Biosensors for Food Mycotoxin Determination: A Comparative and Critical Review
Abstract
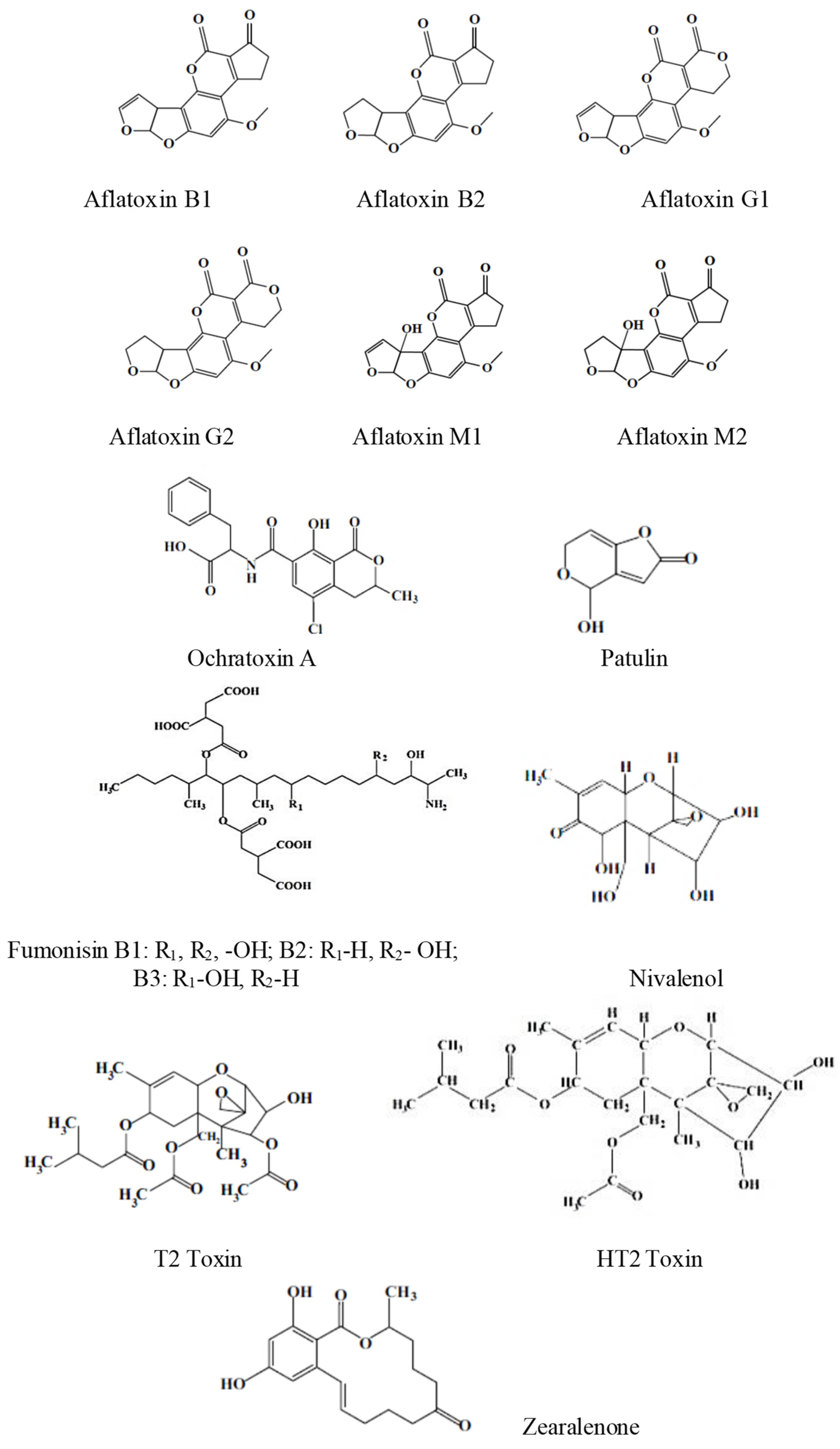
1. Introduction
- -
- Aflatoxins (AFB1, AFB2, AFG1, AFG2, and AFM1) produced by certain Aspergillus molds;
- -
- Ochratoxin A (OTA), produced by certain Aspergillus and Penicillium molds, is the most active of the nine ochratoxins;
- -
- Patulin toxin produced by Penicillium, Aspergillus, and Byssochylamys molds;
- -
- Fumonisins produced by Fusarium molds;
- -
- Trichothecenes (mainly nivalenol-NIV, deoxynivalenol-DON, T-2, and HT-2 toxin) produced by different species belonging to the genera Fusarium, Myrothecium, Trichoderma, Trichothecium, Cephalosporium, Verticimonosporium, and Stachybotrys;
- -
- Zearalenone (ZEN) produced by some species of Fusarium and Gibberella;
- -
- Ergot alkaloids, citrinin (CIT), sterigmatocystin (STC), Alternaria toxins, etc.
- -
- Enzyme sensors [40];
- -
- Immunosensors, which use antibodies, antibody fragments, antigens, or antigen conjugates as biorecognition elements [41];
- -
- Aptasensors (DNA-based biosensors), which use nucleic acid molecules as biorecognition elements [42];
- -
- Biomimetics [43];
- -
- Sensors using synthetic biorecognition elements (molecularly imprinted polymers) [44].
- -
- Electrochemical—based on the variation of electrochemical properties [46];
- -
- Optical—based on the variation of optical properties [47];
- -
- Thermal biosensors—based on thermal energy variation [48];
- -
- Mass sensitive biosensors—based on mass variation [49];
- -
- Acoustic biosensors—the target analyte is detected through the induced variations in the frequency, velocity, or amplitude of the acoustic waves generated by piezoelectric materials [50].
2. Types of Transducers
2.1. Electrochemical Biosensors
- -
- Potentiometric, by measuring potential or charge accumulation; this mode of detection includes, as transducers, ion-specific electrodes or field-effect transistors; the latter rely on measuring the current as a result of a potentiometric effect at a gate electrode;
- -
- Amperometric, by measuring the current intensity at a fixed potential value;
- -
- Voltammetric, by measuring the current—potential dependence at the controlled variation of the potential in time;
- -
- Impedimetric, by measuring impedance (both resistance and reactance);
- -
- Conductometric, by measuring the conductive properties of a medium.
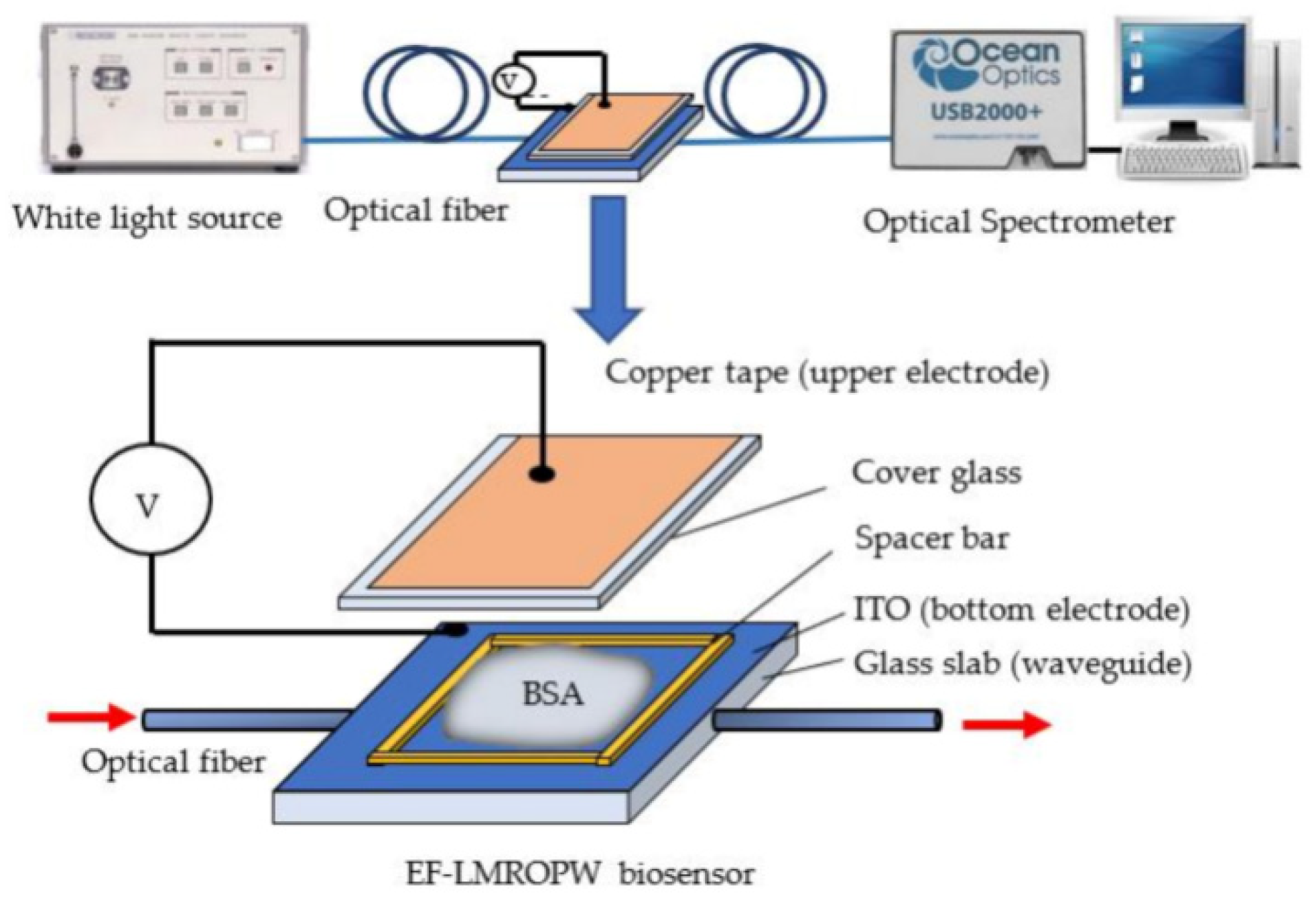
2.2. Optical Biosensors
- -
- The bioreceptor recognizes the analyte, so the analytical signal takes into account the presence of analytes and their amount;
- -
- Conformational changes can result following the biorecognition event, so the measured signal reflects specific alterations in the bioreceptor’s conformation;
- -
- Biocatalyst activity changes can occur, so the signal can also reflect enzyme activity.
2.3. Mass Sensitive Biosensors
2.4. Acoustic Biosensors
2.5. Thermal Biosensors
3. Biomolecule Immobilization
4. Stability and Response Time of Biosensors
5. Analytical Performances of Biosensors Applied to Mycotoxins Quantitation
| No. | Mycotoxin | Matrix | Detection/Bioelement | LOD | RSD% | Linearity | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | AFB1 | milk | colorimetric/aptamer | up to10 nM in spiked samples | 1 pM–1 µM | [126] | |
| 2. | OTA, AFB1 | peanut, barley | colorimetric/aptasensor | 4.7–8.4 ng mL−1 | 5–250 ng mL−1 (AFB1) 0.5–80 ng mL−1 (OTA) | [127] | |
| 3. | AFB1 | rice, flour, seed, maize, pistachio | colorimetric/aptasensor | 0–25 nM | [128] | ||
| 4. | AFB1 | spiked cattle feed samples | colorimetric/aptasensor | 0.88 µg mL−1 (at 1:10 AuNP:aptamer ratio) | 0–10 µg mL−1 | [129] | |
| 5. | AFB1 | maize flour | colorimetric/aptasensors developed through in silico maturation and computational simulation approaches | 0.1 and 0.5 ng mL−1 | 0.1–50 and 0.5–50 ng mL−1 | [130] | |
| 6. | AFB1 | foodstuffs | colorimetric and fluorescence/aptasensor | 7.32 ng mL−1 (colorimetric) 1.48 ng mL−1 (fluorescence) | <13.3 | 10–320 ng mL−1 3–320 ng mL−1 | [131] |
| 7. | AFB1 FB1 | whole grain samples (wheat and maize) | light reflectance spectroscopy/immunosensor | 0.05 ng mL−1 (aflatoxin B1) 1.0 ng mL−1 (fumonisin B1) | 0.1–5.0 ng mL−1 2.0-50 ng mL−1 | [132] | |
| 8. | AFB1 | optical planar waveguide operating as a polarization interferometer/aptasensor | 0.7 pg mL−1 | mean standard deviation of phase shift as ± 0.5 rad | 0.01–100 ng mL−1 | [133] | |
| 9. | ZEN | planar waveguide operating as a polarization interferometer/immunosensor | 0.01 ng mL−1 | 0.01–1000 ng mL−1 | [85] | ||
| 10. | AFB1 OTA | optical planar waveguide operating as polarization interferometer/immunosensor | 2 pg mL−1 | 0.01–1111.11 ng mL−1 | [82] | ||
| 11. | OTA | wheat and corn | fluorescence/aptamer | 2.28 nM | 6.08 | 10–5000 nM | [134] |
| 12. | AFB1 | rice sample extract | fluorescence/aptamer | 0.05 nM | 0.05–5 nM | [135] | |
| 13. | AFM1 | milk powder | fluorescence/aptamer | 0.05 μg kg−1 | 0.2–10 μg kg−1 | [136] | |
| 14. | FB1, OTA | maize | fluorescence/aptamer | 0.019 pg mL−1 (FB1) 0.015 pg mL−1 (OTA) | 0.0001–0.5 ng mL−1 | [137] | |
| 15. | ZEN | cereal samples (maize, wheat, rye flour) | fluorescence/aflatoxin-smartphone-based molecularly imprinted polymer | 1 µg mL−1 | 1–10 µg mL−1 | [138] | |
| 16. | ZEN | spiked maize samples | fluorescence/immunosensor | 20 pg mL−1 | 3.0-9.0 | 0.05–0.5 ng mL−1 as dynamic range | [139] |
| 17. | T2 toxin | barley sample | fluorescence/immunosensor | 0.1 ng mL−1 | 9.42–15.73 | 1–100 ng mL−1 | [140] |
| 18. | PAT | apple juice samples | fluorescence/immunosensor | 8.0 μg L−1 | 0–150 μg L−1 | [141] | |
| 19. | OTA | coffee, tea, and corn | fluorescence/aptasensor | 8 pg mL−1 | 0.01–35 ng mL−1 | [142] | |
| 20. | AFB1 | diluted beer and corn flour | fluorescence/aptasensor | 61 pM | 0.2–31.2 nM | [143] | |
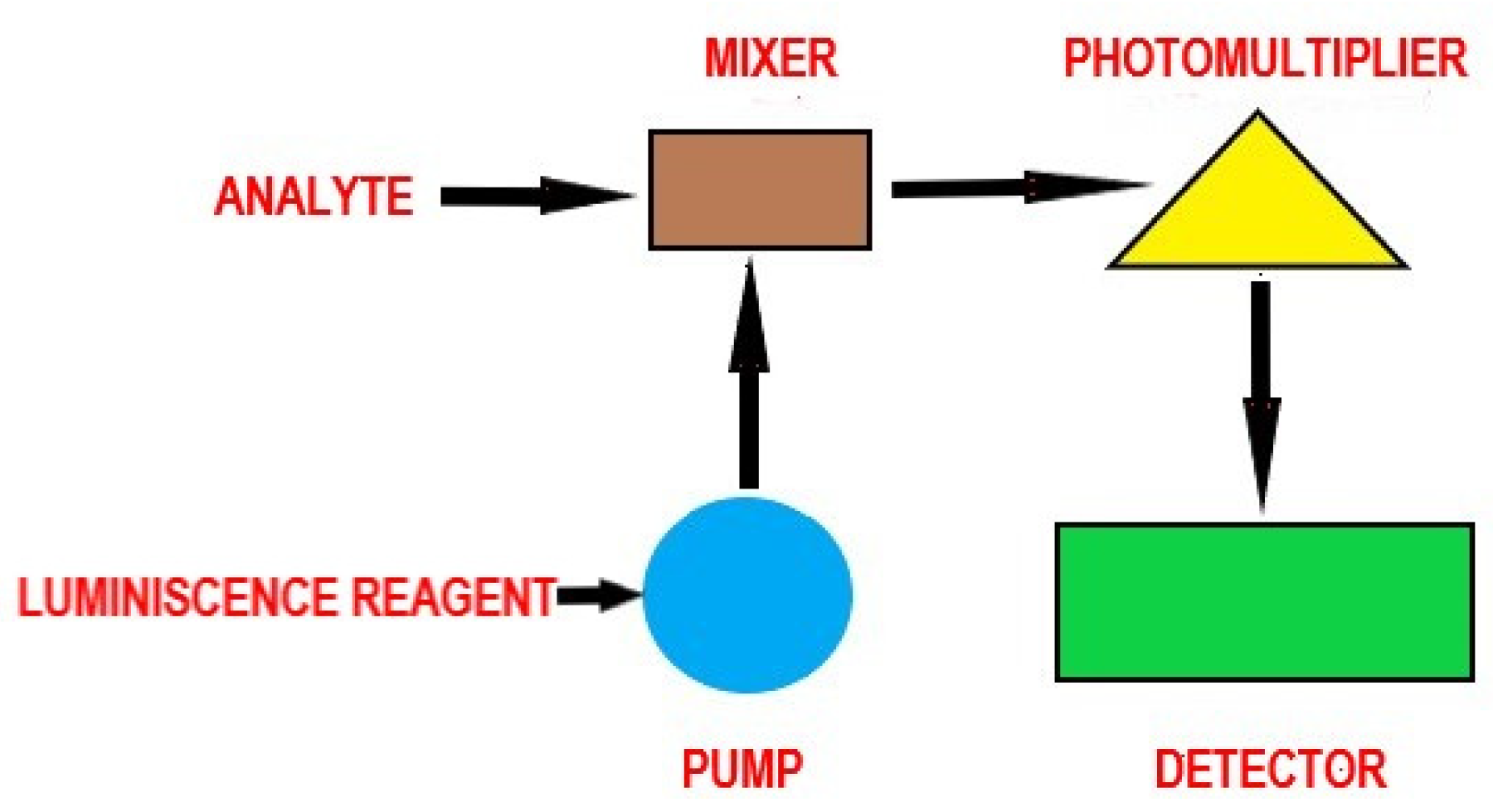
| 21. | OTA | grain samples | luminescence/immunosensor | 0.045 μg L−1 | <4.2 | 0.1–63 μg L−1 | [144] |
| 22. | AFM1, AFB1 | peanuts and pure milk | fluorescence/aptasensor | 6.24 pg mL−1 (AFM1) 9.0 pg mL−1 (AFB1) | 1.8–8.6 | 0.01–200 ng mL−1 (AFM1) 0.01–150 ng mL−1 (AFB1) | [145] |
| 23. | OTA, PAT | apple juice samples | fluorescence/aptasensor | 0.06 ng mL−1 (OTA) 0.09 ng mL−1 (PAT) | 4.3-8.5 (OTA) 4.5–7.8 (PAT) | 0.10–50 ng L−1 (OTA and PAT) | [146] |
| 24. | AFB1, FB1, OTA, ZEN | three infant foods | chemiluminescence optical fiber/aptasensor | 0.032 pg mL−1 0.015 pg mL−1 0.432 pg mL−1 and 0.275 pg mL−1, respectively | <7.2 | 0.3–2 × 104 pg mL−1 0.3–3.2 × 104 pg mL−1 2.5–6 × 104 pg mL−1 2.0–3.5 × 104 pg mL−1 respectively | [87] |
| 25. | OTA | wine, beer | electrochemiluminescence/aptamer | 0.012 nM | 2.25–8.16 | 0.05–5 nM | [147] |
| 26. | OTA | electrochemiluminescence aptasensor | 0.03 ng mL−1 | 0.1–320 ng mL-1 | [148] | ||
| 27. | OTA, STG, ZEN, DON | aqueous solutions | bioluminescence/Photobacte rium phosphoreum cells immobilized in poly(vinyl alcohol) cryogel | 0.017–56 mg L−1, 0.010–33 mg mL−1, 0.009–14 mg L−1, 0.026–177 mg L−1, respectively | [149] | ||
| 28. | ZEN | corn flour extract | nanoscale affinity double layer evanescent wave optical-fiber/aptasensor | 2.31 fM | 1 fM–100 pM | [121] | |
| 29. | OTA | coffee samples | surface plasmon resonance/monoclonal ochratoxin A antibodies | 5.7 ng mL−1 for chitosan and 3.8 ng mL−1 for carboxymethyl chitosan as nanomatrix substrates | coefficient of variance in coffee 16% | 0–50 ng mL−1 | [150] |
| 30. | AFB1, OTA, ZEN, DON | corn and wheat | surface plasmon resonance/mycotoxin antigens | 0.59 ng mL−1, 1.27 ng mL−1, 7.07 ng mL−1 and 3.26 ng mL−1 respectively | 2.52 to 9.8 | 0.99–21.92 ng mL−1 1.98–28.22 ng mL−1 10.37–103.31 ng mL−1 5.31–99.37 ng mL−1 respectively | [151] |
| 31. | AFB1 | milk and groundnut samples | photo-electrochemical/aptasensor | 1 pg mL−1 | 4.5 inter-assay | 0.005–50 ng mL−1 | [152] |
| 32 | OTA | red wine samples | electrochemical-graphene field-effect transistors/aptasensor | 1.4 pM | 6 pM–100 pM | [60] | |
| 33 | AFB1 | amperometry at MWCNTs-modified Pt transducers/enzyme sensor | 0.5 ng mL−1 | 6.1% | 1–225 ng mL−1 | [62] | |
| 34. | AFM1 | spiked milk samples | chronoamperometry at SWCNTs-modified silver transducers/immunosensor | 0.0259 µg L−1 | 0.01–1 µg L−1 | [153] | |
| 35. | OTA | paddy rice-spiked samples | current-voltage measurements at electrospun cellulose acetate-doped 3D-graphene nanofibre transducers/aptasensor | 156 fg mL−1 | 1 fg mL−1–1 ng mL−1 | [154] | |
| 36. | AFB1 | rice milk | cyclic voltammetry at functionalized gold screen-printed transducers/imunosensor (anti-AFB1 antibodies) | 50 fg mL−1 | 50 fg mL−1–5 ng mL−1 | [155] | |
| 37. | OTA | wheat | alternating current voltammetry at modified gold transducers/aptasensor | 3.3 pg mL−1 | 3.9–5.2 | 0.01–10 ng mL−1 | [156] |
| 38. | AFB1, OTA | corn and wheat | alternating current voltammetry at modified gold transducers/aptasensor | 4.3 pg mL−1 13.3 pg mL−1 for AFB1 and OTA | 3.2 (hairpin DNA-based aptasensor) and 6.5 (single stranded DNA-based aptasensor) | 10–3000 pg mL−1 30–10,000 pg mL−1 for AFB1 and OTA | [157] |
| 39. | AFB1 | maize flour samples | differential pulse voltammetry at poly(aniline-anthranilic acid) modified graphite screen-printed transducers/aptamer | 0.086 ng mL−1 | 5–10 | 0.1–10 ng mL−1 | [158] |
| 40. | AFB1 | human blood plasma pasteurized cow milk | differential pulse voltammetry at graphene oxide nanosheets modified glassy carbon transducers/aptasensor | 0.07 nM | 2.9 | 0.5 nM–4 μM | [159] |
| 41. | PAT | patulin solution | differential pulse voltammetry at graphene oxide-gold nanocomposite transducer s/immunosensor | 5 µg L−1 | 10–200 µg L−1 | [160] | |
| 42. | AFB1, FuB1 | spiked corn samples | differential pulse voltammetry at indium tin oxide transducers/molecularly imprinted polymer | 0.313 and 0.322 pg mL−1 for AFB1 and FuB1 | 2.73 | 1 pg mL−1–500 ng mL−1 | [161] |
| 43. | AFB1, OTA | spiked wine samples | differential pulse voltammetry at phosphorene-gold nanocomposite transducers/aptasensor | 0.023 (AFB1), 0.039 ng mL−1 (OTA) | 6.9 | 0.05–10 ng mL−1 | [162] |
| 44. | ZEN | corn powder samples | differential pulse voltammetry at glassy carbon transducers/aptasensor | 3.1 × 10−12 mol L−1 | 10−11–10−6 mol L−1 | [163] | |
| 45. | DON | maize flour samples | differential pulse voltammetry at polyaniline-gold nanoparticles-graphite screen-printed transducers/aptasensor | 3.2 ng mL−1 | 5 | 5.0–30.0 ng mL−1 | [164] |
| 46. | ZEN | semen coicis real samples | differential pulse voltammetry at polyethyleneimine-functionalized multi-walled carbon nanotubes nanocomposite transducers/aptasensor | 1.0 × 10− 5 ng mL−1 | 1.37–1.61% | 5.0 × 10−5 to 50.0 ng mL−1 | [165] |
| 47. | AFB1 | wine and soy sauce | pulse voltammetry at gold thin-film transducers/aptasensor | 0.016 pg mL−1 | <3 | 0.1–10 pg mL−1 | [120] |
| 48. | AFB1 | beer, withe grape wine | square wave voltammetry at gold transducers/aptasensor | 2 nM | ˂4 | 2 nM–4 μM | [122] |
| 49. | ZEN | extract of maize grain | square wave voltammetry at gold transducers/aptasensor | 0.017 ng mL−1 | 0.01–1000 ng mL−1 | [166] | |
| 50. | OTA | red wine | square wave voltammetry at NiCo2S4 nanoparticle-dispersed MoS2 nanosheets transducers/aptasensor | 0.42 pg mL−1 | 0.5 pg mL−1–1 ng mL−1 | [167] | |
| 51. | FB1, AFB1, ZEN, OTA | spiked corn flour samples | impedance spectroscopy at gold nanoparticles—indium tin oxide transducers/aptasensor | 2.47, 3.19, 5.38, 4.87 ng mL−1 for FB1, AFB1, ZEN, OTA, respectively | 7.5, 8.2, 6.6 and 7.2 for FB1, AFB1, ZEN, OTA, respectively | 5–1000, 10–250, 10–1250, 10–1500 ng mL−1 for FB1, AFB1, ZEN, OTA, respectively | [123] |
| 52. | OTA | wine (red and withe), grape juices | impedance spectroscopy at gold nanoparticles-cysteamine-gold transducers/aptasensor | 0.030 ng mL−1 | 1.30–8.20 | 0.1–10 ng mL−1 | [168] |
| 53. | OTA | spiked beer samples | electrochemical impedance spectroscopy at pencil graphite transducers/impedimetric aptasensor | 0.1 ng mL−1 | 3.49–4.95 | 0.1–2.0 ng mL−1 | [169] |
| 54. | OTA | electrochemical impedance spectroscopy at 4-carboxyphenyl monolayer-gold transducers/immunosensor | 0.5 ng mL−1 | 5.8 | 1–20 ng mL−1 | [68] | |
| 55. | AFB1 | conductometric at interdigitated thin film transducers fabricated by gold vapor deposition /enzyme sensor based on acetylcholinesterase | 0.05 µg mL−1 | 7 | 0.25–1 mM | [73] | |
| 56. | T2-toxin, ZEN, FB1 semiquanti tative detection | saliva | mass sensitive/immunosensor | 6.1 ng mL−1, 3.6 ng mL−1, 2.4 ng mL−1 for T2, FB1 and ZEN (sensitivities reported as IC50 values in the multiplex analysis with the portable microfluidic device) | for each toxin coefficients of variation are all below 18 | 0.1–10 ng mL−1 | [49] |
| 57. | AFB1 | contaminated samples (peanut, pistachio, rice, and wheat) | quartz crystal microbalance sensor | 2.8 pg mL−1 | 3.67 | 0.05–75 ng mL−1 | [170] |
6. Comparative Overview and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Dohlman, E. Mycotoxin hazards and regulations-impacts on food and animal feed crop trade. In International Trade and Food Safety Economic Theory and Case Studies; Buzby, J.C., Ed.; Agricultural Economic Report No. 828; United States Department of Agriculture: Washington, DC, USA, 2003; Chapter 6; pp. 97–108. Available online: https://www.ers.usda.gov/webdocs/publications/41603/15644_aer828_1_.pdf?v=0 (accessed on 4 February 2024).
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- da Silva, J.V.B.; de Oliveira, C.A.F.; Ramalho, L.N.Z. An overview of mycotoxins, their pathogenic effects, foods where they are found and their diagnostic biomarkers. Food Sci. Technol. 2022, 42, e48520. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius-Codex General Standard for Contaminants and Toxins in Food and Feed (Codex Stan 193-1995). Available online: https://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf (accessed on 8 March 2024).
- Commission Regulation (EU). 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance). Off. J. Eur. Union 2023, L119, 103–157. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 20 December 2023).
- US FDA—Chemical Contaminants, Metals, Natural Toxins & Pesticides Guidance Documents & Regulations, 2000–2010. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/default.htm (accessed on 15 December 2023).
- Ministry of Health and Family Welfare (Food Safety and Standards Authority of India). Notification, New Delhi, the 7th August, 2020. In The Gazette of India: Extraordinary, Part. III, Sec 4; Ministry of Health and Family Welfare: New Delhi, India, 2020; pp. 18–30. Available online: https://www.fssai.gov.in/upload/notifications/2020/08/5f3d09f97b78aGazette_Notification_Limit_Metal_19_08_2020.pdf (accessed on 29 November 2023).
- Stein, R.A.; Bulboacă, A.E. Mycotoxins. In Foodborne Diseases, 3rd ed.; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 407–446. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef]
- European Commission, Health & Consumer Protection Directorate-General, Scientific Commitee on Food, the 119th Pleanry Meeting, Opinion on Fusarium Toxins. 1999. Available online: https://food.ec.europa.eu/document/download/62e47971-e232-4e39-a9d7-2e3429eb5957_en?filename=cs_contaminants_catalogue_fusarium_out44_en.pdf (accessed on 14 May 2024).
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and Toxicological Effects of Patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Mycotoxins and human health. In Improving Public Health through Mycotoxin Control; Pitt, J.I., Wild, C.P., Baan, R.A., Gelderblom, W.C.A., Miller, J.D., Riley, R.T., Wu, F., Eds.; IARC Scientific Publication No. 158, E, 2012, Chapter 6; International Agency for Research on Cancer: Lyon, France, 2012; pp. 87–104. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Improving-Public-Health-Through-Mycotoxin-Control-2012 (accessed on 16 May 2024).
- Ahmed Adam, M.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (Review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Outcome of a Public Consultation on the Draft Risk Assessment of Aflatoxins in Food; EFSA Supporting Publication: Parma, Italy, 2020; p. EN-1798. 53p. [Google Scholar] [CrossRef]
- Mohebbi, A.; Nemati, M.; Mogaddam, M.R.A.; Farajzadeh, M.A.; Lotfipour, F. Dispersive micro-solid-phase extraction of aflatoxins from commercial soy milk samples using a green vitamin-based metal-organic framework as an efficient sorbent followed by high performance liquid chromatography-tandem mass spectrometry determination. J. Chromatogr. A 2022, 1673, 463099. [Google Scholar] [CrossRef]
- Shuib, N.S.; Saad, B. In-syringe dispersive micro-solid phase extraction method for the HPLC-fluorescence determination of aflatoxins in milk. Food Control 2022, 132, 108510. [Google Scholar] [CrossRef]
- Romero-Sánchez, I.; Ramírez-García, L.; Gracia-Lor, E.; Madrid-Albarrán, Y. Simultaneous determination of aflatoxins B1, B2, G1 and G2 in commercial rices using immunoaffinity column clean-up and HPLC-MS/MS. Food Chem. 2022, 395, 133611. [Google Scholar] [CrossRef]
- Maneeboon, T.; Chuaysrinule, C.; Mahakarnchanakul, W. Optimization and validation of dispersive liquid-liquid microextraction for simultaneous determination of aflatoxins B1, B2, G1, and G2 in Senna leaves and pods using HPLC-FLD with pre-column derivatization. Toxins 2023, 15, 277. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Hou, C.; Dai, Y.Q.; Chu, L.L.; Geng, S.W.; Zheng, S.L.; Kang, X.J. Determination of aflatoxin B1 by novel nanofiber-packed solid-phase extraction coupled with a high performance liquid chromatography-fluorescence detector. Anal. Methods 2023, 15, 472–481. [Google Scholar] [CrossRef]
- Noori, H.; Feizi, J.; Eshaghi, Z. Development of a nanoparticle-assisted fabric phase sorptive extraction technique coupled with high-performance liquid chromatography for sensitive determination of aflatoxins in food samples. Anal. Bioanal. Chem. Res. 2023, 10, 121–134. [Google Scholar] [CrossRef]
- Sartori, A.V.; de Moraes, M.H.P.; dos Santos, R.P.; Souza, Y.P.; Candido, F.S.; da Nobrega, A.W. Determination of aflatoxins M1, M2, B1, B2, G1, G2 and ochratoxin A in infant formulas from Brazil using a modified QuEChERS method and UHPLC-MS/MS. Food Anal. Methods 2023, 16, 841–849. [Google Scholar] [CrossRef]
- Medina, M.L.J.; Lafarga, T.; Frenich, A.G.; Romero-Gonzalez, R. Natural occurrence, legislation, and determination of aflatoxins using chromatographic methods in food: A review (from 2010 to 2019). Food Rev. Int. 2021, 37, 244–275. [Google Scholar] [CrossRef]
- Schincaglia, A.; Aspromonte, J.; Franchina, F.A.; Chenet, T.; Pasti, L.; Cavazzini, A.; Purcaro, G.; Beccaria, M. Current developments of analytical methodologies for Aflatoxins’ determination in food during the last decade (2013–2022), with a particular focus on nuts and nut products. Foods 2023, 12, 527. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Iordache, F.; Stanca, L.; Ionescu Petcu, A.; Purdoiu, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Comprehensive overview and critical perspective on the analytical techniques applied to aflatoxin determination—A review paper. Microchem. J. 2023, 191, 108770. [Google Scholar] [CrossRef]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P.; Pisoschi, A. Ascorbic acid determination by an amperometric ascorbate oxidase-based biosensor. Rev. Chim. 2010, 61, 339–344. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Negulescu, G.P. Ethanol determination by an amperometric bienzyme sensor based on a Clark-type transducer. J. Electroanal. Chem. 2012, 671, 85–91. [Google Scholar] [CrossRef]
- Pisoschi, A.M. Biosensors as bio-based materials in chemical analysis: A Review. J. Biobased Mater. Bioenergy 2013, 7, 19–38. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Q.; Hao, N.; Kun, W. Recent developments of photoelectrochemical biosensors for food analysis. J. Mater. Chem. B 2019, 7, 7283–7300. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Erkmen, C.; Uslu, B. Frontiers in electrochemical enzyme based biosensors for food and drug analysis. TRAC Trends Anal. Chem. 2020, 124, 115809. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, H.; Yin, J.; Guo, Q.; Zhang, Y.; Sun, X.; Guo, Y.; Yang, Q.; Zhang, Y.; Li, F.; et al. Recent advances and future prospects of aptamer-based biosensors in food safety analysis. Int. J. Electrochem. Sci. 2022, 17, 22019. [Google Scholar] [CrossRef]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-based electrochemical biosensors for food safety analysis. Biosensors 2022, 12, 1088. [Google Scholar] [CrossRef]
- Li, W.; Xiao, F.; Bai, X.; Xu, H. Magnetic nanoparticles for food hazard factors sensing: Synthesis, modification and application. J. Chem. Eng. 2023, 465, 142816. [Google Scholar] [CrossRef]
- Sadanandan, S.; Meenakshi, V.S.; Ramkumar, K.; Pillai, N.P.; Anuvinda, P.; Sreelekshmi, P.J.; Devika, V.; Ramanunni, K.; Sankar, R.J.; Sreejaya, M.M. Biorecognition elements appended gold nanoparticle biosensors for the detection of food-borne pathogens-A review. Food Control 2023, 148, 109510. [Google Scholar] [CrossRef]
- Tetyana, P.; Morgan Shumbula, P.; Njengele-Tetyana, Z. Biosensors: Design, development and applications. In Nanopores; Ameen, S., Akhtar, M.S., Shin, H.-S., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Morales, M.A.; Halpern, J.M. Guide to selecting a biorecognition element for biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef]
- Takeda, K.; Nakamura, N. Biosensors: Enzyme Sensors. In Encyclopedia of Sensors and Biosensors; Ikebukuro, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 297–381. [Google Scholar] [CrossRef]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and antibody-derived analytical biosensors. Essays Biochem. 2016, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Ma, J.; Li, D.; Wang, R. DNA-based biosensors for the biochemical analysis: A review. Biosensors 2022, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, K. Biosensors: Biomimetic sensors. In Encyclopedia of Sensors and Biosensors; Ikebukuro, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 393–404. [Google Scholar] [CrossRef]
- Cieplak, M.; Kutner, W. Artificial biosensors: How can molecular imprinting mimic biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Meira, D.I.; Barbosa, A.I.; Borges, J.; Reis, R.L.; Correlo, V.M.; Vaz, F. Recent advances in nanomaterial-based optical biosensors for food safety applications: Ochratoxin-A detection, as case study. Crit. Rev. Food Sci. Nutr. 2023, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, M.; Sichani, S.B.; Cornelis, P.; Wackers, G.; Wagner, P. The hot-wire concept: Towards a one-element thermal biosensor platform. Biosens. Bioelectron. 2021, 179, 113043. [Google Scholar] [CrossRef] [PubMed]
- Nolan, P.; Auer, S.; Spehar, A.; Oplatowska-Stachowiak, M.; Campbell, K. Evaluation of mass sensitive micro-array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 2021, 222, 121521. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Teo, A.J.T.; Li, K.H.H. Acoustic biosensors and microfluidic devices in the decennium: Principles and applications. Micromachines 2021, 13, 24. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-enabled biosensors: A review of fundamentals, design principles, materials, and applications. Biosensors 2023, 13, 40. [Google Scholar] [CrossRef]
- Thevenot, D.; Toth, K.; Durst, R.; Wilson, G. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Voeroes, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, M.; Abdi, M. Recent developments, characteristics, and potential applications of electrochemical biosensors. Anal. Sci. 2004, 20, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Rameil, S.; Schubert, P.; Grundmann, P.; Dietrich, R.; Märtlbauer, E. Use of 3-(4-hydroxyphenyl)propionic acid as electron donating compound in a potentiometric aflatoxin M1-immunosensor. Anal. Chim. Acta 2010, 661, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Stepurska, K.V.; Soldatkin, O.O.; Arkhypova, V.M.; Lagarde, F.; Jaffrezic-Renault, N.; Dzyadevych, S.V. Development of novel enzyme potentiometric biosensor based on pH-sensitive field effect transistors for aflatoxin B1 analysis in real samples. Talanta 2015, 144, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, T.; Kakkar, D.; Wadhwa, G.; Raj, B. Recent advances and progress in development of the field effect transistor biosensor: A review. J. Electron. Mater. 2019, 48, 7635–7646. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent advances in field effect transistor biosensors: Designing strategies and applications for sensitive assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, N.; Jaric, S.; Kireev, D.; Emelianov, A.V.; Orlov, A.V.; Gadjanski, I.; Nikitin, P.I.; Akinwande, D.; Bobrinetskiy, I. Real-time detection of ochratoxin A in wine through insight of aptamer conformation in conjunction with graphene field-effect transistor. Biosens. Bioelectron. 2022, 200, 113890. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Li, S.C.; Chen, J.H.; Cao, H.; Yao, D.S.; Liu, D.L. Amperometric biosensor for aflatoxin B1 based on aflatoxin-oxidase immobilized on multiwalled carbon nanotubes. Food Control 2011, 22, 43–49. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernández, S.; Bertolín, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Tria, S.A.; Lopez-Ferber, D.; Gonzalez, C.; Bazin, I.; Guiseppi-Elie, A. Microfabricated biosensor for the simultaneous amperometric and luminescence detection and monitoring of Ochratoxin A. Biosens. Bioelectron. 2016, 79, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Lazanas, A.C.; Prodomidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Radi, A.E.; Muñoz-Berbel, X.; Lates, V.; Marty, J.L. Label-free impedimetric immunosensor for sensitive detection of ochratoxin A. Biosens. Bioelectron. 2009, 24, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Malvano, F.; Albanese, D.; Crescitelli, A.; Pilloton, R.; Esposito, E. Impedimetric label-free immunosensor on disposable modified screen-printed electrodes for Ochratoxin A. Biosensors 2016, 6, 33. [Google Scholar] [CrossRef]
- Dzyadevych, S.; Jaffrezic-Renault, N. Conductometric biosensors. In Biological Identification; Paul Schaudies, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 153–193. [Google Scholar] [CrossRef]
- Adley, C.C.; Ryan, M.P. Conductometric biosensors for high throughput screening of pathogens in food. In High Throughput Screening for Food Safety Assessment Biosensor Technologies, Hyperspectral Imaging and Practical Applications; Bhunia, A.K., Kim Moon, S., Taitt, C.R.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 315–326. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Electrochemical biosensors for hormone analyses. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Burdak, O.S.; Sergeyeva, T.A.; Arkhypova, V.M.; Dzyadevycha, S.V.; Soldatkin, A.P. Acetylcholinesterase-based conductometric biosensor for determination of aflatoxin B1. Sens. Actuators B Chem. 2013, 188, 999–1003. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Stepurska, K.V.; Arkhypova, V.M.; Soldatkin, A.P.; El’skaya, A.V.; Lagarde, F.; Dzyadevych, S.V. Conductometric enzyme biosensor for patulin determination. Sens. Actuators B Chem. 2017, 239, 1010–1015. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.R.; Jagajjanani Rao, K. A review on label free biosensors. Biosens. Bioelectron. X 2022, 11, 100216. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: A decade in review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, X. Optical ring resonators for biochemical and chemical sensing. Anal. Bioanal. Chem. 2011, 399, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sarkaleh, A.K.; Lahijani, B.V.; Saberkari, H.; Esmaeeli, A. Optical ring resonators: A platform for biological sensing applications. J. Med. Signals Sens. 2017, 7, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, M.; Yan, H. A portable optical fiber biosensor for the detection of zearalenone based on the localized surface plasmon resonance. Sens. Actuators B Chem. 2021, 336, 129752. [Google Scholar] [CrossRef]
- Campell, D.P. Interferometric biosensors. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer: New York, NY, USA, 2008; pp. 169–211. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Rubaye, A.G.; Al-Jawdah, A.M.; Tsargorodska, A.; Marty, J.-L.; Catanante, G.; Szekacs, A.; Takacs, E. Novel optical biosensing technologies for detection of mycotoxins. Opt. Laser Technol. 2019, 109, 212–221. [Google Scholar] [CrossRef]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, L.Y. Subtle application of electrical field-induced lossy mode resonance to enhance performance of optical planar waveguide biosensor. Biosensors 2021, 11, 86. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Jawdah, A.M.; Gémes, B.; Takács, E.; Székács, A. An optical planar waveguide-based immunosensors for determination of fusarium mycotoxin zearalenone. Toxins 2021, 13, 89. [Google Scholar] [CrossRef]
- Al-Jawdah, A.; Nabok, A.; Jarrah, R.; Holloway, A.; Tsargorodska, A.; Takacs, E.; Szekacs, A. Mycotoxin biosensor based on optical planar waveguide. Toxins 2018, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, S.; Li, D.; Yang, J.; Yang, L. Portable chemiluminescence optical fiber aptamer-based biosensors for analysis of multiple mycotoxins. Food Control 2023, 144, 109361. [Google Scholar] [CrossRef]
- Li, S.; Zhong, X.; Xu, Y.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Smartphone-based reading system integrated with phycocyanin-enhanced latex nanospheres immunoassay for on-site determination of aflatoxin b1 in foodstuffs. Food Chem. 2021, 360, 130019. [Google Scholar] [CrossRef] [PubMed]
- Kłos-Witkowska, A. Enzyme-based fluorescent biosensors and their environmental, clinical and industrial applications. Pol. J. Environ. Stud. 2015, 24, 19–25. [Google Scholar] [CrossRef]
- Selvin, P.R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Mol. Biol. 2000, 7, 730–734. [Google Scholar] [CrossRef]
- Das, A.P.; Kumar, P.S.; Swain, S. Recent advances in biosensor based endotoxin detection. Biosens. Bioelectron. 2014, 51, 62. [Google Scholar] [CrossRef]
- Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents. Sensors 2018, 18, 2357. [Google Scholar] [CrossRef]
- Tan, H.; Ma, L.; Guo, T.; Zhou, H.; Chen, L.; Zhang, Y.; Dai, H.; Yu, Y. A novel fluorescence aptasensor based on mesoporous silica nanoparticles for selective and sensitive detection of aflatoxin B1. Anal. Chim. Acta 2019, 1068, 87–95. [Google Scholar] [CrossRef]
- Ebanks, F.; Nasrallah, H.; Garant, T.M.; McConnell, E.M.; DeRosa, M.C. Colorimetric detection of aflatoxins B1 and M1 using aptamers and gold and silver nanoparticles. Adv. Agrochem. 2023, 2, 221–230. [Google Scholar] [CrossRef]
- Dies, H.; Siampani, M.; Escobedo, C.; Docoslis, A. Direct detection of toxic contaminants in minimally processed food products using dendritic surface-enhanced Raman scattering substrates. Sensors 2018, 18, 2726. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman spectroscopic methods in food safety: A review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Monošík, R.; Streďanský, M.; Šturdík, E. Biosensors—Classification, characterization and new trends. Acta Chim. Slovaca 2012, 5, 109–120. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Fogel, R.; Limson, J.; Seshia, A.A. Acoustic biosensors. Essays Biochem. 2016, 60, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Basinskiene, L.; Vidmantiene, D.; Bartkiene, E.; Kunigelis, V.; de Koe, W.J. Rapid acoustic screening of deoxynivalenol (DON) in grain. World Mycotoxin J. 2008, 1, 267–274. [Google Scholar] [CrossRef]
- Ramanathan, K.; Danielsson, B. Principles and applications of thermal biosensors. Biosens. Bioelectron. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Vasuki, S.; Varsha, V.; Mithra, R.; Dharshni, R.A.; Abinaya, S.; Dharshini, R.D.; Sivarajasekar, N. Thermal biosensors and their applications. Am. Int. J. Res. Sci. Technol. Eng. Math. 2019, 1, 262–264. Available online: http://iasir.net/AIJRSTEMpapers/IBCFT-2019.pdf (accessed on 12 January 2024).
- Bhardwaj, T. Review on immobilization techniques of biosensors. Int. J. Eng. Res. Technol. 2014, 3, 294–298. [Google Scholar]
- Liébana, S.; Drago, G.A. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Frutiger, A.; Tanno, A.; Hwu, S.; Tiefenauer, R.F.; Vörös, J.; Nakatsuka, N. Nonspecific binding fundamental concepts and consequences for biosensing applications. Chem. Rev. 2021, 121, 8095–8160. [Google Scholar] [CrossRef] [PubMed]
- Asal, M.; Özen, Ö.; Sahinler, M.; Baysal, H.T.; Polatoglu, I. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Layer-by-layer nano-assembly: A powerful tool for optical fiber sensing applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Vikulina, A.S. Layer-by-layer assemblies of biopolymers: Build-up, mechanical stability and molecular dynamics. Polymers 2020, 12, 1949. [Google Scholar] [CrossRef] [PubMed]
- Iost, R.M.; Crespilho, F.N. Layer-by-layer self-assembly and electrochemistry: Applications in biosensing and bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Gibbson, T.D. Biosensors: The stability problem. Analusis 1999, 27, 630–638. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Panjan, P.; Ohtonen, E.; Tervo, P.; Virtanen, V.; Sesay, A.M. Shelf life of enzymatic electrochemical sensors. Proc. Technol. 2017, 27, 306–308. [Google Scholar] [CrossRef]
- Puggioni, G.; Calia, G.; Arrigo, P.; Bacciu, A.; Bazzu, G.; Migheli, R.; Fancello, S.; Serra, P.A.; Rocchitta, G. Low-temperature storage improves the over-time stability of implantable glucose and lactate biosensors. Sensors 2019, 19, 422. [Google Scholar] [CrossRef] [PubMed]
- Alhamoud, Y.; Yang, D.; Kenston, S.S.F.; Liu, G.; Liu, L.; Zhou, H.; Ahmed, F.; Zhao, J. Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 2019, 141, 111418. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Wei, D.; Cao, M.; Wang, Z.; Wang, M. Biosensors based on core–shell nanoparticles for detecting mycotoxins in food: A review. Food Chem. 2023, 429, 136944. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Wang, E.; Tai, C.S.; Chiu, Y.C.; Li, C.W.; Lin, Y.R.; Lee, T.H.; Huang, C.W.; Chen, J.C.; Chen, W.L. Improving the reproducibility, accuracy, and stability of an electrochemical biosensor platform for point-of-care use. Biosens. Bioelectron. 2020, 155, 112111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, T.; Shen, Y.; Li, H. A high-performance self-supporting electrochemical biosensor to detect Aflatoxin B1. Biosensors 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, S.; Wei, Z.; Lou, X. Evanescent wave optical-fiber aptasensor for rapid detection of zearalenone in corn with unprecedented sensitivity. Biosensors 2022, 12, 438. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.P.; Zhao, Q. A signal-on electrochemical aptasensor for rapid detection of aflatoxin B1 based on competition with complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Y.; Cui, H.; Yang, H.; Hussain, M.; Wang, K.; Wei, J.; Long, L.; Ding, L.; Wang, C. Fabrication of a disposable aptasensing chip for simultaneous label-free detection of four common coexisting mycotoxins. Anal. Chim. Acta 2023, 1282, 341921. [Google Scholar] [CrossRef]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and methods of biosensor interfaces with stability. Front. Mater. 2021, 7, 583739. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, J.; Yang, C.; Jiang, J.; Zhang, Q.; Ping, J.; Li, P. Current trends in biosensors for biotoxins (mycotoxins, marine toxins, and bacterial food toxins): Principles, application, and perspective. TRAC Trends Anal. Chem. 2023, 165, 117144. [Google Scholar] [CrossRef]
- Kasoju, A.; Shrikrishna, N.S.; Shahdeo, D.; Khan, A.A.; Alanazi, A.M.; Gandhi, S. Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay. RSC Adv. 2020, 10, 11843–11850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef] [PubMed]
- Rafati, A.; Dorosti, N.; Gil, P. Smartphone-based technology for nanomolecular detection of aflatoxin B1 by aptamer-conjugated magnetic nanoparticles. World Mycotoxin J. 2022, 15, 159–169. [Google Scholar] [CrossRef]
- Contreras-Trigo, B.; Diaz-Garcia, V.; Oyarzun, P. A novel preanalytical strategy enabling application of a colorimetric nanoaptasensor for on-site detection of AFB1 in cattle feed. Sensors 2022, 22, 9280. [Google Scholar] [CrossRef] [PubMed]
- Mousivand, M.; Javan-Nikkhah, M.; Anfossi, L.; Di Nardo, F.; Salina, M.; Bagherzadeh, K. High performance aptasensing platform development through in silico aptamer engineering for aflatoxin B1 monitoring. Food Control 2023, 145, 109418. [Google Scholar] [CrossRef]
- Xiong, J.; He, S.; Qin, L.; Zhang, S.; Shan, W.; Jiang, H. Aptasensor-based assay for dual-readout determination of aflatoxin B1 in corn and wheat via an electrostatic force-mediated FRET strategy. Microchim. Acta 2023, 190, 80. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, V.; Koukouvinos, G.; Petrou, P.S.; Economou, A.; Dekker, J.; Harjanne, M.; Heimala, P.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Multiplexed mycotoxins determination employing white light reflectance spectroscopy and silicon chips with silicon oxide areas of different thickness. Biosens. Bioelectron. 2020, 153, 112035. [Google Scholar] [CrossRef]
- Al-Jawdah, A.; Nabok, A.; Abu-Ali, H.; Catanante, G.; Marty, J.-L.; Szekacs, A. Highly sensitive label-free in vitro detection of aflatoxin B1 in an aptamer assay using optical planar waveguide operating as a polarization interferometer. Anal. Bioanal. Chem. 2019, 411, 7717–7724. [Google Scholar] [CrossRef]
- Wang, C.; Tan, R.; Li, J. Exonuclease I-assisted fluorescent method for ochratoxin A detection using iron-doped porous carbon, nitrogen-doped graphene quantum dots, and double magnetic separation. Anal. Bioanal. Chem. 2019, 411, 2405–2414. [Google Scholar] [CrossRef]
- Ye, H.; Lu, Q.; Duan, N.; Wang, Z. GO-amplified fluorescence polarization assay for high-sensitivity detection of aflatoxin B1 with low dosage aptamer probe. Anal. Bioanal. Chem. 2019, 411, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wen, F.; Qiao, Q.; Zheng, N.; Saive, M.; Fauconnier, M.-L.; Wang, J. A novel graphene oxide-based aptasensor for amplified fluorescent detection of Aflatoxin M1 in milk powder. Sensors 2019, 19, 3840. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Khan, I.M.; Yan, L.; Khan, M.I.; Mohsin, A.; Duan, N.; Wu, S.; Wang, Z. Simultaneous detection of fumonisin B1 and ochratoxin a using dual-color, time-resolved luminescent nanoparticles (NaYF4: Ce, Tb and NH2-Eu/DPA@SiO2) as labels. Anal. Bioanal. Chem. 2019, 411, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.; Yarynka, D.; Dubey, L.; Dubey, I.; Piletska, E.; Linnik, R.; Antonyuk, M.; Ternovska, T.; Brovko, O.; Piletsky, S.; et al. Sensor based on molecularly imprinted polymer membranes and smartphone for detection of Fusarium contamination in cereals. Sensors 2020, 20, 4304. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Farka, Z.; Mickert, M.J.; Brandmeier, J.C.; Pastucha, M.; Hlaváček, A.; Martínez-Orts, M.; Canales, Á.; Skládal, P.; Benito-Peña, E.; et al. Competitive upconversion-linked immunoassay using peptide mimetics for the detection of the mycotoxin zearalenone. Biosens. Bioelectron. 2020, 170, 112683. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Fu, X.; Liu, H.; Ding, G.B.; Li, L.S.; Zhu, J.; Guo, Y. A competitive immunoassay based on engineered magnetic/fluorescent nanoparticles and biolayer interferometry-based assay for T-2 toxin determination. Microchim. Acta 2020, 187, 514. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, D.; Kim, M. Immunoliposome-based fluorometric patulin assay by using immunomagnetic nanoparticles. Microchim. Acta 2019, 186, 834. [Google Scholar] [CrossRef]
- Song, X.L.; Ding, Q.; Pu, Y.P.; Zhang, J.; Sun, R.L.; Yin, L.H.; Wei, W.; Liu, S.Q. Application of the dimeric G-quadruplex and toehold-mediated strand displacement reaction for fluorescence biosensing of ochratoxin A. Biosens. Bioelectron. 2021, 192, 113537. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, H.; Zhao, Q. A simple structure-switch aptasensor using label-free aptamer for fluorescence detection of aflatoxin B1. Molecules 2022, 27, 4257. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Zhang, H.; Zhu, R.; Meng, Z. Competitive ELISA based on pH-responsive persistent luminescence nanoparticles for autofluorescence-free biosensor determination of ochratoxin A in cereals. Anal. Bioanal. Chem. 2023, 415, 1877–1887. [Google Scholar] [CrossRef]
- Ge, G.; Wang, T.; Liu, Z.; Liu, X.; Li, T.; Chen, Y.; Fan, J.; Bukye, E.; Huang, X.; Song, L. A self-assembled DNA double-crossover-based fluorescent aptasensor for highly sensitivity and selectivity in the simultaneous detection of aflatoxin M1 and aflatoxin B1. Talanta 2023, 265, 124908. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Guo, H.; Li, K.; Zhang, Y.; Guo, H.; Wang, Z. Simultaneous detection of patulin and ochratoxin A based on enhanced dual-color AuNCs modified aptamers in apple juice. Talanta 2024, 266, 124949. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, C.; Xu, E.; Chen, J.; Xu, X.; Wei, W.; Liu, S. A simple and sensitive electrochemiluminescence aptasensor for determination of ochratoxin A based on a nicking endonuclease-powered DNA walking machine. Food Chem. 2019, 282, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Meng, X.; Zhang, Y.; Li, Z.; Zhou, Q.; Jing, X.; Sun, X.; Zhao, W. An “on-off-on” electrochemiluminescence aptasensor based on a self-enhanced luminophore for ochratoxin A detection. Anal. Bioanal. Chem. 2023, 415, 5833–5844. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Stepanov, N.; Maslova, O.; Akhundov, R.; Ismailov, A.; Efremenko, E. Immobilized luminescent bacteria for the detection of mycotoxins under discrete and flow-through conditions. Biosensors 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Rehmat, Z.; Mohammed, W.S.; Sadiq, M.B.; Somarapalli, M.; Kumar Anal, A. Ochratoxin A detection in coffee by competitive inhibition assay using chitosan-based surface plasmon resonance compact system. Colloids Surf. B Biointerfaces 2019, 174, 569–574. [Google Scholar] [CrossRef]
- Wei, T.; Ren, P.P.; Huang, L.L.; Ouyang, Z.C.; Wang, Z.Y.; Kong, X.F.; Li, T.J.; Yin, Y.L.; Wu, Y.N.; He, Q.H. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef]
- Asl, G.B.; Arvand, M.; Habibi, M.F. High affinity aptamers for ultra-sensitive detection of aflatoxin B1 in milk and groundnut samples with label-free photo-electrochemical aptasensor. Food Chem. 2022, 397, 133829. [Google Scholar] [CrossRef] [PubMed]
- Abera, B.D.; Falco, A.; Ibba, P.; Cantarella, G.; Petti, L.; Lugli, P. Development of flexible dispense-printed electrochemical immunosensor for Aflatoxin M1 detection in milk. Sensors 2019, 19, 3912. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M.; Mustapha, M. Electrospun cellulose acetate-doped 3D-graphene nanofibre for enhanced transduction of ochratoxin A determination. Bull. Mater. Sci. 2022, 45, 166. [Google Scholar] [CrossRef]
- Ben Abdallah, Z.; Grauby-Heywang, C.; Beven, L.; Cassagnere, S.; Moroté, F.; Maillard, E.; Sghaier, H.; Bouhacina, T.C. Development of an ultrasensitive label-free immunosensor for fungal aflatoxin B1 detection. Biochem. Eng. J. 2019, 150, 107262. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Shen, X.; Ma, S.; Liu, Y.; You, T. Ratiometric electrochemical aptasensor for ultrasensitive detection of Ochratoxin A based on a dual signal amplification strategy: Engineering the binding of methylene blue to DNA. Biosens. Bioelectron. 2020, 150, 111814. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Ma, S.; Wang, M.; You, T. Hairpin DNA assisted dual-ratiometric electrochemical aptasensor with high reliability and anti-interference ability for simultaneous detection of aflatoxin B1 and ochratoxin A. Biosens. Bioelectron. 2021, 174, 112654. [Google Scholar] [CrossRef]
- Selvolini, G.; Lettieri, M.; Tassoni, L.; Gastaldello, S.; Grillo, M.; Maran, C.; Marrazza, G. Electrochemical enzyme-linked oligonucleotide array for aflatoxin B1 detection. Talanta 2019, 203, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Beheshti-Marnani, A.; Hatefi-Mehrjardi, A.; Es’haghi, Z. A sensitive biosensing method for detecting of ultra-trace amounts of AFB1 based on “Aptamer/reduced graphene oxide” nano-bio interaction. Colloids Surf. B 2019, 175, 98–105. [Google Scholar] [CrossRef]
- Song, X.; Wang, D.; Kimb, M. Development of an immuno-electrochemical glass carbon electrode sensor based on graphene oxide/gold nanocomposite and antibody for the detection of patulin. Food Chem. 2021, 342, 128257. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A simple detection platform based on molecularly imprinted polymer for AFB1 and FuB1 mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, Q.; Liu, Y.; Qin, M.; Bikila, T.L.; Weng, X. A simple paper-based dual-analyte electrochemical nanobiosensor for mycotoxins using phosphorene-gold nanocomposites. IEEE Sens. J. 2022, 22, 19144–19151. [Google Scholar] [CrossRef]
- Liao, Z.; Guo, W.; Ning, G.; Wu, Y.; Wang, Y.; Ning, G. A sensitive electrochemical aptasensor for zearalenone detection based on target-triggered branched hybridization chain reaction and exonuclease I-assisted recycling. Anal. Bioanal. Chem. 2023, 415, 4911–4921. [Google Scholar] [CrossRef]
- Subak, H.; Selvolini, G.; Macchiagodena, M.; Ozkan-Ariksoysal, D.; Pagliai, M.; Procacci, P.; Marrazza, G. Mycotoxins aptasensing: From molecular docking to electrochemical detection of deoxynivalenol. Bioelectrochemistry 2020, 138, 107691. [Google Scholar] [CrossRef]
- Lai, H.; Ming, P.; Wu, M.; Wang, S.; Sun, D.; Zhai, H. An electrochemical aptasensor based on P-Ce-MOF@MWCNTs as signal amplification strategy for highly sensitive detection of zearalenone. Food Chem. 2023, 423, 136331. [Google Scholar] [CrossRef] [PubMed]
- Azri, F.A.; Eissa, S.; Zourob, M.; Chinnappan, R.; Sukor, R.; Yusof, N.A.; Raston, N.H.A.; Alhoshani, A.; Jinap, S. Electrochemical determination of zearalenone using a label-free competitive aptasensor. Microchim. Acta 2020, 187, 266. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yao, J.; Jiang, B.; Yuan, R.; Xiang, Y. NiCo2S4 nanoparticle-dispersed MoS2 nanosheets for catalytic and sensitive electrochemical aptamer sensing of ochratoxin A via cascaded amplifications. Sens. Actuators B Chem. 2022, 371, 132530. [Google Scholar] [CrossRef]
- Nan, M.; Bi, Y.; Xue, H.; Xue, S.; Long, H.; Pu, L.; Fu, G. Rapid determination of Ochratoxin A in grape and its commodities based on a label-free impedimetric aptasensor constructed by layer-by-layer self-assembly. Toxins 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, G.; Ben Aissa, S.; Nemčeková, K.; Catanante, G.; Raouafi, N.; Marty, J.L. Aptamer-modified pencil graphite electrodes for the impedimetric determination of ochratoxin A. Food Control 2020, 115, 107271. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Wu, X.; Pan, M.; Hu, N.; Wang, J.; Wang, S. Quartz crystal microbalance sensor based on covalent organic framework composite and molecularly imprinted polymer of poly(o-aminothiophenol) with gold nanoparticles for the determination of aflatoxin B1. Sens. Actuators B Chem. 2019, 291, 293–297. [Google Scholar] [CrossRef]
- Boshra, M.H.; El-Housseiny, G.S.; Farag, M.M.S.; Aboshanab, K.M. Innovative approaches for mycotoxin detection in various food categories. AMB Express 2024, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, T.B. Detecting mycotoxins in agricultural commodities. Mol. Biotechnol. 2003, 23, 61–71. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Huang, P.; Wei, F.; Ying, G.; Lu, J.; Zhou, L.; Kong, W. Regeneration and reuse of immunoafnity column for highly efficient clean-up and economic detection of ochratoxin A in malt and ginger. Toxins 2018, 10, 462. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Wang, F.; He, P. Recent advances in immunoassays and biosensors for mycotoxins detection in feedstuffs and foods. J. Anim. Sci. Biotechnol. 2021, 12, 108. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, R.; Catanante, G.; Sherazi, T.A.; Bhand, S.; Hayat, A.; Marty, J.L. Designed strategies for fluorescence-based biosensors for the detection of mycotoxins. Toxins 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Y.; Huang, H.; Chen, X.; Leng, Y.; Lai, W.; Hang, X.; Xiong, Y. Engineered gold nanoparticles as multicolor labels for simultaneous multi-mycotoxin detection on the immunochromatographic test strip nanosensor. Sens. Actuators B Chem. 2020, 316, 128107. [Google Scholar] [CrossRef]
- Altunbas, O.; Ozdas, A.; Yilmaz, M.D. Luminescent detection of Ochratoxin a using terbium chelated mesoporous silica nanoparticles. J. Hazard. Mater. 2020, 382, 121049. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, X.; Wang, D.; Zhang, Q.; Li, P.; Ding, X. Rapid on-site sensing aflatoxin B1 in food and feed via a chromatographic time-resolved fluoroimmunoassay. PLoS ONE 2015, 10, e0123266. [Google Scholar] [CrossRef]
- Yugender Goud, K.; Hayat, A.; Satyanarayana, M.; Sunil Kumar, V.; Catanante, G.; Vengatajalabathy Gobi, K.; Marty, J.L. Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim. Acta 2017, 184, 4401–4408. [Google Scholar] [CrossRef]
- Majdinasab, M.; Ben Aissa, S.; Marty, J.L. Advances in colorimetric strategies for mycotoxins detection: Toward rapid industrial monitoring. Toxins 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zeng, L.; Li, N.; Liu, C.; Chen, J. A wash-free and label-free colorimetric biosensor for naked-eye detection of aflatoxin B1 using G-quadruplex as the signal reporter. Food Chem. 2019, 298, 125034. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.; Zherdev, A.; Petrakova, A.; Sadykhov, E.; Koroleva, O.; Dzantiev, B. Rapid multiple immunoenzyme assay of mycotoxins. Toxins 2015, 7, 238–254. [Google Scholar] [CrossRef]
- Sheini, A. Colorimetric aggregation assay based on array of gold and silver nanoparticles for simultaneous analysis of aflatoxins, ochratoxin and zearalenone by using chemometric analysis and paper based analytical devices. Microchim. Acta 2020, 187, 167. [Google Scholar] [CrossRef]
- Kumari Shrestha, Y.; Krishna Shrestha, S. Fundamentals of colorimetry. In Advances in Colorimetry; Samanta, A.K., Ed.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Rene, W.; Lenoble, V.; Chioukh, M.; Branger, C. A turn-on fluorescent ion-imprinted polymer for selective and reliable optosensing of lead in real water samples. Sens. Actuators B Chem. 2020, 319, 128252. [Google Scholar] [CrossRef]
- Jo, E.-J.; Byun, J.-Y.; Mun, H.; Bang, D.; Son, J.H.; Lee, J.Y.; Lee, L.P.; Kim, M.-G. Single-step LRET aptasensor for rapid mycotoxin detection. Anal. Chem. 2018, 90, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ren, Y.; Li, Y.; Li, Y.; Long, F.; Zhu, A. Optical biosensors for microbial toxin detection: Recent advances and future trends. Microchem. J. 2023, 191, 108894. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Sun, C.; Zheng, M.; Zhang, J.; Liu, B.; Wang, Y.; Xie, Z.; Xu, N. Hybrids of carbon dots with subunit B of ricin toxin for enhanced immunomodulatory activity. J. Colloid Interface Sci. 2018, 523, 226–233. [Google Scholar] [CrossRef]
- Farré, M.; Kantiani, L.; Pérez, S.; Barceló, D. Sensors and biosensors in support of EU Directives. TRAC Trends Anal. Chem. 2009, 28, 170–185. [Google Scholar] [CrossRef]
- Kaur, M.; Gaba, J.; Singh, K.; Bhatia, Y.; Singh, A.; Singh, N. Recent advances in recognition receptors for electrochemical biosensing of mycotoxins—A review. Biosensors 2023, 13, 391. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Adányi, N.; Székács, A. Biosensors for Deoxynivalenol and Zearalenone determination in feed quality control. Toxins 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.-H.; Marty, J.L. Nano-aptasensing in mycotoxin analysis: Recent updates and progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Hianik, T. Electrochemical immuno-and aptasensors for mycotoxin determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef]
- Le, V.T.; Vasseghian, Y.; Dragoi, E.-N.; Moradi, M.; Khaneghah, A.M. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, Y.; Hu, X.; Zhang, J.; Chen, Q.; Chen, H. Recent progress in electrochemical nano-biosensors for detection of pesticides and mycotoxins in foods. Biosensors 2023, 13, 140. [Google Scholar] [CrossRef]
- Abd-Ellatief, R.; Abd-Ellatief, M.R. Electrochemical aptasensors: Current status and future perspectives. Diagnostics 2021, 11, 104. [Google Scholar] [CrossRef]
- Guo, X.D.; Wen, F.; Zheng, N.; Saive, M.; Fauconnier, M.L.; Wang, J.Q. Aptamer-based biosensor for detection of mycotoxins. Front. Chem. 2020, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Kesici, E.; Erdem, A. Impedimetric detection of Fumonisin B1 and its biointeraction with fsDNA. Int. J. Biol Macromol. 2019, 139, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Azizi-Lalabadi, M.; Motamedi, P.; Sadeghi, E. Electrochemical determination of T2 toxin by graphite/polyacrylonitrile nanofiber electrode. Food Sci. Nutr. 2021, 9, 1171–1179. [Google Scholar] [CrossRef]
- He, S.; Yuan, Y.; Nag, A.; Feng, S.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C.; Organ, D.R. A review on the use of impedimetric sensors for the inspection of food quality. Int. J. Environ. Res. Public Health 2020, 17, 5220. [Google Scholar] [CrossRef]
- Venton, B.J.; DiScenza, D.J. Voltammetry. In Electrochemistry for Bioanalysis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–50. [Google Scholar] [CrossRef]
- Meliana, C.; Liu, J.; Show, P.L.; Low, S.S. Biosensor in smart food traceability system for food safety and security. Bioengineered 2024, 15, 2310908. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.; Wang, J. Advanced biosensors for mycotoxin detection incorporating miniaturized meters. Biosens. Bioelectron. 2023, 224, 115077. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef] [PubMed]
- Geicu, O.I.; Bilteanu, L.; Stanca, L.; Ionescu Petcu, A.; Iordache, F.; Pisoschi, A.M.; Serban, A.I. Composition-based risk estimation of mycotoxins in dry dog foods. Foods 2023, 12, 110. [Google Scholar] [CrossRef]
- Matson, R.S. ELISA-based biosensors. Methods Mol. Biol. 2023, 2612, 225–238. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, H.; Zhu, M.; Wang, F.; Meng, H.; Feng, L. Electrochemical/visual dual-readout aptasensor for ochratoxin a detection integrated into a miniaturized paper-based analytical device. Biosens. Bioelectron. 2021, 180, 113146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, S.; Zhang, Y.; Wang, S.; Guo, J.; Wang, J. A highly sensitive photothermal immunochromatographic sensor for detection of aflatoxin B1 based on Cu2-xSe-Au nanoparticles. Food Chem. 2024, 401, 134065. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Pan, S.; Hong, F.; Lu, P.; Hu, X.; Jiang, F.; Wu, L.; Chen, Y. Bimetallic nanozyme-bioenzyme hybrid material-mediated ultrasensitive and automatic immunoassay for the detection of aflatoxin B1 in food. Biosens. Bioelectron. 2024, 248, 115992. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Amine, A. Biorecognition elements (Chapter 3). In Wearable Physical, Chemical and Biological Sensors; Morales-Narvaez, E., Dincer, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–70. [Google Scholar] [CrossRef]
- Oliveira, I.S.; da Silva Junior, A.G.; de Andrade, C.A.S.; Oliveira, M.D.L. Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr. Opin. Food Sci. 2019, 29, 64–79. [Google Scholar] [CrossRef]
- Sivasubramanian, S.P.; Vhanbatte, S.; Rajagopalan, S. Biosensor global market potential. Asian Tex. J. 2020, 29, 43–45. [Google Scholar]
- Bhide, A.; Ganguly, A.; Parupudi, T.; Ramasamy, M.; Muthukumar, S.; Prasad, S. Next-generation continuous metabolite sensing toward emerging sensor needs. ACS Omega 2021, 6, 6031–6040. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical biosensors and their applications for the detection of water pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]


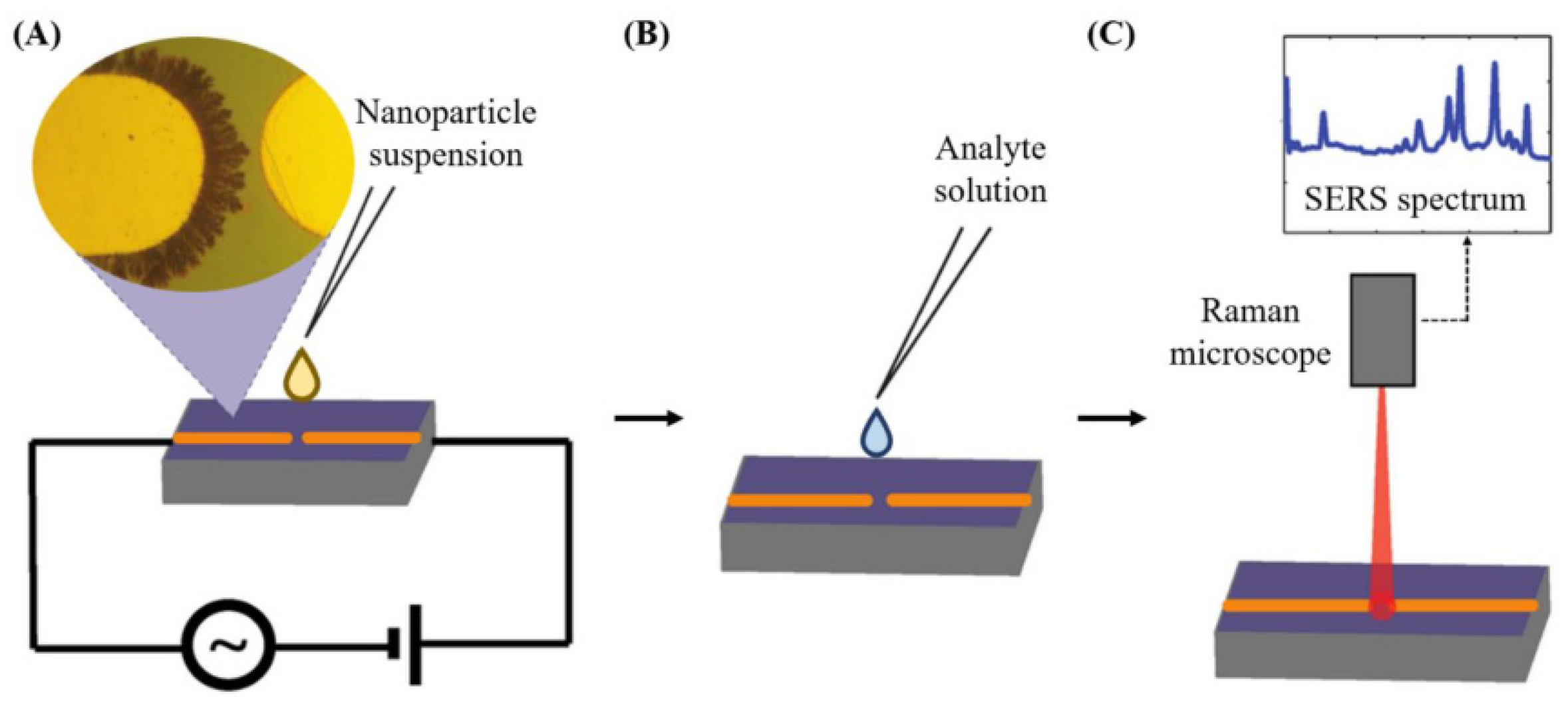
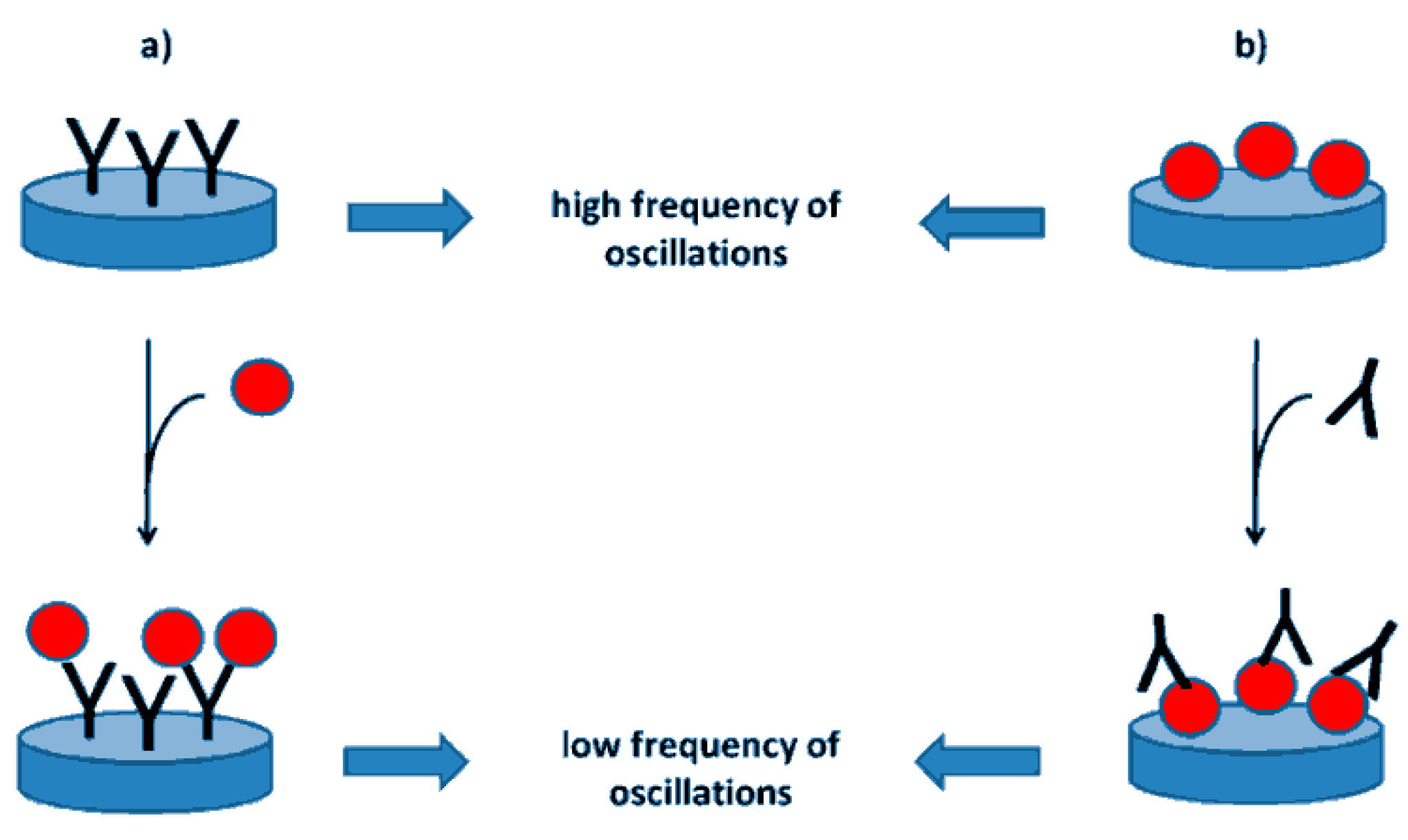
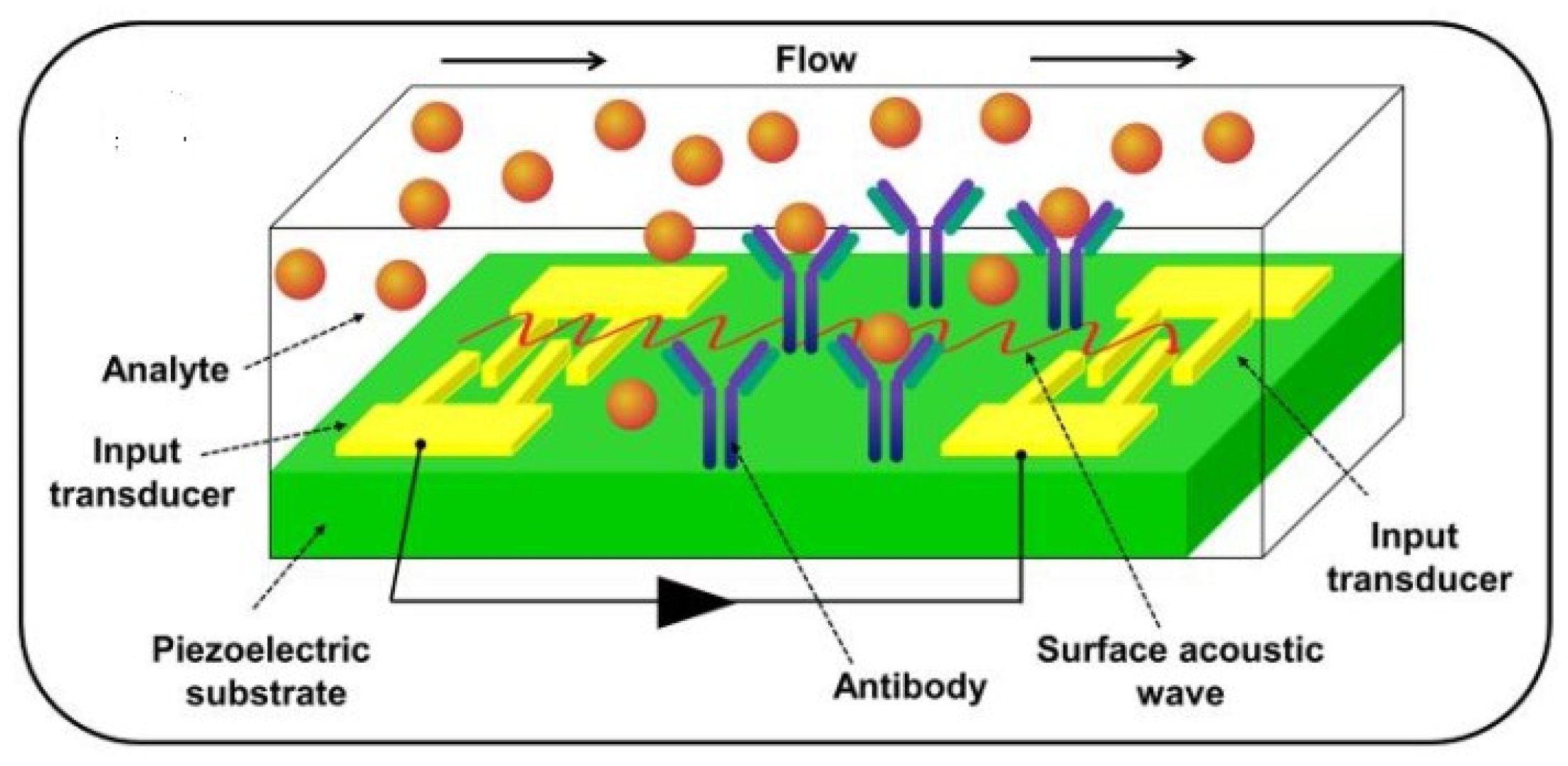
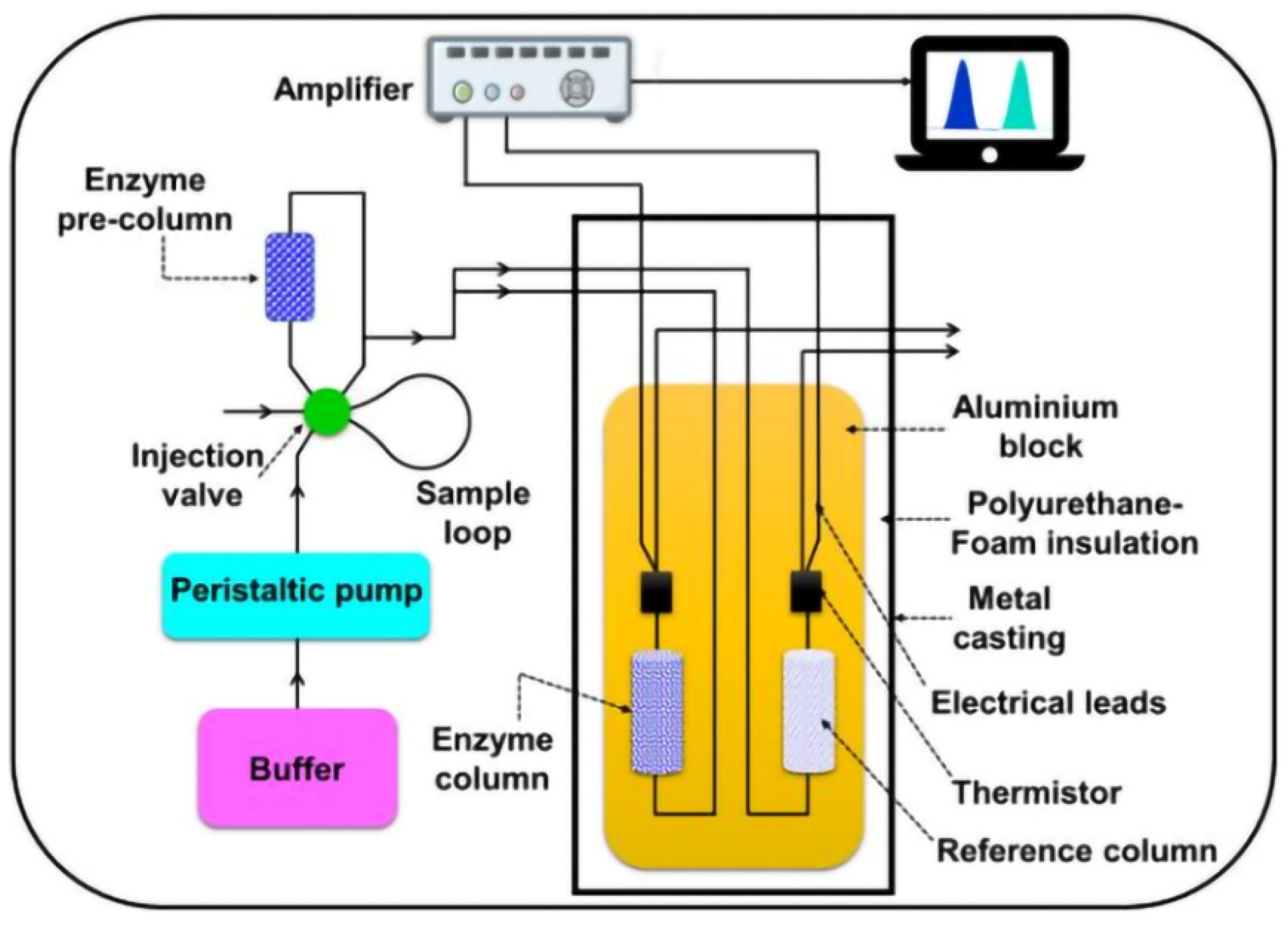
| Aflatoxin | Food Product | Level (μg Kg−1) |
|---|---|---|
| Aflatoxins total | Almonds (ready to eat) | 10 |
| Hazelnuts (ready to eat) | ||
| Pistachios (ready to eat) | ||
| Aflatoxin M1 | Milk | 0.5 |
| Aflatoxin B1 | Cereals and processed cereal products. Exceptions: rice and maize meant to be sorted or to undergo other physical treatments prior to their placing on the market to reach the consumer, or before employing as food ingredients, baby food, food meant for particular medical purposes for young children and infants, food based on processed cereals for young children and infants | 2.0 |
| Food meant for particular medical aims, for young children and infants | 0.1 | |
| Sum of aflatoxins B1, B2, G1 and G2 | Dried fruits meant to be sorted or to undergo other physical treatments prior to their placing on the market to reach the consumer or before employing as food ingredients. Exception: dried figs | 10.0 |
| Groundnuts (peanuts) and other oilseeds meant to be sorted or to undergo other physical treatments prior to their placement on the market to reach the consumer or before employing as food ingredients. Exceptions: groundnuts (peanuts) and other oilseeds to be crushed to obtain refined vegetable oil | 15.0 | |
| Ochratoxin A | Raw wheat | 5.0 |
| Barley | ||
| Rye | ||
| Deoxynivalenol | Pasta | 750 |
| Bread, pastries, biscuits | 500 | |
| Patulin | Fruit juices | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisoschi, A.M.; Iordache, F.; Stanca, L.; Mitranescu, E.; Bader Stoica, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Biosensors for Food Mycotoxin Determination: A Comparative and Critical Review. Chemosensors 2024, 12, 92. https://doi.org/10.3390/chemosensors12060092
Pisoschi AM, Iordache F, Stanca L, Mitranescu E, Bader Stoica L, Geicu OI, Bilteanu L, Serban AI. Biosensors for Food Mycotoxin Determination: A Comparative and Critical Review. Chemosensors. 2024; 12(6):92. https://doi.org/10.3390/chemosensors12060092
Chicago/Turabian StylePisoschi, Aurelia Magdalena, Florin Iordache, Loredana Stanca, Elena Mitranescu, Liliana Bader Stoica, Ovidiu Ionut Geicu, Liviu Bilteanu, and Andreea Iren Serban. 2024. "Biosensors for Food Mycotoxin Determination: A Comparative and Critical Review" Chemosensors 12, no. 6: 92. https://doi.org/10.3390/chemosensors12060092
APA StylePisoschi, A. M., Iordache, F., Stanca, L., Mitranescu, E., Bader Stoica, L., Geicu, O. I., Bilteanu, L., & Serban, A. I. (2024). Biosensors for Food Mycotoxin Determination: A Comparative and Critical Review. Chemosensors, 12(6), 92. https://doi.org/10.3390/chemosensors12060092







