Abstract
Cellular senescence is a recently emerged research topic in modern biology. Often described as a double-edged sword, it encompasses numerous essential biological processes, including beneficial effects such as wound healing and embryonic development, as well as detrimental contributions to chronic inflammation and tumor development. Consequently, there is an increasing need to unravel the intricate networks of senescence and develop reliable detection methods to distinguish it from related phenomena. To address these challenges, a variety of detection methods have been developed. In particular, small-molecule fluorescent probes offer distinct advantages such as suitability for real-time live cell monitoring and in vivo imaging, superior tunable properties, and versatile applications. In this review, we explored recent advancements in the development of small-molecule fluorescent probes toward monitoring cellular senescence by targeting various senescence-related biological phenomena. These phenomena include the upregulation of senescence-associated enzymes, perturbation of the subcellular environment, and increased endogenous ROS levels. Moreover, multi-senescence biomarker-targeting approaches are also discussed to improve their sensitivities and specificities for the detection of cellular senescence. With recent advances in senescence probe development, current challenges in this field are also discussed to facilitate further progress.
1. Introduction
Cell cycle arrest is a fundamental mechanism for preserving bodily homeostasis by preventing the proliferation of damaged or aberrant cells [1,2]. First documented in 1961 by L. Hayflick and P.S. Moorhead, cellular senescence is characterized by irreversible cell cycle arrest [3]. Despite the growth arrest, senescent cells actively secrete a spectrum of signaling molecules, including cytokines, chemokines, and growth factors, collectively termed the senescence-associated secretory phenotype (SASP) [4,5,6]. The SASP plays pivotal roles in biological processes such as tissue regeneration and immune cell migration. However, the accumulation of inflammatory mediators within the SASP can also exert detrimental effects. For example, IL-1β has been implicated in obesity-associated hepatocellular carcinoma [7], while IL-1α and Mcp1 contribute to atherosclerosis development and progression [8]. Consequently, cellular senescence is often likened to a double-edged sword. In addition to the secretion of various signaling molecules, senescent cells play critical roles in homeostasis and disease in organism levels. While senescence aids in the clearance of damaged cells, facilitates wound healing, and suppresses tumor growth, it is also implicated in age-related pathologies, including metabolic disorders, musculoskeletal diseases, and neurodegenerative diseases [9,10,11]. Given its complexity and significant implications for health and disease, the development of senescence detection methods has become spotlighted in biomedical research. Such endeavors hold immense promise for facilitating early disease diagnosis and uncovering novel mechanisms underlying senescence.
One of the primary challenges in identifying cellular senescence stems from the lack of a universal marker, due to the complex and heterogeneous nature of senescent cells [12,13]. As a result, researchers have developed and utilized a variety of techniques to detect senescence-associated biological phenomena. For example, chromogenic chemical probes such as X-gal and Sudan Black B have traditionally served as optical tools to monitor the expression levels of senescence-associated enzymes or related biological granules [14,15]. Telomere shortening, another hallmark of senescent cells, is assessed using in situ DNA hybridization techniques with chemically modified oligonucleotide probes [16]. Immunohistochemical techniques are frequently employed to analyze cell cycle regulatory proteins such as p53, p21Cip1, and p16INK4a [17]. Despite these advancements, the biomedical field continues to seek more precise and accessible methods for identifying cellular senescence along with the discovery of reliable biomarkers.
The small-molecule-based fluorescence techniques for detecting senescent cells are considered as ideal due to their potential for real-time live cell and in vivo imaging, easy tunable properties, and versatile applications [18,19,20,21,22,23]. With an increasing number of studies focusing on novel senescence-related targets and subcellular changes, there have been rapid growing demands in the development of small fluorescent probes capable of targeting these biological markers [24]. This review primarily examines recent advancements in small fluorescent probes designed to monitor various senescence-related biomarkers, including senescence-associated enzymes, subcellular environments, and metabolic species (Figure 1). We also provide an overview of the general features of senescent cells, related biomarkers, and senescence inducers. Along with the conventional detection methods for senescence detection, this review explores recent advancements in fluorescent probes, emphasizing their ability to target senescence-associated events. Finally, the future prospects are discussed for developing advanced senescence probes.
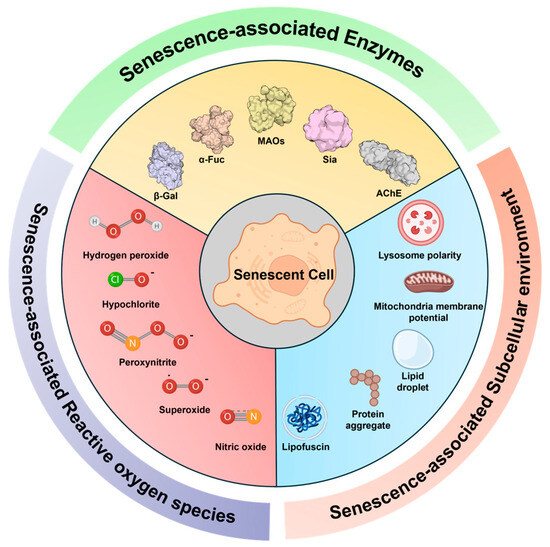
Figure 1.
Representative cellular senescence markers targeted by recently developed small fluorescent probes, which will be mainly discussed in this review. The senescence biomarkers are categorized into three types: senescence-associated enzymes, subcellular environment perturbation, and reactive oxygen species.
2. Characteristics of Senescent Cells
Cellular senescence is characterized by a highly heterogeneous nature, which makes it difficult to distinguish senescent cells from other quiescent or terminally differentiated cells using a single biomarker. Therefore, identifying senescent cells requires a set of multiple simultaneous evidence associated with the cellular senescence process. In this section, we will briefly overview the following: (1) the common features of cellular senescence along with the related biomarkers and (2) the stimuli that induce cellular senescence.
2.1. Representative Features and Biomarkers in Senescent Cells
2.1.1. Changes in Cell Morphology
One of the clearly observable features in cellular senescence is the enlarged and flattened cellular morphology [25,26]. Even though the size of cells can vary depending on the cell type, the size of the same type of cells is known to be tightly regulated [27,28]. However, if the volume of cells is abnormally increased due to senescence-inducing stimuli, the concentration of cytosolic proteins for DNA replication becomes diluted, leading to slower biochemical reactions between DNA and machinery proteins [29]. Consequently, cells with a decreased DNA-to-cytoplasm ratio undergo retarded cell division or growth arrest, thereby exhibiting senescence characteristics. Additionally, an increase in vacuolization or biological granules, such as lipofuscin, is also commonly reported in various senescent cells [30,31,32]. These macroscopic changes can be monitored using light and fluorescence microscopy techniques.
2.1.2. Telomere Shortening
Telomere shortening and its damage are considered as the typical hallmarks of cellular aging [33,34]. The major role of telomeres is to protect chromosomes from damages by forming a highly conserved and repeated DNA cap, known as a TTAGGG unit, at the ends of chromosomes [35]. During cellular division, a phenomenon called the ‘end-replication problem’ gradually induces telomere shortening due to incomplete replication at the DNA ends [36,37]. Thus, the length of telomeres is often considered as a molecular clock to determine the replicative capacity of a given cell. This replicative limitation, which drives cells to permanent cell cycle arrest, is known as replicative senescence [38]. The enzymatic function of telomerase is to add TTAGGG repeats at the ends of chromosomes to protect DNA from damage during each round of DNA replication [39].
2.1.3. Upregulation of Cell Cycle Inhibitory Proteins
Cyclin-dependent kinases (CDKs) play essential regulatory roles in the cell cycle and its progression in response to various cellular signals [40,41,42]. Increased levels of CDK inhibitory proteins, such as p16INK4a and p21Cip1, are therefore considered as important biomarkers of cell cycle arrest [43,44]. DNA damage activates cascade signaling pathways collectively known as the DNA damage response (DDR), which ultimately converge to activate p53, a key tumor suppressor [45]. The following p53-p21Cip1 network can repress cell cycle genes, thereby causing cellular senescence. The increased level of p16INK4a expression is also widely reported in most senescent cells including aged tissues, which inhibits the phosphorylation of the retinoblastoma protein (Rb) by CDKs [46,47]. This inhibitory function leads to the deactivation of a key transcription factor (E2F) in cell proliferation. Consequently, the overexpression of p16INK4a and p21Cip1 served as canonical markers in most senescence studies.
2.1.4. Alterations in Cellular Organelles
During cellular senescence, the physical and chemical properties of various cellular organelles, including the nucleus, lysosomes, and mitochondria, transform in ways that differ from normal cellular conditions. In the nucleus of senescent cells, dense chromatin structures known as senescence-associated heterochromatin foci (SAHF) are often observed due to the rearrangement and condensation of chromatins [48]. In lysosomes, increments in size and contents, as well as changes in microenvironmental properties such as polarity and pH, have been reported [49,50,51,52]. Targeting these properties as markers of senescence using fluorescent probes will be discussed in Section 3.3.1. In addition, a number of evidence indicates that protein leakage, decreased mitochondrial membrane potential, and increased ROS production in mitochondria occur during the aging process [53,54]. Given the importance of its function in cellular metabolism, the dysregulation of mitochondria is presumed to be involved in complex metabolic alterations during the aging process [53,55,56]. In Section 3.3.2, we will present recently reported chemical probes that target mitochondrial changes in senescent models.
2.1.5. Overexpression of Senescence-Associated Enzymes
The overexpression of lysosomal β-galactosidase (β-gal) in senescent cells is the most widely used biomarker in recent senescence studies [57]. This accumulation of β-gal is called “senescence-associated β-galactosidase” (SA-β-gal or SABG). Since a high level of β-gal was reported in aged human skin samples in 1995, it has become a gold standard marker for senescence in numerous studies [58]. However, given that the expression of β-gal is also observed in non-senescent serum-starved cells or overconfluent cells [59], other enzymatic biomarkers need to be further explored. For example, α-L-fucosidase (α-Fuc) has been suggested as another promising biomarker for senescent cells [60]. Another enzyme of interest is monoamine oxidase (MAO), which catalyzes the degradation of biological amines in mitochondria [61]. The upregulation of MAOs in senescent cells, as well as their related effects in producing mitochondrial ROS, has been widely reported [62].
2.1.6. Overabundance of Reactive Oxygen Species (ROS)
During cellular senescence, the dysregulation of mitochondria can lead to an overabundance of reactive oxygen species (ROS) [63]. The upregulation of ROS production has been shown to contribute to the persistent activation of the DNA damage response (DDR) [53,54]. However, considering that ROS signaling also plays contradictory roles in biological processes, including cell proliferation and differentiation [64], the relationship between cellular aging and endogenous ROS could be highly complex and requires more detailed studies. Nonetheless, an increasing number of studies are focusing on detecting endogenous ROS molecules during cellular senescence [65,66]. Recent probes associated with this topic will be discussed in Section 3.4.
2.2. Inducers for Cellular Senescence
DNA damage is considered a major driver of cellular senescence. DNA damage can be triggered by various stimuli including replicative stress, oncogene activation, and chemotherapeutic agents such as doxorubicin and palbociclib. This leads to the activation of a cascade of signaling pathways collectively known as the DNA damage response (DDR). The principal role of the DDR pathway is to block cell cycle progression, thereby preventing the propagation of damaged genetic information to daughter cells. Prolonged activation of DDR has been shown to be a direct cause of cellular senescence.
Replicative stress is directly linked to telomere attrition, where telomeres shorten with repeated cell proliferation, increasing the risk of genetic damage and potentially enhancing the activation of the DDR pathway. Another significant trigger of senescence is oncogene activation, known as oncogene-induced senescence (OIS). Examples of oncogenes that induce OIS include Ras, BRAF, and AKT [67]. OIS promotes cell hyperproliferation, leading to telomere shortening, or it can induce cell cycle arrest through pathways such as MDM2–p53–p21 or p38AMPK–p16. Mitochondrial dysregulation is also a significant trigger of senescence, as it can lead to excessive ROS production. Elevated ROS levels can damage DNA and proteins, thereby inducing senescence through prolonged activation of the DDR pathways [53,54].
3. Detection of Senescent Cells
3.1. Previous Senescence Detection Methods
3.1.1. Colorimetric Analysis
Overexpression of β-gal is the most well-known signature of senescent cells. Colorimetric probes targeting β-gal have been used for the detection of senescent cells. 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal, Figure 2A, top) is one of the easily accessible colorimetric probes for the detection of β-gal activity [14]. X-gal was originally developed by Horwitz et al. in 1964 and is a widely used probe for detecting β-gal encoded by the LacZ gene, which serves as a reporter system in various applications. Due to the strong association between β-gal expression and cellular aging, X-gal is one of the widely used methods for detecting senescent cells to date [68,69]. The O-glycosidic bond of X-gal undergoes hydrolysis by β-gal, leading to the formation of a blue precipitate [58,69]. Similarly, ortho-nitrophenyl-β-galactoside (ONPG) is another colorimetric probe used to detect β-gal activity, particularly in senescent cells [70]. Upon its reaction with β-gal, the glycosidic bond of ONPG is cleaved, generating a yellow ortho-nitrophenol product.
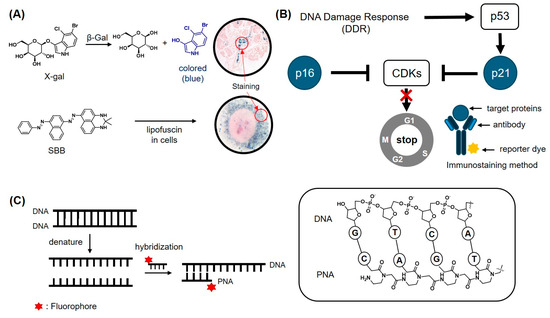
Figure 2.
Conventional detection methods for cellular senescence. (A) Colorimetric probes to detect β-gal activity using X-gal (top) and detecting the accumulation of lipofuscin using Sudan Black B (SBB, bottom). Arrows indicate the staining areas by probes. (B) Signaling pathway of CDK inhibitors (P16 and P21) that promote cell cycle arrest. These protein markers are typically detected using immunostaining methods with antibody–dye conjugates. (C) In situ hybridization method to detect specific DNA sequences. PNA/DNA pairs can exhibit a stronger interaction compared to DNA/DNA, due to reduced electrostatic repulsion.
Lipofuscin is another well-established biomarker for aging cells, characterized by aggregates containing oxidized proteins, lipids, and (oligo)saccharides [71]. Sudan Black B (SBB, Figure 2A, bottom) is a lipophilic dye with diazo groups, known for its ability to detect lipofuscin [72]. When SBB reacts with lipofuscin, it forms blue-black or brown cytoplasmic granules, which are observable under light microscopy [73].
3.1.2. Immunoassay Techniques
Given that senescent cells are in a non-proliferative state, proteins that negatively regulate the cell cycle are often considered as biomarkers of senescent cells. Cyclin-dependent kinases (CDKs) perform essential functions in cell proliferation, and their inhibitor proteins, such as p21cip1 and p16INK4a, are well-known biomarkers of cellular senescence [74,75]. These proteins are overexpressed in senescent cells and trigger apoptosis by promoting cell cycle arrest (Figure 2B) [76,77,78]. They play critical roles in two distinct signaling pathways, the p53/p21cip1 and p16INK4a/Rb pathways. The p53/p21cip1 pathway is primarily involved in the early stages of senescence, while the p16INK4a/Rb pathway functions to maintain senescence [79]. These are supported by the observation of a decrease in p53 levels during cellular senescence, whereas p16 levels remain upregulated throughout the senescence process [80]. These senescence biomarkers (p21cip1 and p16INK4a) are commonly detected by immunohistochemical staining or Western blot [81,82].
3.1.3. Telomere Shortening Detection (FISH Technique)
Telomere shortening, a hallmark of most senescent cells, occurs with each cell division, although it is rare in certain types of cells, such as brain cells [37]. Telomere shortening can be detected using telomere restriction fragment (TRF) analysis or quantitative polymerase chain reaction (qPCR). However, these technologies have their inherent limitations, such as only quantifying the average length of telomere in samples rather than the length of individual telomeres [83]. In 1982, Langer-Safer et al. developed the fluorescence in situ hybridization (FISH) technique utilizing a fluorescent probe conjugated with a specific nucleic acid sequence [84]. This technique enables the visualization and detection of target DNA or RNA sequences with a high degree of precision due to the specific interaction between complementary base pairs. Employing this approach, Q-FISH has been developed to determine the telomere length of each individual chromosome in a single cell using telomere-specific peptide nucleic acid (PNA) (Figure 2C). Due to the absence of negatively charged phosphate groups in the PNA backbone, a repeated CCCTAA sequence of the PNA probe can precisely target its complementary telomere sequence (TTAGGG) with higher affinity compared to that of DNA probe. The quantitative analysis of telomere can be achieved through the digital quantification of Q-FISH microscopic images [16].
3.2. Small Fluorescence Probes Targeting Senescence-Associated Enzymes
Due to their higher cell permeability, ease of use, and outstanding photophysical properties, small-molecule fluorescence probes have been developed to monitor cellular senescence by targeting senescence-associated enzymes, subcellular changes, and metabolites. In this section, we will introduce recent developments of fluorescence probes designed to monitor aging-related phenomena.
3.2.1. Senescence-Associated β-galactosidase (SA-β-gal)
SA-β-gal has been considered as a gold standard biomarker for monitoring cellular senescence. β-gal exhibits its most efficient enzymatic activity at pH 4 in lysosomes, while SA-β-gal maintains its catalytic activity even at pH 6.0 [58]. In general, SA-β-gal-selective fluorescent probes generate a fluorescent signal upon the hydrolysis of the galactose group, which is covalently connected to the signal moiety. Currently developed probes can be categorized into three types: turn-on probes, ratiometric probes, and in situ covalent labeling probes. (ⅰ) The turn-on SA-β-gal probes typically exhibit marginal fluorescence when conjugated with galactose groups. Upon hydrolysis of glycosidic bonds of the probes by SA-β-gal, fluorescence is significantly enhanced (Figure 3A–D). (ⅱ) Ratiometric SA-β-gal probes emit shifted fluorescence signals after the hydrolysis of the galactose group (Figure 4A–C). (ⅲ) Hydrolysis of the galactose group of in situ covalent labeling probes generates an electrophilic functional group for the formation of covalent bonds with proteins nearby, enabling a strong fluorescent signal enhancement and long retention time of the fluorescent probes either in cells or in vivo (Figure 5A–D).
SA-β-gal Turn-on Fluorescence Probe
To develop turn-on fluorogenic probes for SA-β-gal, various types of fluorescent materials, including one-photon, two-photon, and aggregation-induced emission (AIE)-type fluorophores, have been employed (Figure 3A,B). Kim et al. developed a turn-on probe named senescence-responsive fluorescent probe (SRP, 1) for the detection of the senescence of vascular endothelial cells (Figure 3B) [85]. This probe consists of a rhodol-based fluorescence probe conjugated with galactose. Activation of SRP by SA-β-gal conferred maximum emission at 545 nm, with a 27-fold enhancement in fluorescence intensity. SRP also exhibited stronger fluorescence intensity under acidic conditions (pH ≤ 5.0) compared to that under basic conditions (pH ≥ 7.0). SRP successfully imaged senescent cells induced by H2O2 and was co-localized with a lysosomal tracker. Similarly, Qiu et al. reported a near-infrared (NIR) fluorescent probe KSL0401 (2), based on a coumarin–dicyanoisophorone hybrid fluorophore (Figure 3B) [86]. The activated form of KSL0401 exhibits a fluorescent emission maximum at 706 nm, with a large Stokes shift of 218 nm upon the hydrolysis of galactose. In addition, KSL0401 reacts with SA-β-gal within 80 s and selectively images senescent HepG2 cells and A549 cells. In 2021, Fan et al. developed the NIR fluorescent probe DCMCA-β-gal (3) to monitor SA-β-gal activity (Figure 3B) [87]. This probe is designed based on DCM-NH2, a previously reported NIR fluorescent probe [88,89,90]. When DCMCA-β-gal is activated by SA-β-gal, it generates the NIR emission reporter DCM-NH2. The amino group of DCM-NH2 acts as a strong electron donor, facilitating intramolecular charge transfer (ICT), which leads to NIR emission. After DCMCA-β-gal is reacted with SA-β-gal, the emission maxima shift to 676 nm, showing a 12-fold increment in fluorescent intensity compared to its pre-reaction state. This probe can detect the activity of SA-β-gal both in vitro and in vivo. Liu et al. reported the fluorescent probe HBT-gal (4), which is based on the 2-(2′-hydroxyphenyl)-benzothiazoles (HBT) fluorophore (Figure 3B) [91]. The SA-β-gal-mediated hydrolysis of the glycosidic bonds of HBT-gal releases a green fluorophore. Upon activation of the probe, fluorescence emission is increased by over 700-fold at 492 nm. HBT-gal enables the sensitive detection of SA-β-gal with a limit of detection (LOD) of 0.19 mU/mL. HBT-gal successfully imaged the activity of SA-β-gal in senescent HepG2 cells induced by H2O2.
Compared to one-photon fluorescent probes, two-photon fluorescent probes offer advantages such as reduced phototoxicity and deep penetration depth, making them suitable for tissue and in vivo imaging [92,93]. Lozano-Torres et al. reported a naphthalimide-based SA-β-gal probe, AHGa (5), which exhibits two-photon fluorescent properties (Figure 3B,C) [18]. AHGa has an L-histidine methyl ester linker and an acetylated galactose group. When excited with 405 nm light, AHGa has no characteristic fluorescent emission band, but after the hydrolysis of galactose, it shows a 286-fold higher fluorescent emission at 540 nm. In an in vivo demonstration, AHGa exhibited a significant increase in fluorescence intensity in senescent tumors compared to non-senescent tumors in an SK-MEL-103 xenograft mice model. Li et al. also developed a two-photon fluorescent SA-β-gal probe named YDGAL (6) (Figure 3B) [19]. After the release of β-galactose, YDGAL emits a 37-fold enhanced fluorescence at 585 nm via an ICT mechanism due to the strong electron-donating ability of the exposed hydroxyl group. YDGAL was able to detect SA-β-gal both in vitro and in vivo effectively.
In 2001, Tang’s group reported a novel fluorogenic mechanism named aggregation-induced emission (AIE) [94]. AIE-based fluorophores do not emit fluorescence in their single-molecule state but exhibit strong fluorescence when they form aggregates, mainly due to the restriction of intramolecular motions [95]. By employing this mechanism, Dong et al. reported an AIE-based fluorescent probe, TD-Gal6 (7), to detect SA-β-gal activity (Figure 3B,D) [96]. TD-Gal6 is based on dicyano-methylene-4H-pyran conjugated with a tetraphenylethylene core and incorporated with six galactose molecules to enhance its water solubility. The incubation of TD-Gal6 with SA-β-gal at pH 7.4 resulted in a five-fold increment in fluorescence emission, enabling the imaging of senescent cells and ovarian cancer cells without affecting cell viability. It was previously reported that although SA-β-gal can be found in both senescent cells and ovarian cancer cells, the subcellular localization of SA-β-gal in these two types of cells is different [97]. As such, cellular SA-β-gal imaging using TD-Gal6 revealed its distinct localization patterns between senescent cells and cancer cells. When applied together with commercial organelle-targeting probes, TD-Gal6 showed a higher co-localization with lysosomes (0.91) compared to mitochondria (0.71) in H2O2-induced senescent Wi38 cells. In contrast, in ovarian cancer cells (SKOV-3), TD-Gal6 showed a higher co-localization with both lysosomes (0.95) and mitochondria (0.92), which demonstrated the cell type-dependent subcellular localization of SA-β-gal.
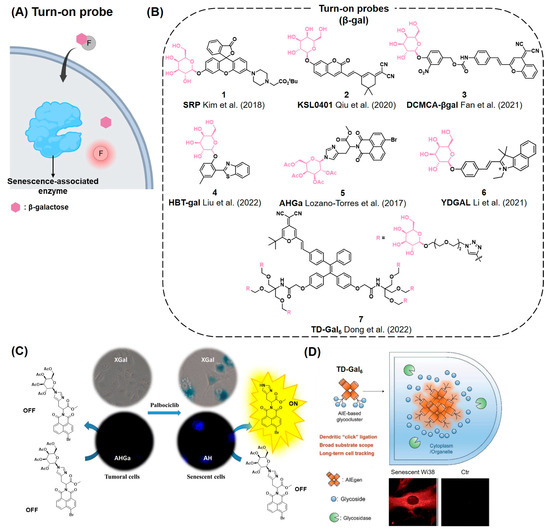

Figure 3.
(A) Schematic of the turn-on probe for the detection of senescence-associated enzymes such as SA-β-gal. The probes become fluorescent after hydrolysis of β-galactose by SA-β-gal. (B) Chemical structures of representative turn-on probes that can react with SA-β-gal [18,19,85,86,87,91,96]. (C) An example of a two-photon turn-on probe, AHGa, which is activated upon hydrolysis of the N-glycosidic bond by SA-β-gal. Reprinted with permission from [18]. Copyright 2017 American Chemical Society. (D) AIE-dependent senescence turn-on probe, TD-Gal6. When the β-gal groups (blue) from TD-Gal6 are cleavage by SA-β-gal (green), it aggregates and emits fluorescence through the AIE mechanism (fluorescent red). Reproduced from [96] with permission from the Royal Society of Chemistry.
SA-β-gal Ratiometric Fluorescence Probe
A ratiometric fluorescence probe exhibits a shifted emission wavelength of fluorescence signals after reacting with a target analyte (Figure 4A) [98]. Compared to turn-on type probes, ratiometric fluorescence probes show more accurate analytical power by measuring the intensity ratio between the original emission wavelength and the shifted emission wavelength induced by analytes. Using this concept, ratiometric fluorescence SA-β-gal probes have been reported for the detection of senescent cells (Figure 4B). In 2014, Lee et al. reported the ratiometric two-photon fluorescent probe SG1 (8), capable of detecting the activity of SA-β-gal in senescent cells (Figure 4B) [99]. SG1 was based on 6-(benzo[d]thiazol-2′-yl)-2-(methylamino)naphthalene conjugated with a galactose group via a self-immolative linker. After activation, SG1 exhibited a maximum emission wavelength shift (460 nm→540 nm) concomitant with a decrease in intensity at 460 nm. SG1 successfully detected SA-β-gal activity in aged human diploid fibroblasts (HDFs) and skin tissues. Chen et al. also developed an NIR fluorescent probe, TZ-Br (9), to detect SA-β-gal in both senescent and cancer cells [100]. TZ-Br showed a fast response to SA-β-gal within 15 min, a large Stokes shift (170 nm), and an 80-fold enhancement in fluorescence emission in response to SA-β-gal. Due to its long wavelength emission in the NIR region, TZ-Br could successfully image SA-β-gal not only in senescent cells but also in aged zebrafish. In 2024, Liu et al. reported a ratiometric fluorescent probe, P1 (10), for the real-time detection of SA-β-gal in lysosomes (Figure 4B,C) [20]. P1 is embedded with cyanovinylidene dye (BMZ) as a signal fluorophore, which can migrate into lysosomes due to the presence of the diethylamino functional group. Fluorescence of P1 undergoes a blue shift in fluorescent emission maxima from 620 nm to 533 nm upon its activation by SA-β-gal. Using P1, the SA-β-gal activity was successfully monitored in drug-induced senescence in HepG2 cells and in a senescence rat in vivo model.
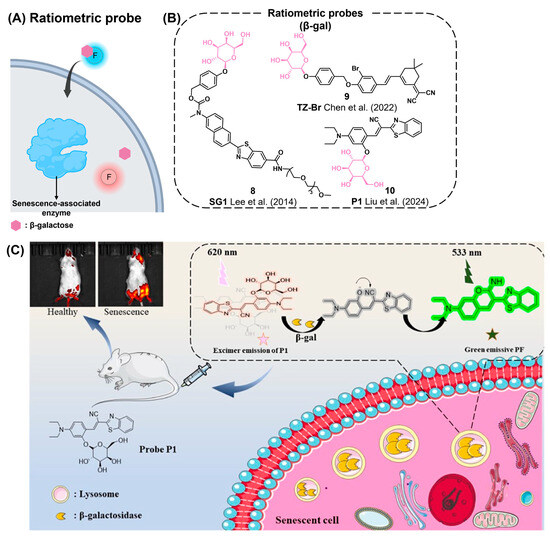

Figure 4.
(A) Schematic of the ratiometric probe (blue circle) for senescence-associated enzymes exhibiting shifted emission (red circle) after enzymatic cleavage reaction. (B) Chemical structures of representative ratiometric probes [20,99,100]. (C) The ratiometric probe P1 reacting with SA-β-gal and exhibiting a change in fluorescence from red to green. The probe is applicable for a senescent mouse model, enabling detection of SA-β-gal activity in vivo. Reprinted with permission from [20]. Copyright 2024 American Chemical Society.
In Situ Covalent Labeling of SA-β-gal Fluorescence Probes
After the signal enhancement in fluorescent probes by analytes, the probes can freely move out from the intracellular region of interest, resulting in an unclear fluorescent cellular image. To visualize the intracellular location of analytes, fluorescent probes should remain exactly at the location where they react with the analytes. To resolve this issue, in 2016, Urano’s group reported a pioneering strategy employing quinone methide chemistry to develop a reactive fluorescent probe capable of forming covalent labels with adjacent proteins after enzyme-mediated probe activation (Figure 5A) [101]. They designed SPiDER-βGal-1 (11), a novel probe for SA-β-gal activity, consisting of a galactose group, a spirocyclic fluorophore, and a fluoride leaving group (Figure 5B). Using this probe, lacZ(+) cells and targeted neurons in living brains were imaged by achieving over a 650-fold enhancement in fluorescence intensity with an excellent resolution upon SA-β-gal-mediated activation (Figure 5C). Due to the high correlation between β-gal and cellular senescence, SPiDER-βGal-1 has become one of the most frequently applied fluorogenic tools in recent senescence studies. Recently, Liu et al. reported NIR-BG2 (12) by conjugating an (E)-2-(2-(6-hydroxy-2,3-dihydro-1H-xanthen-4-yl)-vinyl)-3,3-dimethyl-1-propyl-3H-indol-1-ium (HXPI) NIR fluorophore with a quinone methide-based self-immobilizing group (Figure 5D) [102]. After the cleavage of β-galactose by SA-β-gal, in situ-generated electrophilic quinone methide enables the probe to generate covalent bonds with proteins nearby, which helps to monitor the exact cellular location of SA-β-gal with fluorescent imaging. Compared to the control probe lacking a self-immobilizing group, NIR-BG2 exhibited stronger fluorescence and a longer retention of fluorescence after its activation in senescent cells. The authors applied this probe in mouse xenograft models to assess its efficiency and non-invasive imaging capabilities through the in situ labeling strategy. In 2023, Yu et al. reported on the NIR fluorescent probe Gal-Cy-Gd-1 (13), which is capable of labeling and visualizing SA-β-gal (Figure 5B) [103]. Similar to the previous two examples, when Gal-Cy-Gd-1 reacts with β-gal, it forms a quinone methide intermediate that enables in situ covalent labeling with nearby enzymes or proteins. Additionally, the probe is conjugated to a paramagnetic DOTA-Gd chelate for magnetic resonance imaging. Upon activation, Gal-Cy-Gd-1 exhibits a 42-fold increase in fluorescence at 717 nm, allowing the detection of SA-β-gal activity in living cells and mouse models.
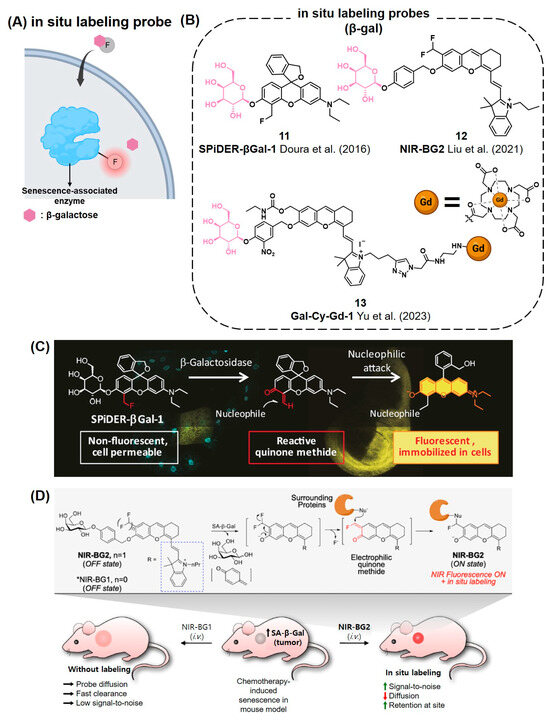

Figure 5.
(A) Schematic of the in situ covalent labeling probe for senescence-associated enzymes. These probes are hydrolyzed by SA-β-gal to form reactive quinone methide intermediate, which is capable of forming covalent bonds with proteins nearby. (B) Chemical structures of in situ labeling probes that can detect SA-β-gal [101,102,103]. (C) SPiDER-βGal-1 is an in situ covalent labeling probe for the detection of β-Gal. The ortho quinone methide formed after hydrolysis enables covalent binding to proteins nearby. Reprinted with permission from [101]. Copyright 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany. (D) A recent in situ labeling probe, NIR-BG2, that demonstrates applicability in in vivo imaging. Reprinted with permission from [102]. Copyright 2021 American Chemical Society.
3.2.2. Senescence-Associated α-fucosidase (SA-α-fuc)
Along with SA-β-gal as the most well-known biomarker for senescent cells, recent studies reported the upregulated expression levels of other hydrolases in cellular senescence, such as α-fucosidase (α-fuc), which is involved in the metabolism of glycolipids or glycoproteins [60,104].
In 2021, Koo et al. developed the probe QM-NHαfuc (14) for the detection of α-L-fucosidase as a cellular senescence imaging agent (Figure 6A,E) [21]. Once QM-NHαfuc is activated by α-fucosidase, the resulting fluorophore exhibits AIE properties with a maximum emission at 586 nm. They demonstrated that QM-NHαfuc could detect cellular senescence even in SA-β-gal lacking aged cells. Furthermore, the probe was able to detect senescent cells induced by various stimuli such as reactive oxygen species (ROS), UVA, and drugs. QM-NHαfuc was also utilized as an in vivo senescence imaging agent in a drug-induced senescence xenograft mouse model.
3.2.3. Monoamine Oxidases (MAOs)
Monoamine oxidases (MAOs) are mitochondrial enzymes oxidizing biological amines. There are two isoforms of MAOs: monoamine oxidase-A (MAO-A) and monoamine oxidase-B (MAO-B). MAO-A and MAO-B exhibit different substrate preferences. MAO-A has a preference for dopamine, tyramine, serotonin, and norepinephrine, whereas MAO-B exhibits a preference for dopamine, tyramine, phenylethylamine, and benzylamine [105]. It is known that the expression levels of MAOs increase in senescent cells, and they are strongly associated with degenerative diseases such as Alzheimer’s and Parkinson’s [61]. Furthermore, MAOs generate ROS, which has recently been revealed as a potential contributor to senescence [62].
Wang et al. reported a ratiometric probe, MitoCy-NH2 (15), which is selective for MAO-B (Figure 6B,F) [106]. This probe consists of a propylamine group as a substrate for MAO-B, a cyanine fluorophore as a signal unit, and triphenylphosphonium as an organelle-targeting moiety for mitochondria. Upon reacting with MAO-B, MitoCy-NH2 exhibits a decreased fluorescent emission maximum at 803 nm and increased fluorescent emission maximum at 750 nm in H2O2-induced senescent cells and in aging mouse models.
3.2.4. Sialidase
Sialic acid is typically located at glycoproteins on the cell surface [107,108]. One of the critical roles of sialic acid is related to host–pathogen interaction for immune responses [107]. Moreover, sialic acid is reported to contribute to the cellular surface charge due to its carboxylic acid moiety and is associated with the cellular senescence in erythrocyte [109]. The level of sialic acid is significantly lower in aged erythrocyte compared to younger erythrocyte. Treatment of young erythrocyte with sialidase resulted in the reduction of sialic acid on the cell surface and accelerated cellular senescence [110].
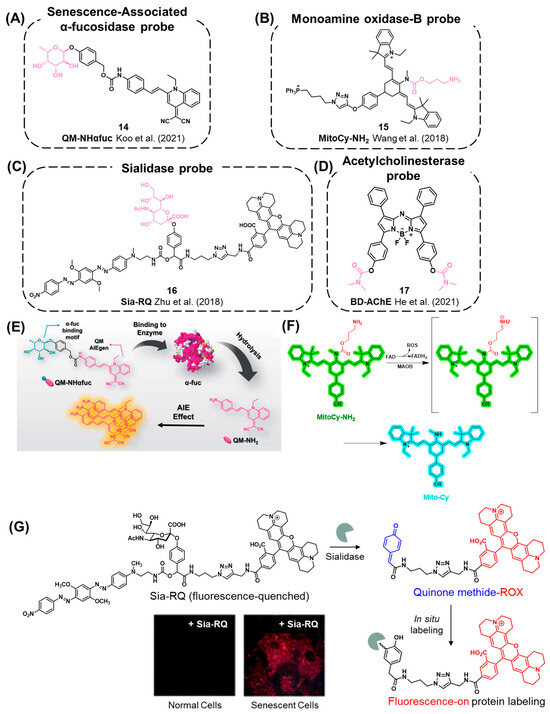

Figure 6.
(A–D) Chemical structures of probes for senescence-associated enzymes other than β-gal: (A) SA-α-fuc [21], (B) MAO-B [106], (C) sialidase [111], (D) AChE [112]. (E) A recent SA-α-fuc probe, QM-NHαfuc, which exhibits fluorogenic AIE phenomena after reacting with α-fuc. Reproduced from [21] with permission from the Royal Society of Chemistry. (F) Detection of MAO-B activity using MitoCy-NH2 that undergoes oxidative deamination, β-elimination, followed by self-immolative discharge of CO2 in senescent cells. Reprinted with permission from [106]. Copyright 2021 American Chemical Society. (G) Chemical mechanism of Sia-RQ. After the reaction of Sia-RQ with sialidase, a reactive quinone methide is generated and formed in situ covalent bonds with proteins nearby. Reproduced from [111] with permission from the Royal Society of Chemistry.
In this context, Zhu et al. reported a fluorescent probe, Sia-RQ (16), to elucidate the correlation between sialidase and cellular senescence (Figure 6C,G) [111]. Sia-RQ was designed by conjugating a sialic acid residue with self-immolative 4-hydroxymandelic acid, which is further conjugated to a fluorophore (rhodamine-X) and a quenching moiety (blackhole fluorescence quencher, BHQ). Initially, the fluorescence signal of Sia-RQ is suppressed due to the quenching effect of BHQ. Upon the reaction of Sia-RQ with sialidase, the self-immolation and release of BHQ generate the fluorescence signal. Additionally, the in situ formation of a reactive quinone methide group allows for the capture of adjacent sialidase through the formation of a covalent linkage (Figure 6G). Furthermore, Sia-RQ successfully monitored the significantly enhanced activity of sialidase in palbociclib-induced senescent cells, suggesting sialidase as a potential biomarker of senescence.
3.2.5. Acetylcholinesterase
A recent study by Yue’s group suggests that acetylcholinesterase (AChE) can serve as a potential biomarker to monitor the aging process, particularly in neuronal senescence [112]. They developed an acetylcholinesterase (AChE)-targeting probe, BD-AChE (17), based on the NIR aza-BODIPY moiety, to evaluate the level of AChE in the brains of mice during dietary restriction (Figure 6D). Using BD-AChE, they monitored the activity of acetylcholinesterase in vivo in 1-, 6-, 12-, and 18-month-old mice and found the correlation between the level of AChE and age. Additionally, they observed a decreased level of AChE in old mice after long-term dietary restriction, demonstrating the close relationship between dietary restriction and AChE levels.
3.3. Small Fluorescence Probes Monitoring Senescence-Associated Subcellular Environment
3.3.1. Lysosomal Polarity
During cellular senescence, alterations in the characteristics of lysosome such as size, number of lysosomes, and internal pH have been observed, which could be potential targets for monitoring the aging process (Figure 7A) [49,50,51,52]. Shi et al. reported a curcumin-based near-IR fluorescent probe, KSLP1 (18), exhibiting polarity-dependent fluorescent signal emission (Figure 7B,C) [113]. KSLP1 exhibits red fluorescence (~700 nm) in a low-polar environment, while the fluorescence is dissipated in the polar environment due to the presence of rotatable bonds. Using this probe, they monitored increased polarity in lysosomes in drug-induced senescent MRC-5 cells and aged C. elegans. They proposed the relationship between aging and lysosomal polarity as follows: During the process of senescence, decreased v-ATPase activity causes an increase in lysosomal pH. Consequently, the activity of lysosomal hydrolases decreases, which facilitates the proteolysis of the hydrolases. As a result, hydrophilic peptides are generated and contribute to the hydrophilic environment of lysosomes.
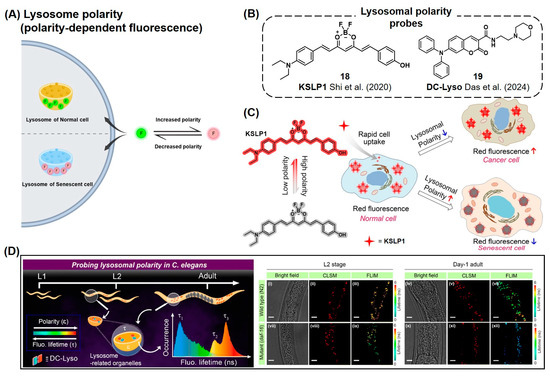
Figure 7.
(A) Schematic of fluorescent probes for the detection of lysosome polarity during cellular senescence. (B) Chemical structures of lysosome polarity detection probes [113,114]. (C) KSLP1 shows decreased fluorescence as lysosomal polarity increases. KSLP1 emits significant red fluorescence signals in normal cells, while its fluorescent signal was reduced in senescent cells. The star symbol represents the red fluorescence signal from KSLP1 in a low-polarity environment. Reprinted from [113]. Copyright 2020, with permission from Elsevier. (D) Application of DC-Lyso probe to monitor the senescence cells in C. elegans with fluorescent lifetime analysis. DC-Lyso showed a decrease in fluorescence lifetime, confirming the increased polarity of lysosome during the senescence process. CLSM: confocal laser scanning microscopy. FLIM: fluorescence lifetime imaging microscopy. Reproduced from [114] with permission from the Royal Society of Chemistry.
Recently, Das et al. reported a thermally activated delayed fluorescent (TADF) probe named DC-Lyso (19) (Figure 7B,D) [114]. DC-Lyso was designed to have a lysosome-targeting morpholine group, along with diphenylamine as a donor and coumarin as an acceptor for charge transfer properties. In polar environments, DC-Lyso preferably undergoes a nonradiative decay process, resulting in a reduced fluorescence lifetime. In the senescent cells, DC-Lyso showed a decrease in the fluorescence lifetime, confirming the increased polarity of lysosomes during the senescence process. Exploiting this property of DC-Lyso, they further monitored the autophagy process with real-time imaging in various cell lines and also observed the senescence process in C. elegans through lifetime imaging analysis.
3.3.2. Mitochondria Membrane Potential
Recently, mitochondria with decreased membrane potential have been suggested to serve as a potential marker for cellular senescence (Figure 8A) [115,116]. Ang et al. utilized a diversity-oriented fluorescence library approach (DOFLA) to discover a cyanine derivative fluorescent probe CyBC9 (20) specifically targeting senescent human mesenchymal stromal cells (MSCs, Figure 8B,C) [115]. This probe showed rapid staining capability within 2 h, non-toxicity, and high reproducibility in targeting live senescent MSCs. CyBC9 exhibited higher accumulation in mitochondria compared to other organelles, such as the Golgi apparatus and endoplasmic reticulum. The accumulation of CyBC9 in the mitochondrial region was increased by the treatment of a mitochondrial oxidative phosphorylation uncoupling reagent, carbonyl cyanide m-chlorophenyl hydrazone (CCCP). This observation demonstrated that the targeting mechanism of CyBC9 is due to the reduction in mitochondrial potential. They proposed that during the cellular senescence process, the depolarization of mitochondria is induced by elevated levels of ROS, which may lead to a reduction in mitochondrial membrane potential and the specific accumulation of CyBC9 in senescent mitochondria. Tian et al. reported a spiropyran-based probe, TANG (21), capable of monitoring membrane potential perturbation during cellular senescence (Figure 8B,D) [116]. Due to the switchable characteristics of the spiropyran structure, TANG exhibits two different fluorescence characteristics: red fluorescence in the open form (573 nm) and blue fluorescence in the closed form (459 nm), which can be switched depending on the environmental conditions. During the real-time monitoring of HepG2 cell senescence, a reduction in the fluorescence ratio (IRed/IBlue) of TANG from 17 to 1.9 was observed. Similar to CyBC9, the mechanism of action of TANG is also believed to be associated with the reduction in mitochondrial membrane potential in senescent cells.
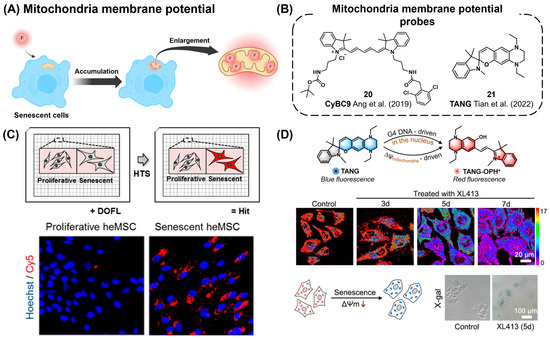
Figure 8.
(A) Schematic of probes targeting changes in mitochondrial membrane potential for senescence cell detection. F (red circle) represents a fluorescent reporter. (B) Chemical structures of mitochondrial membrane potential probes [115,116]. (C) Discovery of CyBC9 using high-throughput screening (top panel) and specific fluorescent labeling of senescent heMSCs by CyBC9 (bottom panel). Reprinted from [115]. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (D) A recent membrane potential probe, TANG, exhibiting ratiometric emission changes due to its intramolecular cyclization, which is sensitive to the level of membrane potential. Reprinted from [116]. Copyright 2022, with permission from Elsevier.
3.3.3. Accumulation of Lipids and Protein Aggregations
The accumulation of protein and lipid aggregates has been reported as disease-relevant and aging-associated events at the subcellular level [117,118]. Specifically, oxidized proteins and lipid droplets can be accumulated and form lipofuscin, which is strongly related to the aging process. Therefore, targeting these cellular aggregates has been suggested as a potential biomarker for senescent cells (Figure 9A).
Long et al. reported a dual-functional fluorescent probe, LW-1 (22), for monitoring the lipid droplets (LDs) and protein aggregates simultaneously (Figure 9B,C) [119]. The fluorescence properties of LW-1 were regulated by the formation of an intramolecular hydrogen bond, which results in the restriction of C-N bond rotation or that of intramolecular charge transfer. With those fluorescent emission mechanisms, LW-1 emits either red fluorescence in aprotic lipid droplets (LDs) or NIR fluorescence in protic and viscous protein aggregates. Using this probe, they investigated the relationship between LDs and protein aggregates in the intestinal tissues of aged mice. Given that protein aggregates can gradually accumulate during the aging process, they suggested that the formation of intracellular LDs can be augmented to encapsulate the protein aggregates, eventually leading to the lipophagy process for the degradation of lipid–protein aggregates.
Lipofuscin, a lipid-containing pigment found in the lysosomes of post-mitotic cells, is known to accumulate in senescent tissues [120,121]. As mentioned in Section 3.1.1., a conventional method for detecting lipofuscin is using histochemical lipophilic dyes such as Sudan Black B (SBB) [15]. Lozano-Torres et al. reported a hybrid approach for the in situ fluorescent labeling of lipofuscin by combining histochemical techniques with bioorthogonal chemistry (Figure 9B,D) [122]. Lipofuscin is targeted using azide-conjugated SSB (SBB-N3, 23), followed by a strain-promoted azide–alkyne cycloaddition (SPAAC) reaction with a cyclooctyne-containing BODIPY fluorophore. This method successfully labeled senescent melanoma SK-MEL-103 cells, triple-negative breast cancer MDA-MB-231 cells, and senescent fibroblast WI-38 cells, demonstrating selective senescent cell detection via binding with lipofuscin using an in situ bioorthogonal approach.
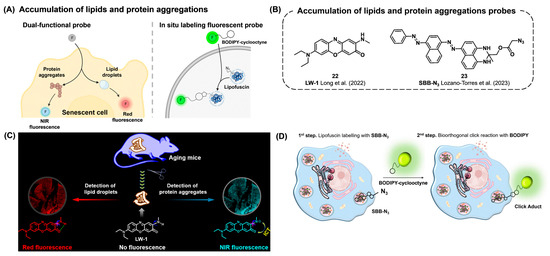

Figure 9.
(A) Schematic of dual-functional and in situ labeling fluorescent probes to monitor the accumulation of lipids and protein aggregates. (B) Chemical structures of probes capable of detecting lipids and protein aggregates [119,122]. (C) The dual-functional probe LW-1 that can detect both lipid droplets and protein aggregates. LW-1 exhibits red fluorescence when interacting with lipid droplets and NIR fluorescence when interacting with protein aggregates. Reprinted with permission from [119]. Copyright 2022 American Chemical Society. (D) The SBB-N3 and BODIPY-cyclooctyne probe pair enables two-step in situ bioorthogonal labeling of lipofuscin via SPAAC. Reprinted with permission from [122]. Copyright 2022 The Authors. The FEBS Journal published by John Wiley & Sons Ltd. on behalf of Federation of European Biochemical Societies.
3.4. Small Fluorescence Probes Detecting Senescence-Associated ROS
An elevated level of reactive oxygen species (ROS) is one of the general features in senescent cells. Therefore, monitoring various ROS levels is reported in a number of senescence cell detection studies.
Govindaraju’s group developed QCy-BA (24) by conjugating phenyl boronic acid with a quinone–cyanine group for the detection of in-cell generated H2O2 (Figure 10A) [123]. Upon the cleavage of a p-quinone-methide group from QCy-BA by H2O2, the resulting fluorophore QCy-DT intercalates into the minor groove of DNA, thereby emitting its NIR fluorescence. In both primary and cancer cells, genotoxic stress can trigger the in situ generation of H2O2 during cellular senescence, which can be detected using the QCy-BA probe (Figure 10B). Dong et al. reported an AIE-based H2O2 probe, b-PyTPA (25), for the detection of senescent oocytes (Figure 10A) [124]. Initially, the fluorescent characteristics of the probe were quenched through a twisted intramolecular charge transfer (TICT) process. Upon interaction with H2O2 and subsequent release of the boronatebenzyl unit, the probe forms aggregates and starts to emit its fluorescence. During the quality assessment of oocytes, older ones can be differentiated from younger ones by monitoring higher fluorescent signals emitted by b-PyTPA, attributed to higher levels of H2O2 in senescent oocytes.
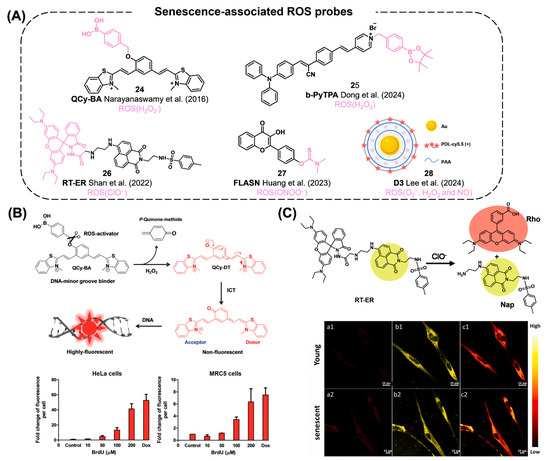
Figure 10.
(A) Chemical structures of senescence-associated ROS probes [22,23,123,124,125]. (B) ROS detection mechanism by an H2O2 sensor, QCy-BA. After its reaction with intracellular H2O2, the resulting product is intercalated to the DNA minor groove and exhibiting enhanced fluorescent intensity. The bottom tables show the fluorescence intensity of QCy-BA induced by H2O2 generation after treatment with BrdU or doxorubicin in HeLa cells (left) and MRC5 cells (right). Reproduced from [123] with permission from the Royal Society of Chemistry. (C) The senescence cell detection mechanism of RT-ER. It reacts selectively with ClO− among various ROS to detect ER stress and senescence. Upon reaction, the probe cleaves into two distinct fluorophores: ER targeting Nap and lysosome targeting Rho. The bottom confocal images are hDPMSC cells treated with RT-ER. a1–a2: red channel, b1–b2: yellow channel, c1–c2: ratiometric images. Reprinted from [22]. Copyright 2022, with permission from Elsevier.
Shan et al. reported a ClO− reporter, RT-ER (26), by conjugating 1,8-naphthalimide and rhodamine B (Figure 10A,C) [22]. Using this probe, they monitored ClO− levels under ER stress induced by tunicamycin (an ER stress inducer). The accumulation of ClO− was also detected by RT-ER in replicative senescent human dental pulp mesenchymal stem cells (hDPMSCs). Their findings suggest the in vivo accumulation of ClO− during the cellular senescence process. Huang et al. reported a flavonol-based sensor, FLASN (27), for the detection of endogenous ONOO− levels in senescent models (Figure 10A) [23]. By using a dimethylaminothioformyl group as a reacting unit, the probe can detect endogenous ONOO− levels in senescence cells and in vivo zebrafish. It is worth mentioning that this study marks the first report of the elevated ONOO− levels in senescent cells.
Lee et al. reported a nanoprobe, D3 (28), consisting of poly-D-lysine (PDL)-Cy5.5 and polyacrylic acid (PAA) assembled through charge–charge interactions on nanoparticles (Figure 10A) [125]. In their proposed mechanism, the probe can be activated and turned on by the sensitive properties of PDL to reactive oxygen species (ROS), such as O2−, H2O2, and NO. In senescent cells induced by palbociclib, D3 successfully detects elevated concentrations of ROS. Furthermore, they performed in vivo imaging in an MDA-MB-231 breast tumor xenograft model, demonstrating a three-fold increment in the fluorescent signal from D3 compared to that in the non-senescent tumors.
3.5. Multi-Biomarker-Targeting Fluorescent Probes
Given the heterogeneity of senescent cells, it has been suggested that targeting multiple senescence markers can significantly enhance the accuracy and specificity toward senescent cells over others (Figure 11A). Recently, the development of multi-biomarker-targeting probes successfully demonstrated their enhanced specificity for cellular senescence (Figure 11B) [126,127,128,129,130].
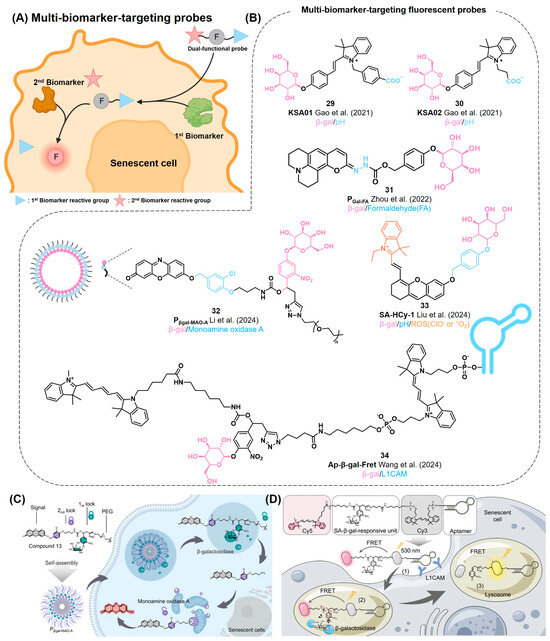
Figure 11.
(A) Schematic of dual-functional probes for the detection of senescence cells. In general, the dual-functional probe can produce a fluorescent reporter (F, red circle) through the dual activation of the 1st and 2nd biomarkers. (B) Chemical structures of multi-biomarker-targeting probes [126,127,128,129,130]. (C) Pβgal-MAO-A sequentially reacts with SA-β-gal and MAO-A in cellular senescence. The probe is activated only in the presence of both enzymes. Reprinted with permission from [128]. Copyright 2023 American Chemical Society. (D) Ap-β-gal-Fret as a FRET-based dual-targeting probe. Due to the presence of an anti-L1CAM aptamer, the probe exhibits superior selectivity for senescent cells along with β-gal reactive functionality. Reprinted with permission from [130]. Copyright 2023 American Chemical Society.
In 2021, Gao et al. reported dual-biomarker-targeting probes, KSA01 and KSA02 (29, 30), to monitor both SA-β-gal activity and local pH perturbation in lysosomes, which are the general hallmarks of the cellular senescence process (Figure 11B) [126]. The probes were designed by coupling a carboxylic acid-containing merocyanine with a β-D-galactosyl group. Enzymatic cleavage of KSA01 and KSA02 by β-gal generates green fluorophores, KSAP1 and KSAP2 (λem = ~530 nm), followed by the senescent-related basic environment in lysosomes further promoting red shifts in fluorescence (560–570 nm), due to their pH-sensitive carboxylic acid groups. Using these dual-responding characteristics, the authors were able to distinguish senescent HL-7702 cells (red fluorescence) from non-senescent ovarian cancer cells (SKOV-3) with a high endogenous β-gal level (green fluorescence). They also successfully monitored kidney tissues with different levels of senescence using KSA01 and KSA02 for ratiometric fluorescence analysis.
Zhou et al. reported a dual-targeting probe, PGal-FA (31), that can be activated by sequential reactions with β-Gal and formaldehyde (FA) (Figure 11B) [127]. FA was chosen as a senescence biomarker considering that it is a stress-related metabolite. PGal-FA is synthesized by conjugating a coumarin fluorophore with a self-immolative galactose unit. Upon reacting with β-Gal, the probe generates a nucleophilic hydrazonate group, which further reacts with FA for exhibiting turn-on green fluorescence (526 nm). They applied the probe to monitor senescent cells in pulmonary fibrosis tissues.
In 2024, Li et al. reported an amphiphilic dual-enzyme-targeting probe, Pβgal-MAO-A (32), that is activated by sequential reactions with SA-β-gal and MAO-A, given the high expression levels of both in senescent cells. (Figure 11B,C) [128]. Pβgal-MAO-A has a resorufin signal unit, which is masked by two cleavable linkers containing galactose and alkylamine moieties, respectively. Conjugation with PEG-2000 allows the formation of water-soluble micelles of Pβgal-MAO-A for its efficient delivery into live cells. By sequential activation by SA-β-gal in lysosomes and MAO-A in the mitochondria, the probe produces far-red fluorescence signaling (575–700 nm) in the senescent cells by the resorufin signal moiety. Notably, this probe showed its capability to distinguish senescence-associated SA-β-gal/MAO-A from cancer-related β-gal/MAO-A. Similar to KSA01 and KSA02, this probe serves as a compelling example that highlights the advantage of using dual-biomarker-targeting probes over single-targeting probes for a more precise determination of cellular senescence.
Liu et al. reported an NIR multi-dimensional reporter, SA-HCy-1 (33), by conjugating a cyanine and a xanthene derivative with a galactose moiety through a self-immolative linker (Figure 11B) [129]. This probe aims to detect both SA-β-gal activity and ROS levels, which are simultaneously increased in senescent cells. After releasing the galactose residue by β-gal, the probe emits strong NIR fluorescence (713 nm). Its subsequent reaction with ROS promotes the oxidative cleavage of the olefin linker, inducing the further shifting of the emission wavelength toward the blue area (468 nm) due to the presence of the xanthene moiety. Using this multi-responding characteristic, they monitored replicative senescent Raw 264.7 cells as well as skin aging by UV irradiation in a mouse model.
Wang et al. developed an aptamer-conjugated ratiometric fluorescent probe (Ap-β-gal-Fret, 34) for imaging therapy-induced cancer senescence (Figure 11B,D) [130]. Ap-β-gal-Fret is embedded with two cyanine fluorophores (Cy3 and Cy5) as a FRET pair, which are connected by a self-immolative linker containing β-galactose. This structure enables the ratiometric change in fluorescent emission after its reaction with β-gal. To further enhance its selectivity, Ap-β-gal-Fret was conjugated with an anti-L1CAM aptamer. L1CAM is known as a membrane biomarker of senescent cancer cells [131]. Conjugation of Ap-β-gal-Fret with anti-L1CAM aptamer facilitates its accumulation in senescent cancer cells, thereby significantly enhancing the sensitivity and selectivity of the probe. Ap-β-gal-Fret exhibited a fluorescent maximum emission at 670 nm, corresponding to the emission wavelength of Cy5, while it emits minimal fluorescence at 568 nm, corresponding to that of Cy3. The ratio of its fluorescence intensities at 568 and 670 nm (F568/F670) was initially 0.14. After its activation by β-gal, Ap-β-gal-Fret released Cy5 and exhibited a 59-fold increment in the ratio of F568/F670, reaching 8.3. Coupled with the L1CAM-targeting moiety, the probe was selectively delivered to senescent cancer cells and was found to accumulate in the lysosomes. They demonstrated the successful application of this dual-targeting probe in chemically induced senescent cells and 3D tumor spheroid models, showing excellent spatiotemporal specificity.
3.6. Pros and Cons of Small-Molecule Fluorescent Probes for Cellular Senescence
Here, we summarize the fluorescent senescence probes described in this review (Table 1). Most of the current senescence probes employ a reaction-based approach, involving either enzymatic or chemically driven cleavage processes. Since the mode of action for these probes is straightforward, the development of new cellular senescence probes has a clear direction to achieve better sensitivity. However, the major drawback of those individual probes arises from the heterogeneous characteristics of cellular senescence, making it difficult to use only a single probe to evaluate complex cellular senescence exactly. In this regard, multi-dimensional probes can be an effective solution for the minimization of off-target events and more reliable detection of cellular senescence.

Table 1.
Summary of key features for small-molecule senescent probes described in this article.
Notably, a few recent environmentally sensitive probes, especially those targeting microenvironment changes in cellular organelles, have emerged as alternative tools for monitoring senescence-associated organelle changes [113,114,115,116]. However, the limitation of this approach lies in the unclear targeting mechanisms and the limited number of available fluorescent probes. Considering recent examples, novel molecular systems such as rotatable building blocks [113,114] or lipophilic cationic scaffolds [115,116] could serve as promising candidates for developing future senescent probes to monitor changes in lysosomes or mitochondria, respectively.
4. Conclusions and Perspective
In this review, we have highlighted recent advancements in fluorescent small molecules capable of targeting senescence-associated phenomena, including the upregulation of senescence-associated enzymes, perturbation of the subcellular environment, and increase in endogenous ROS levels. Although a number of fluorescent senescent cell probes have been developed so far, the sensitivity and specificity of these probes need to be improved for the accurate detection of senescent cells [128,132]. In this context, the development of multi-biomarker-targeting fluorescent probes is specifically emphasized due to their superior selectivity for senescent cells. Given that the identification of senescent cells requires the simultaneous detection of multiple hallmarks due to their highly heterogeneous nature, we foresee that future advancement in senescent cell detection could be facilitated by coupling multi-targeting probes with high-throughput or high-content screening techniques to enable more reliable, statistics-based analysis. Additionally, pattern-based analysis could be achieved by developing fluorescent sensor arrays composed of various biomarker-targeting probes. In addition, the combination of the fluorescent probe with nanomaterials or inorganic materials is expected to significantly enhance both the sensitivity and selectivity of senescence detection, especially for in vivo applications.
In addition to the current biomarkers for senescent cells, intracellular metal accumulations and their related metabolic proteins have been reported as new biomarkers for cellular senescence [133,134,135,136]. Cellular iron levels were found to be positively correlated with cellular senescence, while ferritin, an iron-storage protein, showed a negative correlation [133,134]. Additionally, iron accumulation in cells appears to contribute to mitochondrial dysregulation, which further drives the inflammatory senescence-associated secretory phenotype (SASP) [135]. Similarly, elevated levels of cellular copper and the high-affinity copper uptake protein 1 (CTR1) are positively associated with cellular senescence, while copper-transporting ATPase 1 (Atp7a) exhibits a negative correlation [136]. We believe that these findings could be applied for the monitoring of senescent cells using fluorescent metal chelators or protein-specific probes in future studies.
Author Contributions
J.P. (Junyoung Park), Y.L. and J.P. (Jongmin Park) contributed to writing the original manuscript. Y.L. and J.P. (Jongmin Park) contributed to conceptualization. J.P. (Jongmin Park) contributed funding acquisition and supervision. All authors contributed to editing and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Basic Science Research Program (2023R1A2C1007899 to J.P.) and Priority Research Institute Program (RS-2023-00271205 to J.P.) through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning. This work is also supported by the Korea Basic Science Institute (KBSI) National Research Facilities & Equipment Center (NFEC) grant (2019R1A6C1010006 to J.P.) and Global-Learning & Academic research institution for Master’s·PhD students, and Postdocs (LAMP) program of the National Research Foundation of Korea (NRF) grant (RS-2023-00301850 to J.P.) funded by the Ministry of Education, Republic of Korea. This work is supported by a 2019 Research Grant from Kangwon National University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pellegata, N.S.; Antoniono, R.J.; Redpath, J.L.; Stanbridge, E.J. DNA damage and p53-mediated cell cycle arrest: A reevaluation. Proc. Natl. Acad. Sci. USA 1996, 93, 15209–15214. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.E.; Levine, A.J. Tumor-suppressor p53 and the cell cycle. Curr. Opin. Genet. Dev. 1993, 3, 50–54. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Acosta, J.C.; O’Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Da Costa, M.; Brown, C.; Popov, N.; et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef]
- Binet, R.; Ythier, D.; Robles, A.I.; Collado, M.; Larrieu, D.; Fonti, C.; Brambilla, E.; Brambilla, C.; Serrano, M.; Harris, C.C. WNT16B is a new marker of cellular senescence that regulates p53 activity and the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2009, 69, 9183–9191. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; Van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Peng, N.; Kang, H.H.; Feng, Y.; Minikes, A.M.; Jiang, X. Autophagy inhibition signals through senescence to promote tumor suppression. Autophagy 2023, 19, 1764–1780. [Google Scholar] [CrossRef] [PubMed]
- Nehlin, J.O. Biomarkers of replicative senescence revisited. In Cellular Ageing and Replicative Senescence; Rattan, S.I.S., Hayflick, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 203–239. [Google Scholar]
- Gil, J. The challenge of identifying senescent cells. Nat. Cell Biol. 2023, 25, 1554–1556. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.P.; Chua, J.; Curby, R.J.; Tomson, A.J.; Da Rooge, M.A.; Fisher, B.E.; Mauricio, J.; Klundt, I. Substrates for cytochemical demonstration of enzyme activity. I. Some substituted 3-indolyl-β-D-glycopyranosides1a. J. Med. Chem. 1964, 7, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, J.B.; Moussa, T.A. The sudan black B technique in cytology. J. R. Microsc. Soc. 1949, 69, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Iourov, I.Y. Quantitative Fluorescence In Situ Hybridization (QFISH). Methods Mol. Biol. 2017, 1541, 143–149. [Google Scholar] [PubMed]
- Giatromanolaki, A.; Kouroupi, M.; Balaska, K.; Koukourakis, M.I. Immunohistochemical detection of senescence markers in human sarcomas. Pathol. Res. Pract. 2020, 216, 152800. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Galiana, I.; Rovira, M.; Garrido, E.; Chaib, S.; Bernardos, A.; Munoz-Espin, D.; Serrano, M.; Martinez-Manez, R.; Sancenon, F. An OFF–ON two-photon fluorescent probe for tracking cell senescence in vivo. J. Am. Chem. Soc. 2017, 139, 8808–8811. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, J.; Huang, L.; Li, W.; Zhao, Y.; Lin, W. Aging Diagnostic Probe for Research on Aging and Evaluation of Anti-aging Drug Efficacy. Anal. Chem. 2021, 93, 13800–13806. [Google Scholar] [CrossRef]
- Liu, C.; Mei, Y.; Yang, H.; Zhang, Q.; Zheng, K.; Zhang, P.; Ding, C. Ratiometric Fluorescent Probe for Real-Time Detection of beta-Galactosidase Activity in Lysosomes and Its Application in Drug-Induced Senescence Imaging. Anal. Chem. 2024, 96, 3223–3232. [Google Scholar]
- Koo, S.; Won, M.; Li, H.; Kim, W.Y.; Li, M.; Yan, C.; Sharma, A.; Guo, Z.; Zhu, W.H.; Sessler, J.L.; et al. Harnessing alpha-l-fucosidase for in vivo cellular senescence imaging. Chem. Sci. 2021, 12, 10054–10062. [Google Scholar] [CrossRef]
- Shan, Y.M.; Yu, K.K.; Wang, N.; Yu, F.Y.; Li, K.; Liu, Y.H.; Yu, X.Q. Assessing ClO-level during ER stress and cellular senescence through a ratio fluorescent probe with dual organelle targeting ability. Sens. Actuators B Chem. 2022, 358, 131383. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Nong, L.; Cheng, J.; Lin, W. A therapeutic probe for detecting and inhibiting ONOO− in senescent cells. J. Mater. Chem. B 2023, 11, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Finney, N.S. Small-molecule fluorescent probes and their design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef] [PubMed]
- Brauer, E.; Lange, T.; Keller, D.; Gorlitz, S.; Cho, S.; Keye, J.; Gossen, M.; Petersen, A.; Kornak, U. Dissecting the influence of cellular senescence on cell mechanics and extracellular matrix formation in vitro. Aging Cell 2023, 22, e13744. [Google Scholar] [CrossRef]
- Lengefeld, J.; Cheng, C.-W.; Maretich, P.; Blair, M.; Hagen, H.; McReynolds, M.R.; Sullivan, E.; Majors, K.; Roberts, C.; Kang, J.H. Cell size is a determinant of stem cell potential during aging. Sci. Adv. 2021, 7, eabk0271. [Google Scholar] [CrossRef]
- Liu, X.; Yan, J.; Kirschner, M.W. Cell size homeostasis is tightly controlled throughout the cell cycle. PLoS Biol. 2024, 22, e3002453. [Google Scholar] [CrossRef]
- Tan, C.; Ginzberg, M.B.; Webster, R.; Iyengar, S.; Liu, S.; Papadopoli, D.; Concannon, J.; Wang, Y.; Auld, D.S.; Jenkins, J.L. Cell size homeostasis is maintained by CDK4-dependent activation of p38 MAPK. Dev. Cell 2021, 56, 1756–1769.e1757. [Google Scholar] [CrossRef] [PubMed]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive Cell Growth Causes Cytoplasm Dilution and Contributes to Senescence. Cell 2019, 176, 1083–1097. [Google Scholar] [CrossRef]
- Höhn, A.; Jung, T.; Grimm, S.; Grune, T. Lipofuscin-bound iron is a major intracellular source of oxidants: Role in senescent cells. Free Radic. Biol. Med. 2010, 48, 1100–1108. [Google Scholar] [CrossRef]
- Aravinthan, A.; Verma, S.; Coleman, N.; Davies, S.; Allison, M.; Alexander, G. Vacuolation in hepatocyte nuclei is a marker of senescence. J. Clin. Pathol. 2012, 65, 557–560. [Google Scholar] [CrossRef]
- Lian, X.J.; Gallouzi, I.E. Oxidative Stress Increases the Number of Stress Granules in Senescent Cells and Triggers a Rapid Decrease in p21waf1/cip1 Translation. J. Biol. Chem. 2009, 284, 8877–8887. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sfeir, A.; Gryaznov, S.M.; Shay, J.W.; Wright, W.E. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol. Biol. Cell 2004, 15, 3709–3718. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.-R. A highly conserved repetitive DNA sequence, (TTAGGG) n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Chang, E.; Kashefi-Aazam, M.; Rogaev, E.I.; Piatyszek, M.A.; Shay, J.W.; Harley, C.B. Telomere shortening is associated with cell division in vitro and in vivo. Exp. Cell Res. 1995, 220, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Cross, F.R. Multiple levels of cyclin specificity in cell-cycle control. Nat. Rev. Mol. Cell Biol. 2007, 8, 149–160. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Orlando, D.A.; Lin, C.Y.; Bernard, A.; Wang, J.Y.; Socolar, J.E.; Iversen, E.S.; Hartemink, A.J.; Haase, S.B. Global control of cell-cycle transcription by coupled CDK and network oscillators. Nature 2008, 453, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, D.A.; Xiong, Y.; Phelps, D.; Hannon, G.; Beach, D.; Barrett, J.C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA 1996, 93, 13742–13747. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Garcia-Barros, M.; Wen, S.; Li, F.; Lin, C.L.; Hannun, Y.A.; Obeid, L.M.; Mao, C. Tumor suppressor p53 links ceramide metabolism to DNA damage response through alkaline ceramidase 2. Cell Death Differ. 2018, 25, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001, 2, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Dalgarno, A.; Neretti, N. The functional impact of nuclear reorganization in cellular senescence. Brief. Funct. Genom. 2022, 21, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cai, S.-Z.; Zhou, Y.; Zhang, X.-P.; Liu, D.-F.; Jiang, R.; Wang, Y.-P. Senescence as A Consequence of Ginsenoside Rg 1 Response on K562 Human Leukemia Cell Line. Asian. Pac. J. Cancer Prev. 2012, 13, 6191–6196. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kawaguchi, K.; Tanaka, S.; He, C.; Maeshima, Y.; Suzuki, E.; Toi, M. Cellular senescence triggers intracellular acidification and lysosomal pH alkalinized via ATP6AP2 attenuation in breast cancer cells. Commun. Biol. 2023, 6, 1147. [Google Scholar] [CrossRef]
- Rovira, M.; Sereda, R.; Pladevall-Morera, D.; Ramponi, V.; Marin, I.; Maus, M.; Madrigal-Matute, J.; Diaz, A.; Garcia, F.; Munoz, J.; et al. The lysosomal proteome of senescent cells contributes to the senescence secretome. Aging Cell 2022, 21, e13707. [Google Scholar] [CrossRef]
- Curnock, R.; Yalci, K.; Palmfeldt, J.; Jäättelä, M.; Liu, B.; Carroll, B. TFEB-dependent lysosome biogenesis is required for senescence. EMBO J. 2023, 42, e111241. [Google Scholar] [CrossRef] [PubMed]
- Borodkina, A.; Shatrova, A.; Abushik, P.; Nikolsky, N.; Burova, E. Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging 2014, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Nelson, G.; Wang, C.; Richter, T.; Simillion, C.; Proctor, C.J.; Miwa, S.; Olijslagers, S.; Hallinan, J.; Wipat, A. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, T.E.S.; Kauppila, J.H.K.; Larsson, N.G. Mammalian Mitochondria and Aging: An Update. Cell Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.C.; Hu, M.L. The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp. Gerontol. 2005, 40, 813–819. [Google Scholar] [CrossRef]
- Hildebrand, D.; Lehle, S.; Borst, A.; Haferkamp, S.; Essmann, F.; Schulze-Osthoff, K. α-Fucosidase as a novel convenient biomarker for cellular senescence. Cell Cycle 2013, 12, 1922–1927. [Google Scholar] [CrossRef]
- Santin, Y.; Resta, J.; Parini, A.; Mialet-Perez, J. Monoamine oxidases in age-associated diseases: New perspectives for old enzymes. Ageing Res. Rev. 2021, 66, 101256. [Google Scholar] [CrossRef]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019, 38, e100492. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [PubMed]
- Victorelli, S.; Passos, J.F. Reactive Oxygen Species Detection in Senescent Cells. Methods Mol. Biol. 2019, 1896, 21–29. [Google Scholar] [PubMed]
- Fan, L.M.; Li, J.M. Evaluation of methods of detecting cell reactive oxygen species production for drug screening and cell cycle studies. J. Pharmacol. Toxicol. Methods 2014, 70, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Ding, J.; Meng, L.H. Oncogene-induced senescence: A double edged sword in cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef]
- De Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Is senescence-associated β-galactosidase a reliable in vivo marker of cellular senescence during embryonic development? Front. Cell Dev. Biol. 2021, 9, 623175. [Google Scholar] [CrossRef]
- Burn, S.F. Detection of beta-galactosidase activity: X-gal staining. Methods Mol. Biol. 2012, 886, 241–250. [Google Scholar]
- Lederberg, J. The beta-d-galactosidase of Escherichia coli, strain K-12. J. Bacteriol. 1950, 60, 381–392. [Google Scholar] [CrossRef]
- Jung, T.; Hohn, A.; Grune, T. Lipofuscin: Detection and quantification by microscopic techniques. Methods Mol. Biol. 2010, 594, 173–193. [Google Scholar]
- Evangelou, K.; Gorgoulis, V.G. Sudan Black B, The Specific Histochemical Stain for Lipofuscin: A Novel Method to Detect Senescent Cells. Methods Mol. Biol. 2017, 1534, 111–119. [Google Scholar]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Munoz-Espin, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Estepa-Fernández, A.; Rovira, M.; Orzaez, M.; Serrano, M.; Martinez-Manez, R.; Sancenon, F. The chemistry of senescence. Nat. Rev. Chem. 2019, 3, 426–441. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Rahman, M.; Ayad, A.A.; Warrington, A.E.; Burns, T.C. P21 overexpression promotes cell death and induces senescence in human glioblastoma. Cancers 2023, 15, 1279. [Google Scholar] [CrossRef] [PubMed]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Kamal, N.S.M.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Park, M.H.; Choi, J.E.; Kim, J.R.; Bae, Y.K. Immunohistochemical Expressions of Senescence-Associated Secretory Phenotype and Its Association with Immune Microenvironments and Clinicopathological Factors in Invasive Breast Cancer. Pathol. Oncol. Res. 2021, 27, 1609795. [Google Scholar] [CrossRef]
- Herrmann, J.; Babic, M.; Tolle, M.; Eckardt, K.U.; van der Giet, M.; Schuchardt, M. A Novel Protocol for Detection of Senescence and Calcification Markers by Fluorescence Microscopy. Int. J. Mol. Sci. 2020, 21, 3475. [Google Scholar] [CrossRef] [PubMed]
- Ourliac-Garnier, I.; Londono-Vallejo, A. Telomere length analysis by quantitative fluorescent in situ hybridization (Q-FISH). Methods Mol. Biol. 2011, 735, 21–31. [Google Scholar] [PubMed]
- Langer-Safer, P.R.; Levine, M.; Ward, D.C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc. Natl. Acad. Sci. USA 1982, 79, 4381–4385. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Podder, A.; Maiti, M.; Lee, J.M.; Chung, B.G.; Bhuniya, S. Selective monitoring of vascular cell senescence via β-galactosidase detection with a fluorescent chemosensor. Sens. Actuators B Chem. 2018, 274, 194–200. [Google Scholar] [CrossRef]
- Qiu, W.; Li, X.; Shi, D.; Li, X.; Gao, Y.; Li, J.; Mao, F.; Guo, Y.; Li, J. A rapid-response near-infrared fluorescent probe with a large Stokes shift for senescence-associated β-galactosidase activity detection and imaging of senescent cells. Dye. Pigment. 2020, 182, 108657. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, L.; Zhou, X.; Mu, F.; Shi, G. A sensitive fluorescent probe for beta-galactosidase activity detection and application in ovarian tumor imaging. J. Mater. Chem. B 2021, 9, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, W.; Tian, H. Dicyanomethylene-4H-pyran chromophores for OLED emitters, logic gates and optical chemosensors. Chem. Commun. 2012, 48, 6073–6084. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, X.; Guo, Z.; Tang, J.; Shen, Y.; James, T.D.; Tian, H.; Zhu, W. In vivo and in situ tracking cancer chemotherapy by highly photostable NIR fluorescent theranostic prodrug. J. Am. Chem. Soc. 2014, 136, 3579–3588. [Google Scholar] [CrossRef]
- Gu, K.; Liu, Y.; Guo, Z.; Lian, C.; Yan, C.; Shi, P.; Tian, H.; Zhu, W.H. In Situ Ratiometric Quantitative Tracing of Intracellular Leucine Aminopeptidase Activity via an Activatable Near-Infrared Fluorescent Probe. ACS Appl. Mater. Interfaces 2016, 8, 26622–26629. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Z.; Chen, A.; Zhang, P. A turn on fluorescent assay for real time determination of beta-galactosidase and its application in living cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 120345. [Google Scholar] [CrossRef]
- Bush, P.G.; Wokosin, D.L.; Hall, A.C. Two-versus one photon excitation laser scanning microscopy: Critical importance of excitation wavelength. Front. Biosci. 2007, 12, 2646–2657. [Google Scholar] [CrossRef]
- Squirrell, J.M.; Wokosin, D.L.; White, J.G.; Bavister, B.D. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat. Biotechnol. 1999, 17, 763–767. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. ACS Appl. Bio Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, M.Y.; Han, H.H.; Zang, Y.; Chen, G.R.; Li, J.; He, X.P.; Vidal, S. A general strategy to the intracellular sensing of glycosidases using AIE-based glycoclusters. Chem. Sci. 2021, 13, 247–256. [Google Scholar] [CrossRef]
- Chai, X.; Han, H.H.; Sedgwick, A.C.; Li, N.; Zang, Y.; James, T.D.; Zhang, J.; Hu, X.L.; Yu, Y.; Li, Y.; et al. Photochromic Fluorescent Probe Strategy for the Super-resolution Imaging of Biologically Important Biomarkers. J. Am. Chem. Soc. 2020, 142, 18005–18013. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Lee, H.W.; Heo, C.H.; Sen, D.; Byun, H.O.; Kwak, I.H.; Yoon, G.; Kim, H.M. Ratiometric two-photon fluorescent probe for quantitative detection of beta-galactosidase activity in senescent cells. Anal. Chem. 2014, 86, 10001–10005. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Ma, X.; Wu, Y.; Hou, S. Kill two birds with one stone: A near-infrared ratiometric fluorescent probe for simultaneous detection of β-galactosidase in senescent and cancer cells. Sens. Actuators B Chem. 2022, 367, 132061. [Google Scholar] [CrossRef]
- Doura, T.; Kamiya, M.; Obata, F.; Yamaguchi, Y.; Hiyama, T.Y.; Matsuda, T.; Fukamizu, A.; Noda, M.; Miura, M.; Urano, Y. Detection of LacZ-Positive Cells in Living Tissue with Single-Cell Resolution. Angew. Chem. Int. Ed. Engl. 2016, 55, 9620–9624. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Cui, C.; Chen, Z.; Wang, Y.; Deenik, P.R.; Cui, L. Noninvasive NIR Imaging of Senescence via In Situ Labeling. J. Med. Chem. 2021, 64, 17969–17978. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, L.; Jiang, M.; Xiao, L.; Xiang, Y.; Wang, R.; Liu, Z.; Zhou, R.; Yang, M.; Li, C.; et al. An NIR Fluorescence Turn-on and MRl Bimodal Probe for Concurrent Real-time in vivo Sensing and Labeling of beta-Galactosidase. Angew. Chem. Int. Ed. Engl. 2023, 62, e202313137. [Google Scholar] [CrossRef]
- Singh, M.; Piekorz, R.P. Senescence-associated lysosomal alpha-L-fucosidase (SA-alpha-Fuc): A sensitive and more robust biomarker for cellular senescence beyond SA-beta-Gal. Cell Cycle 2013, 12, 1996. [Google Scholar] [CrossRef]
- Jones, D.N.; Raghanti, M.A. The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J. Chem. Neuroanat. 2021, 114, 101957. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Han, X.; You, J.; Yu, F.; Chen, L. Ratiometric near-infrared fluorescent probe for synergistic detection of monoamine oxidase B and its contribution to oxidative stress in cell and mice aging models. Anal. Chem. 2018, 90, 4054–4061. [Google Scholar] [CrossRef]
- Chen, X.; Varki, A. Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef]
- Schmidt, C.; Stehling, P.; Schnitzer, J.; Reutter, W.; Horstkorte, R.d. Biochemical Engineering of Neural Cell Surfaces by the SyntheticN-Propanoyl-substituted Neuraminic Acid Precursor. J. Biol. Chem. 1998, 273, 19146–19152. [Google Scholar] [CrossRef]
- Durocher, J.R.; Payne, R.C.; Conrad, M.E. Role of sialic acid in erythrocyte survival. Blood 1975, 45, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Landaw, S.A.; Tenforde, T.; Schooley, J.C. Decreased surface charge and accelerated senescence of red blood cells following neuraminidase treatment. J. Lab. Clin. Med. 1977, 89, 581–591. [Google Scholar]
- Zhu, R.; Wang, S.; Xue, Z.; Han, J.; Han, S. Senescence-associated sialidase revealed by an activatable fluorescence-on labeling probe. Chem. Commun. 2018, 54, 11566–11569. [Google Scholar] [CrossRef]
- He, N.; Yu, L.; Xu, M.; Huang, Y.; Wang, X.; Chen, L.; Yue, S. Near-infrared fluorescent probe for evaluating the acetylcholinesterase effect in the aging process and dietary restriction via fluorescence imaging. J. Mater. Chem. B 2021, 9, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Hu, L.; Li, X.; Liu, W.; Gao, Y.; Li, X.; Jiang, B.; Xia, C.; Guo, Y.; Li, J. Lysosomal polarity increases with aging as revealed by a lysosome-targetable near-infrared fluorescent probe. Sens. Actuators B Chem. 2020, 319, 128302. [Google Scholar] [CrossRef]
- Das, S.; Batra, A.; Kundu, S.; Sharma, R.; Patra, A. Unveiling autophagy and aging through time-resolved imaging of lysosomal polarity with a delayed fluorescent emitter. Chem. Sci. 2023, 15, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.; Lee, Y.A.; Raghothaman, D.; Jayaraman, P.; Teo, K.L.; Khan, F.J.; Reuveny, S.; Chang, Y.T.; Kang, N.Y.; Oh, S. Rapid detection of senescent mesenchymal stromal cells by a fluorescent probe. Biotechnol. J. 2019, 14, 1800691. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Zhang, Y.; Gao, Y.; Afzal, M.W.; Wang, A.; James, T.D.; Bai, Y.; Guo, Y. A spiropyran with low pKa for tracking DNA G-quadruplexes and revealing the dissipation of ΔΨm with senescence using an in-situ switching strategy. Sens. Actuators B Chem. 2022, 359, 131618. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Schulz, J.; Mukherjee, A.; Park, K.W.; Armijo, E.; Soto, C. Extensive accumulation of misfolded protein aggregates during natural aging and senescence. Front. Aging Neurosci. 2022, 14, 1090109. [Google Scholar] [CrossRef]
- Chen, X.; Chen, K.; Hu, J.; Dong, Y.; Zheng, M.; Jiang, J.; Hu, Q.; Zhang, W. Palmitic acid induces lipid droplet accumulation and senescence in nucleus pulposus cells via ER-stress pathway. Commun. Biol. 2024, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Liu, W.; Ruan, P.; Yang, X.; Chen, X.; Li, L.; Yuan, F.; He, D.; Huang, P.; Gong, A.; et al. Visualizing the Interplay of Lipid Droplets and Protein Aggregates During Aging via a Dual-Functional Fluorescent Probe. Anal. Chem. 2022, 94, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Strehler, B.L.; Mark, D.D.; Mildvan, A.S. Rate and magnitude of age pigment accumulation in the human myocardium. J. Gerontol. 1959, 14, 430–439. [Google Scholar] [CrossRef]
- Sohal, R.S.; Wolfe, L.S. Lipofuscin: Characteristics and significance. Prog. Brain Res. 1986, 70, 171–183. [Google Scholar]
- Lozano-Torres, B.; Blandez, J.F.; García-Fernández, A.; Sancenón, F.; Martínez-Máñez, R. Lipofuscin labeling through biorthogonal strain-promoted azide-alkyne cycloaddition for the detection of senescent cells. FEBS J. 2023, 290, 1314–1325. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Narra, S.; Nair, R.R.; Saini, D.K.; Kondaiah, P.; Govindaraju, T. Stimuli-responsive colorimetric and NIR fluorescence combination probe for selective reporting of cellular hydrogen peroxide. Chem. Sci. 2016, 7, 2832–2841. [Google Scholar] [CrossRef]
- Dong, X.; Ouyang, H.; Lou, X.; Xia, F.; Jin, L.; Wang, S.; Dai, J. Dual-Activated H2O2-Responsive AIE Probes for Oocyte Quality Assessment. Anal. Chem. 2024, 96, 5960–5967. [Google Scholar] [CrossRef]
- Lee, S.K.; Han, M.S.; Tung, C.H. In vivo senescence imaging nanoprobe targets the associated reactive oxygen species. Nanoscale 2024, 16, 1371–1383. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Y.; Liu, Q.; Li, X.; Li, X.; Kim, C.Y.; James, T.D.; Li, J.; Chen, X.; Guo, Y. Two-dimensional design strategy to construct smart fluorescent probes for the precise tracking of senescence. Angew. Chem. 2021, 133, 10851–10860. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Dong, Y.; Pan, Y.; Li, J.; Zang, Y.; Li, X. A Tandemly Activated Fluorescence Probe for Detecting Senescent Cells with Improved Selectivity by Targeting a Biomarker Combination. ACS Sens. 2022, 7, 1958–1966. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Luo, X.; Xia, Y.; Xie, Y.; Liu, Y.; Tan, W. Dual-Parameter Recognition-Directed Design of the Activatable Fluorescence Probe for Precise Imaging of Cellular Senescence. Anal. Chem. 2023, 95, 3996–4004. [Google Scholar] [CrossRef]
- Liu, H.; Lv, R.; Song, F.; Yang, Y.; Zhang, F.; Xin, L.; Zhang, P.; Zhang, Q.; Ding, C. A near-IR ratiometric fluorescent probe for the precise tracking of senescence: A multidimensional sensing assay of biomarkers in cell senescence pathways. Chem. Sci. 2024, 15, 5681–5693. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhao, Z.; Xia, Y.; Xie, Y.; Hong, D.; Liu, Y.; Tan, W. Aptamer Conjugate-Based Ratiometric Fluorescent Probe for Precise Imaging of Therapy-Induced Cancer Senescence. Anal. Chem. 2024, 96, 154–162. [Google Scholar] [CrossRef]
- Mrazkova, B.; Dzijak, R.; Imrichova, T.; Kyjacova, L.; Barath, P.; Dzubak, P.; Holub, D.; Hajduch, M.; Nahacka, Z.; Andera, L.; et al. Induction, regulation and roles of neural adhesion molecule L1CAM in cellular senescence. Aging 2018, 10, 434–462. [Google Scholar] [CrossRef]
- Gonzalez-Gualda, E.; Baker, A.G.; Fruk, L.; Munoz-Espin, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Wei, T.-T.; Zhuang, M.; Tan, C.-Y.; Xie, T.-H.; Cai, J.; Yao, Y.; Zhu, L. Iron derived from NCOA4-mediated ferritinophagy causes cellular senescence via the cGAS-STING pathway. Cell Death Discov. 2023, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Clatworthy, S.A.; Gamell, C.; Meggyesy, P.M.; Rigopoulos, A.-T.; Haupt, S.; Haupt, Y.; Denoyer, D.; Adlard, P.A.; Bush, A.I. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018, 14, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.; Lopez-Polo, V.; Mateo, L.; Lafarga, M.; Aguilera, M.; De Lama, E.; Meyer, K.; Sola, A.; Lopez-Martinez, C.; Lopez-Alonso, I.; et al. Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype. Nat. Metab. 2023, 5, 2111–2130. [Google Scholar] [CrossRef]
- Masaldan, S.; Clatworthy, S.A.; Gamell, C.; Smith, Z.M.; Francis, P.S.; Denoyer, D.; Meggyesy, P.M.; La Fontaine, S.; Cater, M.A. Copper accumulation in senescent cells: Interplay between copper transporters and impaired autophagy. Redox Biol. 2018, 16, 322–331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).