Influence of P(V3D3-co-TFE) Copolymer Coverage on Hydrogen Detection Performance of a TiO2 Sensor at Different Relative Humidity for Industrial and Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
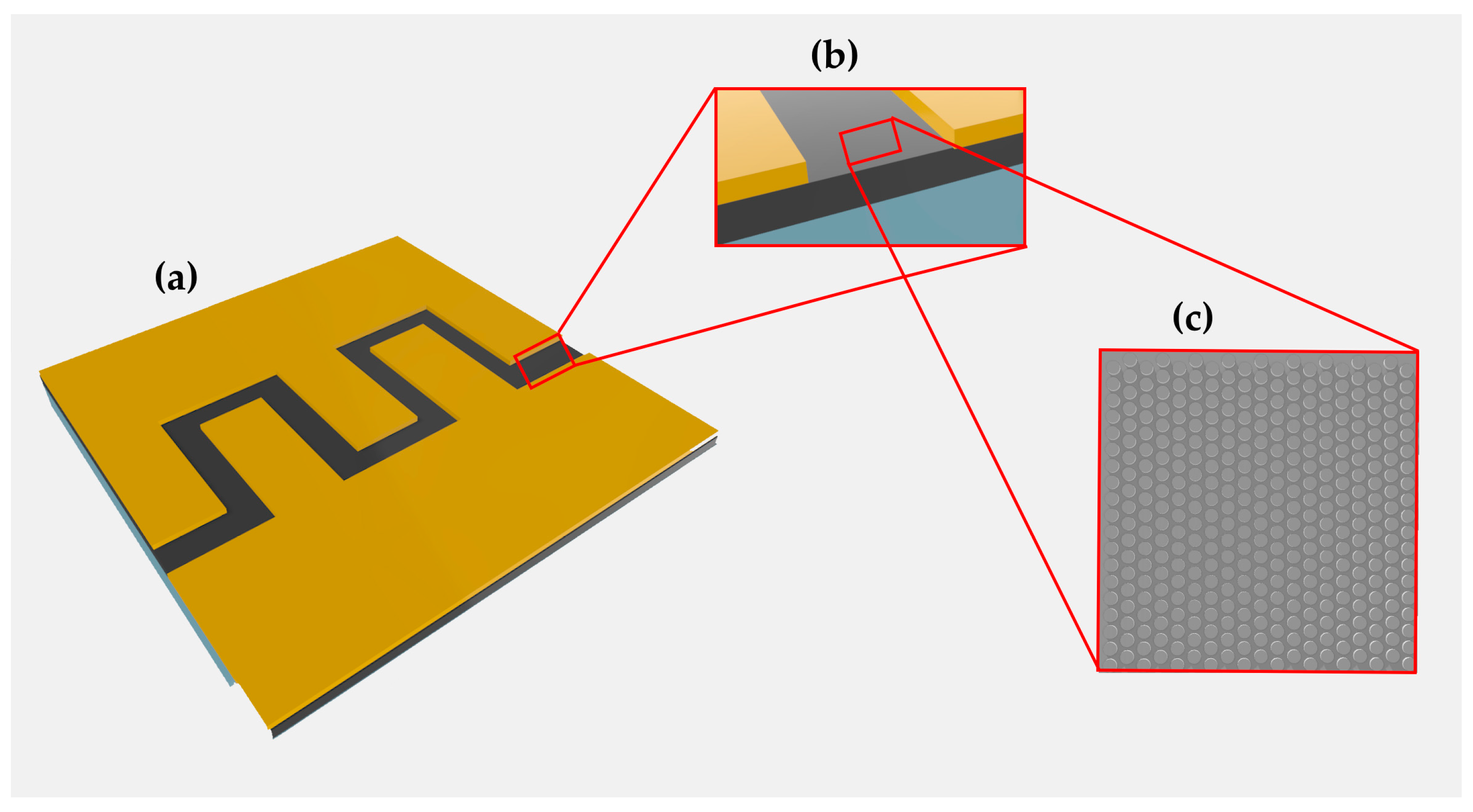
2.1. Sample Production
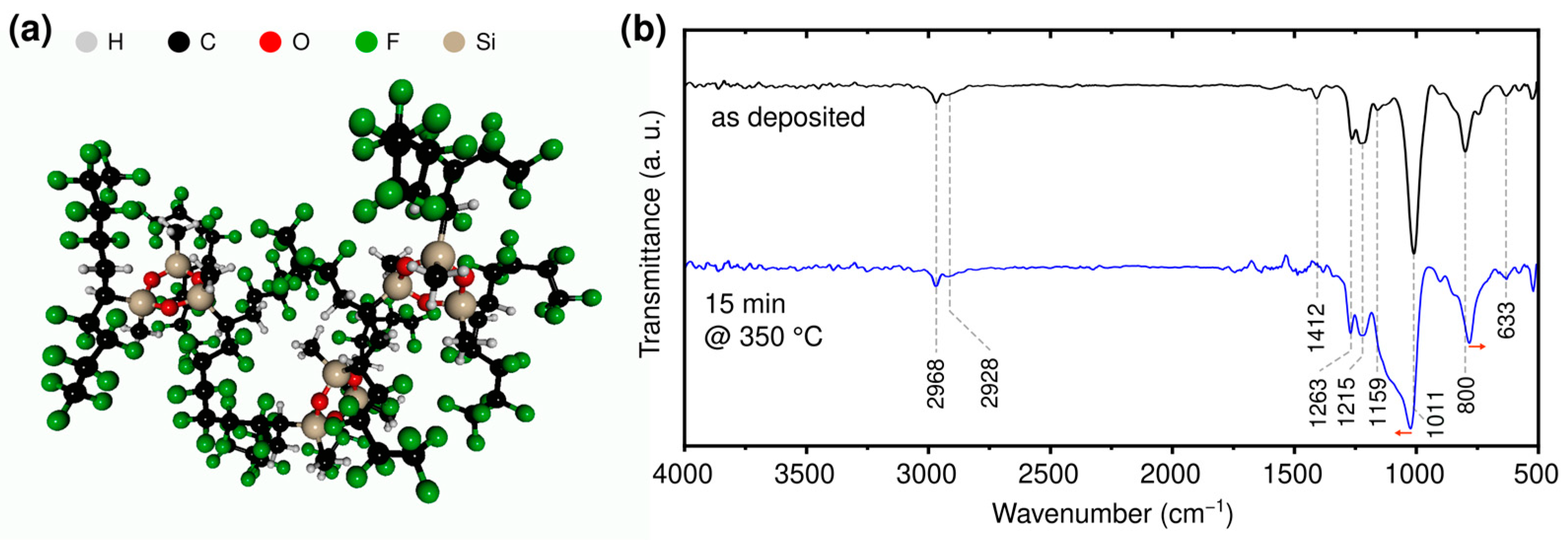
2.2. Computational
2.3. Sample Characterization
3. Results and Discussion
3.1. Chemical Characterization of the Fabricated Hybrid Sensors
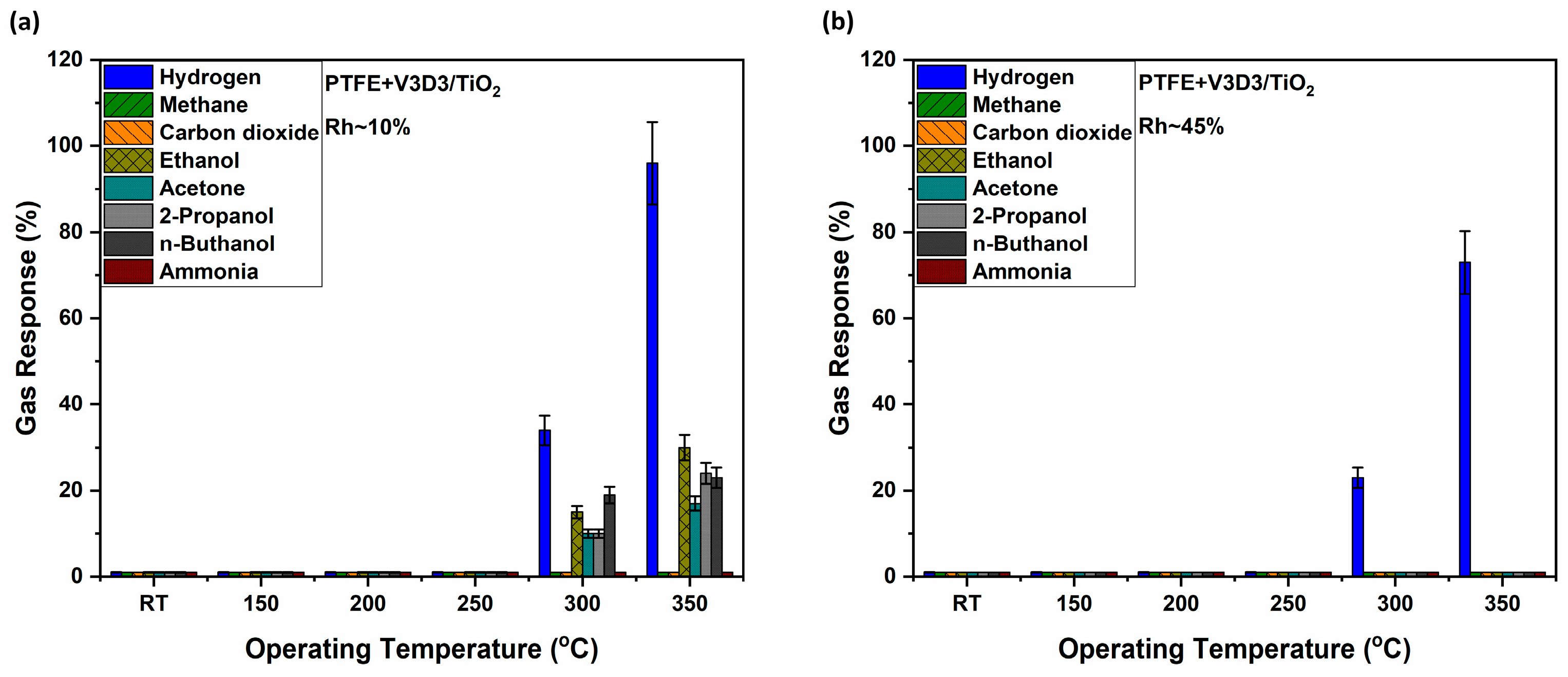
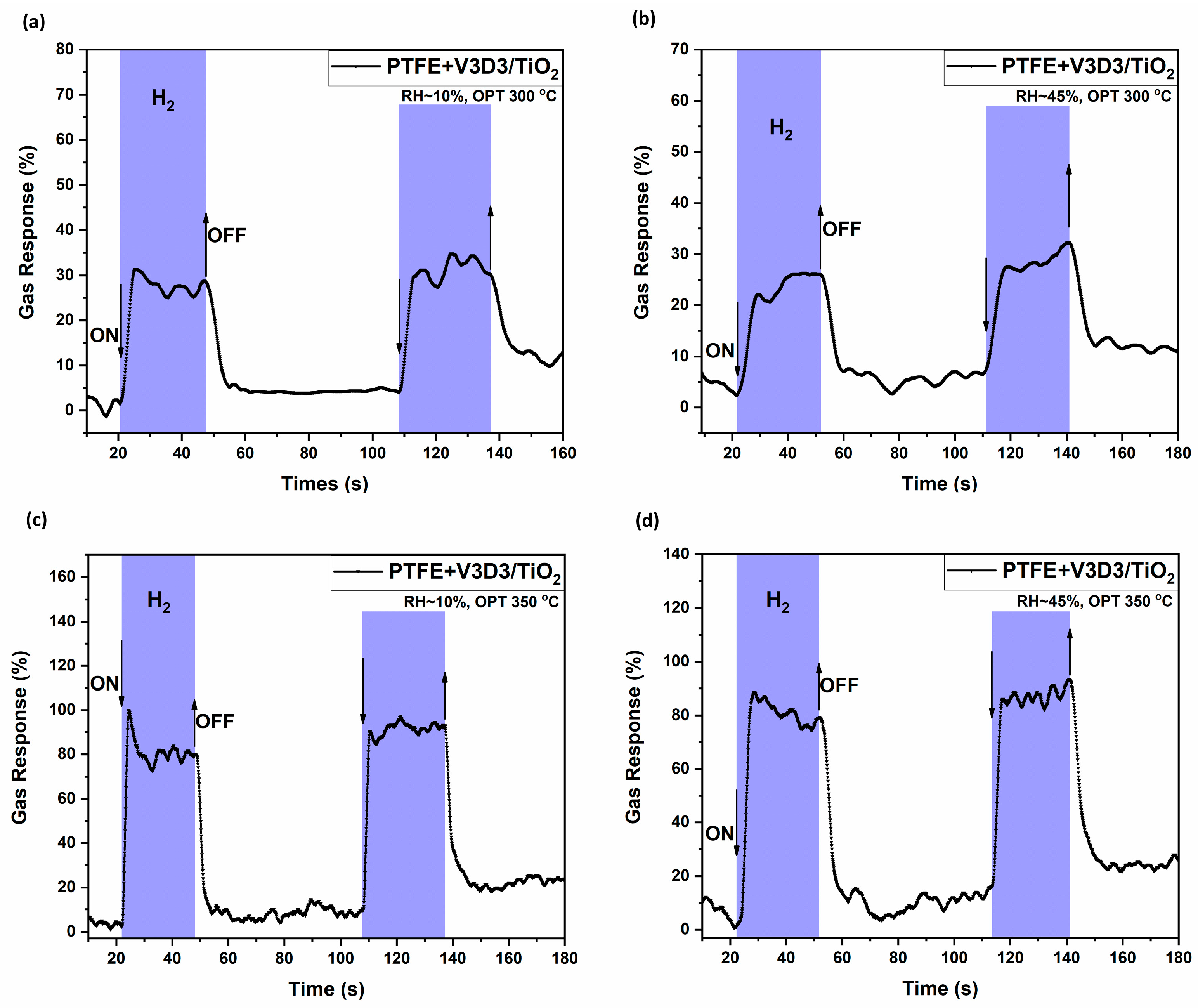
3.2. Gas Sensing Measurements and Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meixner, H.; Lampe, U. Metal Oxide Sensors. Sens. Actuators B Chem. 1996, 33, 198–202. [Google Scholar] [CrossRef]
- Lenz, J.; Edelstein, S. Magnetic Sensors and Their Applications. IEEE Sens. J. 2006, 6, 631–649. [Google Scholar] [CrossRef]
- Loock, H.-P.; Wentzell, P.D. Detection Limits of Chemical Sensors: Applications and Misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Okolie, J.A.; Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Futuristic Applications of Hydrogen in Energy, Biorefining, Aerospace, Pharmaceuticals and Metallurgy. Int. J. Hydrogen Energy 2021, 46, 8885–8905. [Google Scholar] [CrossRef]
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen Economy and Sustainable Development Goals: Review and Policy Insights. Curr. Opin. Green Sustain. Chem. 2021, 31, 100506. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen Storage: Recent Improvements and Industrial Perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V.G.; Natte, K.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Catalytic Reductive Aminations Using Molecular Hydrogen for Synthesis of Different Kinds of Amines. Chem. Soc. Rev. 2020, 49, 6273–6328. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen Peroxide: A Key Chemical for Today’s Sustainable Development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef]
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The Clinical Application of Hydrogen as a Medical Treatment. Acta Med. Okayama 2016, 70, 331–337. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liu, C.; Zhou, L.; Qu, K.; Wang, R.; Tai, M.; Lei Lei, J.W.; Wu, Q.F.; Wang, Z. A Review of Hydrogen as a New Medical Therapy. Hepatogastroenterology 2012, 59, 1026. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S. Molecular Hydrogen in Sports Medicine: New Therapeutic Perspectives. Int. J. Sports Med. 2014, 36, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent Advances in Hydrogen Research as a Therapeutic Medical Gas. Free Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Recent Progress Toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Brinza, M.; Schröder, S.; Ababii, N.; Gronenberg, M.; Strunskus, T.; Pauporté, T.; Adelung, R.; Faupel, F.; Lupan, O. Two-in-One Sensor Based on PV4D4-Coated TiO2 Films for Food Spoilage Detection and as a Breath Marker for Several Diseases. Biosensors 2023, 13, 538. [Google Scholar] [CrossRef]
- Ghawanmeh, A.A.; A.Tanash, S.; Al-Rawashdeh, N.A.F.; Albiss, B. The Recent Progress on Nanomaterial-Based Chemosensors for Diagnosis of Human Exhaled Breath: A Review. J. Mater. Sci. 2024, 59, 8573–8605. [Google Scholar] [CrossRef]
- Kerlin, P.; Wong, L. Breath Hydrogen Testing in Bacterial Overgrowth of the Small Intestine. Gastroenterology 1988, 95, 982–988. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, C.; Van de Maele, K.; Hauser, B.; Vandenplas, Y. Hydrogen and Methane Breath Test in the Diagnosis of Lactose Intolerance. Nutrients 2021, 13, 3261. [Google Scholar] [CrossRef]
- Marton, A.; Xue, X.; Szilagyi, A. Meta-analysis: The Diagnostic Accuracy of Lactose Breath Hydrogen or Lactose Tolerance Tests for Predicting the North European Lactase Polymorphism C/T-13910. Aliment. Pharmacol. Ther. 2012, 35, 429–440. [Google Scholar] [CrossRef]
- Lupan, O.; Brinza, M.; Piehl, J.; Ababii, N.; Magariu, N.; Zimoch, L.; Strunskus, T.; Pauporté, T.; Adelung, R.; Faupel, F.; et al. Influence of Silsesquioxane-Containing Ultra-Thin Polymer Films on Metal Oxide Gas Sensor Performance for the Tunable Detection of Biomarkers. Chemosensors 2024, 12, 76. [Google Scholar] [CrossRef]
- Lupan, O.; Brinza, M.; Schroder, S.; Ababii, N.; Strunskus, T.; Viana, B.; Pauporté, T.; Adelung, R.; Faupel, F. Sensors Based on Hybrid Materials for Environmental, Industrial and Biomedical Applications. In Proceedings of the 2024 IEEE 14th International Conference “Nanomaterials: Applications and Properties” (NAP 2024), Riga, Latvia, 8–13 September 2024; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Ababii, N.; Reimer, T.; Shree, S.; Hoppe, M.; Polonskyi, O.; Sontea, V.; Chemnitz, S.; Faupel, F.; et al. Ultra-Thin TiO2 Films by Atomic Layer Deposition and Surface Functionalization with Au Nanodots for Sensing Applications. Mater. Sci. Semicond. Process. 2018, 87, 44–53. [Google Scholar] [CrossRef]
- Fan, G.; Guan, J.; Yu, H.; Zhu, Q.; Han, N.; Mo, J.; Chen, Y. Highly Sensitive Formaldehyde Gas Sensor Based on SnO2/Zn2SnO4 Hybrid Structures. Build. Environ. 2024, 262, 111781. [Google Scholar] [CrossRef]
- Sakalley, S.; Saravanan, A.; Kathiravan, D.; Tang, J.-C.; Cheng, W.-C.; Chen, S.-C.; Sun, H.; Huang, B.-R. Enhanced Hydrogen Gas Sensing through the Utilization of a Hybrid Nanostructure Combining ZnO Nanotubes and HiPIMS Cu3N Thin Film. Sens. Actuators B Chem. 2024, 402, 135107. [Google Scholar] [CrossRef]
- Nirmala Devi, K.; Vadivel, S. Room Temperature Fiber Optic Gas Sensor Technology Based Zn3(VO4)2/PMMA Hybrid Composites via Facile Hydrothermal Route. Opt. Fiber Technol. 2024, 82, 103642. [Google Scholar] [CrossRef]
- Lupan, O.; Santos-Carballal, D.; Ababii, N.; Magariu, N.; Hansen, S.; Vahl, A.; Zimoch, L.; Hoppe, M.; Pauporté, T.; Galstyan, V.; et al. TiO2/Cu2O/CuO Multi-Nanolayers as Sensors for H2 and Volatile Organic Compounds: An Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2021, 13, 32363–32380. [Google Scholar] [CrossRef]
- Sari, Y.; Gareso, P.L.; Armynah, B.; Tahir, D. A Review of TiO2 Photocatalyst for Organic Degradation and Sustainable Hydrogen Energy Production. Int. J. Hydrogen Energy 2024, 55, 984–996. [Google Scholar] [CrossRef]
- Seremak, W.; Jasiorski, M.; Baszczuk, A.; Winnicki, M. Durability Assessment of Low-Pressure Cold-Sprayed TiO2 Photocatalytic Coatings: Photocatalytic and Mechanical Stability. Surf. Coat. Technol. 2025, 497, 131740. [Google Scholar] [CrossRef]
- Li, M.; Zhao, N.; Wang, C.; Zou, Y.; Chen, X.; Mei, Y. Improving Thermal Stability and Electrochemical Performance of Polymer Solid Electrolyte Membranes with a Novel TiO2@PA-APP Composite Additive. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135640. [Google Scholar] [CrossRef]
- Ferroni, M.; Guidi, V.; Martinelli, G.; Faglia, G.; Nelli, P.; Sberveglieri, G. Characterization of a Nanosized TiO2 Gas Sensor. Nanostruct. Mater. 1996, 7, 709–718. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-Type Hydrogen Gas Sensor Based on TiO2: A Review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Malallah Rzaij, J.; Mohsen Abass, A. Review on: TiO2 Thin Film as a Metal Oxide Gas Sensor. J. Chem. Rev. 2020, 2, 114–121. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Mishra, A.K.; Bodduluri, M.T.; Magariu, N.; Vahl, A.; Krüger, H.; Wagner, B.; Faupel, F.; Adelung, R.; et al. Heterostructure-Based Devices with Enhanced Humidity Stability for H2 Gas Sensing Applications in Breath Tests and Portable Batteries. Sens. Actuators A Phys. 2021, 329, 112804. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Wicker, S.; Guiltat, M.; Weimar, U.; Hémeryck, A.; Barsan, N. Ambient Humidity Influence on CO Detection with SnO2 Gas Sensing Materials. A Combined DRIFTS/DFT Investigation. J. Phys. Chem. C 2017, 121, 25064–25073. [Google Scholar] [CrossRef]
- Enachi, M.; Lupan, O.; Braniste, T.; Sarua, A.; Chow, L.; Mishra, Y.K.; Gedamu, D.; Adelung, R.; Tiginyanu, I. Integration of Individual TiO 2 Nanotube on the Chip: Nanodevice for Hydrogen Sensing. Phys. Status Solidi-Rapid Res. Lett. 2015, 9, 171–174. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahbarpour, S.; Hosseini-Golgoo, S.-M.; Jamaati, H. Reducing the Destructive Effect of Ambient Humidity Variations on Gas Detection Capability of a Temperature Modulated Gas Sensor by Calcium Chloride. Sens. Actuators B Chem. 2021, 331, 129091. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Fundamentals of Semiconductor Gas Sensors. In Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–38. [Google Scholar] [CrossRef]
- Schröder, S.; Ababii, N.; Lupan, O.; Drewes, J.; Magariu, N.; Krüger, H.; Strunskus, T.; Adelung, R.; Hansen, S.; Faupel, F. Sensing Performance of CuO/Cu2O/ZnO:Fe Heterostructure Coated with Thermally Stable Ultrathin Hydrophobic PV3D3 Polymer Layer for Battery Application. Mater. Today Chem. 2022, 23, 100642. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, D.; Li, D.; Zhao, W.; Wang, Y.; Pang, L.; Shi, Z.; Zhou, T.; Sun, S.; Singh, C.; et al. Improved Energy Storage Performance of Sandwich-Structured P(VDF-HFP)-Based Nanocomposites by the Addition of Inorganic Nanoparticles. J. Mater. Chem. C 2023, 11, 6999–7009. [Google Scholar] [CrossRef]
- Lupan, C.; Kohlmann, N.; Petersen, D.; Bodduluri, M.T.; Buzdugan, A.; Jetter, J.; Quandt, E.; Kienle, L.; Adelung, R.; Lupan, O. Hydrogen Nanosensors Based on Core/Shell ZnO/Al2O3 and ZnO/ZnAl2O4 Single Nanowires. Mater. Today Nano 2025, 29, 100596. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.O.; Bobrikov, I.A.; Trukhanov, S.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal Structure and Magnetic Properties of the BaFe12−XAlXO19 (X=0.1–1.2) Solid Solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Troyanchuk, I.O.; Pushkarev, N.V.; Szymczak, H. Magnetic Properties of Anion-Deficient La1−xBaxMnO3−x/2 (0≤x≤0.30) Manganites. J. Exp. Theor. Phys. 2003, 96, 110–117. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Stepin, S.G.; Szymczak, H.; Botez, C.E. Effect of the Size Factor on the Magnetic Properties of Manganite La0.50Ba0.50MnO3. Phys. Solid State 2008, 50, 886–893. [Google Scholar] [CrossRef]
- Schröder, S.; Ababii, N.; Brînză, M.; Magariu, N.; Zimoch, L.; Bodduluri, M.T.; Strunskus, T.; Adelung, R.; Faupel, F.; Lupan, O. Tuning the Selectivity of Metal Oxide Gas Sensors with Vapor Phase Deposited Ultrathin Polymer Thin Films. Polymers 2023, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Brinza, M.; Cretu, V.; Zimoch, L.; Gronenberg, M.; Ababii, N.; Railean, S.; Strunskus, T.; Pauporté, T.; Adelung, R.; et al. A New Approach in Detection of Biomarker 2-Propanol with PTFE-Coated TiO2 Nanostructured Films. Sontea, V., Tiginyanu, I., Railean, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 75–83. ISBN 978-3-031-42782-4. [Google Scholar] [CrossRef]
- Baker, B.B.; Kasprzak, D.J. Thermal Degradation of Commercial Fluoropolymers in Air. Polym. Degrad. Stab. 1993, 42, 181–188. [Google Scholar] [CrossRef]
- Conesa, J.A.; Font, R. Polytetrafluoroethylene Decomposition in Air and Nitrogen. Polym. Eng. Sci. 2001, 41, 2137–2147. [Google Scholar] [CrossRef]
- Hirai, D.; Sawai, O.; Nunoura, T.; Hiroi, Z. Facile Synthetic Route to Transition Metal Oxyfluorides via Reactions between Metal Oxides and PTFE. J. Fluor. Chem. 2018, 209, 43–48. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Gleason, K.K. Initiated and Oxidative Chemical Vapor Deposition of Polymeric Thin Films: ICVD and OCVD. Adv. Funct. Mater. 2008, 18, 979–992. [Google Scholar] [CrossRef]
- Wang, P.; Shao, Z.; Ulfa, M.; Pauporté, T. Insights into the Hole Blocking Layer Effect on the Perovskite Solar Cell Performance and Impedance Response. J. Phys. Chem. C 2017, 121, 9131–9141. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Santos-Carballal, D.; Terasa, M.-I.M.-I.; Magariu, N.; Zappa, D.; Comini, E.; Pauporté, T.; Siebert, L.; Faupel, F.; et al. Tailoring the Selectivity of Ultralow-Power Heterojunction Gas Sensors by Noble Metal Nanoparticle Functionalization. Nano Energy 2021, 88, 106241. [Google Scholar] [CrossRef]
- Hoppe, M.; Ababii, N.; Postica, V.; Lupan, O.; Polonskyi, O.; Schütt, F.; Kaps, S.; Sukhodub, L.F.; Sontea, V.; Strunskus, T.; et al. (CuO-Cu2O)/ZnO:Al Heterojunctions for Volatile Organic Compound Detection. Sens. Actuators B Chem. 2018, 255, 1362–1375. [Google Scholar] [CrossRef]
- Schröder, S.; Polonskyi, O.; Strunskus, T.; Faupel, F. Nanoscale Gradient Copolymer Films via Single-Step Deposition from the Vapor Phase. Mater. Today 2020, 37, 35–42. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Pauporté, T.; Viana, B.; Terasa, M.-I.; Adelung, R. Room Temperature Gas Nanosensors Based on Individual and Multiple Networked Au-Modified ZnO Nanowires. Sens. Actuators B Chem. 2019, 299, 126977. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Lee, S.Y.; Sohn, W.; Lee, J.-H.J.E.; Kim, D.H.; Shim, Y.-S.; Kwon, K.C.; Choi, K.S.; Yoo, H.J.; et al. Highly Selective and Sensitive Chemoresistive Humidity Sensors Based on RGO/MoS 2 van Der Waals Composites. J. Mater. Chem. A 2018, 6, 5016–5024. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Mishra, A.K.; Gronenberg, O.; Vahl, A.; Schürmann, U.; Duppel, V.; Krüger, H.; Chow, L.; Kienle, L.; et al. Single CuO/Cu2O/Cu Microwire Covered by a Nanowire Network as a Gas Sensor for the Detection of Battery Hazards. ACS Appl. Mater. Interfaces 2020, 12, 42248–42263. [Google Scholar] [CrossRef]
- Postica, V. Proprietăţile Senzoriale Ale Structurilor Hibride În Bază de Oxizi Metalici Şi a Reţelelor Lor. Ph.D. Thesis, Technical Univeristy of Moldova, Chișinău, Moldova, 2020. [Google Scholar]
- Ding, K.; Zhang, D.; Jiang, L.; Chen, J.; Qu, R.; Shi, F.; Li, B.; He, X.; Wang, L.; Wang, H. An Ionic Liquid-Present Immersion Method for Preparing Cotton Fiber-Shaped Cu2O Nanoparticles at Room Temperature. J. Appl. Electrochem. 2022, 52, 55–65. [Google Scholar] [CrossRef]
- Nakate, U.; Yu, Y.-T.; Park, S. Hierarchical CuO Nanostructured Materials for Acetaldehyde Sensor Application. Microelectron. Eng. 2022, 251, 111662. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Z.-Y.; Deng, S.; Hou, M.; Zhang, X.; Jiang, Z.; Lai, N.-C. Morphology-Controlled Synthesis of Cu2O Encapsulated Phase Change Materials: Photothermal Conversion and Storage Performance in Visible Light Regime. Chem. Eng. J. 2023, 454, 140089. [Google Scholar] [CrossRef]
- Helwig, N.; Schüler, M.; Bur, C.; Schütze, A.; Sauerwald, T. Gas Mixing Apparatus for Automated Gas Sensor Characterization. Meas. Sci. Technol. 2014, 25, 055903. [Google Scholar] [CrossRef]
- Luo, S.; Shen, Y.; Wu, Z.; Cao, M.; Gu, F.; Wang, L. Enhanced Ethanol Sensing Performance of Mesoporous Sn-Doped ZnO. Mater. Sci. Semicond. Process. 2016, 41, 535–543. [Google Scholar] [CrossRef]
- Brunetti, B.; Desimoni, E. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta 2015, 06, 1–4. [Google Scholar] [CrossRef]
- Cretu, V.; Postica, V.; Mishra, A.K.; Hoppe, M.; Tiginyanu, I.; Mishra, Y.K.; Chow, L.; de Leeuw, N.H.; Adelung, R.; Lupan, O. Synthesis, Characterization and DFT Studies of Zinc-Doped Copper Oxide Nanocrystals for Gas Sensing Applications. J. Mater. Chem. A 2016, 4, 6527–6539. [Google Scholar] [CrossRef]
- Biadasz, A.; Kotkowiak, M.; Łukawski, D.; Jadwiżak, J.; Rytel, K.; Kędzierski, K. A Versatile Gas Transmission Device with Precise Humidity Control for QCM Humidity Sensor Characterizations. Measurement 2022, 200, 111674. [Google Scholar] [CrossRef]
- O’Shaughnessy, W.S.; Gao, M.; Gleason, K.K. Initiated Chemical Vapor Deposition of Trivinyltrimethylcyclotrisiloxane for Biomaterial Coatings. Langmuir 2006, 22, 7021–7026. [Google Scholar] [CrossRef]
- Piwowarczyk, J.; Jędrzejewski, R.; Moszyński, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR Studies of Polytetrafluoroethylene Thin Films Obtained by Physical Methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef]
- Du, W.; Tu, J.; Qiu, M.; Zhou, S.; Luo, Y.; Ong, W.-L.; Zhao, J. Temperature-Mediated Structural Evolution of Vapor–Phase Deposited Cyclosiloxane Polymer Thin Films for Enhanced Mechanical Properties and Thermal Conductivity. Int. J. Extrem. Manuf. 2023, 5, 025101. [Google Scholar] [CrossRef]
- Ziegler, V.; Paraginski, R.T.; Ferreira, C.D. Grain Storage Systems and Effects of Moisture, Temperature and Time on Grain Quality—A Review. J. Stored Prod. Res. 2021, 91, 101770. [Google Scholar] [CrossRef]
- Esrafili, A.; Wagner, A.; Inamdar, S.; Acharya, A.P. Covalent Organic Frameworks for Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2002090. [Google Scholar] [CrossRef]
- Wolkoff, P.; Azuma, K.; Carrer, P. Health, Work Performance, and Risk of Infection in Office-like Environments: The Role of Indoor Temperature, Air Humidity, and Ventilation. Int. J. Hyg. Environ. Health 2021, 233, 113709. [Google Scholar] [CrossRef]
- Ma, Z.; Fei, T.; Zhang, T. An Overview: Sensors for Low Humidity Detection. Sens. Actuators B Chem. 2023, 376, 133039. [Google Scholar] [CrossRef]
- Sofia Paulino Mendes, A.; Rutgersson, A.; Paulsson, M. Outdoor Environmental Effects on Cleanrooms—A Study from a Swedish Hospital Pharmacy Compounding Unit. Eur. J. Pharm. Biopharm. 2022, 177, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Sikka, M.P.; Mondal, M. A Critical Review on Cleanroom Filtration. Res. J. Text. Appar. 2022, 26, 452–467. [Google Scholar] [CrossRef]
- Meng, H.; Shiue, A.; Wang, C.; Liu, J.; Jia, L.; Leggett, G. Particle and Bacterial Colony Emissions from Garments and Humans in Pharmaceutical Cleanrooms. J. Build. Eng. 2024, 97, 110829. [Google Scholar] [CrossRef]
- Lobanchykova, N.M.; Vakaliuk, T.A.; Korbut, V.P.; Lobanchykov, S.M.; Krasnov, Y.B. Features of Designing Systems for the Formation of an Internal Microclimate of a High Class of Cleanliness of Operating Rooms of Medical Institutions. IOP Conf. Ser. Earth Environ. Sci. 2024, 1415, 012124. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Shin, W.; Hong, S.; Jeong, Y.; Jung, G.; Park, J.; Kim, D.; Choi, K.; Shin, H.; Koo, R.-H.; Kim, J.-J.; et al. Low-Frequency Noise in Gas Sensors: A Review. Sens. Actuators B Chem. 2023, 383, 133551. [Google Scholar] [CrossRef]
- Chaoumead, A.; Joo, B.-H.; Kwak, D.-J.; Sung, Y.-M. Structural and Electrical Properties of Sputtering Power and Gas Pressure on Ti-Dope In2O3 Transparent Conductive Films by RF Magnetron Sputtering. Appl. Surf. Sci. 2013, 275, 227–232. [Google Scholar] [CrossRef]
- Cao, R.; Ding, H.; Kim, K.-J.; Peng, Z.; Wu, J.; Culp, J.T.; Ohodnicki, P.R.; Beckman, E.; Chen, K.P. Metal-Organic Framework Functionalized Polymer Coating for Fiber Optical Methane Sensors. Sens. Actuators B Chem. 2020, 324, 128627. [Google Scholar] [CrossRef]
- Sankar, S.; George, A.; Ramesan, M.T. Copper Alumina @ Poly (Aniline- Co -Indole) Nanocomposites: Synthesis, Characterization, Electrical Properties and Gas Sensing Applications. RSC Adv. 2022, 12, 17637–17644. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, P.; Jithin, K.; Meera, K.; Ramesan, M.T. Synthesis, Characterization, Magnetoelectric Properties and Gas Sensing Application of Poly(Anthranilic Acid- Co- Indole)/ Magnetite Nanocomposites. J. Thermoplast. Compos. Mater. 2023, 36, 2523–2542. [Google Scholar] [CrossRef]
- Arjmand, F.; Mohamadnia, Z. Fabrication of a Light-Responsive Polymer Nanocomposite Containing Spiropyran as a Sensor for Reversible Recognition of Metal Ions. Polym. Chem. 2022, 13, 937–945. [Google Scholar] [CrossRef]
- Sankar, S.; Ramesan, M.T. Synthesis, Characterization, Conductivity, and Gas-sensing Performance of Copolymer Nanocomposites Based on Copper Alumina and Poly(Aniline-Co-pyrrole). Polym. Eng. Sci. 2022, 62, 2402–2410. [Google Scholar] [CrossRef]
- Li, G.; Lian, J.; Zheng, X.; Cao, J. Electrogenerated Chemiluminescence Biosensor for Glucose Based on Poly(Luminol–Aniline) Nanowires Composite Modified Electrode. Biosens. Bioelectron. 2010, 26, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Hia, I.L.; Snyder, A.D.; Turicek, J.S.; Blanc, F.; Patrick, J.F.; Therriault, D. Electrically Conductive and 3D-Printable Copolymer/MWCNT Nanocomposites for Strain Sensing. Compos. Sci. Technol. 2023, 232, 109850. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Jiang, F.; Liu, J.; Liu, G.; Ma, X.; Liu, P.; Huang, R.; Xu, J.; Wang, L. Flexible Fiber-Shaped Hydrogen Gas Sensor via Coupling Palladium with Conductive Polymer Gel Fiber. J. Hazard. Mater. 2021, 411, 125008. [Google Scholar] [CrossRef] [PubMed]
- Sonwane, N.D.; Maity, M.D.; Kondawar, S.B. Conducting Polyaniline/SnO2 Nanocomposite for Room Temperature Hydrogen Gas Sensing. Mater. Today Proc. 2019, 15, 447–453. [Google Scholar] [CrossRef]
- Sharma, H.J.; Jamkar, D.V.; Kondawar, S.B. Electrospun Nanofibers of Conducting Polyaniline/Al-SnO2 Composites for Hydrogen Sensing Applications. Procedia Mater. Sci. 2015, 10, 186–194. [Google Scholar] [CrossRef]
- Pippara, R.K.; Chauhan, P.S.; Yadav, A.; Kishnani, V.; Gupta, A. Room Temperature Hydrogen Sensing with Polyaniline/SnO2/Pd Nanocomposites. Micro Nano Eng. 2021, 12, 100086. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, P.; Vincent, A.; McCormack, R.; Kalyanaraman, R.; Cho, H.J.; Seal, S. Hydrogen Selective Gas Sensor in Humid Environment Based on Polymer Coated Nanostructured-Doped Tin Oxide. Sens. Actuators B Chem. 2011, 155, 884–892. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Liu, F.; Gu, T.; Wang, B.; He, J.; Wang, C.; Meng, X.; Sun, P.; Lu, G. Multilayer Fluorine-Free MoBT x MBene with Hydrophilic Structural-Modulating for the Fabrication of a Low-Resistance and High-Resolution Humidity Sensor. Adv. Sci. 2024, 11, 2404178. [Google Scholar] [CrossRef]


| No. | Sensing Material | Polymer | Target | OPT, °C | Concentration | Response | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | ZIF-8/PDMS | PDMS | CH4 | - | 50% | 16% | [82] |
| 2. | PANI-co-PIN /Cu–Al2O3 | PANI-co-PIN | Ammonia | RT | - | 8 μΩ * | [83] |
| 3. | (PANA-Co-PIN): Fe3O4- | (PANA-Co-PIN) | Ammonia | - | - | 7 μΩ * | [84] |
| 4. | TiO2-g-P(SPEA-co-GMA) | P(SPEA-co-GMA) | Light Responsiveness | - | - | - | [85] |
| 5. | PANI-co-PPy/Cu-Al2O3 | (PANI-co-PPy) | Ammonia | RT | 100 ppm | 5 μΩ * | [86] |
| 6. | PLANC-GOD | PLANC | Glucose | - | 1; 3; 5; 7; 10−6 mol L | - | [87] |
| 7. | (EMAA)/(MWCNT) | EMAA | Strain sensing | 220 | - | 41.1 S m −1 | [88] |
| D1 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | H2 | 300 | 100 ppm | 153% | T.w. |
| D1 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | H2 | 350 | 100 ppm | 123% | T.w. |
| No. | Sensing Material | Polymer | H2 Concentration | RH, ~% | OPT, °C | Response | Time, s | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Response | Recovery | ||||||||
| 1. | TiO2 | PV4D4 | 100 ppm | - | 300 | 100% | 3 | 44 | [15] |
| 2. | TiO2 | PV4D4 | 100 ppm | - | 350 | 709.07% | 3.02 | 23.23 | [20] |
| 3. | PEDOT:PSS@Pd | PEDOT: PSS | 4 % | - | RT | 31.6% | 19 (±4) | 73 (±11) | [89] |
| 4. | PANI/SnO2 | PANI | 6000 ppm | - | RT | 42% | 11 | 7 | [90] |
| 5. | PANI/Al-SnO2 | PANI | 1000 ppm | - | 48 | - | 2 | 2 | [91] |
| 6. | PANI/Al-SnO2 | PANI | 100 ppm | - | 340 | - | 3 | 2 | [91] |
| 7. | PANI/SnO2 + Pd | PANI | 50 ppm | - | RT | 19.2% | 39 | 53 | [92] |
| 8. | PANI/SnO2 + Pd | PANI | 350 ppm | - | RT | 353.7% | 141 | 76 | [92] |
| 9. | PMMA/SnO2:In2O3 | PMMA | 600 ppm | 14 | RT | 1.05 × 103 Ω | 196 | 282 | [93] |
| 10. | PMMA/SnO2:In2O3 | PMMA | 600 ppm | 65 | RT | 1.34 × 102 Ω | 842 | 387 | [93] |
| 11. | Cytop/SnO2:In2O3 | Cytop | 600 ppm | 14 | RT | 1.49 × 101 Ω | 1550 | 35 | [93] |
| 12. | Cytop/SnO2:In2O3 | Cytop | 600 ppm | 65 | RT | 7.52 × 101 Ω | 322 | 44 | [93] |
| 13. | Fluoropel/SnO2:In2O3 | fluoropolymer | 600 ppm | 14 | RT | 1.55 × 102 Ω | 134 | 30 | [93] |
| 14. | Fluoropel/SnO2:In2O3 | fluoropolymer | 600 ppm | 65 | RT | 9.79 × 101 Ω | 356 | 56 | [93] |
| D2 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 10 | 300 | 34% | 3 | 4.9 | T.w. |
| D2 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 10 | 350 | 96% | 1.3 | 2.1 | T.w. |
| D2 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 45 | 300 | 23% | 4.2 | 4.4 | T.w. |
| D2 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 45 | 350 | 73% | 2.3 | 3.3 | T.w. |
| D3 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 10 | 300 | 28% | 1.5 | 3.1 | T.w. |
| D3 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 10 | 350 | 75% | 2.5 | 3.2 | T.w. |
| D3 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 45 | 300 | 23% | 1.7 | 2.1 | T.w. |
| D3 | P(V3D3-co-TFE)/TiO2 | P(V3D3-co-TFE) | 100 ppm | 45 | 350 | 48% | 1.7 | 3.5 | T.w. |
| Sensing Structure | Polymer Coating | Enhancement | OPT, °C | Detected Gas | Gas Response, % | Ref. |
|---|---|---|---|---|---|---|
| TiO2 | Uncoated | TA 450 °C | 250 | Hydrogen | 600 | [22] |
| TiO2 | PTFE | - | 350 | 2-propanol | 64 | [46] |
| TiO2 | PV4D4 | - | 400 | 2-propanol | 225 | [45] |
| TiO2 | PV4D4 | TA 610 °C for TiO2 | 300/RT | Hydrogen/Ammonia | 100/52 | [15] |
| TiO2 | PV4D4 | TA 450 °C for PV4D4 | 350 | Hydrogen | 709 | [20] |
| CuO/Cu2O/ZnO:Fe | PV3D3 | RTA 650 °C | 350 | Hydrogen | 191 | [39] |
| TiO2 | PV3D3 | - | - | - | - | To be published |
| TiO2 | P(V3D3 + TFE) | Copolymer structure | 300 | Hydrogen | 153 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinza, M.; Schwäke, L.; Zimoch, L.; Strunskus, T.; Pauporté, T.; Viana, B.; Ameri, T.; Adelung, R.; Faupel, F.; Schröder, S.; et al. Influence of P(V3D3-co-TFE) Copolymer Coverage on Hydrogen Detection Performance of a TiO2 Sensor at Different Relative Humidity for Industrial and Biomedical Applications. Chemosensors 2025, 13, 150. https://doi.org/10.3390/chemosensors13040150
Brinza M, Schwäke L, Zimoch L, Strunskus T, Pauporté T, Viana B, Ameri T, Adelung R, Faupel F, Schröder S, et al. Influence of P(V3D3-co-TFE) Copolymer Coverage on Hydrogen Detection Performance of a TiO2 Sensor at Different Relative Humidity for Industrial and Biomedical Applications. Chemosensors. 2025; 13(4):150. https://doi.org/10.3390/chemosensors13040150
Chicago/Turabian StyleBrinza, Mihai, Lynn Schwäke, Lukas Zimoch, Thomas Strunskus, Thierry Pauporté, Bruno Viana, Tayebeh Ameri, Rainer Adelung, Franz Faupel, Stefan Schröder, and et al. 2025. "Influence of P(V3D3-co-TFE) Copolymer Coverage on Hydrogen Detection Performance of a TiO2 Sensor at Different Relative Humidity for Industrial and Biomedical Applications" Chemosensors 13, no. 4: 150. https://doi.org/10.3390/chemosensors13040150
APA StyleBrinza, M., Schwäke, L., Zimoch, L., Strunskus, T., Pauporté, T., Viana, B., Ameri, T., Adelung, R., Faupel, F., Schröder, S., & Lupan, O. (2025). Influence of P(V3D3-co-TFE) Copolymer Coverage on Hydrogen Detection Performance of a TiO2 Sensor at Different Relative Humidity for Industrial and Biomedical Applications. Chemosensors, 13(4), 150. https://doi.org/10.3390/chemosensors13040150










