Simple and Sensitive Electrochemical Sensor-Based Three-Dimensional Porous Ni-Hemoglobin Composite Electrode
Abstract
:1. Introduction
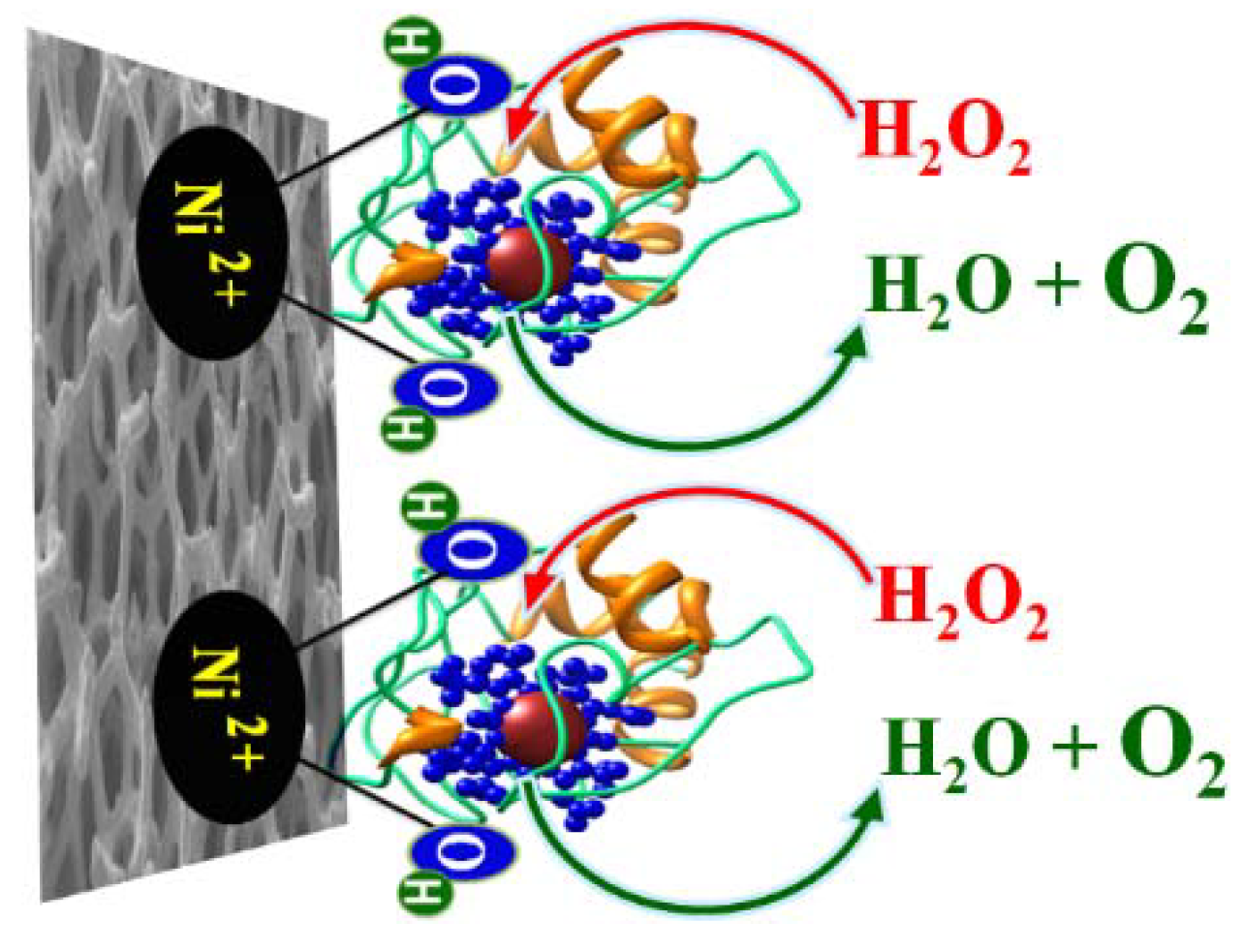
2. Experimental Section
2.1. Chemicals
2.2. Preparation of the Hb-Modified Ni Foam Electrode
2.3. Instrumentation
2.4. Materials Characterization
2.5. Determination of H2O2 in a Real Sample
3. Results and Discussion
3.1. Structural Features of Hb-Modified Ni Foam
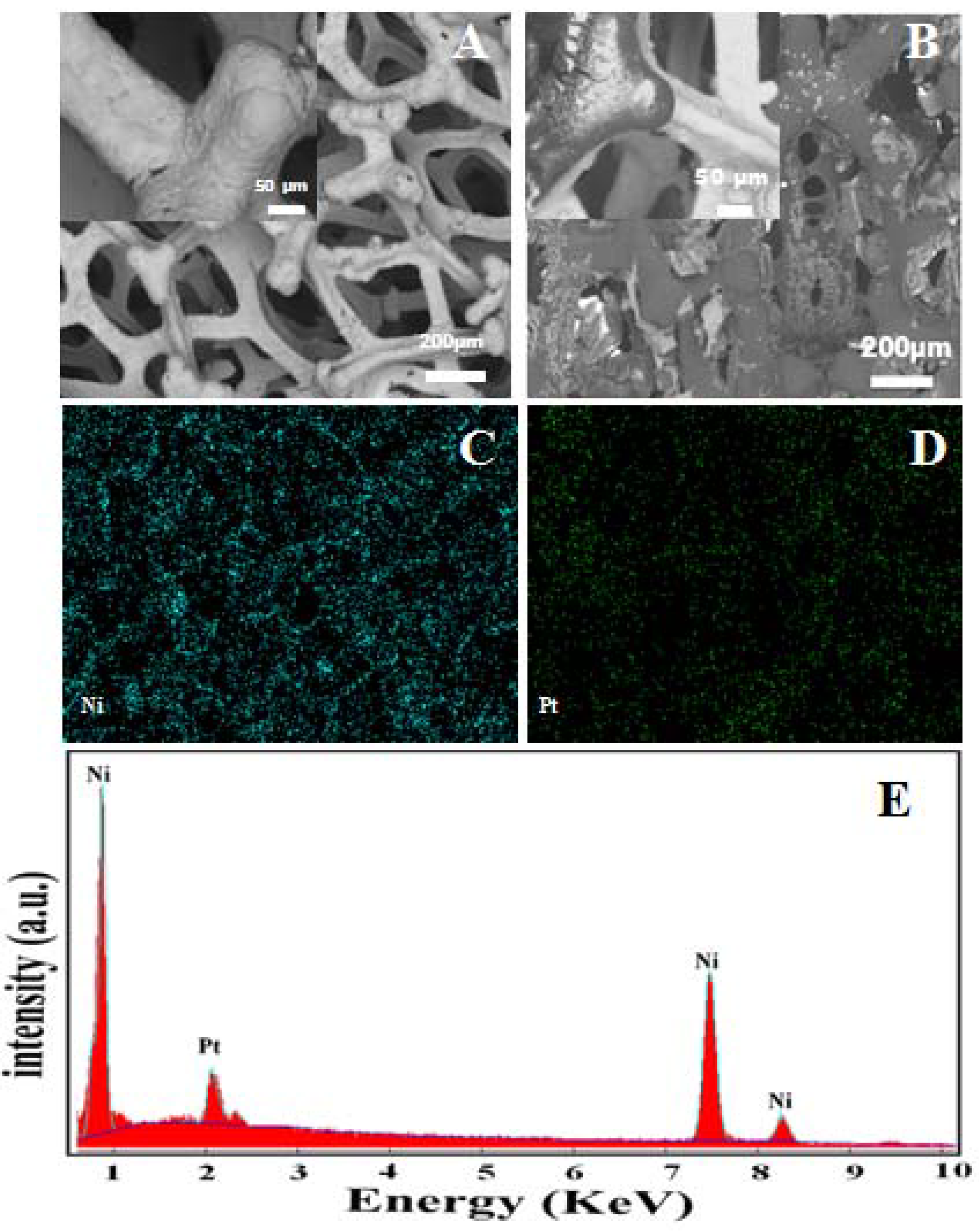
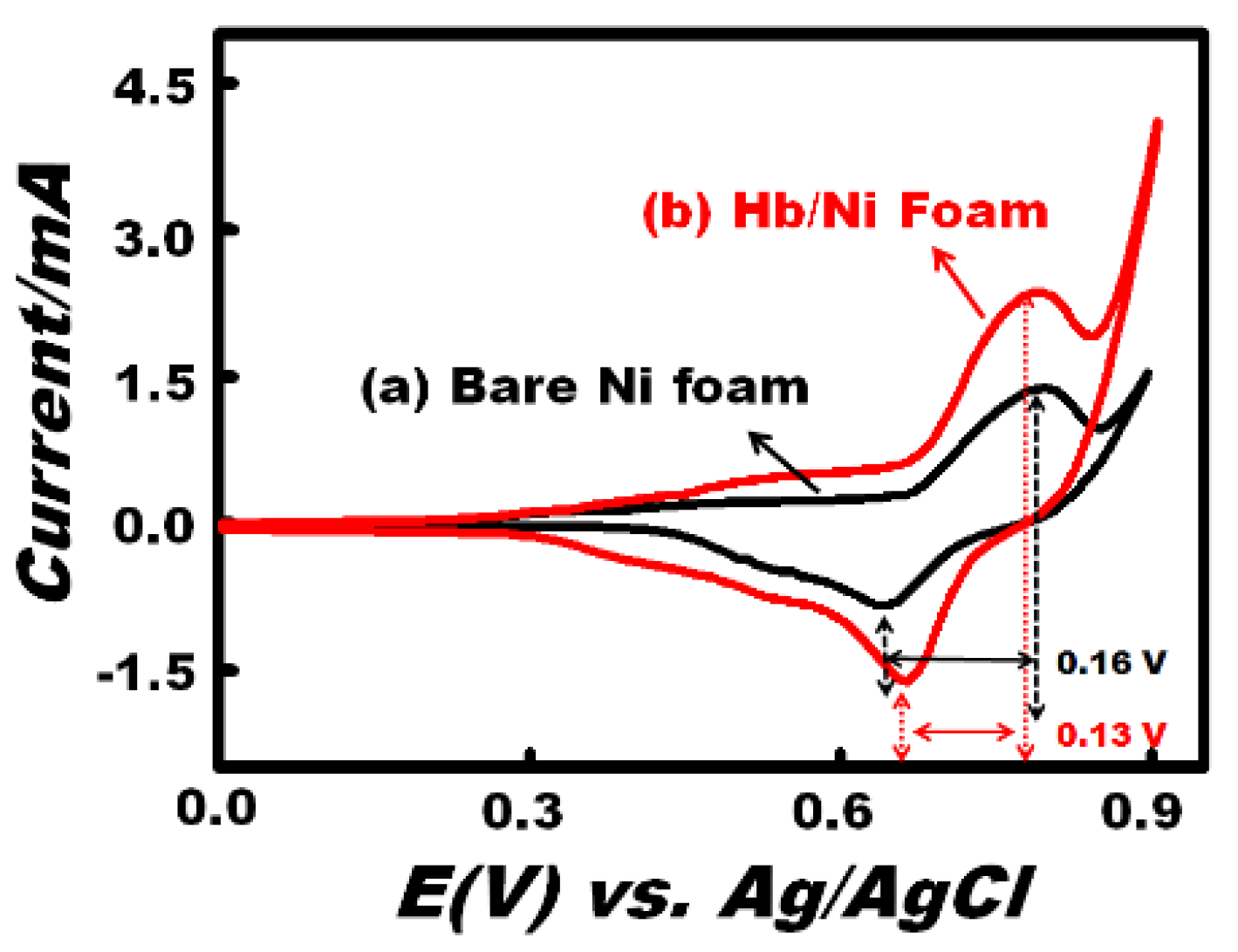
3.2. Electrochemical Mechanism of the Working Electrode
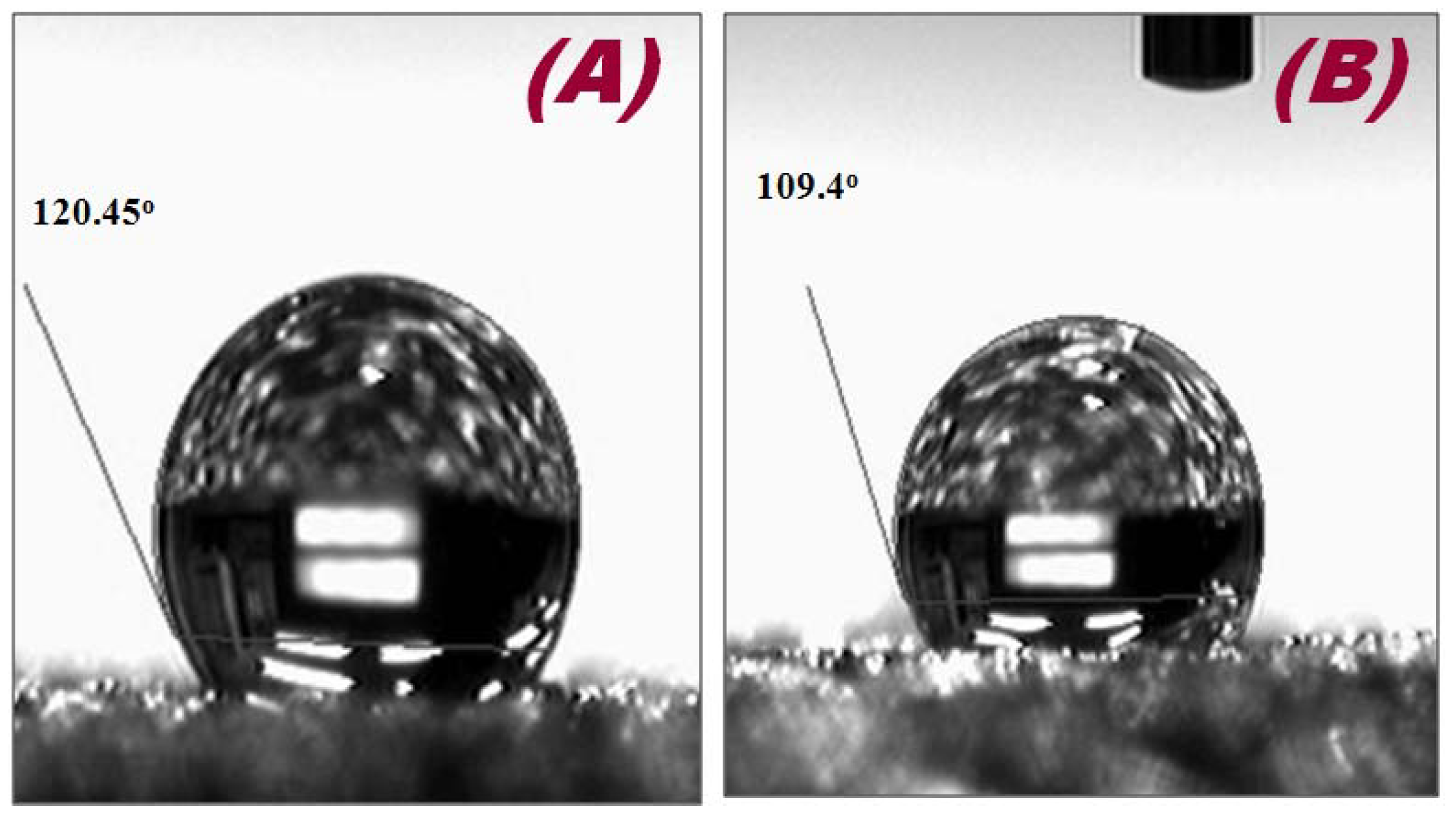
| Sensor | Contact Angle | Surface Energy (m·Jm−2) | Surface Tension (N/m) |
|---|---|---|---|
| Ni foam | 120.45° | 4.40 | 14.40 |
| Hb/Ni foam | 109.45° | 8.06 | 19.71 |
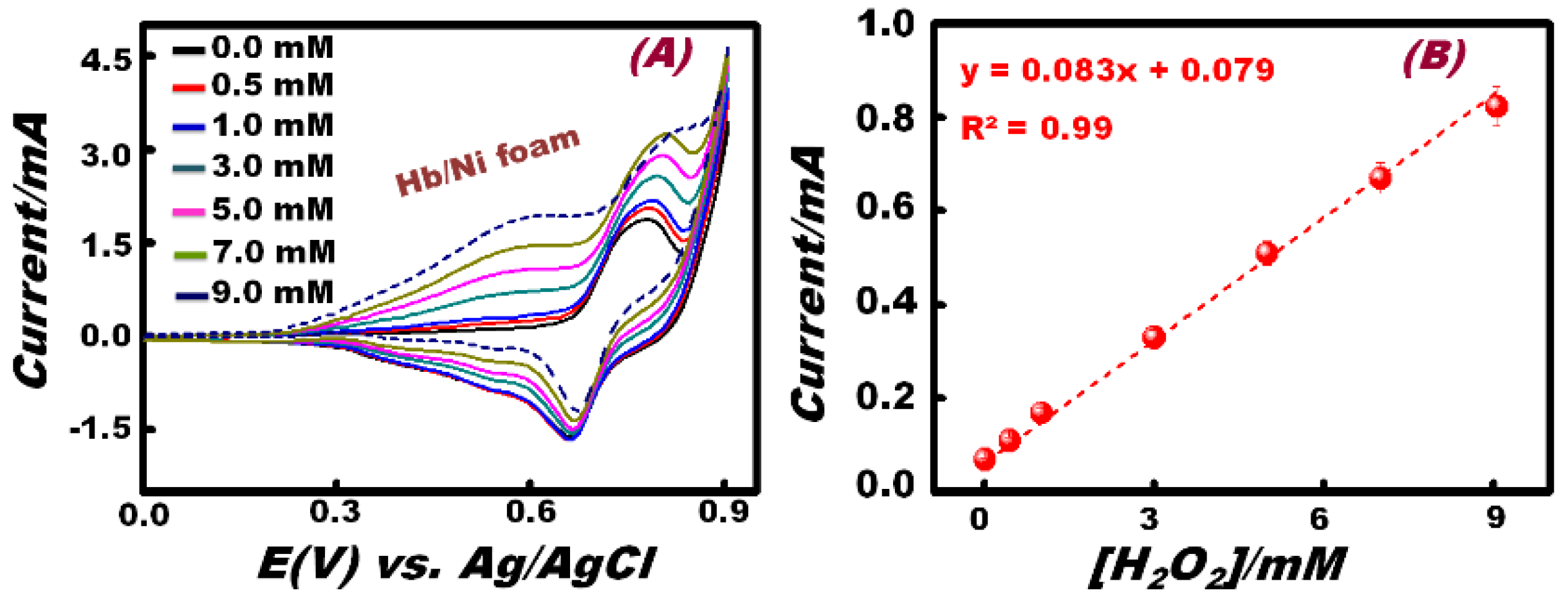
3.3. Sensitivity of the Working Electrode
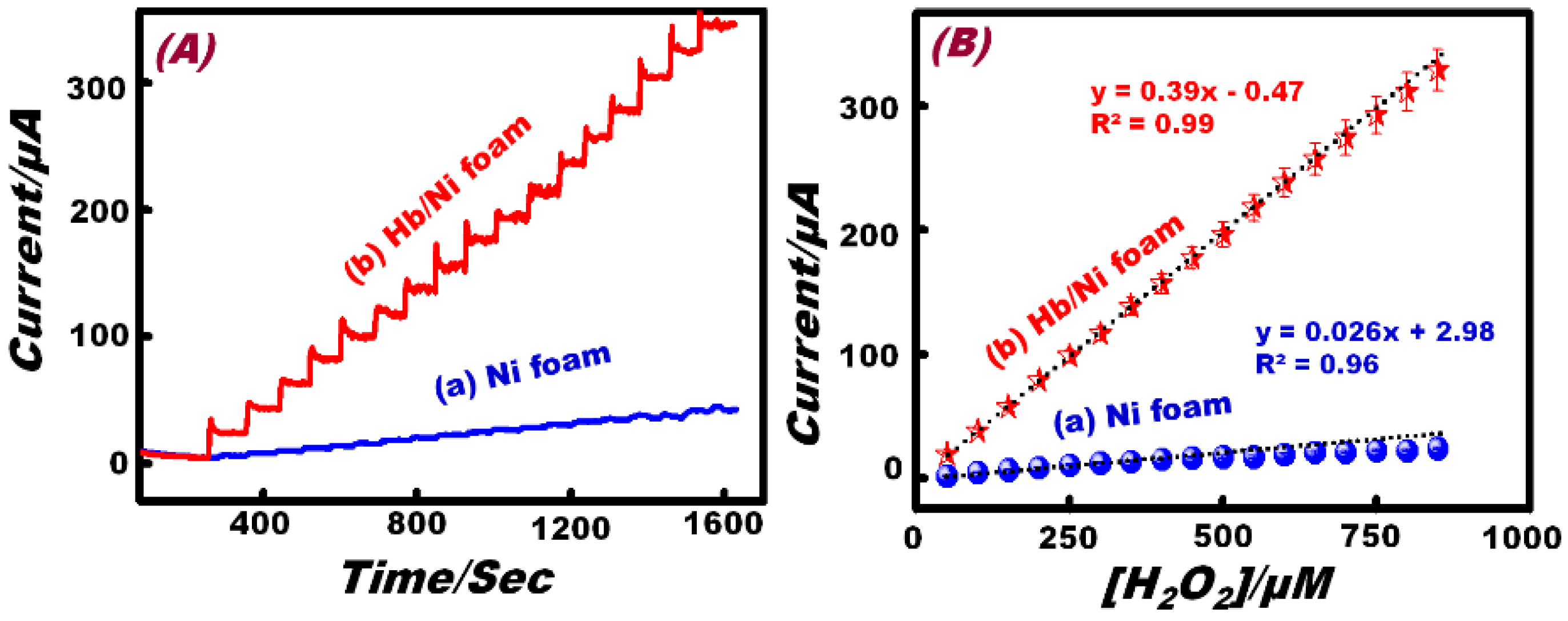
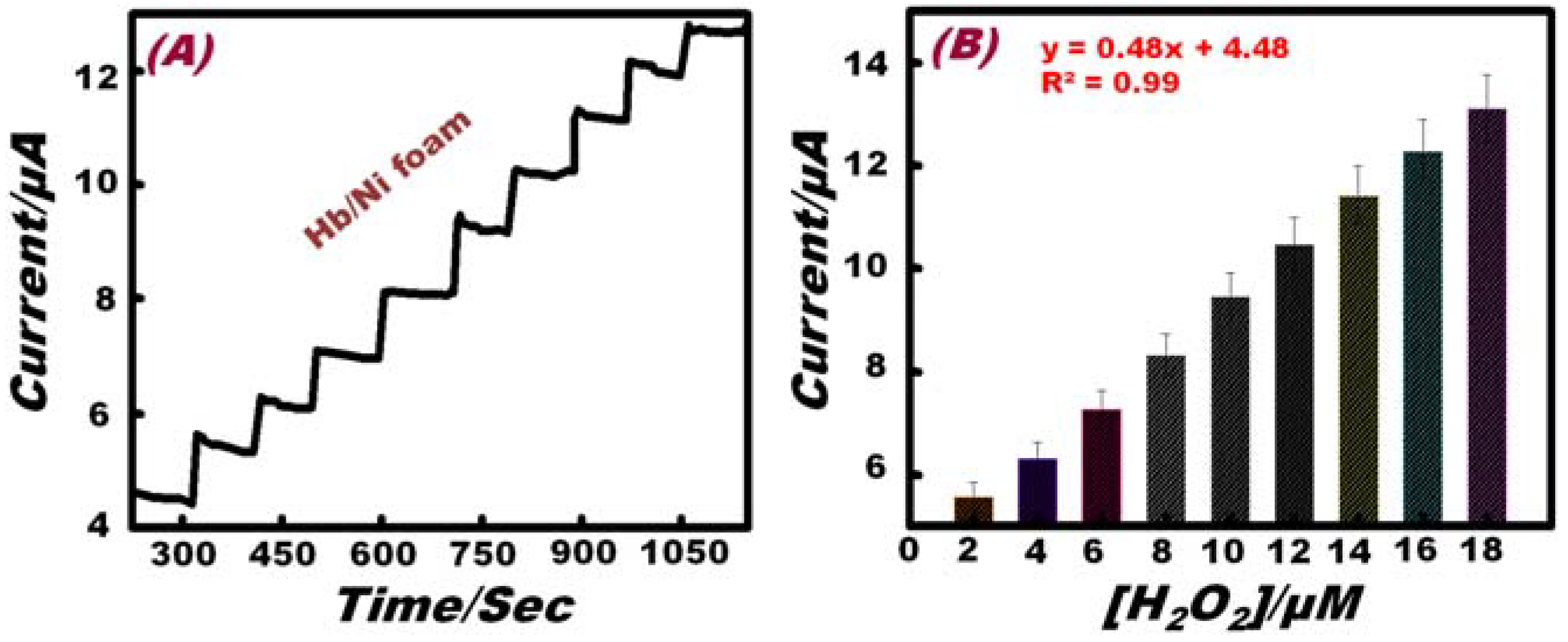
| Sensing Material | Electrolyte | Linear Range (M) | Limit of Detection (M) | References |
|---|---|---|---|---|
| PdNPs/PEDOT * | PBS | 2.5 × 10−6~1.0 × 10−3 | 2.84 × 10−6 | [39] |
| nano-CuO/Nf-coated Pt | 0.1 M NaOH | 1.5 × 10−7~9 × 10−3 | 0.6 × 10−7 | [40] |
| NiO/CPE* | 0.1 M NaOH | 0.6~6 × 10−3 | 0.34 × 10−3 | [41] |
| NiO-NPs/cMWCNT/PANI* | PBS | 3 × 10−6~7 × 10−3 | 0.2 × 10−6 | [42] |
| Hb/Ni foam | 0.1 M NaOH | 5 × 10−6~9 × 10−3 | 0.41 × 10−6 | Present study |
3.4. Selectivity of the Working Electrode

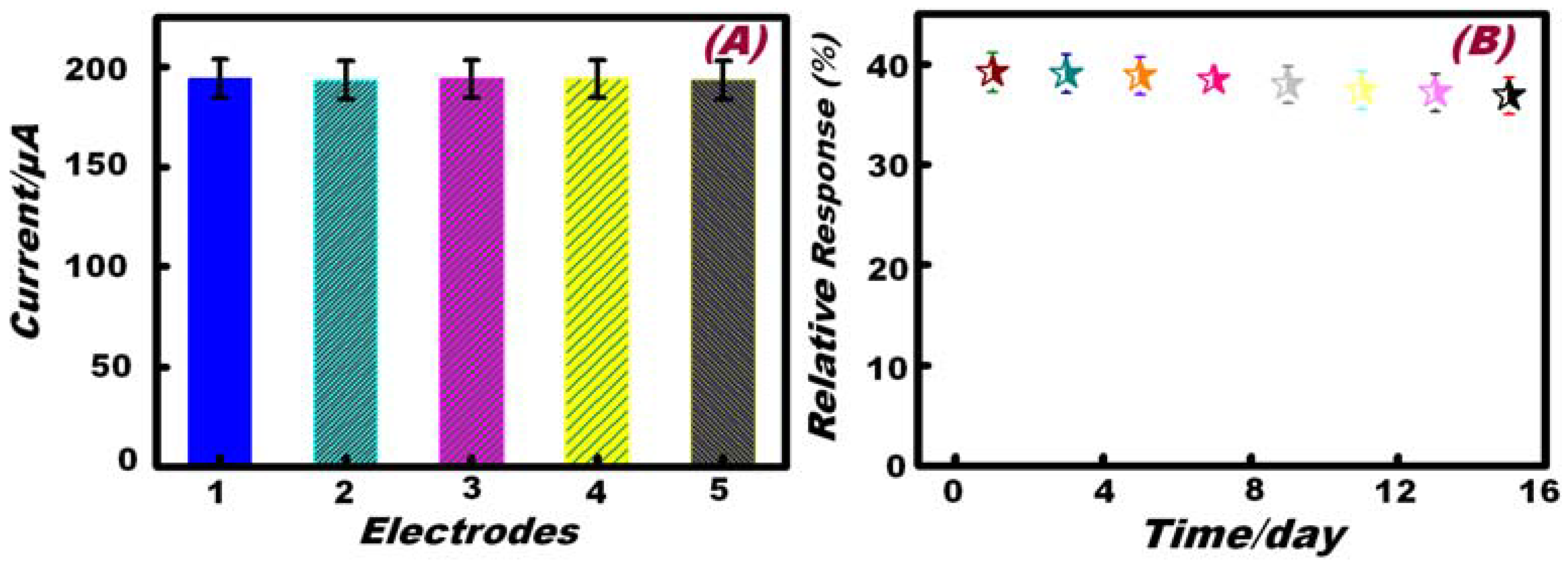
3.5. Reproducibility and Long-Term Stability of the Working Electrode
3.6. Determination of H2O2 in a Real Sample

4. Conclusions
Author Contributions
Conflicts of Interest
References
- El-Safty, S.A.; Shenashen, M.A.; Ismael, M.; Khairy, M. Mesocylindrical Aluminosilica Monolith Biocaptors for Size-Selective Macromolecule Cargos. Adv. Funct. Mater. 2012, 22, 3013–3021. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Ismail, A.A.; Matsunaga, H.; Hanaoka, T.; Mizukami, F. Optical Nanoscale Pool-on-Surface Design for Control Sensing Recognition of Multiple Cations. Adv. Funct. Mater. 2008, 18, 1485–1500. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Ismael, M.; Khairy, M.; Awual, M.R. Mesoporous aluminosilica sensors for the visual removal and detection of Pd(II) and Cu(II) ions. Microporous Mesoporous Mater. 2013, 166, 195–205. [Google Scholar] [CrossRef]
- El-Safty, S.A. Designs for Size-Exclusion Separation of Macromolecules by Densely Engineered Mesofilters. Trends Anal. Chem. 2011, 30, 447–458. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, X.; (David) Lou, X.W. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887. [Google Scholar] [CrossRef]
- Das, S.K.; El-Safty, S.A. Development of Mesoscopically Assembled Sulfated Zirconia Nanoparticles as Promising Heterogeneous and Recyclable Biodiesel Catalysts. ChemCatChem 2013, 5, 3050–3059. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A.; Ismael, M.; Kawarada, H. Mesoporous NiO nanomagnets as catalysts and separators of chemical agents. Appl. Catal. B Environ. 2012, 127, 1–10. [Google Scholar] [CrossRef]
- Balaji, T.; El-Safty, S.A.; Matsunaga, H.; Hanaoka, T.; Mizukami, F. Optical Sensors Based on Nanostructured Cage Materials for the Detection of Toxic Metal Ions. Angew. Chem. Int. Ed. 2006, 45, 7202–7208. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S. Mesoporous NiO nanoarchitectures for electrochemical energy storage: Influence of size, porosity, and morphology. RSC Adv. 2013, 3, 23801–23809. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, E. A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized on colloidal Au modified ITO electrode. Electrochem. Commun. 2004, 6, 225–229. [Google Scholar] [CrossRef]
- Zhou, M.; Diwu, Z.; Panchuk-Voloshina, N.; Haugland, R.P. A Stable Nonfluorescent Derivative of Resorufin for the Fluorometric Determination of Trace Hydrogen Peroxide: Applications in Detecting the Activity of Phagocyte NADPH Oxidase and Other Oxidases. Anal. Biochem. 1997, 253, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Demirata-Ozturk, B.; Ozen, G.; Filik, H.; Tor, I.; Afsar, H. Spectrofluorometric Determination of Hydrogen Peroxide. J. Fluoresc. 1998, 8, 185–189. [Google Scholar] [CrossRef]
- Han, J.H.; Jang, J.; Kim, B.K.; Choi, H.N.; Lee, W. Detection of hydrogen peroxide with luminol electrogenerated chemiluminescence at mesoporous platinum electrode in neutral aqueous solution. J. Electroanal. Chem. 2011, 660, 101–107. [Google Scholar] [CrossRef]
- Tanner, P.A.; Wong, A.Y.S. Spectrophotometric determination of hydrogen peroxide in rainwater. Anal. Chim. Acta 1998, 370, 279–287. [Google Scholar] [CrossRef]
- Luo, B.; Li, X.; Yang, J.; Li, X.; Xue, L.; Li, X.; Gu, J.; Wang, M.; Jiang, L. Non-enzymatic electrochemical sensors for the detection of hydrogen peroxide based on Cu2O/Cu nanocomposites. Anal. Methods 2014, 6, 1114–1120. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, R.; Chai, Y.; Li, W.; Ling, S. A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on a Polypyrrole Nanowire-Copper Nanocomposite Modified Gold Electrode. Sensors 2008, 8, 5141–5152. [Google Scholar] [CrossRef]
- Ansari, A.A.; Solanki, P.R.; Malhotra, B.D. Nanostructured metal oxides based enzymatic electrochemical biosensors. J. Biotechnol. 2009, 142, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Li, J.; Dong, S.J. Hemin functionalized graphene nanosheets based dual biosensor platforms for hydrogen peroxide and glucose. Sens. Actuators B Chem. 2011, 160, 295–300. [Google Scholar] [CrossRef]
- Haghighi, B.; Nikzad, R. Prussian blue modified carbon ionic liquid electrode: Electrochemical characterization and its application for hydrogen peroxide and glucose measurements. Electroanalysis 2009, 16, 1862–1868. [Google Scholar] [CrossRef]
- Song, M.J.; Hwang, S.W.; Whang, D. Non-enzymatic electrochemical CuO nanoflowers sensor for hydrogen peroxide detection. Talanta 2010, 80, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Wang, G.; Gu, J.; Zhang, X.; Fang, B. An unusual H2O2 electrochemical sensor based on Ni(OH)2 nanoplates grown on Cu substrate. Electrochim. Acta 2010, 55, 7182–7187. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S. Hemoproteins-nickel foam hybrids as effective supercapacitors. Chem. Commun. 2014, 50, 1356–1358. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A. Nanosized rambutan-like nickel oxides as electrochemical sensor and pseudocapacitor. Sens. Actuators B Chem. 2014, 193, 644–652. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Khairy, M. Bioadsorption of proteins on large mesocage-shaped mesoporous alumina monoliths. Colloids Surf. B Biointerfaces 2013, 103, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.R.; Williams, R.J.P. Nuclear-Magnetic-Resonance Studies of Ferrocytochrome C, pH and Temperature Dependence. Eur. J. Biochem. 1980, 103, 513–521. [Google Scholar] [CrossRef]
- Schlereth, D.; Mantele, W. Redox-induced conformational changes in myoglobin and hemoglobin: Electrochemistry and ultraviolet visible and fourier transform infrared difference spectroscopy at surface modified gold electrodes in an ultra thin layer spectroelectrochemical cell. Biochemistry 1992, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; El-Safty, S.; Ismael, M. Mesoporous nanomagnet supercaptors for selective heme proteins from human cells. Chem. Commun. 2012, 48, 10832–10834. [Google Scholar] [CrossRef]
- Lu, X.; Xiao, X.; Li, Z.; Xu, F.; Tan, H.; Sun, L.; Wang, L. A novel nonenzymatic hydrogen peroxide sensor based on three-dimensional porous Ni foam modified with a Pt electrocatalyst. Anal. Methods 2014, 6, 235–241. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, Z.; Zhang, J.; Peng, H.; Chen, X.; Hou, H.; Liu, C. Nickel hydroxide modified silicon nanowires electrode for hydrogen peroxide sensor applications. Electrochim. Acta 2012, 61, 148–153. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chem. Interfacial Electrochem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, K.; Xue, X.; Yin, J.; Wang, G.; Cao, D. Three-dimensional porous Ni film electrodeposited on Ni foam: High performance and low-cost catalytic electrode for H2O2 electrooxidation in KOH solution. Electrochim. Acta 2013, 107, 194–199. [Google Scholar] [CrossRef]
- Bunea, A.; Pavel, I.; David, S.; Gaspar, S. Modification with hemeproteins increases the diffusive movement of nanorods in dilute hydrogen peroxide solutions. Chem. Commun. 2013, 49, 8803–8805. [Google Scholar] [CrossRef]
- Murata, K.; Suzuki, M.; Nakamura, N.; Ohno, H. Direct evidence of electron flow via the heme c group for the direct electron transfer reaction of fructose dehydrogenase using a silver nanoparticle-modified electrode. Electrochem. Commun. 2009, 11, 1623–1626. [Google Scholar] [CrossRef]
- Van Oss, C.J. A Review of: “Wettability”; Berg, J.C., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 717–718. [Google Scholar]
- Kim, D.Y.; Kim, J.Y.; Chang, H.; Kim, M.S.; Leem, J.; Ballato, J.; Kim, S. Low-temperature growth of multiple-stack high-density ZnO nanoflowers/nanorods on plastic substrates. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Sanli, A.E.; Aytac, A. Response to disselkamp direct peroxide/peroxide fuel cell as a novel type fuel cell. Int. J. Hydrog. Energy 2011, 36, 869–875. [Google Scholar] [CrossRef]
- Epee, N.S.; Palacìn, M.R.; Delahaye-Vidal, A.; Chabre, Y.; Tarâscon, J.M. Evidence for direct γ-NiOOH ↔ β-Ni(OH)2 transitions during electrochemical cycling of the nickel hydroxide electrode. J. Electrochem. Soc. 1998, 145, 1434–1441. [Google Scholar]
- Jiang, F.; Yue, R.; Du, Y.; Xu, J.; Yang, P. A one-pot “green” synthesis of pd-decorated PEDOT nanospheres for nonenzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 2013, 44, 127–131. [Google Scholar]
- Miao, X.; Yuan, R.; Chai, Y.; Shi, Y.; Yuan, Y. Direct electrocatalytic reduction of hydrogen peroxide based on nafion and copper oxide nanoparticles modified pt electrode. J. Electroanal. Chem. 2008, 612, 157–163. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.B.; Norouzi, B. An Efficient Sensor for Determination of Concentrated Hydrogen Peroxide Based on Nickel Oxide Modified Carbon Paste Electrod. Int. J. Electrochem. Sci. 2012, 7, 1852–1863. [Google Scholar]
- Lata, S.; Batra, B.; Karwasra, N.; Pundir, C.S. An amperometric H2O2 biosensor based on cytochrome c immobilized onto nickel oxide nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode. Process Biochem. 2012, 47, 992–998. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Hoa, N.D.; Shenashen, M.A. Topical Developments of Nanoporous Membrane Filters for Ultrafine Noble Metal Nanoparticles. Eur. J. Inorg. Chem. 2012, 33, 5439–5450. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A.; Shenashen, M. Environmental Remediation and Monitoring of Cadmium. TrAC Trends Anal. Chem. 2014, 62, 56–68. [Google Scholar] [CrossRef]
- Liu, X.; Zweier, J.L. A real-time electrochemical technique for measurement of cellular hydrogen peroxide generation and consumption: Evaluation in human polymorphonuclear leukocytes. Free Radic. Biol. Med. 2001, 31, 894–901. [Google Scholar] [CrossRef] [PubMed]
- El-Safty, S.A.; Shenashen, M.A. Mercury-Ion Optical Sensors. Trends Anal. Chem. 2012, 48, 98–115. [Google Scholar] [CrossRef]
- Khairy, M.; El-Safty, S.A.; Shenashen, M.A.; Elshehy, E.A. Simultaneous Detection and Removal of Cadmium Ions from Different Environmental Matrices. J. Life Cycle Assess. 2014, 10, 126–141. [Google Scholar]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, Morphological Control, and Properties of Silver Nanoparticles in Potential Applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Abdellatef, S.; Ismael, M.; Shahat, A. Optical Nanosphere Sensor Based on Shell-by-Shell Fabrication for Removal of Toxic Metals from Human Blood. Adv. Healthc. Mater. 2013, 2, 854–862. [Google Scholar] [CrossRef] [PubMed]
- El-Safty, S.A.; Shenashen, M.A. Optical Mesosensor for Capturing of Fe(III) and Hg(II) Ions from Water and Physiological fluids. Sens. Actuator B Chem. 2013, 183, 58–70. [Google Scholar] [CrossRef]
- Elshehy, E.A.; El-Safty, S.A.; Shenashen, M.A. Reproducible Design for the Optical Screening and Sensing of Hg(II) Ions. Chemosensors 2014, 2, 219–234. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, N.; El-Safty, S.A.; Khairy, M. Simple and Sensitive Electrochemical Sensor-Based Three-Dimensional Porous Ni-Hemoglobin Composite Electrode. Chemosensors 2014, 2, 235-250. https://doi.org/10.3390/chemosensors2040235
Akhtar N, El-Safty SA, Khairy M. Simple and Sensitive Electrochemical Sensor-Based Three-Dimensional Porous Ni-Hemoglobin Composite Electrode. Chemosensors. 2014; 2(4):235-250. https://doi.org/10.3390/chemosensors2040235
Chicago/Turabian StyleAkhtar, Naeem, Sherif A. El-Safty, and Mohamed Khairy. 2014. "Simple and Sensitive Electrochemical Sensor-Based Three-Dimensional Porous Ni-Hemoglobin Composite Electrode" Chemosensors 2, no. 4: 235-250. https://doi.org/10.3390/chemosensors2040235
APA StyleAkhtar, N., El-Safty, S. A., & Khairy, M. (2014). Simple and Sensitive Electrochemical Sensor-Based Three-Dimensional Porous Ni-Hemoglobin Composite Electrode. Chemosensors, 2(4), 235-250. https://doi.org/10.3390/chemosensors2040235





