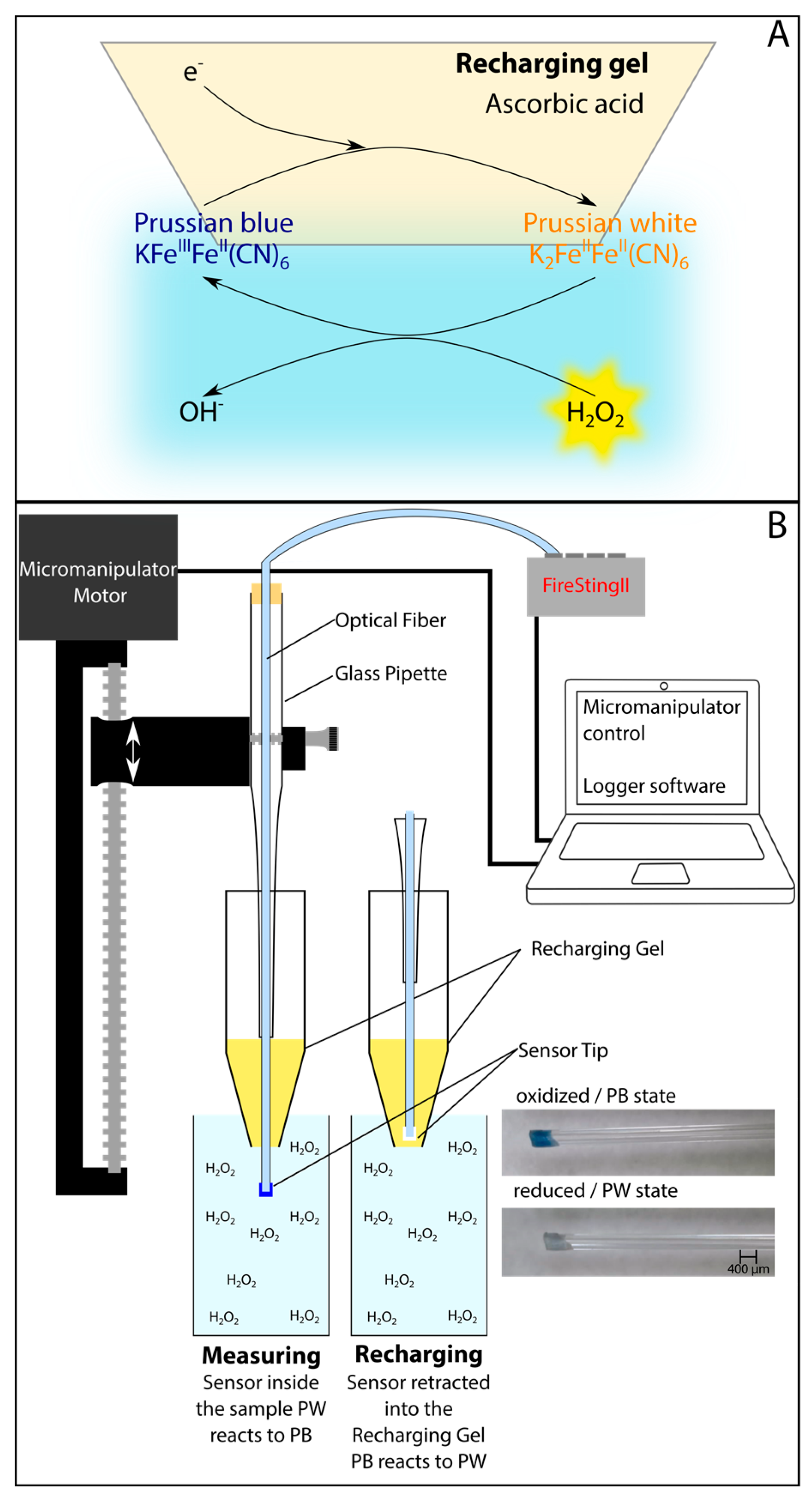
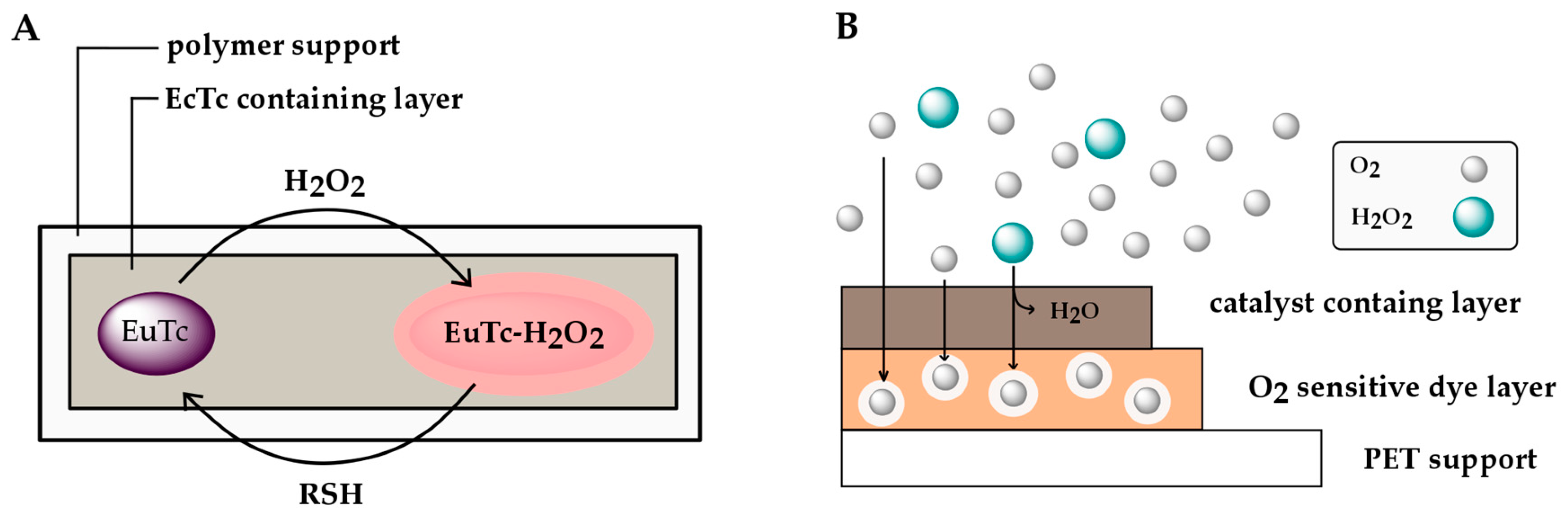
| Technique | Advantages | Disadvantages/Limitations |
|---|---|---|
| Intracellular H2O2 Measurements | ||
| Oxidative Cleavage-Based Probes | Easily tunable emission spectrum (by changing the dye backbone or substituents), Accumulative H2O2 measurement, Can show hotspots, Can be used for comparison of treatments, Fast response times, High signal-to-background ratios. | Irreversible Cross-sensitivities (e.g., other ROS, pH, temperature) No absolute concentrations, Cannot be calibrated in situ, No in situ referencing, In situ measurements do not start at a defined time point, Issue of cytotoxicity and chemical perturbation effects. |
| Nanomaterials | Highly and easily tunable properties, Do not necessarily rely on diffusion into the cell, Can be water-soluble or insoluble, Long fluorescent lifetimes, High photo-stability. | Irreversible, Some QDs contain heavy metals (cytotoxicity and problematic for environment), Cross-sensitivities to other species, temperature or pH (dependent on material), Cannot be calibrated in situ, In situ measurements do not start at a defined time point. |
| Genetically Encoded Probes | Reversible reaction, Dynamic changes can be monitored, Can be tailored to target specific subcellular compartments Allow ratiometric read-out. | pH sensitive, No absolute concentration, Cannot be calibrated in situ, Can only be used intracellular, Some show cross-sensitivities to other oxidants, Needs to be genetically encoded. |
| Extracellular H2O2 Measurements | ||
| Chemiluminescent Reactions | High signal-to-background ratio, Spectral properties can be easily adjusted. | Irreversible, Temperature dependency, Reaction dependent interferences (e.g., Ag(I), Cu(II), Co(II), Fe(II), pH, ROS, hydroperoxides…). |
| Assays | Well established for biological fluids, Allows monitoring of H2O2 dynamics over time. | Irreversible, Cross-sensitivities (e.g., NADH, reduced glutathione, ROS, pH, phenols…), Low temporal-resolution, Samples need to be taken prior to measurement. |
| Intermediate H2O2 Sensing Systems | ||
| Flow Injection Analysis (FIA) | Allows continuous measurement, Can counterbalance e.g., pH or Fe(II) cross-sensitivity of normal chemiluminescent reactions, Allows quantitative H2O2 measurement. | Point measurement (limited spatial and temporal resolution), Sample needs to be free of aggregates (filtering, or other sample treatment required), Confined to liquid samples, Sample is used up (relatively large sample volume needed), Temperature dependency. |
| Redox Systems | Can be reversed externally, Allow pseudo-continuous measurements, Sensor material is not used up, Insight into redox processes, Allow quantitative measurements. | Can react with other oxidizing/reducing species, Other species can interfere within the system Possible pH dependencies. |
| Quasi Reversible Optical H2O2 Sensors | ||
| (tetracycline)europium(III) (EuTc) | Reversible, Sensor material is not used up (coordination of H2O2). | External source needed to reverse, Reversible with water or thiosulfate Slow response time (~10 min), Quenched by Cu2+, Interferences from phosphate and citrate. |
| Indirect H2O2 Determination via O2 Measurements | Fully reversible, Allows continuous monitoring, Well established chemistry, Allows quantitative H2O2 measurement. | Low signal against a high background, Fluctuations in O2 need to be accounted for. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moßhammer, M.; Kühl, M.; Koren, K. Possibilities and Challenges for Quantitative Optical Sensing of Hydrogen Peroxide. Chemosensors 2017, 5, 28. https://doi.org/10.3390/chemosensors5040028
Moßhammer M, Kühl M, Koren K. Possibilities and Challenges for Quantitative Optical Sensing of Hydrogen Peroxide. Chemosensors. 2017; 5(4):28. https://doi.org/10.3390/chemosensors5040028
Chicago/Turabian StyleMoßhammer, Maria, Michael Kühl, and Klaus Koren. 2017. "Possibilities and Challenges for Quantitative Optical Sensing of Hydrogen Peroxide" Chemosensors 5, no. 4: 28. https://doi.org/10.3390/chemosensors5040028
APA StyleMoßhammer, M., Kühl, M., & Koren, K. (2017). Possibilities and Challenges for Quantitative Optical Sensing of Hydrogen Peroxide. Chemosensors, 5(4), 28. https://doi.org/10.3390/chemosensors5040028




