Low-Cost Wireless Device for DNA Sensing Using Square Wave Voltammetry
Abstract
1. Introduction
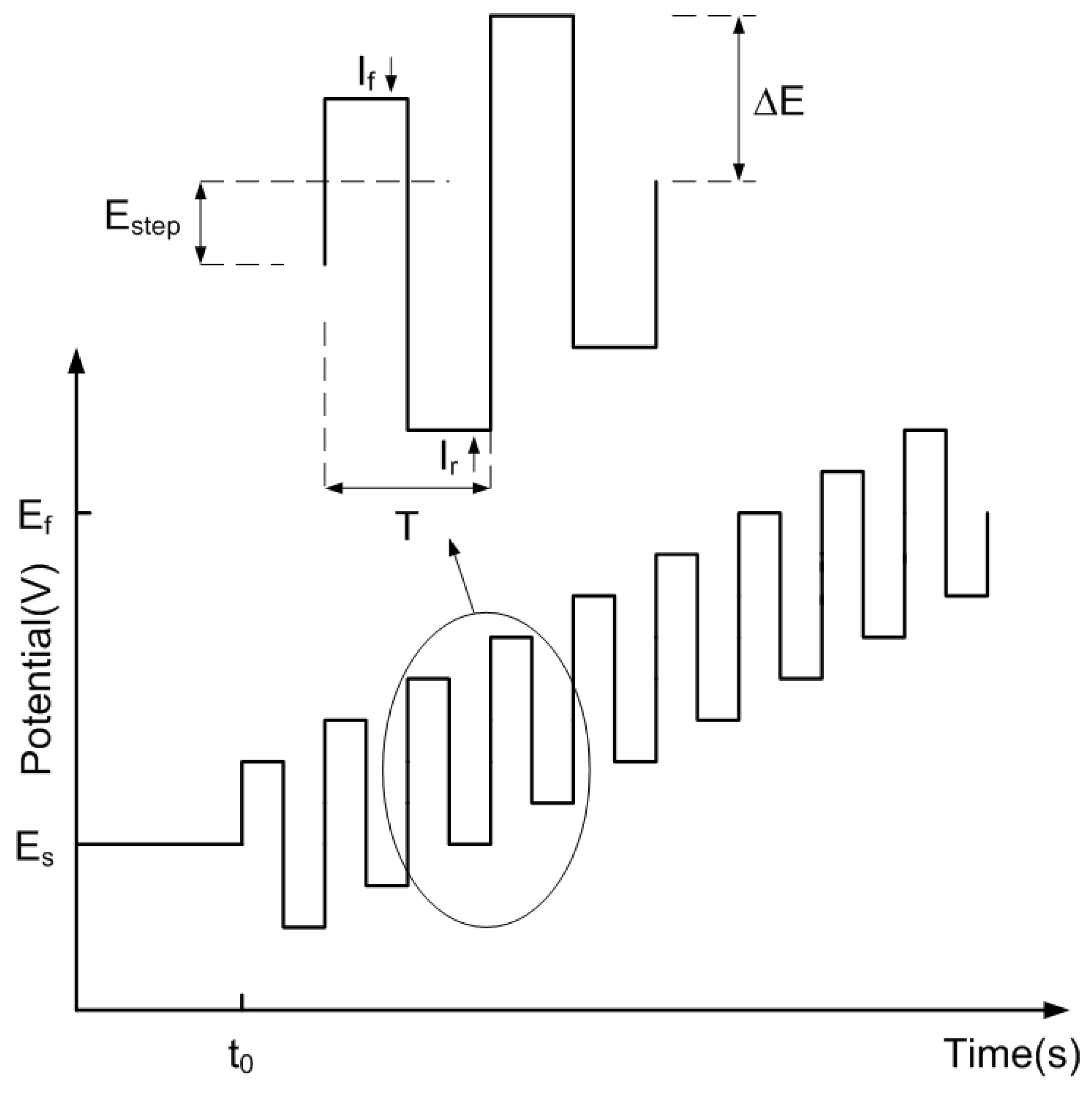
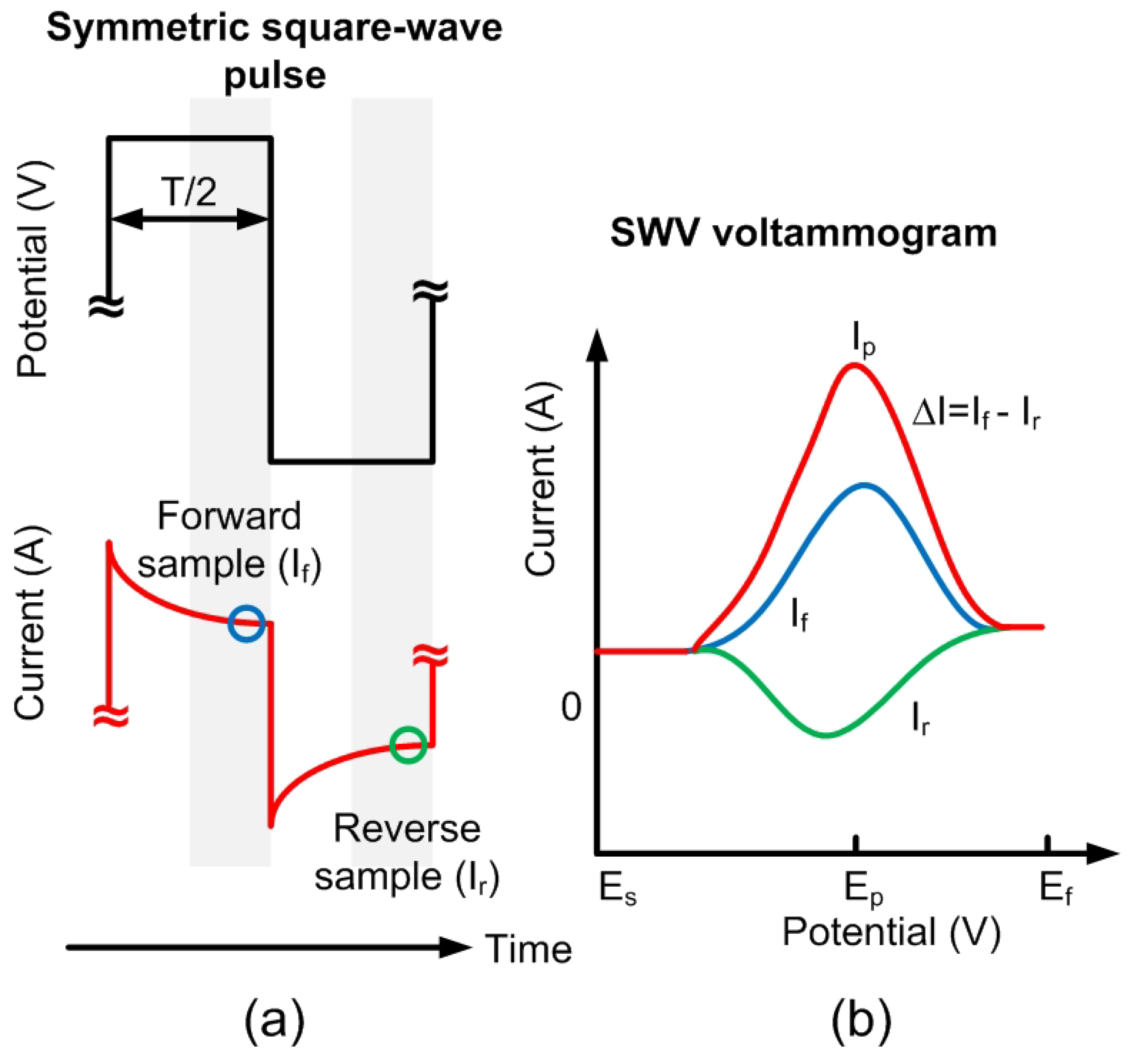
Square Wave Voltammetry Principles
2. Materials and Methods
2.1. Materials and Reagents
- Components and reagents: All chemicals were purchased from Sigma–Aldrich Co. and used as received, unless otherwise noted. All aqueous solutions were prepared using Milli–Q water (from a Milli–Q Direct purification system, resistivity = 18 M). A phosphate buffer saline solution (1X PBS) containing 137 mM sodium chloride, 2.7 mM potassium chloride, and 10 mM phosphate buffer was prepared by dissolving five tablets in 1 L of Milli–Q water. HPLC-purified oligonucleotides were purchased from Biosearch Technologies (UK). Upon receipt, the oligonucleotides were dissolved in TE buffer (10 mM Tris, 0.1 mM EDTA, pH = 8, obtained from Integrated DNA Technologies, Inc., Leuven, Belgium) at a concentration of 100 M and then aliquoted and stored at −20 °C. Table 1 shows the sequences of oligonucleotides.
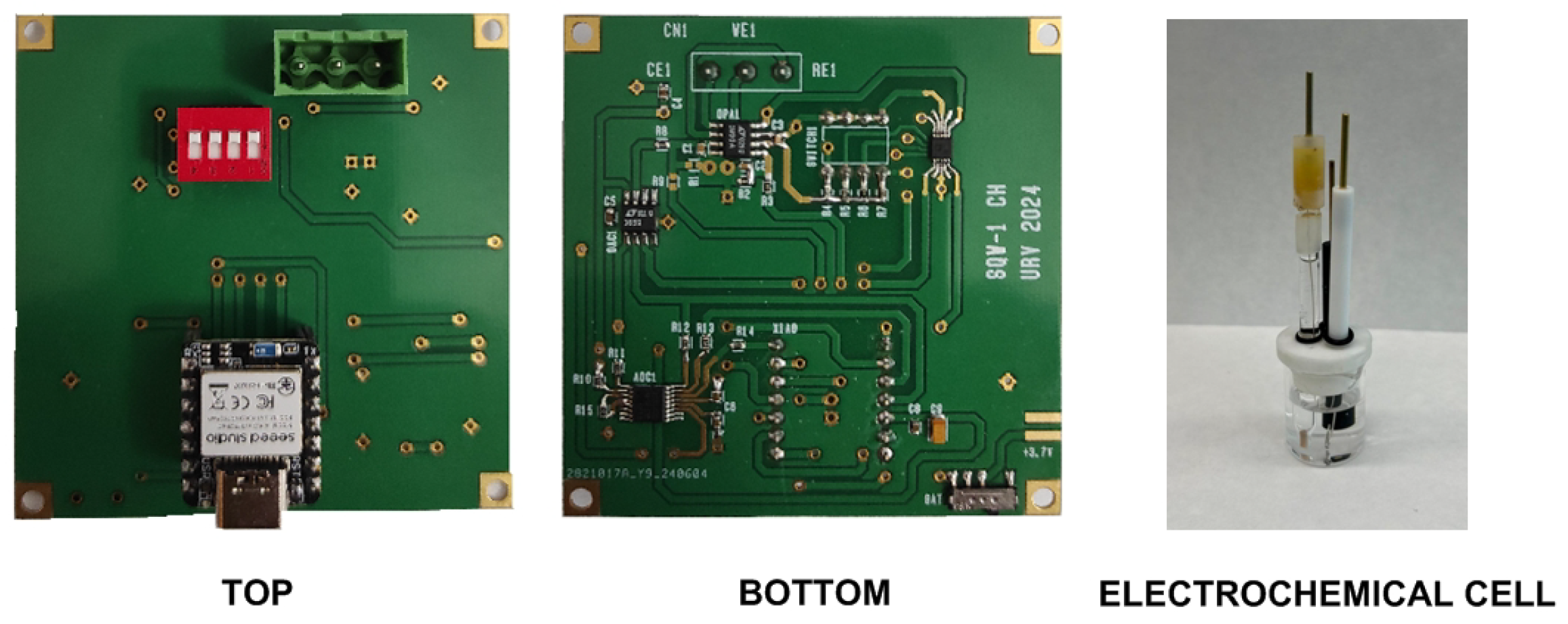
- Electrochemical cell: Electrochemical measurements are performed at room temperature using simultaneously an IVIUM CompactStat potentiostat (Ivium Technologies, Netherlands) and the low-cost prototype proposed in this work using a three-electrode cell system containing a platinum counter electrode, an Ag/AgCl reference electrode (TianjinAida Co., Tianjin, China), and a gold rod electrode (2 mm diameter, CH Instruments, Inc., Austin, TX, USA) as the working electrode.
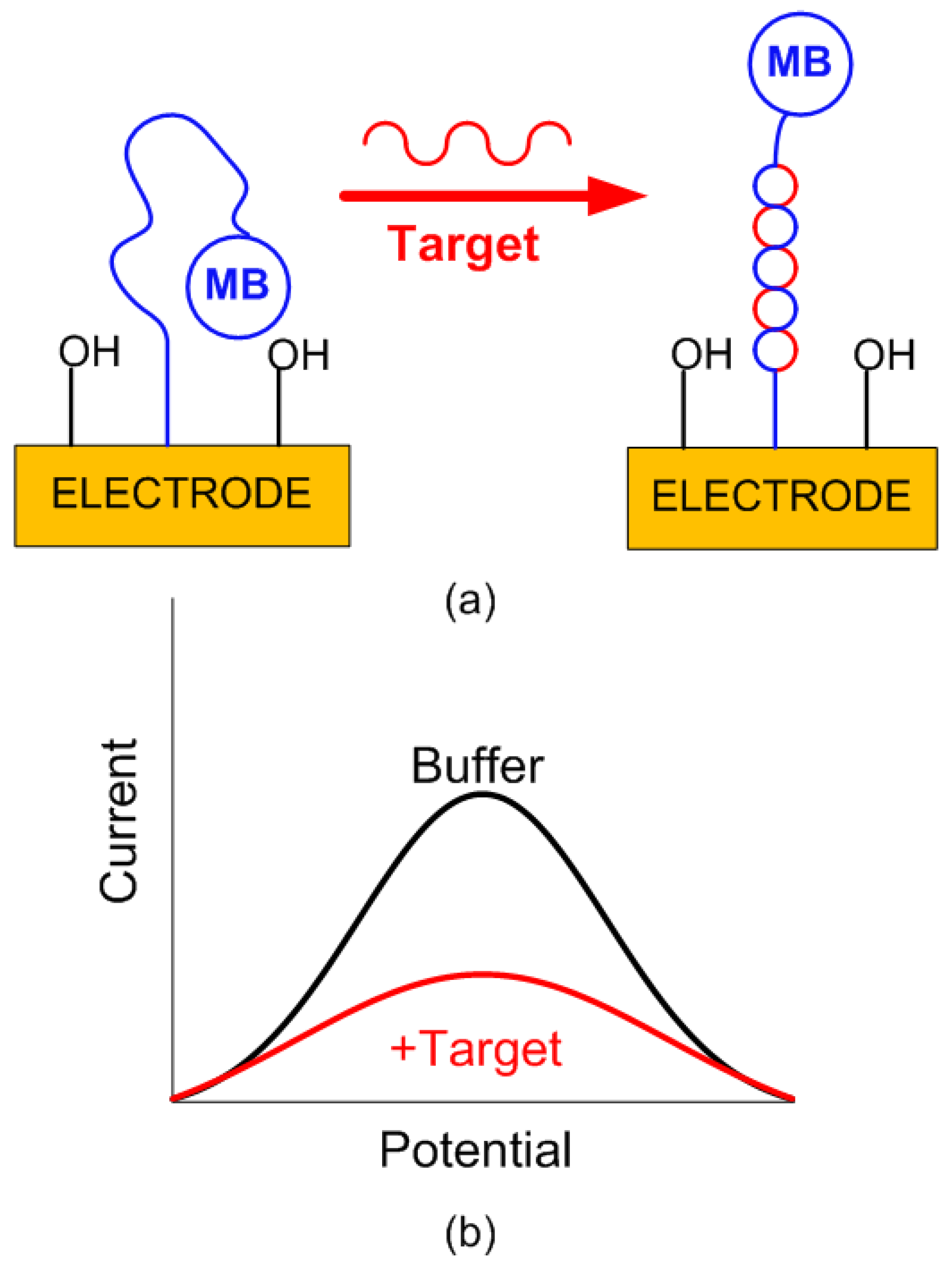
- DNA sensor fabrication: The gold electrodes were first cleaned by immersing them in a freshly prepared piranha’s solution (1:3, 30% and concentrated , respectively) for 5 min, followed by rinsing with deionized water. The electrodes were then subjected to an electrochemical pretreatment consisting of varying the electrode potential with respect to that of the Ag/AgCl reference electrode in cycles between −0.1 and 1.6 V, at a scan rate of 0.1 for 40 scans in a 0.5 M solution. Then, the electrodes were rinsed with deionized water and dried using pure nitrogen gas [20]. For capture probe immobilization, an aliquot of the capture probe is thawed and reduced with a 100-fold molar excess of tris(2–carboxyethyl) phosphine (TCEP) for 1 h at room temperature in the dark. The reduced probe was diluted to a final concentration of 1 M in PBS, and the cleaned gold electrodes were incubated in this solution for 2 h in a humid chamber. Following this, the electrodes were rinsed with Milli–Q water and incubated overnight at room temperature in a freshly prepared 1 mM solution of 6–mercaptohexanol (MCH) in PBS. After a final rinse with Milli–Q water, the electrodes were ready for biosensing measurements [21].
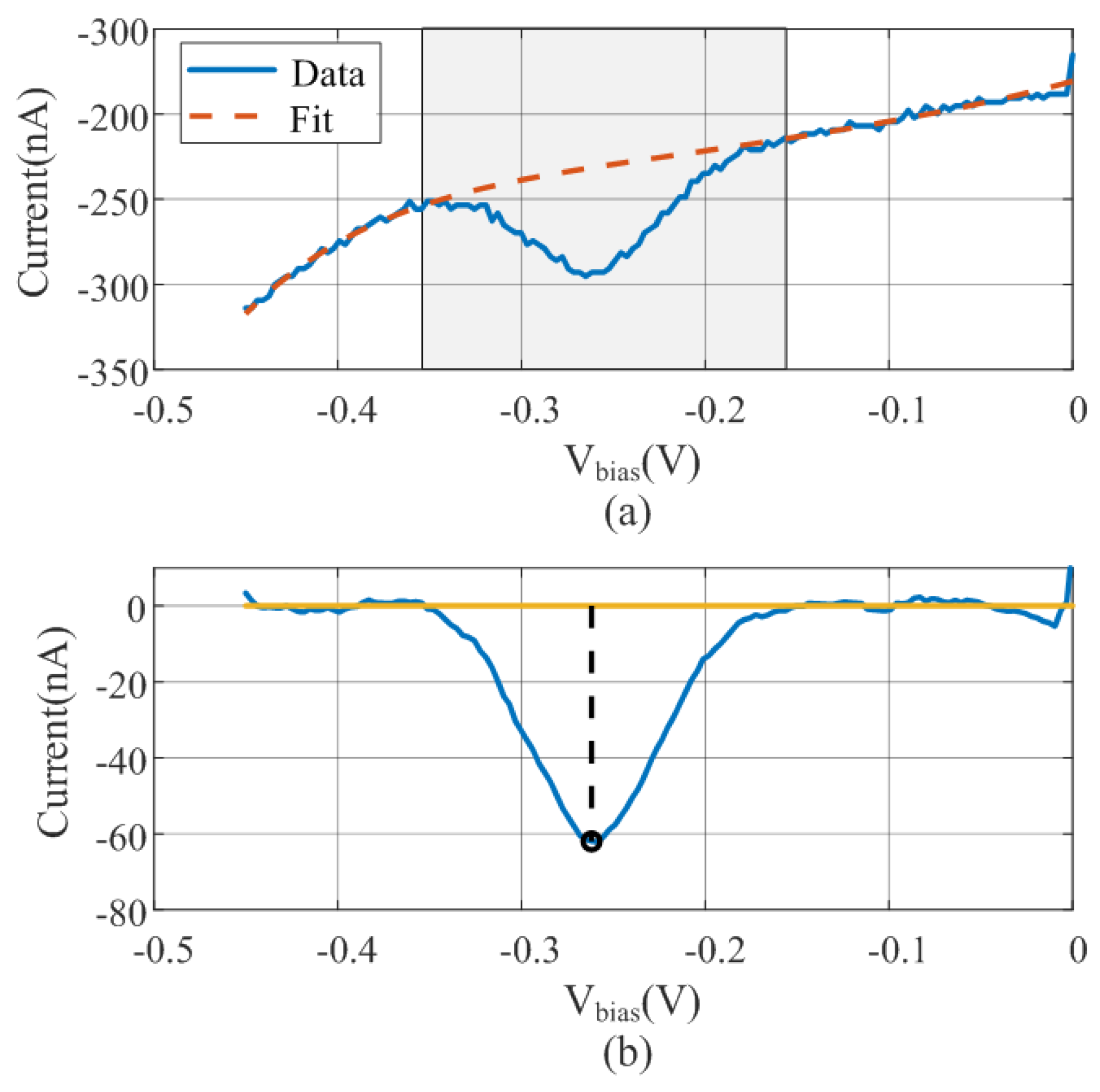
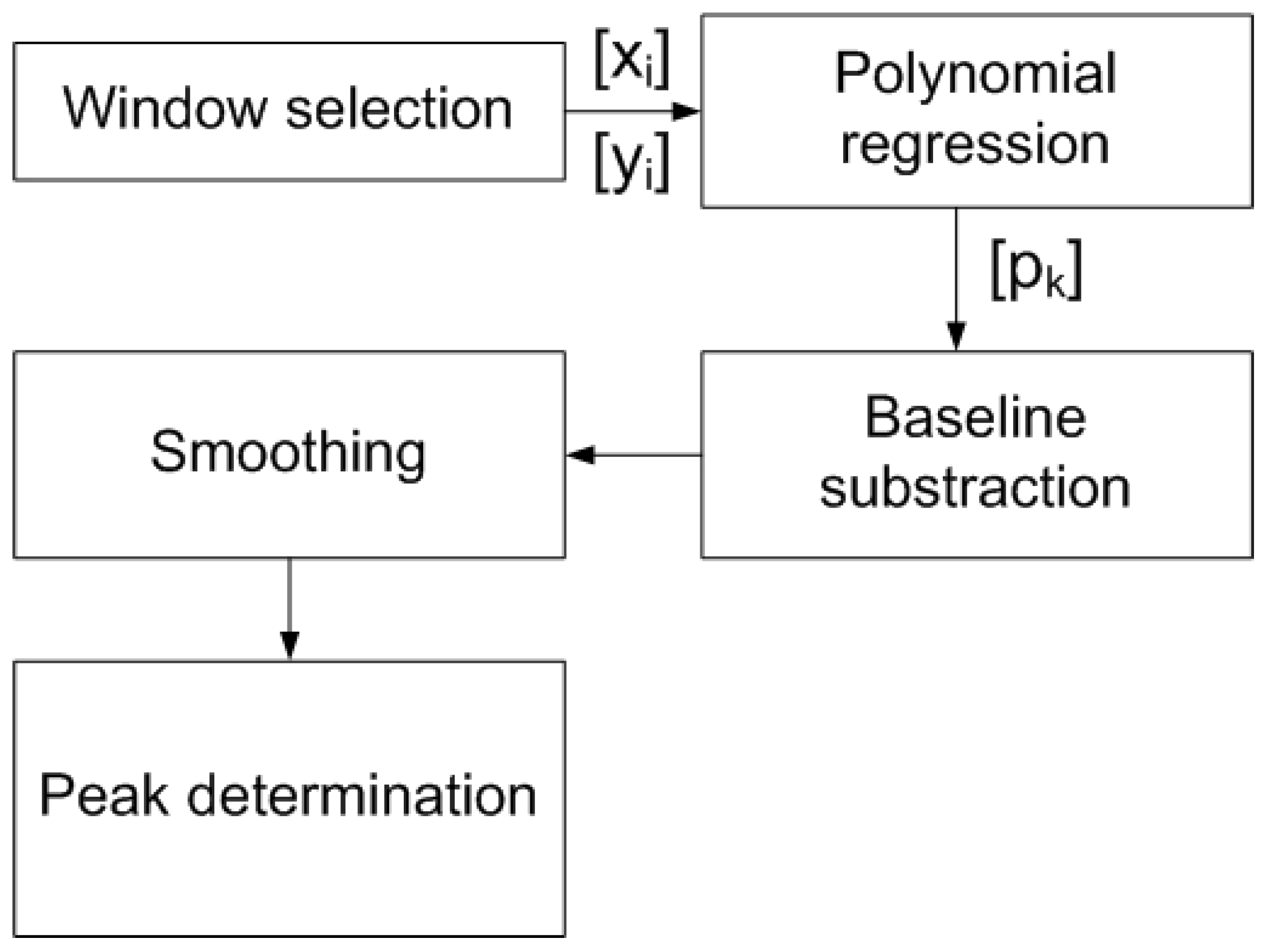
2.2. Measurement Method: Signal Processing
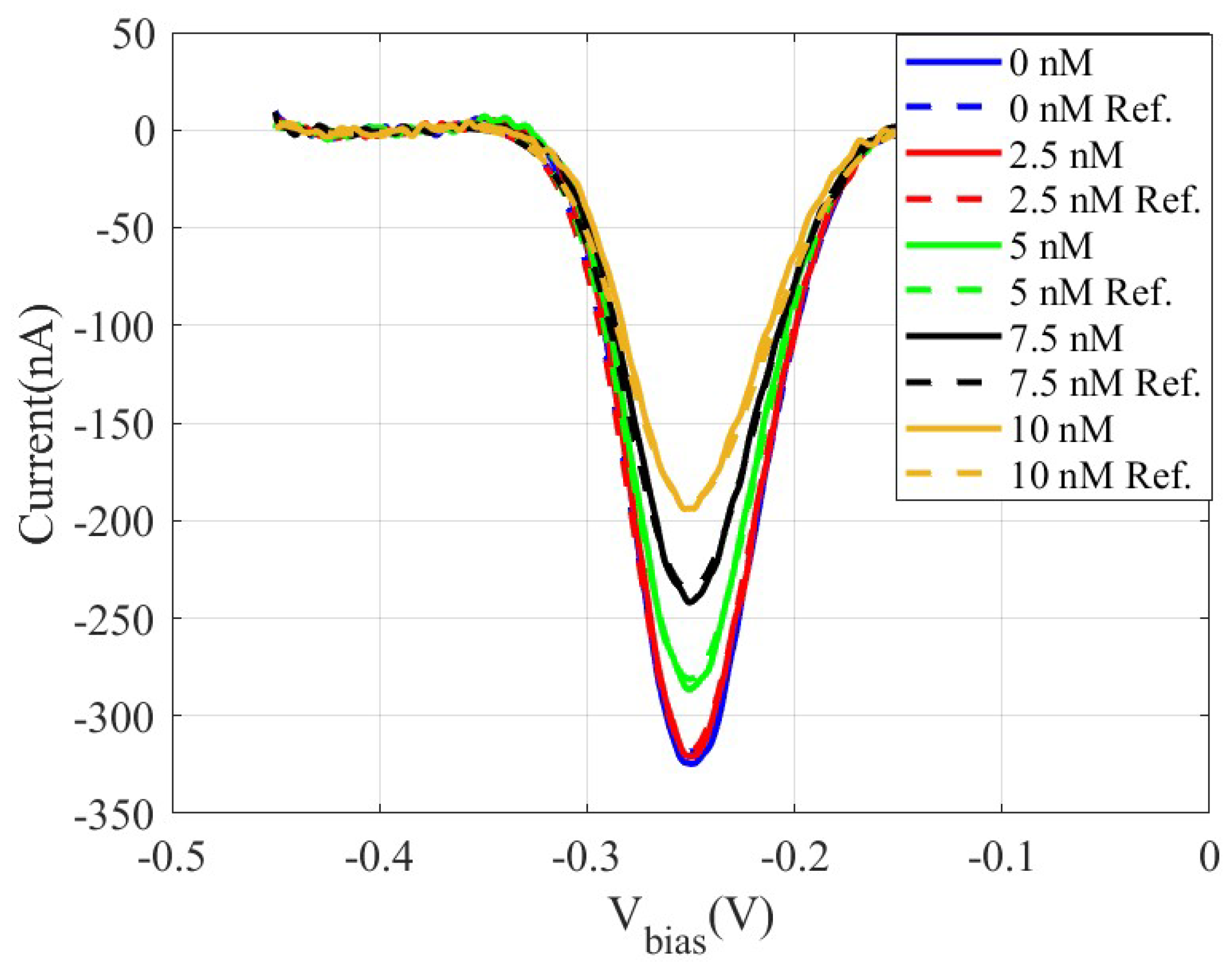
2.3. DNA Quantification Using SWV
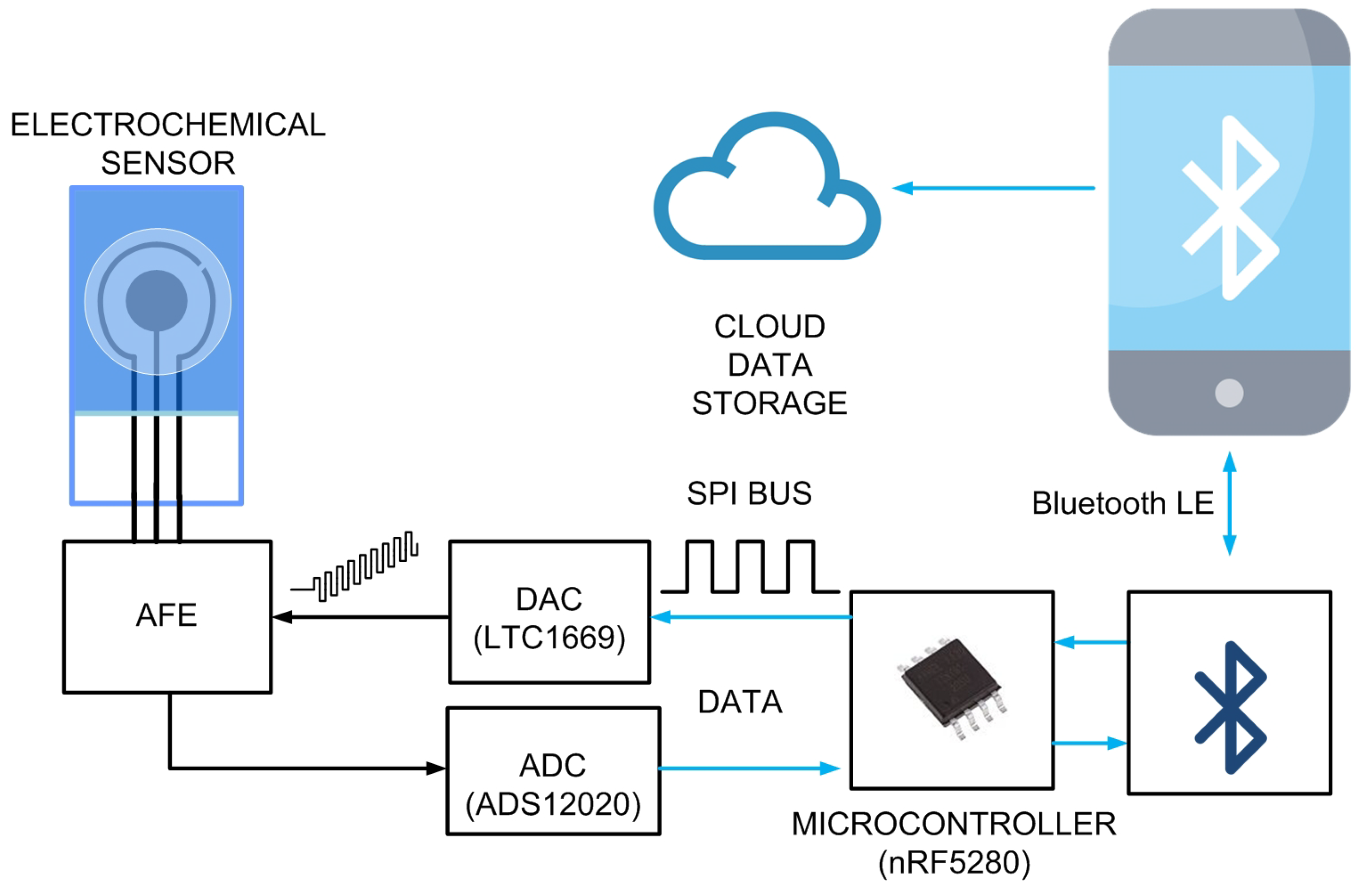
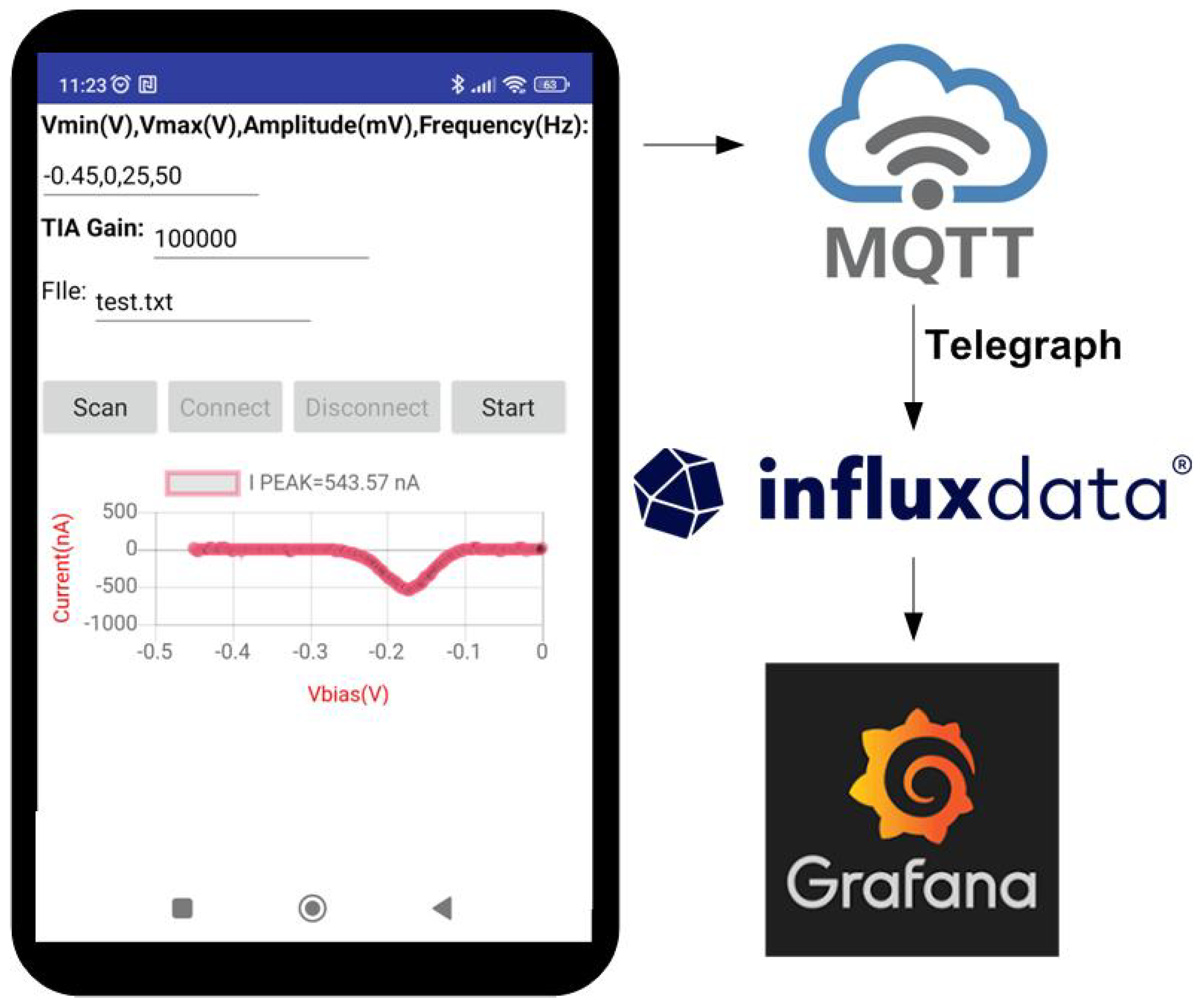
2.4. System Description
2.4.1. System Overview
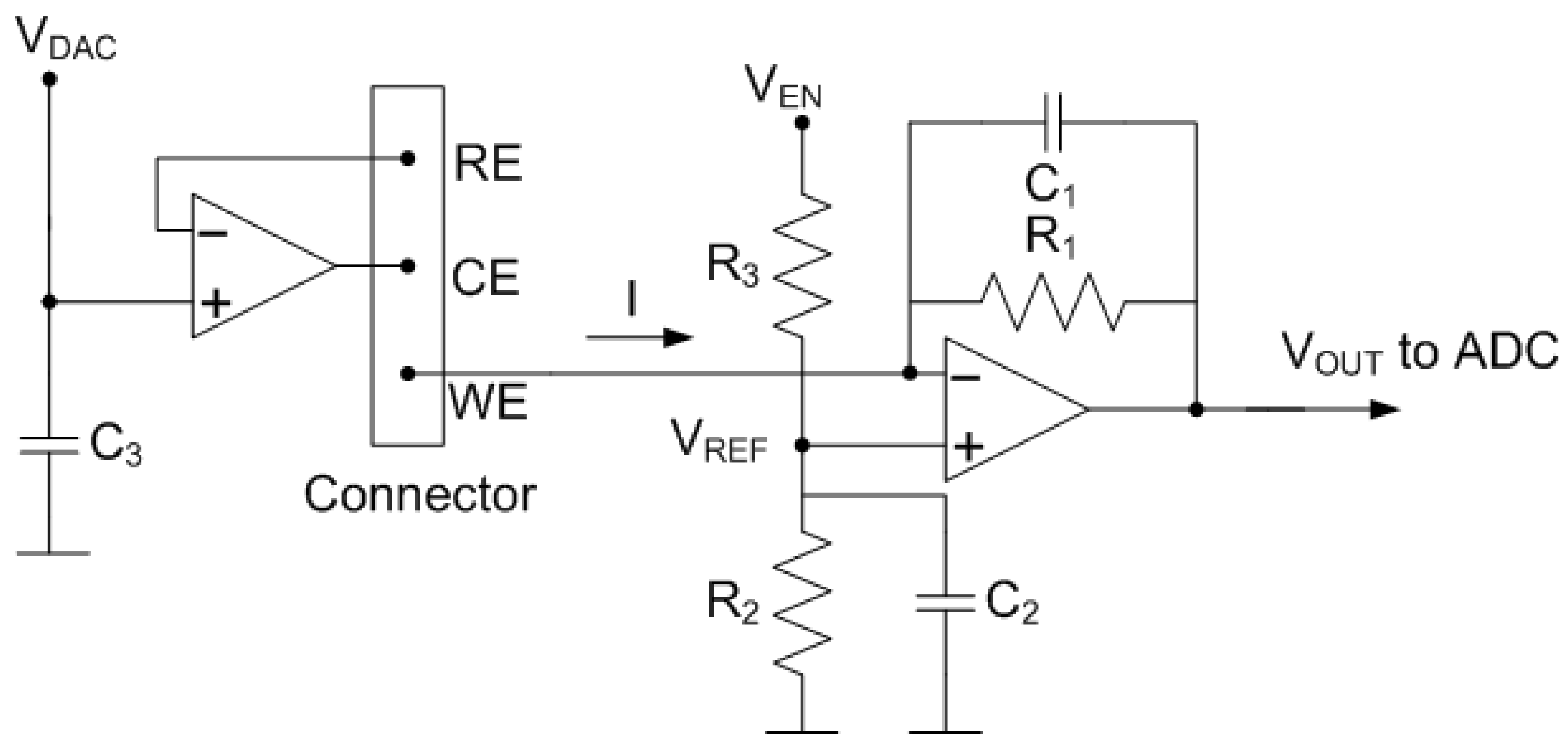
2.4.2. Analog Front-End
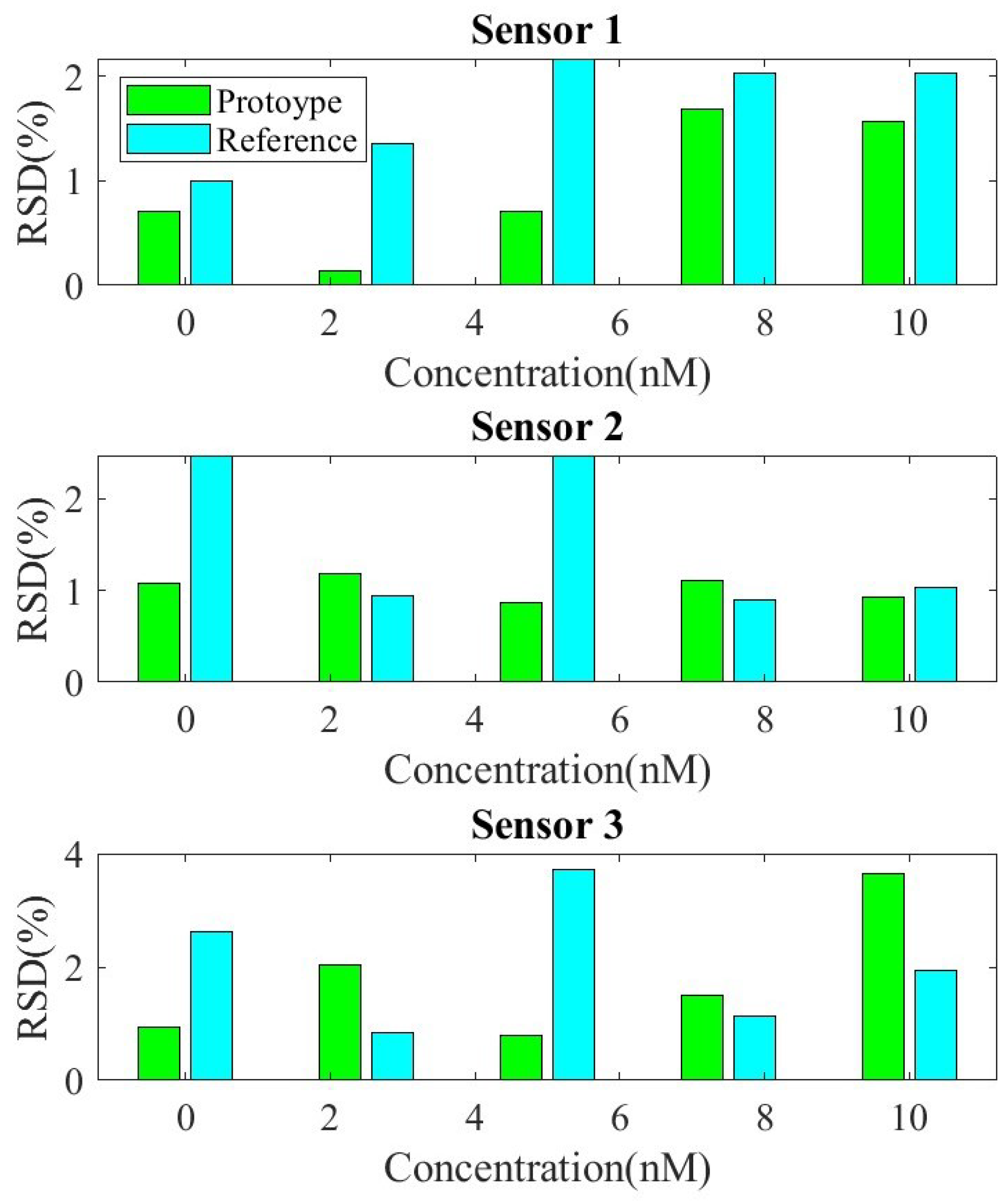
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirceski, V.; Komorsky-Lovric, S.; Lovric, M. Square-Wave Voltammetry: Theory and Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Mirceski, V.; Gulaboski, R. Recent achievements in square-wave voltammetry (a review). Maced. J. Chem. Chem. Eng. 2014, 33, 1–12. [Google Scholar] [CrossRef]
- Abu Shama, N.; Aşır, S.; Ozsoz, M.; Göktürk, I.; Türkmen, D.; Yılmaz, F.; Denizli, A. Gold-modified molecularly imprinted N-Methacryloyl-(L)-phenylalanine-containing electrodes for electrochemical detection of dopamine. Bioengineering 2022, 9, 87. [Google Scholar] [CrossRef]
- Guillem, P.; Bustos, R.H.; Garzon, V.; Munoz, A.; Juez, G. A low-cost electrochemical biosensor platform for C-reactive protein detection. Sens. Bio-Sens. Res. 2021, 31, 100402. [Google Scholar] [CrossRef]
- Msagati, T.A.; Ngila, J. Voltammetric detection of sulfonamides at a poly(3-methylthiophene) electrode. Talanta 2002, 58, 605–610. [Google Scholar] [CrossRef]
- Shaw, J.L. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Rajagopal, S.; Prieto-Simón, B.; Pogue, B.W. Recent advances in smart wearable sensors for continuous human health monitoring. Talanta 2024, 272, 125817. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Ai, J.; Wang, N.; Pu, Q. Recent progress of smartphone-assisted microfluidic sensors for point of care testing. TrAC Trends Anal. Chem. 2022, 157, 116792. [Google Scholar] [CrossRef]
- Lazaro, A.; Villarino, R.; Lazaro, M.; Canellas, N.; Prieto-Simon, B.; Girbau, D. Recent Advances in Batteryless NFC Sensors for Chemical Sensing and Biosensing. Biosensors 2023, 13, 775. [Google Scholar] [CrossRef]
- Gürsan, K.A.E.; Şentürk, M.H.; Göz, E.Y.; Maral, M.; Yildirim, A.; Bozoğlu, A.; Kivrak, B.; Ay, N.C. Electrochemical DNA biosensors developed for the monitoring of biointeractions with drugs: A review. Turk. J. Chem. 2023, 47, 864–887. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Ricci, F.; Plaxco, K.W. E-DNA sensors for convenient, label-free electrochemical detection of hybridization. Microchim. Acta 2008, 163, 149–155. [Google Scholar] [CrossRef]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628. [Google Scholar] [CrossRef] [PubMed]
- Chin, G.P.; Guo, K.; Vasani, R.; Voelcker, N.H.; Prieto-Simón, B. Carbon-stabilized porous silicon biosensor for the ultrasensitive label-free electrochemical detection of bacterial RNA gene fragments. Biosens. Bioelectron. X 2024, 16, 100438. [Google Scholar] [CrossRef]
- Osteryoung, J.G.; Osteryoung, R.A. Square wave voltammetry. Anal. Chem. 1985, 57, 101–110. [Google Scholar] [CrossRef]
- Ramaley, L.; Krause, M.S. Theory of square wave voltammetry. Anal. Chem. 1969, 41, 1362–1365. [Google Scholar] [CrossRef]
- Mirceski, V.; Skrzypek, S.; Stojanov, L. Square-wave voltammetry. ChemTexts 2018, 4, 17. [Google Scholar] [CrossRef]
- Yi, Z.; Kun-Lin, Y. Quantitative detection of phenol in wastewater using square wave voltammetry with pre-concentration. Anal. Chim. Acta 2021, 1178, 338788. [Google Scholar] [CrossRef] [PubMed]
- Guziejewski, D. Electrode mechanisms with coupled chemical reaction—Amplitude effect in square-wave voltammetry. J. Electroanal. Chem. 2020, 870, 114186. [Google Scholar] [CrossRef]
- Haji-Hashemi, H.; Norouzi, P.; Safarnejad, M.R.; Ganjali, M.R. Label-free electrochemical immunosensor for direct detection of Citrus tristeza virus using modified gold electrode. Sens. Actuators B Chem. 2017, 244, 211–216. [Google Scholar] [CrossRef]
- Kang, D.; Zuo, X.; Yang, R.; Xia, F.; Plaxco, K.W.; White, R.J. Comparing the properties of electrochemical-based DNA sensors employing different redox tags. Anal. Chem. 2009, 81, 9109–9113. [Google Scholar] [CrossRef]
- Golub, G.H.; Van Loan, C.F. Matrix Computations; Johns Hopkins Studies in the Mathematical Sciences; Johns Hopkins University Press: Baltimore, MD, USA, 2013. [Google Scholar]
- Somerson, J.; Plaxco, K.W. Electrochemical aptamer-based sensors for rapid point-of-use monitoring of the mycotoxin ochratoxin a directly in a food stream. Molecules 2018, 23, 912. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Liu, Y.; Guan, X.; Li, Y. DNA nanosensors based on the use of single gold nanowire electrodes and methylene blue as an intercalator. Microchim. Acta 2018, 185, 152. [Google Scholar] [CrossRef]
- Rowe, A.A.; Bonham, A.J.; White, R.J.; Zimmer, M.P.; Yadgar, R.J.; Hobza, T.M.; Honea, J.W.; Ben-Yaacov, I.; Plaxco, K.W. Cheapstat: An open-source,“do-it-yourself” potentiostat for analytical and educational applications. PLoS ONE 2011, 6, e23783. [Google Scholar] [CrossRef]
- Ainla, A.; Mousavi, M.P.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernandez-Abedul, M.T.; Whitesides, G.M. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef]
- Aznar-Poveda, J.; Lopez-Pastor, J.A.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Otero, T.F. A cots-based portable system to conduct accurate substance concentration measurements. Sensors 2018, 18, 539. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Huertas, G.; Maldonado-Jacobi, A.; Martín, M.; Serrano, J.A.; Olmo, A.; Daza, P.; Yúfera, A. Sensing cell-culture assays with low-cost circuitry. Sci. Rep. 2018, 8, 8841. [Google Scholar] [CrossRef]
- Lazaro, A.; Villarino, R.; Lazaro, M.; Canellas, N.; Prieto-Simon, B.; Girbau, D. Battery-Less NFC Potentiostat for Electrochemical Point-of-Care Sensors Based on COTS Components. Sensors 2022, 22, 7213. [Google Scholar] [CrossRef]
- Dieffenderfer, J.; Wilkins, M.; Hood, C.; Beppler, E.; Daniele, M.A.; Bozkurt, A. Towards a sweat-based wireless and wearable electrochemical sensor. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- da Costa, J.P.d.C.; Bastos, W.B.; Da Costa, P.I.; Zaghete, M.A.; Longo, E.; Carmo, J.P. Portable laboratory platform with electrochemical biosensors for immunodiagnostic of hepatitis C virus. IEEE Sens. J. 2019, 19, 10701–10709. [Google Scholar] [CrossRef]
- Lopin, P.; Lopin, K.V. PSoC-Stat: A single chip open source potentiostat based on a Programmable System on a Chip. PLoS ONE 2018, 13, e0201353. [Google Scholar] [CrossRef]
- Ahmad, R.; Surya, S.G.; Sales, J.B.; Mkaouar, H.; Catunda, S.Y.C.; Belfort, D.R.; Lei, Y.; Wang, Z.L.; Baeumner, A.; Wolfbeis, O.S.; et al. KAUSTat: A wireless, wearable, open-source potentiostat for electrochemical measurements. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Sarkar, S.; Bhattacharya, M. SStat: Wi-Fi and Bluetooth integrated Multimodal “Do-It-Yourself” Electrochemical Potentiostat. In Proceedings of the IECON 2020 The 46th Annual Conference of the IEEE Industrial Electronics Society, Singapore, 18–21 October 2020; pp. 5249–5254. [Google Scholar] [CrossRef]

| Oligonucleotide | Sequence (From 5′ to 3′) |
|---|---|
| DNA capture probe | SH(CH2)6–TAACCGATTTCAAATGGTGCTA–MB |
| Target DNA | TAGCACCATTTGAAATCGGTTA |
| Non-complementary 1 | TGAGAACTGAATTCCATAGGCTGT |
| Non-complementary 2 | TGT CAG TTT GTC AAA TAC CCC |
| 2-base mismatch (2 bmm) | TAACACCATTTGAAATCAGTTA |
| Part | Current Consumption | Price(USD) | Comment |
|---|---|---|---|
| ADS1220 | 500 A | 5 | 24-bit, 2-kSPS ADC |
| LTC1659 | 240 A | 8 | 12-bit DAC |
| OPA2314 | 300 A | 1 | Dual Op.Amplifier |
| ADG704 | 1 A | 1.6 | 4 Channel multiplexer |
| XIAO BLE | 30 mA | 9 | transmitting at 8 dBm |
| LiPo Battery | - | 3 | 1000 mAh, 3.7 V |
| PCB | - | 5 | PCB and other components |
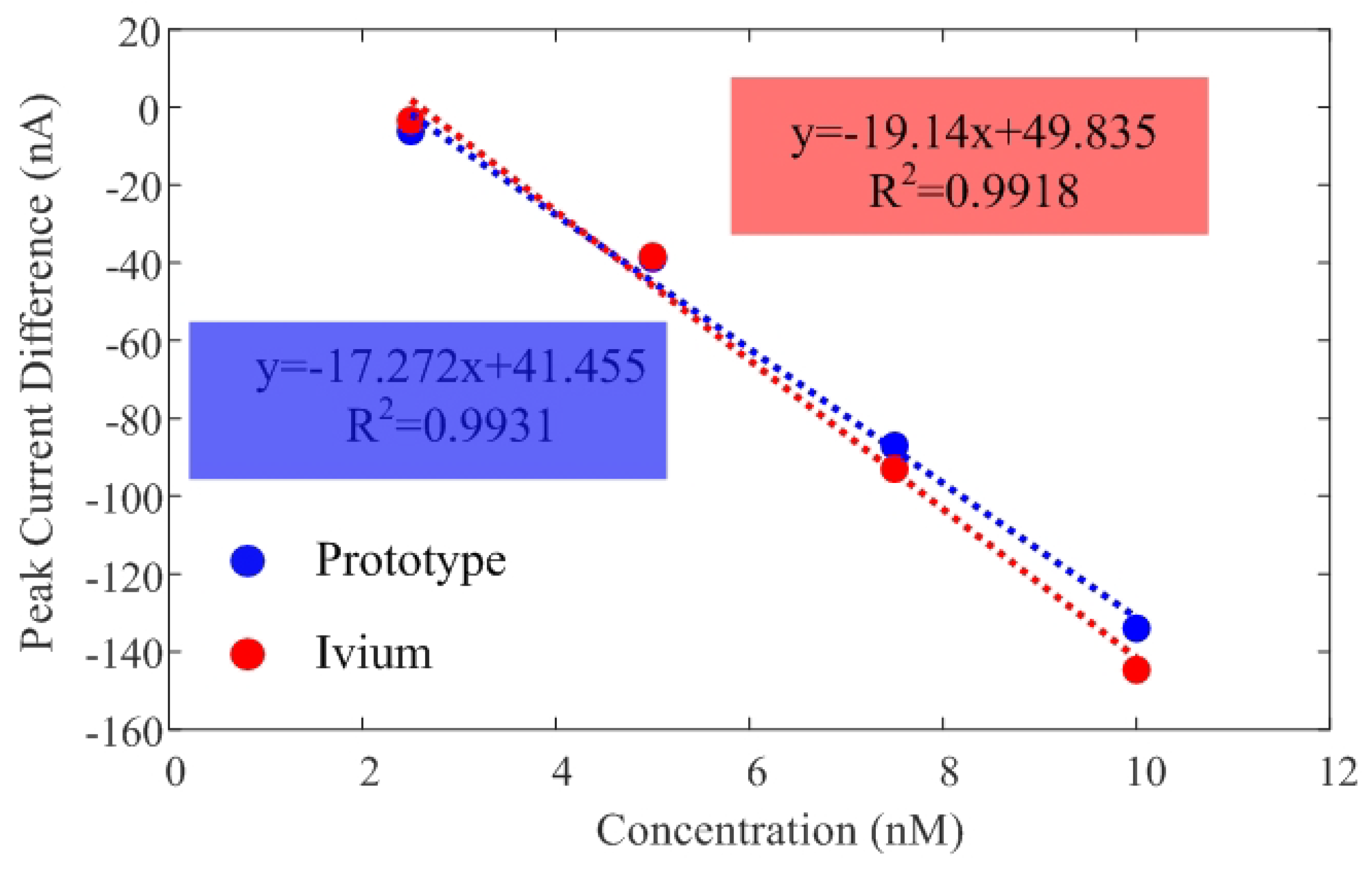
| System | Sensitivity | R2 | LOD |
|---|---|---|---|
| Ivium CompactStat | 19.146 nA/nM | 0.992 | 2.43 nM |
| Prototype | 17.272 nA/nM | 0.993 | 2.27 nM |
| Reference | [31] | [30] | [32] | [33] | [26] | [34] | This Work |
|---|---|---|---|---|---|---|---|
| Microcontroller | Teensy 3.2 | CC2541 | CY8CKIT-059 | RFDUINO | RFDUINO | ESP32 | XIAO BLE |
| Frequency (MHz) | 96 | 32 | 80 | 16 | 16 | 120 | 64 |
| ADC | 12 bit MCU | 16 bit ADS1115 | 20 bit | 12 bit MCU | 12 bit | 12 bit | 24 bit |
| DAC | 12 bit MCU | 6 bit | 12 bit | 12 bit | 16 bit MCU | 16 bit | 12 bit |
| Voltage range | ±1.5 | ±1.2 | ±2 | ±1.5 | ±1.5 | ±1.5 | ±1.5 |
| Current range (A) | 0.1–1000 | 10 | 100 | 10–500 | 10–180 | 10–180 | 0.01–1000 |
| Amplifier | AD8609 | LMP9100 | NA | NA | AD8608 | AD8609 | OPA2314 |
| Technique | CA, CV | CA, CV | CA, CV | CA, CV | CA, CV | CA, CV | CA, CV, SWV |
| Communication | Bluetooth (HC05) | BLE | USB | BLE | BLE | BLE, WIFI | USB, BLE |
| Analyte | Hepatitis C | Lactate | Vitamin, glucose, lead | Ferricyanide | Ferricyanide | Lactate | DNA |
| LOD | 1 ng/L | 0.1 mM | 0.1 mg/mL | 5 mM | 10 M | 0.1 mM | 2.5 nM |
| Price (USD) | NA | 92 | NA | NA | 61.5 | 50 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaro, A.; Villarino, R.; Girbau, D.; Haji-Hashemi, H.; Prieto-Simon, B. Low-Cost Wireless Device for DNA Sensing Using Square Wave Voltammetry. Chemosensors 2025, 13, 119. https://doi.org/10.3390/chemosensors13040119
Lazaro A, Villarino R, Girbau D, Haji-Hashemi H, Prieto-Simon B. Low-Cost Wireless Device for DNA Sensing Using Square Wave Voltammetry. Chemosensors. 2025; 13(4):119. https://doi.org/10.3390/chemosensors13040119
Chicago/Turabian StyleLazaro, Antonio, Ramon Villarino, David Girbau, Hedieh Haji-Hashemi, and Beatriz Prieto-Simon. 2025. "Low-Cost Wireless Device for DNA Sensing Using Square Wave Voltammetry" Chemosensors 13, no. 4: 119. https://doi.org/10.3390/chemosensors13040119
APA StyleLazaro, A., Villarino, R., Girbau, D., Haji-Hashemi, H., & Prieto-Simon, B. (2025). Low-Cost Wireless Device for DNA Sensing Using Square Wave Voltammetry. Chemosensors, 13(4), 119. https://doi.org/10.3390/chemosensors13040119





