Abstract
The need for performant analytical methodologies to assess mycotoxins is vital, given the negative health impact of these compounds. Biosensors are analytical devices that consist of a biological element for recognizing the analyte and a transducer, which translates the biorecognition event into a signal proportional to the analyte concentration. The biorecognition elements can be enzymes, antibodies, or DNA fragments. The modalities of detection can be optical, electrochemical, thermal, or mass-sensitive. These analytical tools represent viable alternatives to laborious, expensive traditional methods and are characterized by specificity given by the biorecognition element, sensitivity, fast response, portability, multi-modal detection, and the possibility of in situ application. The present paper focuses on a comprehensive view, enriched with a critical, comparative perspective on mycotoxin assay using biosensors. The use of different biorecognition elements and detection modes are discussed comparatively. Nanomaterials with optical and electrochemical features can be exploited in association with a variety of biorecognition elements. Analytical parameters are reviewed along with a broad range of applications.
1. Introduction
Mycotoxins are natural products that occur as a result of the metabolism of some fungi and are found in some foods like cereals, dried fruits, dried beans, coffee, animal feed, various agricultural products, and many others. The toxicity of mycotoxins has been largely documented [1,2,3,4,5,6], and as a result, international legislation was introduced [7,8] as well as at the national level [9,10]. Legislation requires mycotoxin contents in food products to be as low as possible to protect consumers from the risk of illness (establishing tolerable daily intakes). The main mycotoxins are as follows:
- -
- Aflatoxins (AFB1, AFB2, AFG1, AFG2, and AFM1) produced by certain Aspergillus molds;
- -
- Ochratoxin A (OTA), produced by certain Aspergillus and Penicillium molds, is the most active of the nine ochratoxins;
- -
- Patulin toxin produced by Penicillium, Aspergillus, and Byssochylamys molds;
- -
- Fumonisins produced by Fusarium molds;
- -
- Trichothecenes (mainly nivalenol-NIV, deoxynivalenol-DON, T-2, and HT-2 toxin) produced by different species belonging to the genera Fusarium, Myrothecium, Trichoderma, Trichothecium, Cephalosporium, Verticimonosporium, and Stachybotrys;
- -
- Zearalenone (ZEN) produced by some species of Fusarium and Gibberella;
- -
- Ergot alkaloids, citrinin (CIT), sterigmatocystin (STC), Alternaria toxins, etc.
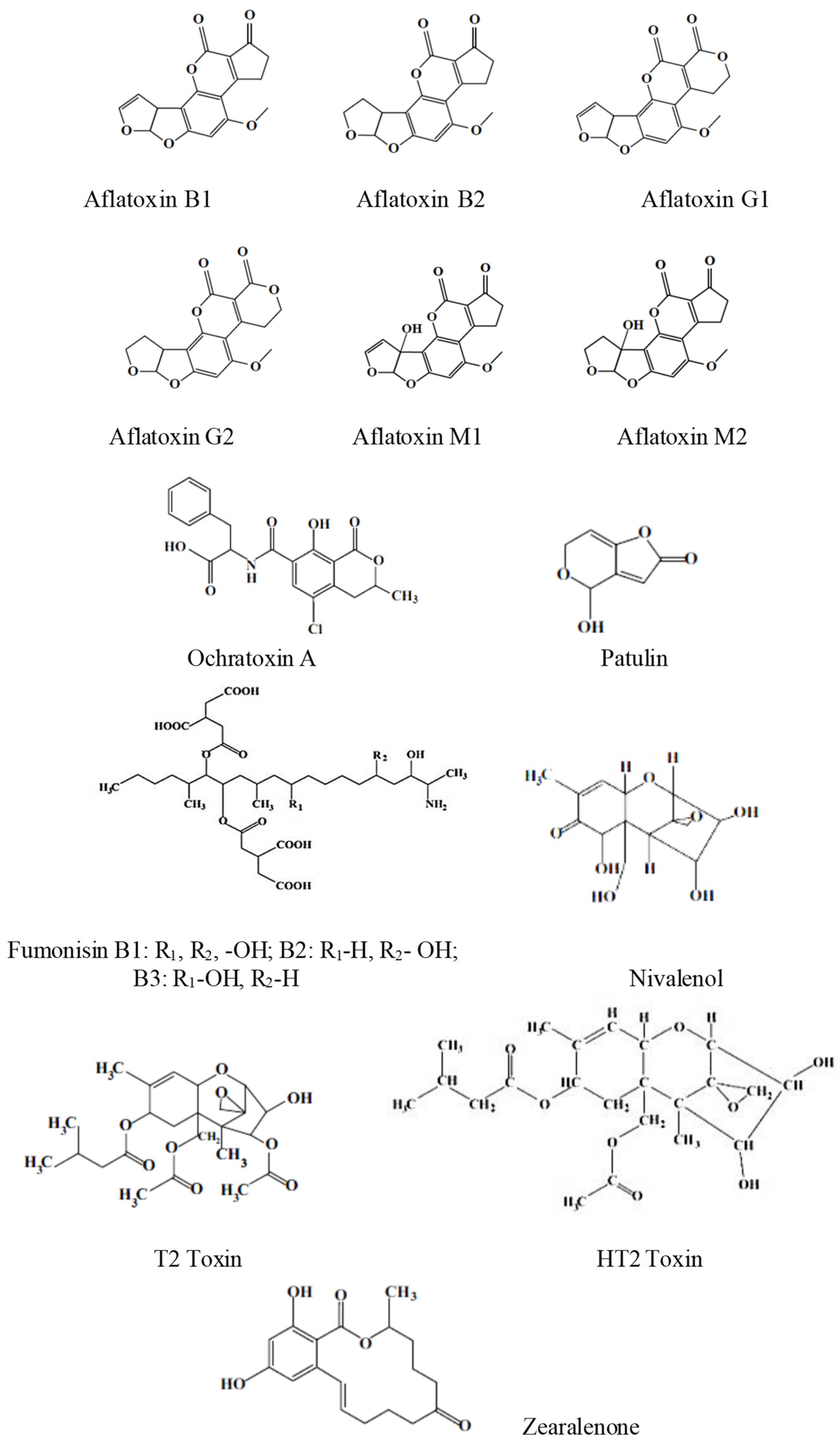
The most representative mycotoxins are illustrated in Scheme 1.
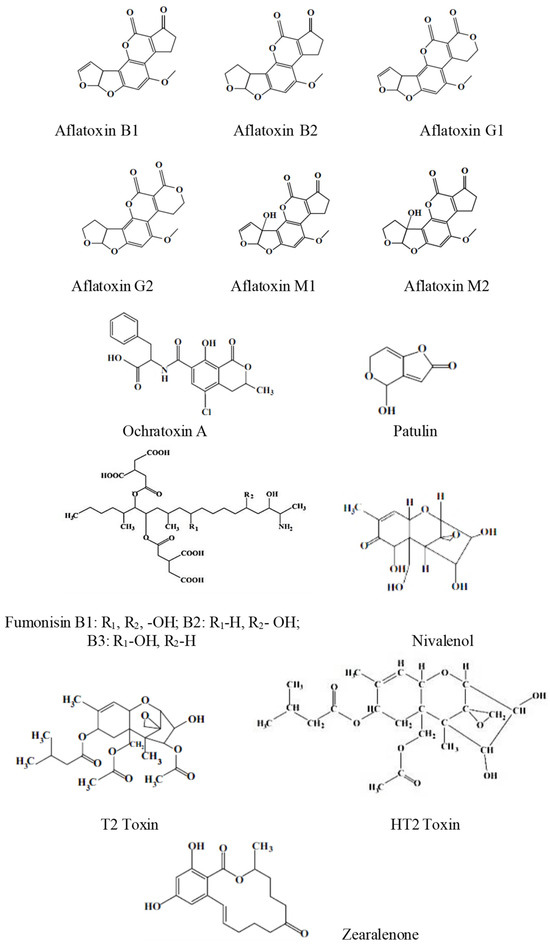
Scheme 1.
The structures of some of the most representative mycotoxins.
More than 500 mycotoxins have been identified, and new mycotoxins are expected to be discovered [11].
The lethal dose (LD50, as milligrams of substance per kilogram of body weight) is defined as the amount of an ingested compound inducing the death of one-half of a tested sample (population). The LD50 values for aflatoxins are found within the range of 0.5 to 10 mg/kg. The liver is mainly affected, with centrilobular necrosis, bile duct proliferation, fatty acid infiltration, and hepatic lesions, which can result in liver cancer [12]. For most animal species, the oral LD50 value for aflatoxin B1 ranges between 0.3 and 17.9 mg/kg body weight. Oral LD50 values of 78 and 46 mg/kg body weight have been reported for deoxynivalenol in B6C3F1 and DDY mice, respectively [13]. The oral LD50 of patulin in rodents ranges between 29 and 55 mg/kg body weight [14].
Mycotoxins have been investigated for their genotoxic, mutagenic, and carcinogenic potential. Aflatoxin B1, due to the presence of a double bond at the 8,9 position, can be converted to the reactive AFB1-8,9-epoxide, which interacts with key biomolecules including DNA. The main DNA adduct resulted is AFB1-N7-guanine, and this pro-mutagenic insult leads to a G → T transversion mutation [15]. This mutation will impact the cell cycle by affecting the P53 gene, which encodes a tumor suppressor protein that counteracts tumorigenesis and carcinogenesis [16].
In the case of FB1, the DNA damage was assigned to the stimulation of oxidative decay and lipid peroxidation. In vitro genotoxicity studies revealed micronuclei and chromosomal aberrations [15]. It inhibits protein synthesis and DNA synthesis at elevated concentrations. Its carcinogenic potential was ascribed to its potential to stimulate 3H thymidine incorporation. Following animal studies, it was reported as an inductor of liver, oesophageal, kidney cancer, and neurodegeneration [16].
Ochratoxin A inhibits protein and nucleic acid synthesis due to competitive inhibition of phenylalanine-tRNA ligase. Co-administration of phenylalanine in animals could lower the acute toxic effects of OTA. The formation of DNA adducts induced by OTA was linked to its carcinogenic potential. It was found that the OTA phenoxy radical resulted from metabolic activation, and the associated DNA damage resulting from reactive oxygenated species represents the source of adduct formation [15]. Moreover, Ochratoxin A is considered strong hepatotoxic and nephtotoxic and was reported as a promoter of lipid peroxidation and an inhibitor of mitochondrial ATP synthesis [16].
Deoxynivalenol exerts toxicity via its epoxide moiety without necessitating metabolic activation. Trichothecene exposure at low doses led to the modulation of the expression of several cytokines and chemokines, hence immune function, in animal studies. DON is involved in the modulation of physiological processes controlled by mitogen-activated protein kinases: control of cell growth, differentiation, and apoptosis, which are essential for signal transduction in the immune response. Along with alterations in cytokine expression, alterations in MAPK expression also result in the immune imbalance and toxicity linked to DON and other trichothecenes [15].
Zearalenone represents a competitive substrate for enzymes present in steroid synthesis pathway and metabolism and, therefore, has the potential to act as an endocrine disruptor. So, it was stipulated that ZEA could exert intense effects on gene expression as a result of the altered activity of nuclear transcription factors [15]. Zearalenone is genotoxic, induces DNA alterations, is reported as carcinogenic in mice, and is an inductor of hepatocellular adenomas and pituitary tumors [16].
Patulin was characterized as carcinogenic, mutagenic, and teratogenic and as an inductor of intestinal injuries. Its involvement in carcinogenesis and tumorigenesis was ascribed to its ability to impair cellular DNA. The inactivation of the active site of protein tyrosine phosphatase, a regulator of intestinal epithelial barrier function, was considered the source of patulin toxicity for intestinal cells [16].
The toxicity of aflatoxins is also proved by EFSA, which evaluated over 200,000 analytical results, assessing that the risk of poisoning with AFM1 comes from milk and fermented milk products and the risk for AFB1 comes from the consumption of grains and grain-based products [17].
Because of their toxicity, the levels allowed in food products are very low (as shown in Table 1) [7,8], especially in milk and baby food products.

Table 1.
Maximum mycotoxin levels allowed for some food products [7,8].
Biosensors approached in this review, by their analytical parameters, show their ability to detect such low levels in the analyzed food products.
There are numerous recent articles on the determination of aflatoxins (B1, B2, G1, G2, M1) from different food products [18,19,20,21,22,23,24,25,26,27]. Among the determination methods, the immunochemical and chromatographic techniques in tandem with mass spectrometry are mentioned. Immunochemical methods are fast and, therefore, used in routine controls and on-site detection. Chromatographic methods ensure a sensitive and selective analysis [28].
Traditional techniques such as HPLC, gas chromatography, mass spectrometry, and enzyme-linked immunosorbent assay, though accurate and sensitive, require training, accessibility, costs, and the sample pre-treatment is time-consuming.
Biosensors, as broadly used analytical tools for determining the quality of food products, aim at circumventing these drawbacks [29,30,31,32,33,34,35,36,37]. They consist of two important parts: a biological element for recognizing the analyte and a transducer, which translates the biorecognition event into a signal proportional to the analyte concentration. Therefore, biosensors are classified according to the biological recognition element and according to the type of transducer [38]. The recognition element is either a biomolecule or a molecular assembly capable of recognizing an analyte (a substrate). According to the biological element of recognition (bioreceptor), they are the following [39]:
- -
- Enzyme sensors [40];
- -
- Immunosensors, which use antibodies, antibody fragments, antigens, or antigen conjugates as biorecognition elements [41];
- -
- Aptasensors (DNA-based biosensors), which use nucleic acid molecules as biorecognition elements [42];
- -
- Biomimetics [43];
- -
- Sensors using synthetic biorecognition elements (molecularly imprinted polymers) [44].
So, biosensors can be developed by incorporating a variety of biorecognition elements, including enzymes, aptamers, antibodies, nucleic acids, cells, tissues, and molecularly imprinted polymers [39].
According to the type of transducer, the biosensors can be classified as follows [45]:
- -
- Electrochemical—based on the variation of electrochemical properties [46];
- -
- Optical—based on the variation of optical properties [47];
- -
- Thermal biosensors—based on thermal energy variation [48];
- -
- Mass sensitive biosensors—based on mass variation [49];
- -
- Acoustic biosensors—the target analyte is detected through the induced variations in the frequency, velocity, or amplitude of the acoustic waves generated by piezoelectric materials [50].
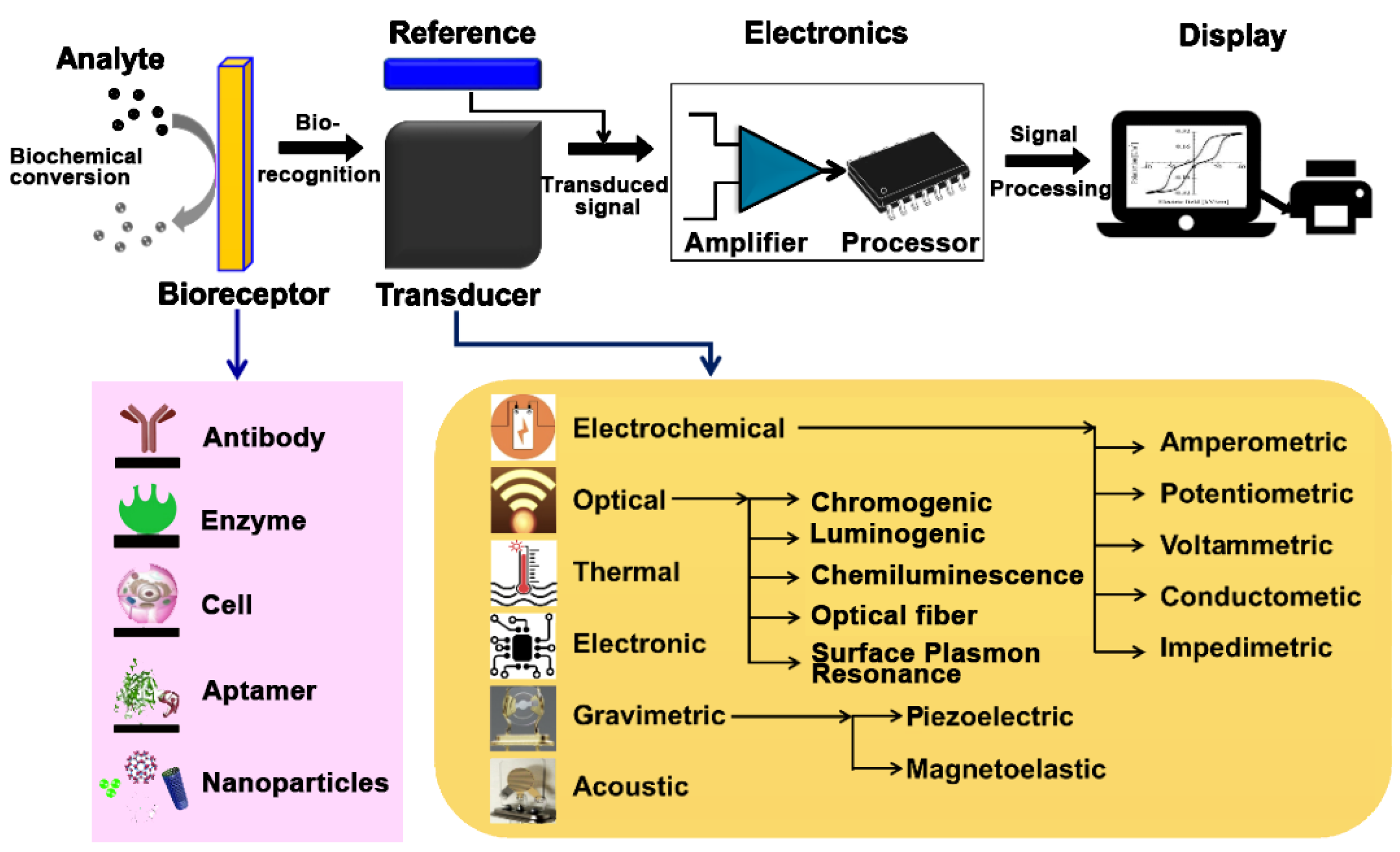
M. Ramesh et al. [51] developed an illustrative general scheme regarding the components and the functioning of biosensors.
The general scheme of a biosensor, encompassing various biorecognition elements and transduction principles, is shown in Figure 1 [45,51].
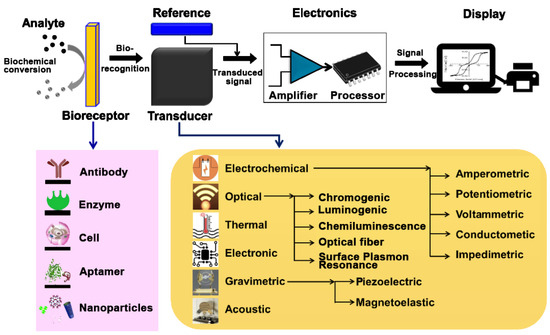
Figure 1.
General diagram of a biosensor from [45,51], MDPI 2021, 2023.
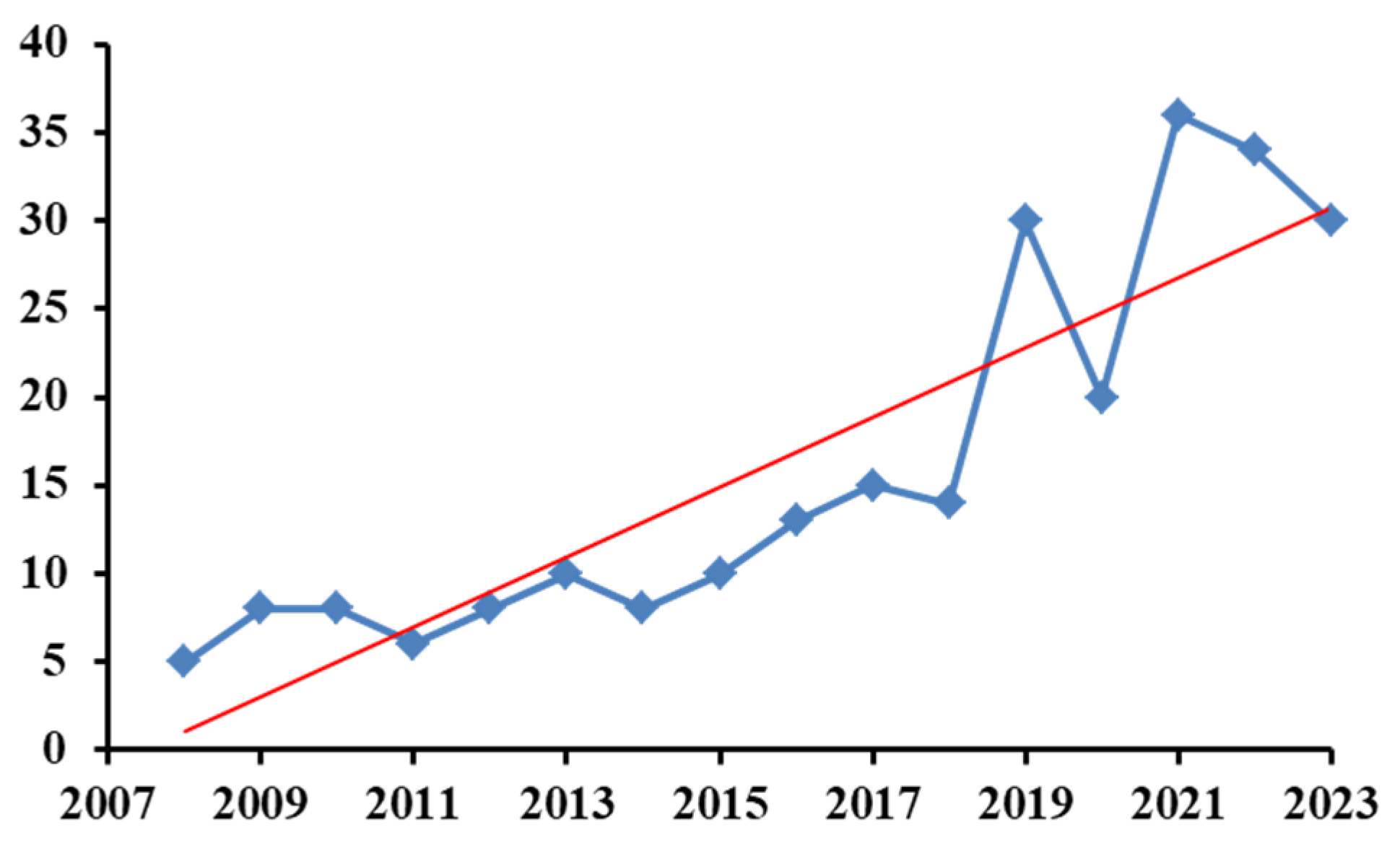
The number of articles published yearly and indexed in Clarivate Analytics-Web of Science Core Collection related to the use of biosensors in the analysis of mycotoxins is presented in Figure 2. The search dealt with the last 15 years and was performed according to the keywords “biosensors for mycotoxins” (271 indexations in total). These keywords gave the most results when compared to “biosensors for analysis of mycotoxins” (126 indexations in total) or “biosensors for determination of mycotoxins” (84 indexations in total). The data were collected on 26 January 2024.
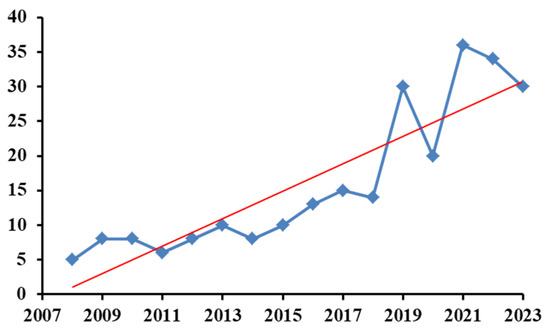
Figure 2.
Number of articles indexed yearly in Clarivate Analytics-Web of Science Core Collection database.
As can be seen, the tendency to use biosensors for mycotoxins is increasing, demonstrating the potential and viability of this analytical method.
The proposed paper aims to present an overview enriched by a critical perspective on biosensing techniques using various biorecognition elements and modes of detection. Enzyme sensors, immunosensors, and aptamers with different transduction modes are comparatively reviewed and critically discussed. The databases accessed for collecting information were Web of Science Core Collection, ScienceDirect Freedom Collection Elsevier, Springer Journals, Wiley, Google Scholar, etc.
Section 2 and Section 3 present the most relevant development and detection principles relying on different kinds of transducers, as well as methods of immobilizing bioelements. The applications of food assay (presented in Table 2), as well as the recent developments depicted in the section dealing with the comparative overview and future perspective, are focused on the last five years.
The present paper is distinguished by a quasi-exhaustive analytical approach, which integrates the comprehensive view that details the variety of biorecognition elements (antibodies, enzymes, aptamers) and transducers (electrochemical, optical, thermal, piezoelectrical). The critical, comparative perspective deals with the advantages and disadvantages of each biosensor type, as well as recent trends and authors’ views.
2. Types of Transducers
In this section, the biosensing applications are analyzed depending on the type of transducer that transforms the recognition reaction into a measurable signal proportional to the concentration value.
2.1. Electrochemical Biosensors
These sensors are characterized by the fact that the biochemical reaction between the analyte and the substrate is transformed into an electrical signal; the latter can be measured by several techniques [52,53].
- -
- Potentiometric, by measuring potential or charge accumulation; this mode of detection includes, as transducers, ion-specific electrodes or field-effect transistors; the latter rely on measuring the current as a result of a potentiometric effect at a gate electrode;
- -
- Amperometric, by measuring the current intensity at a fixed potential value;
- -
- Voltammetric, by measuring the current—potential dependence at the controlled variation of the potential in time;
- -
- Impedimetric, by measuring impedance (both resistance and reactance);
- -
- Conductometric, by measuring the conductive properties of a medium.
Electrical measurements are performed with the use of electrodes, their name deriving from the role played in the measurement, working electrode, reference electrode, and counter electrode. The most common reference electrode is Ag/AgCl, which has the role of maintaining a stable (fixed) and known (established) potential. The working electrode converts the biochemical reaction into an electrical signal and is made of Au, Pt, carbon, or silicon. Working electrodes should impart good conductivity and be chemically stable. The counter electrode establishes a connection to the electrolytic solution so that a current can pass through the working electrode [53]. Mehrvar and Abdi [54] described the biosensor types depending on the afore-mentioned transduction modes. For the first-generation biosensors, the reaction product of the biocatalyzed reaction directly diffuses to the transducer. The second-generation or mediated biosensors make use of specific mediators between the biorecognition reaction and the transducer, enhancing sensitivity [54].
Potentiometric biosensors are based on measuring the potential between the working electrode and the reference electrode and use ion-selective electrodes (ISE) and ion-sensitive field effect transistors (ISFET). These biosensors involve ion-selective electrodes in the detection of an electrical signal determined by the reaction of the biological element with the analyte. The biological recognition element (enzyme) is immobilized on a semi-permeable membrane that surrounds the working electrode (for instance, the glass membrane electrode) or is immobilized with gels (ion-selective electrodes).
Rameil et al. [55] developed a potentiometric biosensor for the determination of Aflatoxin M1, composed of a polypyrrole-surface-working electrode coated with a polyclonal anti-M1 antibody (20 μg mL−1) and an HRP-aflatoxin B1 conjugate. 3-(4-hydroxyphenyl)propionic acid functioned as an electron-donating compound for the enzyme. Ag/AgCl electrode served as a reference. The limit of detection (LOD) was 40 pg mL−1. The working time was less than 10 min without sample treatment in different milk matrices. Aflatoxin B1 was determined from real samples of sesame, walnut, and pea with a potentiometric biosensor based on pH-sensitive field-effect transistors and acetylcholinesterase. The obtained calibration curve was between 0.2 μg mL−1 and 40 μg mL−1 [56].
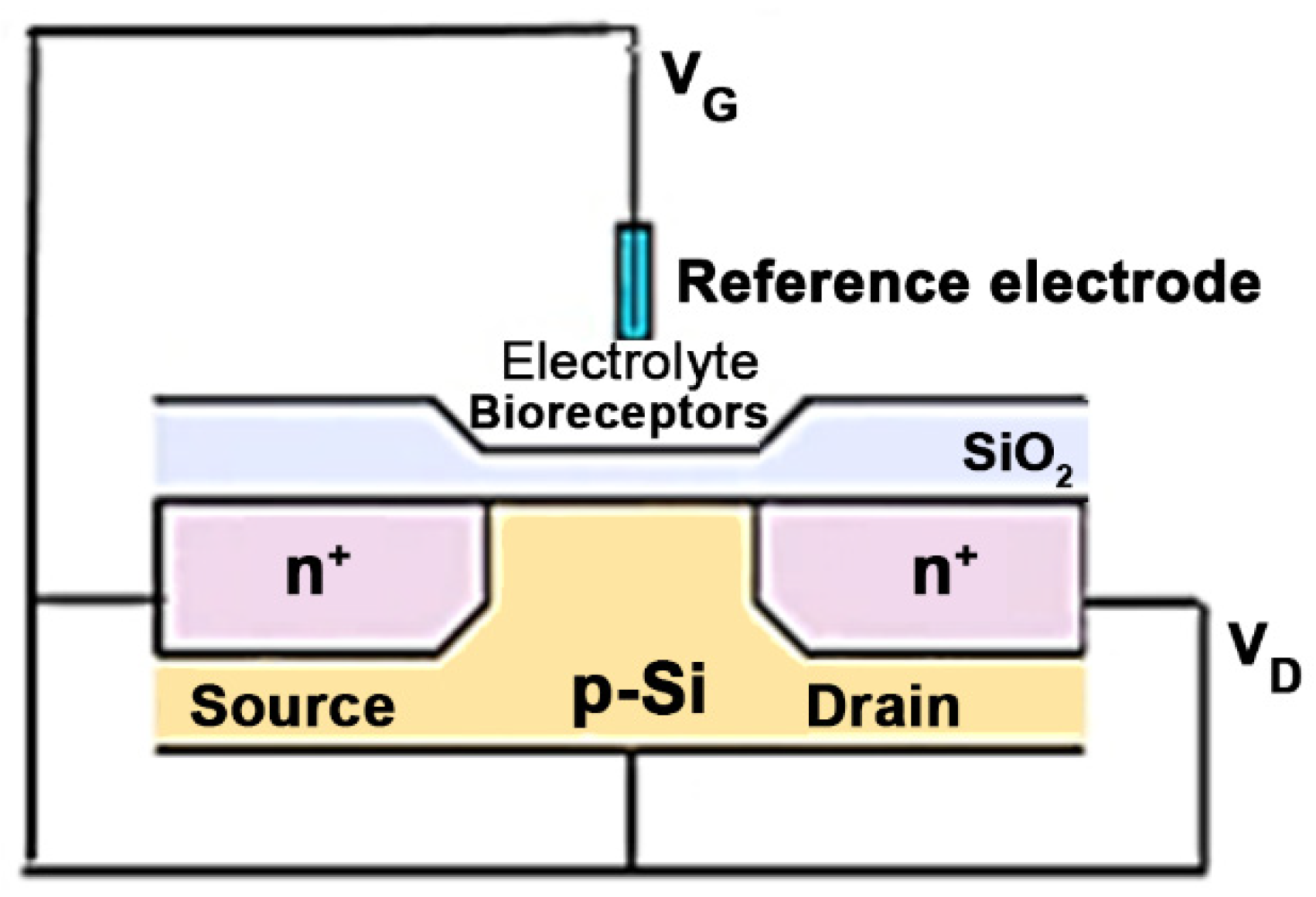
Field-effect transistor biosensors are semiconductor electronic devices with a general structure consisting of three components—source, gate, and drain—which represent the three electrodes (Figure 3). The biosensor has an insulating layer and a semiconductor layer. The gate on which the biorecognition element is fixed is placed between the drain and the source. It interacts with the analyte, transforming the chemical information into a charge change, recorded as a measurable electrical signal [57,58,59].
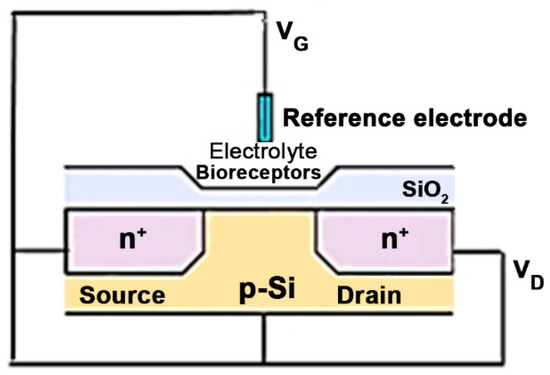
Figure 3.
Schematic representation of an ISFET-based biosensor (VD-voltage drain-source, VG-voltage gate-source).
Ochratoxin A was determined from white and red wines using a biosensor based on a graphene field-effect transistor integrated into a single silicon chip. A specifically designed aptamer against ochratoxin A was chosen as a biorecognition element. 1-pyrenebutyric acid N-hydroxysuccinimide ester was employed to covalently attach the aptamer to the graphene surface. An electric field stimulation was employed to induce more efficacious π-π stacking of pyrenebutanoic acid succinimidyl ester to graphene. The G-rich aptamer exhibited π-π stacking on graphene and reconfiguration in G-quadruplex arrangement in the presence of OTA. The real aptamer behavior became sensitive to the ionic force of the analyte solution. Hence, a specific, sensitive, and fast assay was achieved. The LOD was 1.4 pM, and the response time was as low as 10 s [60]. It should be mentioned that electrochemical methods can be combined with gravimetric techniques (like quartz crystal microbalance with dissipation monitoring) or microscopic techniques (like scanning probe microscopies and fluorescent microscopies). These methods promote increased sensitivity in the proximity of the surface of the electrochemical/optical transducer (electrode, waveguide, sensing tip, etc.), enabling delineation of the interfacial phenomena, acquiring a broader range of information and exerting control on the biosensing environment [53].
Amperometric and voltammetric biosensors are based on the measurement of current intensity at controlled, for amperometry, or variable potential, in the case of voltammetry. Pt, Au, Ag, Hg, graphite, glassy carbon, carbon paste, carbon nanotubes, screen-printed electrodes, metallic oxide nanoparticles, boron-doped diamond, and composites are used as electrodes. The recognition biomolecules are fixed on the surface of the transducer.
Second-generation biosensors make use of mediators (functioning as electron acceptors) instead of molecular oxygen to perform electron transport to the electrode and to hamper oxygen dependence of the analytical signal. In voltammetric or amperometric assay, mediators allow diminution of the working potential versus bare electrodes, minimizing the influence of interferent species. Mediators are represented by low-molecular-weight redox active molecules (e.g., ferrocyanide, ferrocene derivatives, quinones, or conducting organic salts). They react at the enzyme active site and then at the electrode’s surface, transferring electrons. For instance, if the biocatalyzed reaction is an oxidation one, the mediator is reduced following the enzyme reaction, is subsequently transported to the electrode, and converted to its oxidized form. So, it gives rise to electron transfer at the electrode’s surface and yields a measurable current proportional to the analyte’s concentration [61].
Aflatoxin B1 was determined by cyclic voltammetry with aflatoxin-oxidase as a biorecognition element, which is embedded in sol-gel, coupled with a multiwalled carbon nanotubes-modified Pt transducer. The modification resulted in optimized surface area, electrocatalytic effect, and sensitivity. The linearity ranged between 3.2 nmol L−1 and 721 nmol L−1 with a detection limit of 1.6 nmol L−1. The silica sol-gel afforded stabilization of the enzyme activity, prevented biocatalyst leakage, and ensured a biocompatible environment [62]. Vidal et al. [63] describe the determination of mycotoxins by amperometry based on electrodes made of inert metals (Pt, Au), carbon (graphite, glassy-carbon), or screen-printed electrodes.
For the determination of Ochratoxin A (OTA), an amperometric microfabricated biosensor was used, employing the immobilized synthetic peptide NFO4 as a biorecognition element, characterized by enhanced affinity and enabling OTA molecular recognition by simulation of the specific toxin antibody. Horseradish peroxidase-conjugated OTA was added to the free toxin solutions, followed by incubation on the specific biotransducer. The amperometric responses versus hydrogen peroxide were taken after 5 min of enzyme reaction, post H2O2/luminol addition. Simultaneously, the recording of light emission signals enabled a direct comparison of the amperometric and luminescence assay modes of the biochip. OTA concentrations up to 10 μg L−1 were measured, the authors reporting a broader dynamic range in amperometry [64].
Impedimetric biosensors [65] are based on the measurement of circuit impedance. Electrochemical impedance biosensors measure the current passing through a cell to which an alternating current potential, a sinusoidal potential, and a small excitation signal that causes an impedance response are applied. The biorecognition elements are fixed on the surface of the electrode. Electrochemical impedance spectroscopy (EIS) is an effective technique employed for the analysis of interfacial features connected to bio-recognition events occurring at the surface of the electrode, such as antibody-antigen interaction, substrate-enzyme interaction, or whole-cell capture [66].
Electrochemical impedance spectroscopy relies on a perturbation induced in an electrochemical system found in equilibrium. A sinusoidal signal (ac voltage or ac current) is applied on a wide frequency domain, and the sinusoidal response (current or voltage) of the system is eventually recorded as a consequence of the induced perturbation [67]. The applied frequency can vary from less than 1mHz to over 1 MHz, and the impedance of the circuit is measured in ohms [66]. The technique is non-destructive and sensitive, obtaining a detection limit of 0.5 ng mL−1 for ochratoxin A [68].
For the determination of OTA, Malvano et al. [69] applied an alternating sinusoidal potential of 10 mV in the frequency range from 0.1 to 104 Hz, which was superimposed on 0.00 mV DC potential (considered vs. reference electrode). They obtained a LOD value between 0.37 and 5.42 ng mL−1, depending on the biosensor used, with 5 or 10 μg mL−1 OTA monoclonal antibody for biorecognition. The linear range of analytical response was between 0.3–20.0 and 5.0–40.0 ng mL−1, depending on the biosensor used, with 5 μg mL−1 anti-OTA or 10 μg mL−1 anti-OTA, respectively.
Conductometric biosensors [70,71] are based on measuring, using two electrodes, the conductivity of a solution, which is directly dependent on the amount of ions in the solution. The biosensitive element can be embedded in a film that is deposited on the surface of the electrode. An ohmmeter measures the conductance change caused by the biorecognition reaction [72]. Soldatkin et al. [73] determined Aflatoxin B1 using acetylcholinesterase immobilized onto the surface of a conductometric transducer. The detection error was less than 7%, and the minimum limit of detection was 0.05 μg mL−1. Patulin was determined with a conductometric biosensor, with urease being used as a biorecognition element, and it was co-immobilized with bovine serum albumin by cross-linking with glutaraldehyde. The linear range was 1–50 μM [74].
Characterized as novel electrochemical analytical tools, photoelectrochemical biosensors integrate the benefits of biological and optical analysis [32]. The biorecognition event takes place under illumination and induces a signal variation in the photoactive nanomaterial. The reduction of the background signal and the increased sensitivities were ascribed to the thorough separation between the source of excitation (light) and the detected signal (current) [32].
2.2. Optical Biosensors
These analytical tools are viewed as the most widespread and frequently used class of biosensors. The property measured following the reaction of the biomolecule with the analyte is of optical nature. These sensors were divided into two general classes: label-free and label-based. In label-free sensors, the quantified signal is directly produced by the interaction between the analyte and the transducer. Label-based sensors employ labels, and the analytical signal is quantified via a colorimetric, fluorescent, or luminescent technique [75].
Optical sensing makes use of surface plasmon resonators, interferometers, optical waveguides, and photonic crystals, quantifying various properties like absorption, refraction, phosphorescence, fluorescence, and Raman scattering [76,77].
An optical fiber biosensor comprises a fiber core, a metal coating, and a layer containing the bioelement. Part of the covering region will encompass the metal coating and the bioelement, thus enabling the immobilization of the biocatalytic component on the fiber core and its integration with the optical transducing system. Optical fiber biosensors are small-sized, cost-effective, and easy to use [77].
In surface plasmon resonance optical biosensors, analyte molecules and biomolecules (proteins, bacteria, cells, DNA, etc.) determine the modification of the refractive index of the surrounding medium, which leads to deviation of resonance conditions of the resonator [78,79]. Zearalenone was determined with a relative standard deviation (RSD) of 2.81% [80].
In interferometric biosensors, the difference in the change in the refractive index between two light beams is measured. The assay is based on a single input beam and two output beams, one for reference and another influenced by the interaction of biomolecules. The difference in refractive index between the two beams shows the modification brought into the system by the biorecognition reaction [81]. AFB1, OTA, ZON, and B1 were quantified with a polarization interferometer biosensor, with a LOD of 0.002 ng mL−1 (estimated by linear extrapolation of the graph) [82].
Optical waveguide biosensors are constituted of a sensitive layer, cladding, and waveguide layers located on a substrate. The evanescent wave scattering results in an optical change in the vicinity of the guiding surface, which is exploited as a mechanism of detection in optical waveguide biosensors [77].
In most cases, planar waveguides are placed on a thin layer of stable substrate and are commonly constituted of low refractive index and glassy materials (e.g., silica or polymers). Relying on their geometrical design, we can classify planar waveguides into slab waveguides and channel waveguides [83].
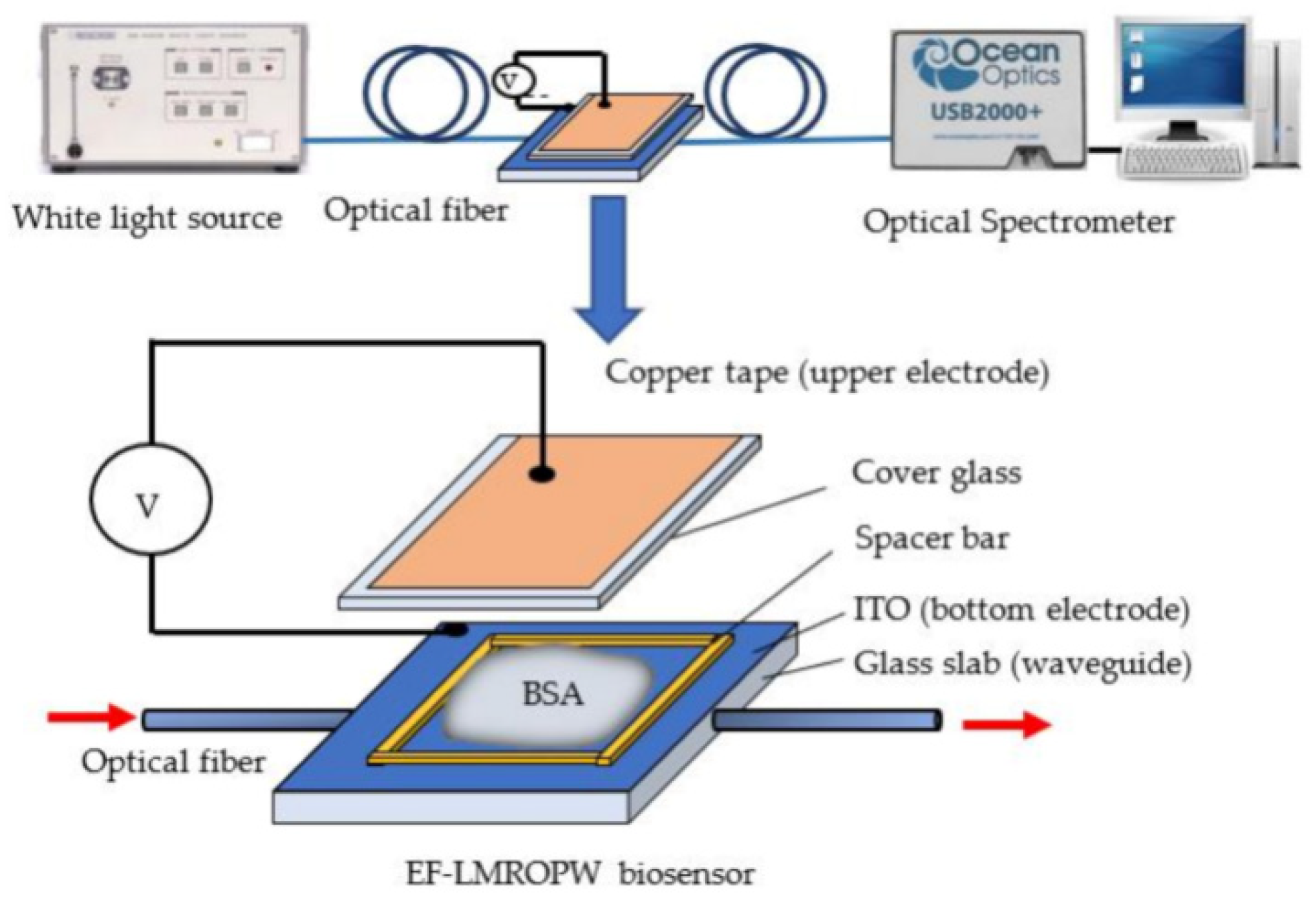
Figure 4 shows the representation of a waveguide biosensor with an optical alignment platform [84].
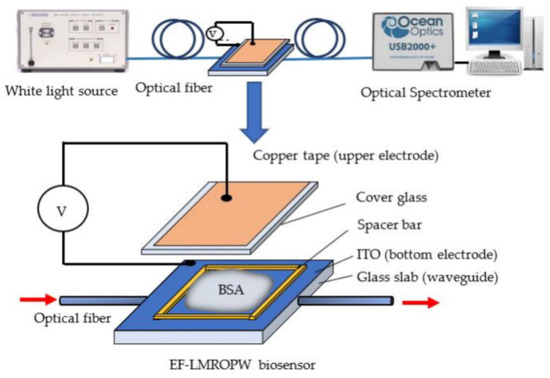
Figure 4.
A representation of an electrical field-induced lossy mode resonance-based optical planar waveguide sensor (experimental set-up and photo), from [84] MDPI 2021.
Zearalenone was determined with an immunosensor that functioned as a polarization interferometer with a detection limit of 0.01 ng mL−1 [85]. Novel planar waveguide-based sensors operate by relying on the polarization interferometry principle, measuring a phase shift between p- and s-components of the polarized light obtained during analyte binding. The sensor functions similarly to a Mach–Zehnder interferometer, but its principle is much simpler, not requiring the splitting of the waveguide into two arms. The refractive index sensitivity corresponded to 5200 radians per refractive index unit. Ochratoxin A was determined using the optical planar waveguide in a broad concentration range between 0.01 and 100 ng mL−1, corresponding to an analytical response increase [86]. Surface plasmon resonance, evanescent wave fluorescence, and optical waveguide interferometry exploit the evanescent field in the proximity of the surface of the biosensor to detect the biorecognition reaction. Evanescent wave fluorescent and luminescent optical fiber biosensors can be subsequently developed [75].
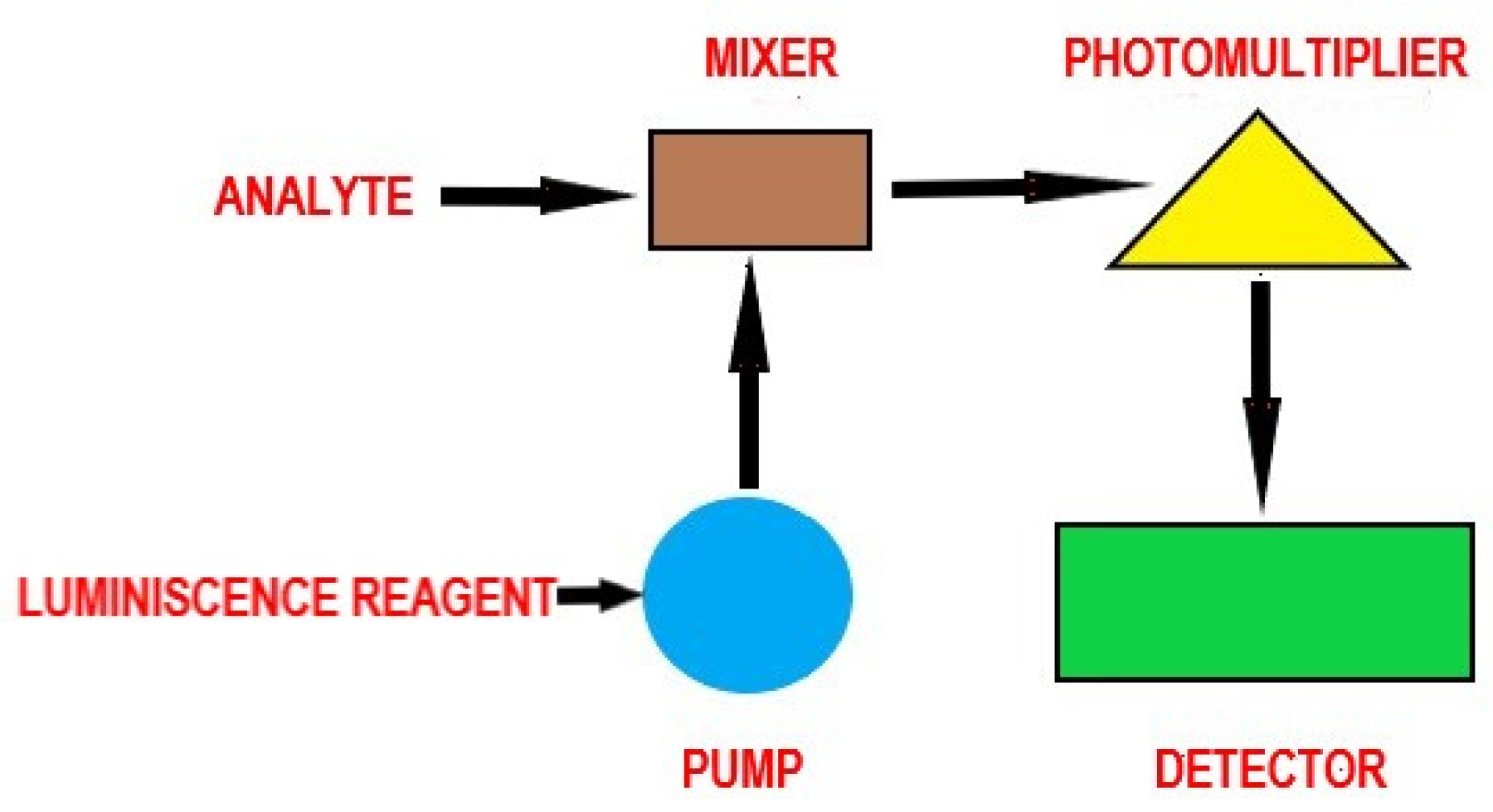
Chemiluminescence is distinguished from fluorescence or phosphorescence in that the electronic excited state results from a chemical reaction rather than the absorption of a quantum (photon). Figure 5 illustrates the principle and main components of a chemiluminescent assay.
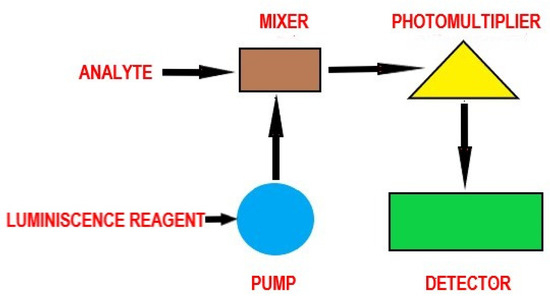
Figure 5.
The principle of chemiluminescence—general scheme.
Jia et al. [87] developed a portable chemiluminescence optical fiber aptasensor aiming at the detection of four mycotoxins (aflatoxin B1, FB1, ZEN, and OTA). A single-stranded binding protein was chosen to be responsible for specific biorecognition. The LOD obtained at mycotoxin assay was between 0.015 pg mL−1 and 0.423 pg mL−1. The sensor proved successful in the determination of four mycotoxins in infant foods (baby rice flour and baby rice cake) [87].
Fluorescent biosensors are based on the phenomenon of fluorescence, which is considered a form of chemiluminescence. Fluorescence is an emission of photons that occurs when electromagnetic radiation of a certain wavelength is absorbed by molecules (fluorophores or fluorescently labeled). An immunochromatographic assay for the quantitative detection of AFB1 in foodstuffs was developed by Li et al., based on AFB1 antibody and phycocyanin-labeled latex nanospheres. The assay enabled obtaining a LOD of 0.16 ng mL−1. Phycocyanin and anti-AFB1 monoclonal antibodies on the surface of latex nanospheres enhanced the fluorescent signal and sensitivity [88].
In fluorescence resonance energy transfer or Förster resonance energy transfer (based on a non-radiative dipole-dipole coupling), the energy is transferred from an excited fluorophore donor to a fluorophore acceptor, whose excitation spectrum overlaps with the donor’s emission spectrum. The donors and acceptors are generally dye molecules. The transfer depends on the distance between the donor and the acceptor, and eventually, the emission of photons from the acceptor can be detected [89,90].
Given the loss of energy through non-radiative processes, the fluorescent photons have lower energy compared to the energy of the photons responsible for the generation of the excited state. So, the wavelength of fluorescent emission is higher than that of the excitation one, the Stokes shift being the difference (in wavenumber, frequency, or energy) between the positions of absorption and emission maxima. The emitted radiation may have a smaller wavelength or the same wavelength as the excitation radiation, and then the phenomenon is called resonance fluorescence [91].
When fluorescent emission occurs, an optical measurable signal is generated from the sample. The signal occurrence relies on the three most commonly encountered mechanisms [89]:
- -
- The bioreceptor recognizes the analyte, so the analytical signal takes into account the presence of analytes and their amount;
- -
- Conformational changes can result following the biorecognition event, so the measured signal reflects specific alterations in the bioreceptor’s conformation;
- -
- Biocatalyst activity changes can occur, so the signal can also reflect enzyme activity.
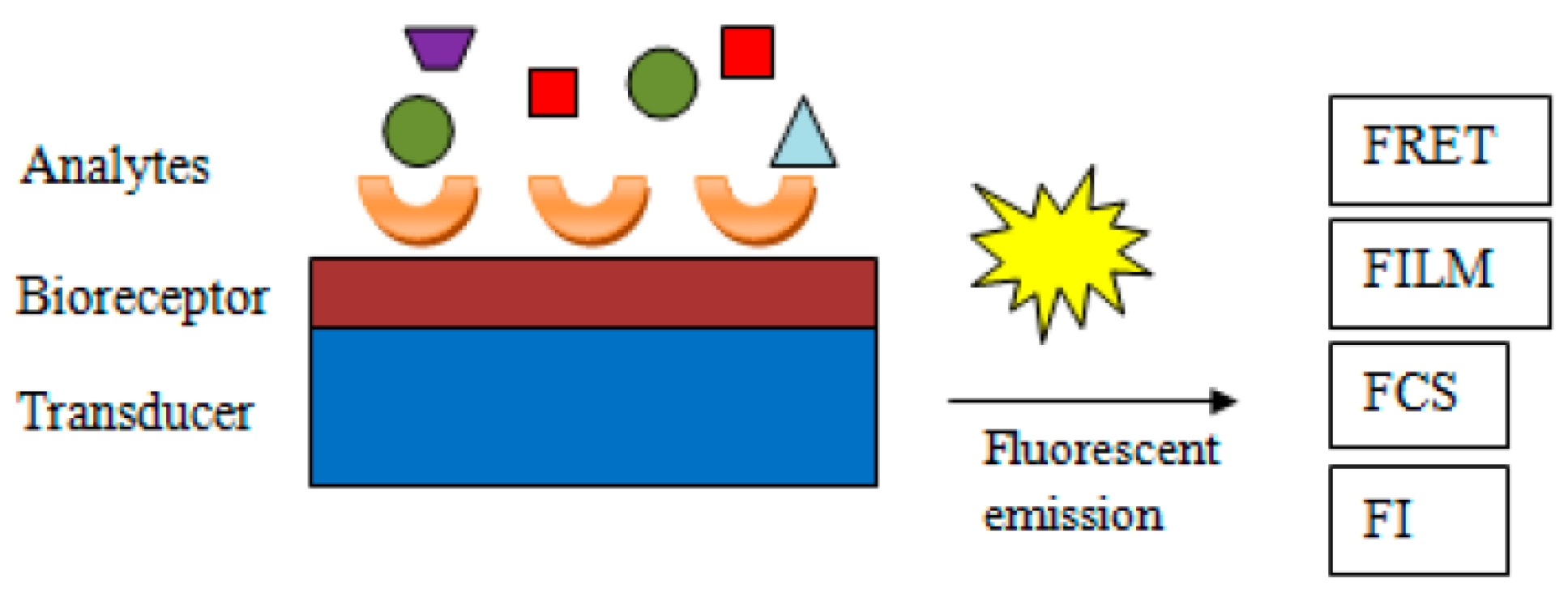
The following methods can be applied to detect the generated analytical signal: FRET (Förster resonance energy transfer, previously detailed), FLIM (fluorescence lifetime imaging, which relies on the differences in the exponential decay rate of photon emission of a fluorophore present in a sample), FCS (fluorescence correlation spectroscopy, which performs correlation analysis of the time-related fluctuations of the fluorescence intensity), or mere change in fluorescence intensity. Figure 6 represents the key components and mechanisms of a fluorescent assay [92].
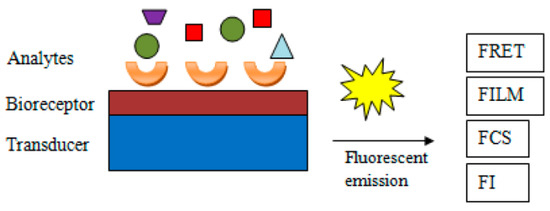
Figure 6.
General scheme of fluorescent biosensors and underlying mechanisms from [92], MDPI 2018.
Tan et al. [93] determined Aflatoxin B1 with a detection limit of 0.13 ng mL−1. The linearity range was 0.5–50 ng mL−1. The biosensor was constituted of mesoporous silica nanoparticles, the fluorescence being imparted by the presence of Rhodamine 6G [93].
ELISA (Enzyme-linked immunosorbent assay) was developed as an enzyme-competitive technique for aflatoxin quantitation relying on fluorescent or, more often, on colorimetric detection. The sample and an enzyme-coupled mycotoxin conjugate are mixed and then added to antibody-coated microtiter wells. After the addition of the enzyme substrate, the blue color develops. Alkaline phosphatase, horseradish peroxidase, β-galactosidase, acetylcholinesterase, and catalase function as bioreceptors for biological recognition [27]. Also, exploiting the rapid and simple colorimetric detection in solution or on paper-based strips, novel DNA aptamers as biorecognition elements were used in combination with the optical transducing properties of gold and silver nanoparticles for AFB1 and AFM1. Thiolated DNA aptamers can be covalently conjugated to the surface of golden nanoparticles via gold–thiol bonds or by physisorption to the surface of the nanoparticles. For non-thiolated DNA aptamers, chemical interaction forces (such as hydrophobic effects and van der Waals forces) between the atoms of the DNA bases lead to strong adsorption on the surface of the nanoparticles [94].
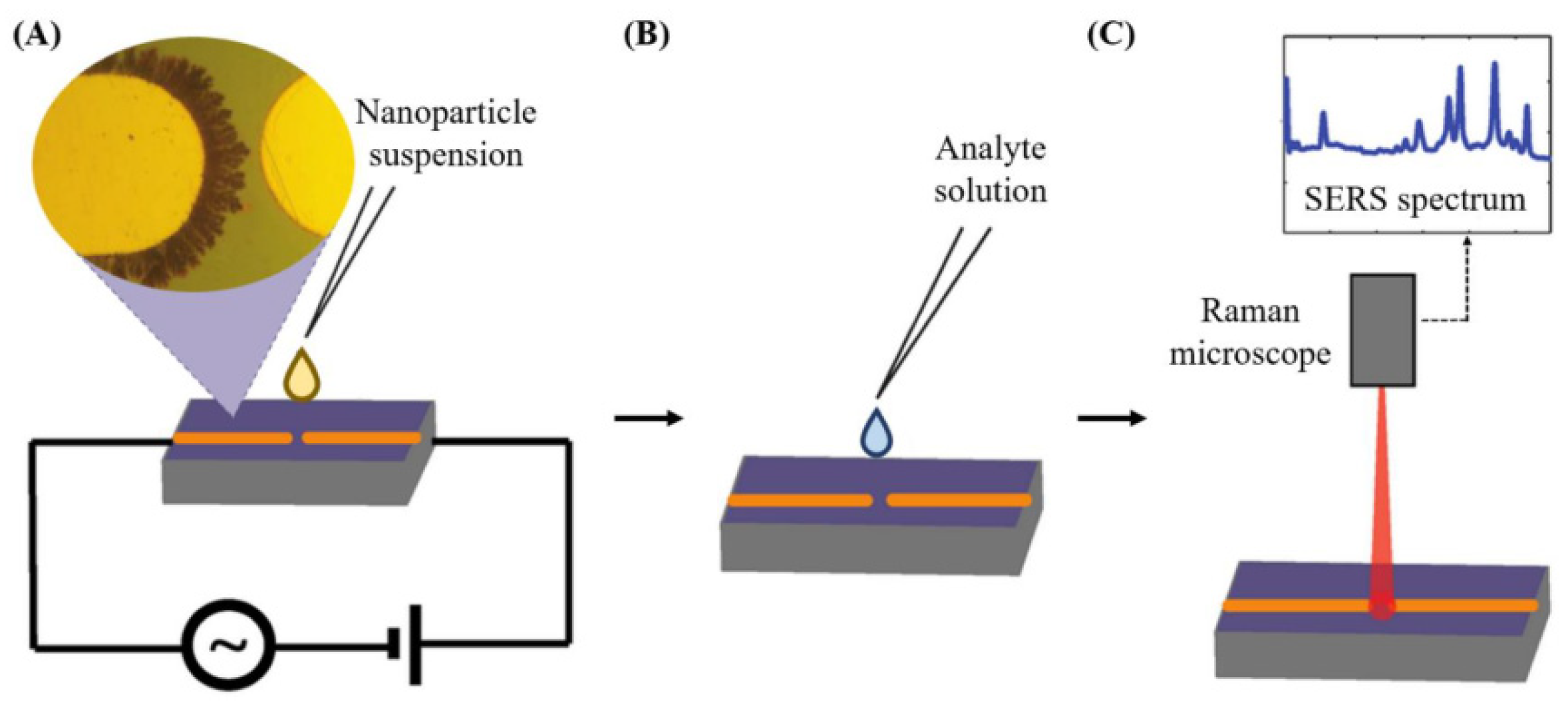
Vibrational spectroscopy can also be applied as a nondestructive analytical detection methodology for assessing mycotoxins. Surface-enhanced Raman scattering as a biosensing method relies on the amplification of the intensity of the vibration signals of a molecule by several orders of magnitude in the proximity of a gold or silver metallic nano-structure surface [75]. The plasmonic resonance frequency influences the choice of the metal. Visible and near-infrared radiation are applied to promote Raman mode excitation. Silver and gold, having particularly their plasmon resonance frequency values placed within these wavelength ranges, enable amplification of visible and near infrared light, so they are considered ideal for use in surface-enhanced Raman scattering spectroscopy. Figure 7 shows the application of surface-enhanced Raman scattering spectroscopy for the detection of contaminants present in a liquid food sample [95,96].
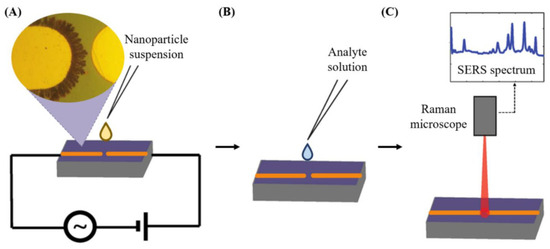
Figure 7.
(A) Preparation of nanostructures. (B) Addition of the analyte solution to the microelectrodes’ surface (C) Analyte detection by SERS, from [95,96], MDPI 2018, 2021.
2.3. Mass Sensitive Biosensors
These analytical devices use quartz crystal microbalance to record mass variation. They rely on the measurement of the changes occurring in the resonance frequency of a piezoelectric crystal due to mass variations in the crystal structure [97].
To develop these biosensors, the bioelement is coupled with a piezoelectric transducer, usually a gold electrode-coated quartz-crystal. Many types of materials (quartz, tourmaline, lithium tantalate or niobate, oriented zinc oxide, or aluminum nitride) can function as viable piezoelectric transducers. The vibration of these crystals can be induced at a specific frequency by applying an electrical signal of a specific frequency. Consequently, the oscillation frequency depends on the electrical frequency applied to the crystal and on the crystal’s mass [98].
Nolan et al. [49] determined several mycotoxins (T2 mycotoxin, Fumonisin B1, and ZEN) with a mass sensitive biosensor. The lowest and highest T2 content in food ranged between 15 and 1000 ppb, for Fumonisin B1 between 200 and 4000 ppb, and for ZEN between 50 and 400 ppb. The most sensitive result obtained with the mass sensitive micro-array analytical tool was 1.3 ng mL−1 for T2 in singleplex analysis based on the portable microfluidic device [49].
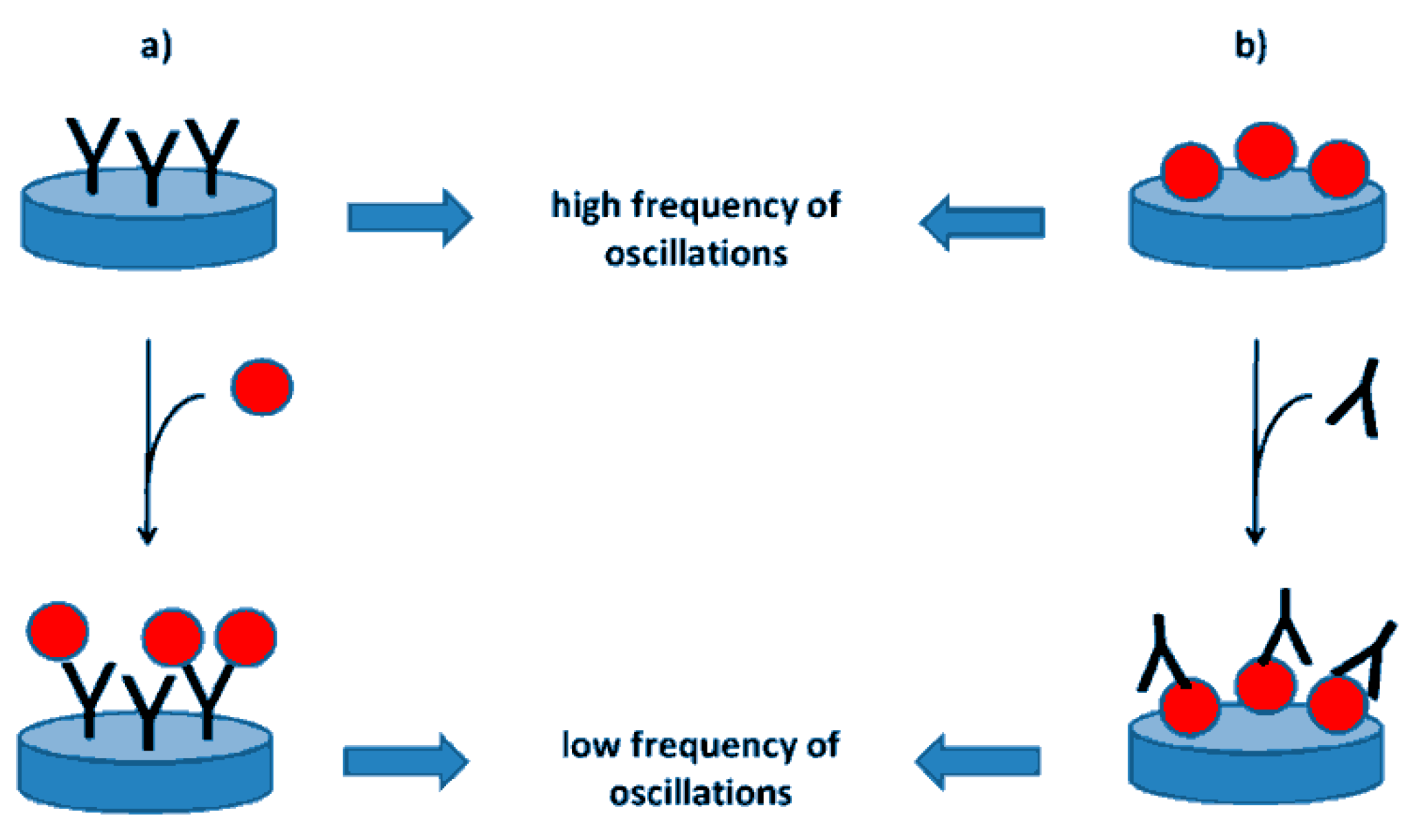
Figure 8 depicts an immunosensor relying on the use of a piezoelectric crystal as a transducer [99].
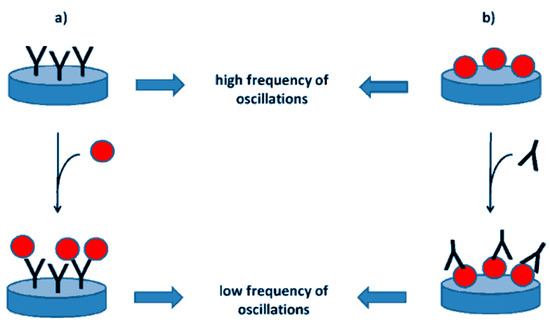
Figure 8.
Representation of piezoelectric immunosensors for the determination of an antigen (a) or an antibody (b). The blue disc represents the piezoelectric crystal. The antibodies are depicted as Y letter, following the typical immunoglobulin shape; the red ball represents the antigen from [99], MDPI 2018.
2.4. Acoustic Biosensors
The functioning of acoustic wave sensors relies on the monitoring of the variations occurring in the physical characteristics of an acoustic wave imparted by the analyte’s presence [50]. Piezoelectric materials are constituted of crystals devoid of a centre of inversion symmetry and presenting an intensified coupling between mechanical strain and electrical polarization. Such transducers currently exploited in acoustic sensors generate acoustic waves across solid materials using adequately balanced electrical fields. The acoustic waves are detected by the charge produced through induced mechanical deformation. Quartz is often employed, as it is available in high amounts, amenable to cost-effective manufacturing, and characterized by high mechanical properties and good chemical stability.
The functioning of these devices relies on the modulation of a physical property of the acoustic wave (acoustic velocity, resonant frequency, dissipation) that can consequently be related to the amount of adsorbed analyte. Acoustic wave devices are sensitive analytical tools that can detect a series of physical parameters (mass, pressure, temperature, etc.) [100]. Acoustic waves can be essentially classed into two main types, bulk acoustic waves (BAW) and surface acoustic waves (SAW), relying on the propagation of mechanical waves in piezoelectric materials [50].
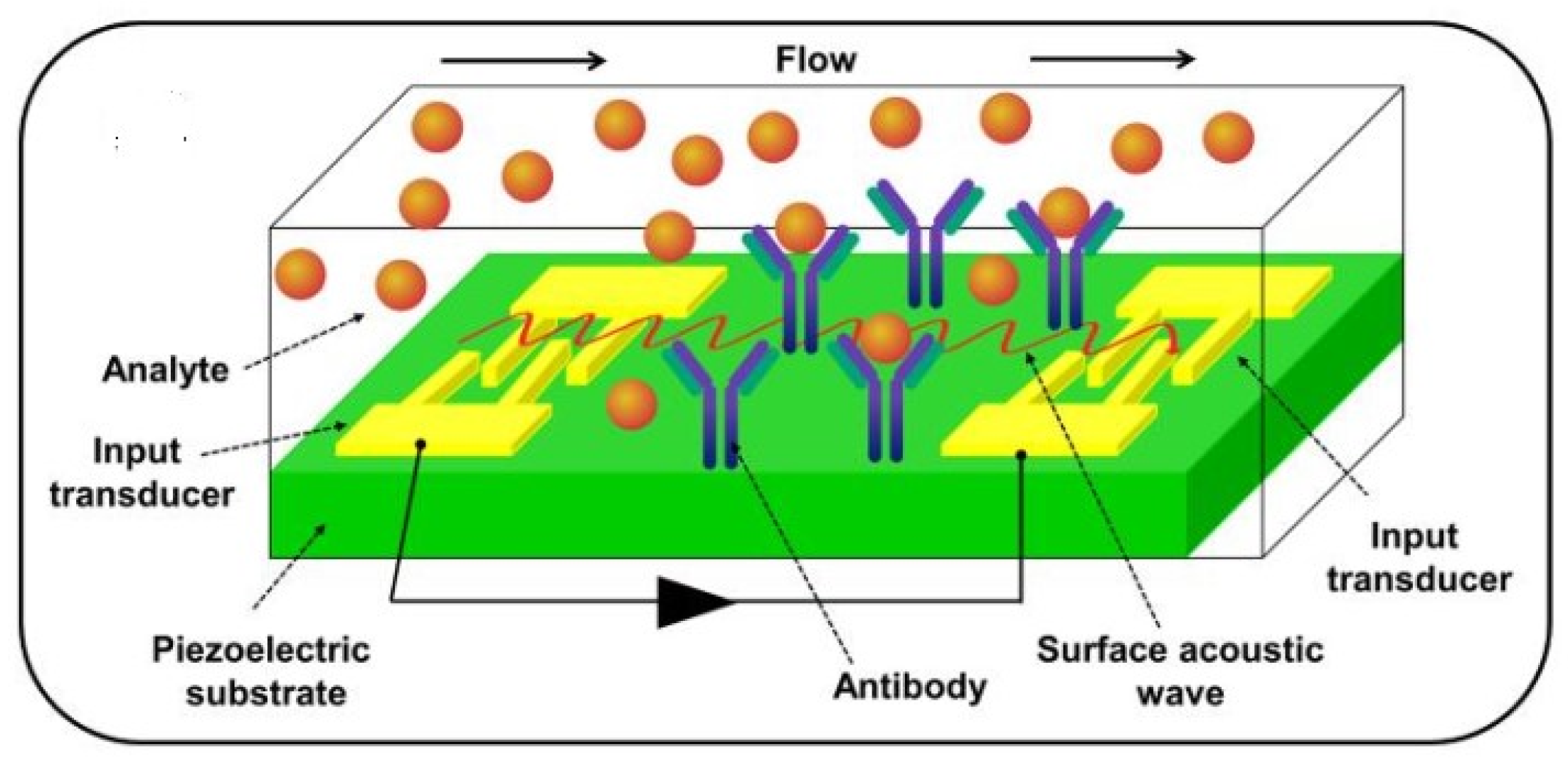
In BAW sensors, the acoustic wave is propagated from one crystal surface to another, whereas in SAW sensors, it is propagated along a single crystal face from one location to another. Figure 9 presents a surface acoustic wave-based biosensor [45].
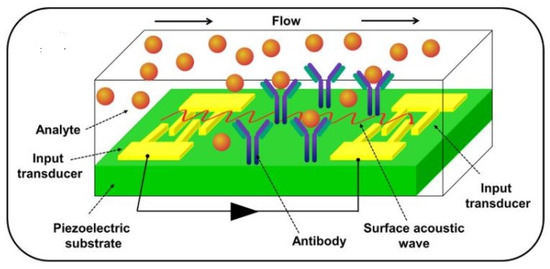
Figure 9.
A surface acoustic wave (SAW)-based biosensor, from [45], MDPI 2021.
Juodeikiene et al. [101] used an acoustic spectrometer with transmission and an acoustic impulse spectrometer with transmission and reflection to assess the content of deoxynivalenol. The range of frequencies of 4.95 to 35.70 kHz was applied for the operation of the acoustic spectrometer, and the amplitude of the acoustic signal crossing the sample was monitored. A similar model has been exploited by employing the acoustic impulse spectrometer, where the amplitude of the reflected acoustic signal diminished with increasing DON concentration. The authors determined performance analytical characteristics such as relative standard deviation of repeatability for deoxynivalenol, with results ranging from 4.03 to 16.48%.
2.5. Thermal Biosensors
These sensors are based on the measurement of the temperature variations that occur during the biochemical reaction. Temperature sensors can be of two types: thermistors or thermopiles. Thermistors change their electrical resistance depending on the temperature, while thermopiles (metal or semiconductor-based structures) measure the temperature differences between two distinct areas [102,103].
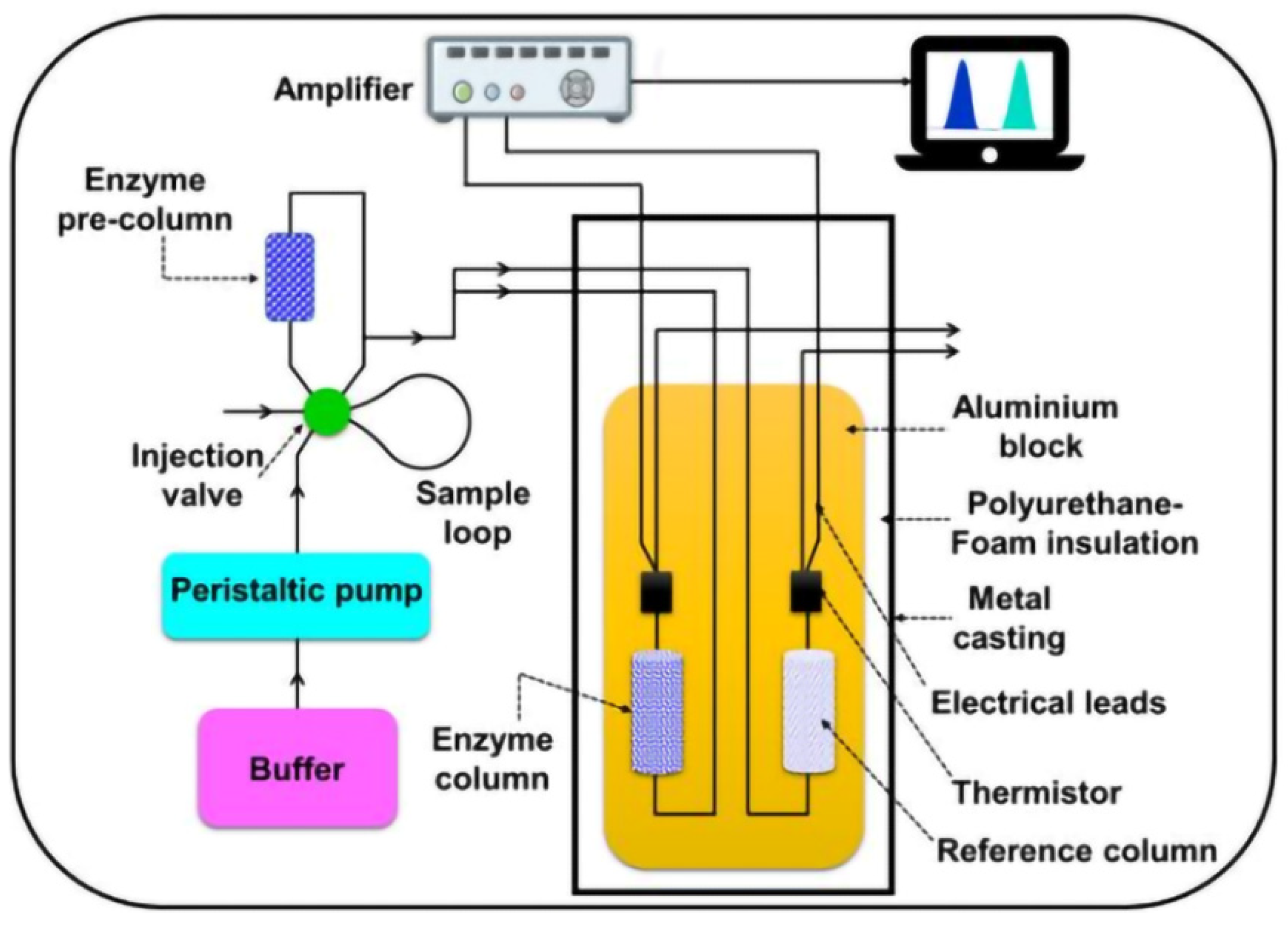
In Figure 10, an enzymatic thermistor-based sensor is presented [45].
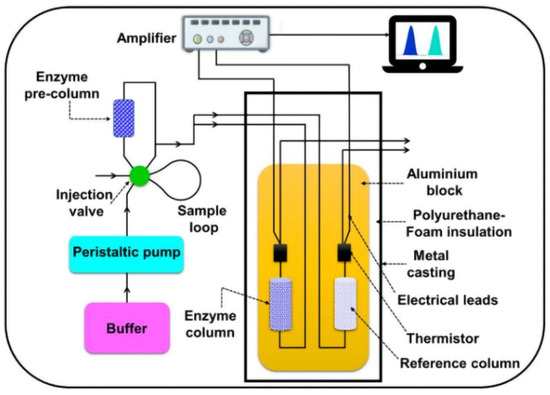
Figure 10.
The detailed component parts of an enzyme thermistor-based biosensor, from [45], MDPI 2021.
3. Biomolecule Immobilization
Immobilizing and recognizing biomolecules can be performed directly on the transducer or on a support that is fixed on the transducer. This can be performed reversibly through adsorption, encapsulation, affinity binding, or irreversibly through entrapment, cross-linking, and covalent bonding. Another classification is determined by the method of immobilization: physical or chemical immobilization. The fixation on the surface of the support material must be stable and ensure the diffusion and exchange of electrons. In the case of adsorption, the interactions established can be Van der Waals, electrostatic, or other noncovalent bonds like hydrophobic interactions. Adsorption and encapsulation are classed as physical methods, whereas crosslinking and covalent bonding are chemical immobilization techniques [104]. Covalent binding, cross-linking, and entrapment are irreversible and impart stability, whereas adsorption and bioaffinity binding are reversible and do not bring cross-linker toxicity. Moreover, adsorption binding occurs fast but is less reproducible, and affinity interaction, though highly specific, can involve high costs [105].
Physical immobilization is much weaker than chemical immobilization due to weaker bonds established with the support. The support on which the biological recognition element is fixed can be inorganic (e.g., alumina, silica, zeolites, etc.), organic (polymers like polyketone polymers, acrylic resins, chitosan, cellulose, starch, carrageenans, etc.), or can be represented by nanoparticles (e.g., noble metals, magnetic nanoparticles, etc.). The support must be chemically and mechanically stable and insoluble [53,106]; its nature depends on the type of transducer, the immobilization method, the surface stability, etc. Following the immobilization of the recognition element on the support, an efficient transfer of electrons must be ensured.
Encapsulation is a method that uses the inclusion of the recognition element in a porous polymer network, in a sol-gel [104], or in lipids (liposomes) [106]. Sol-gel matrixes enable the entrapment of large enzyme amonts, are thermally and chemically stable, and are characterized by the easiness of preparation [104,107]. Affinity binding consists in binding the recognition element to a ligand through Van der Waals, hydrogen bridges, or electrostatic bonds [108]. Entrapment is based on the embedding of biomolecules in a polymeric gel network. Polyacrylamide, chitosan, starch, nylon, silastic gel, sol-gel-like polymers, etc. were used as polymers [109]. Cross-linking consists of fixing biomolecules via cross-linking agents, such as glutaraldehyde or hexamethylene diamine. A widely used immobilization technique is the fixation of biomolecules on the surface of the transducer through the layer-by-layer technique, which allows the deposition of a wide range of substances in thin films, layer by layer (alternatively), positively and negatively charged [110,111]. Incorporation of a broad variety of materials (metals, metal oxides, luminescent compounds, dyes) or biocompounds (proteins, aptamers, antibodies, antigens) into the multilayer structure, can lead to optimization of the sensing films’ characteristics: selectivity, sensitivity, response time and dynamic range [110]. Essentially, the promotion of biosensing performance needs a transducer material to impart a good signal, and effective communication/connection between the biomolecules and the electrode surface must be obtained to enhance signal processing at the electrode surface [112].
4. Stability and Response Time of Biosensors
The stability of biosensors is a particularly important characteristic; the analyst should make use of reliable instruments, which, among other things, ensure good reproducibility over time, especially when dealing with commercial sensors. It was found that the analytical response of biosensors decreases over time, the stability being considered as shelf-life and as operational stability, depending mainly on the immobilization mode of the biological recognition element. Shelf life is related to the improvement in the activity retention of a biocatalyst under specific conditions of storage. Operational stability refers to the activity retention of a biocatalyst during its usage [113]. In addition to the immobilization method, other factors that determine stability are storage temperature, working environment (acidic or basic), ionic concentration, nature, and degradation of the biological element [114,115,116].
Improving the immobilization of the biological recognition element leads to an increase in the stability of the biosensor as well as an increase in sensitivity [117,118]. Also, depending on the nature of the biological element, Y. Alhamoud et al. found that the antibody is sensitive to pH, temperature, and ionic strength, with poor stability, the peptides are quite stable, and the aptamer is much more stable and has a longer life at room temperature [117]. These authors consider molecular imprinted polymers to be the most stable at high temperatures and pressures, with a long life.
Some electrochemical semiconductor biosensors have been improved in immobilization and the use of the most suitable mediator. The stability was evaluated for 9 weeks, the variation of the analytical signal in this interval being 6%. It was inferred that the semiconductor manufacturing electrochemical biosensor platform had a 94% stability [119].
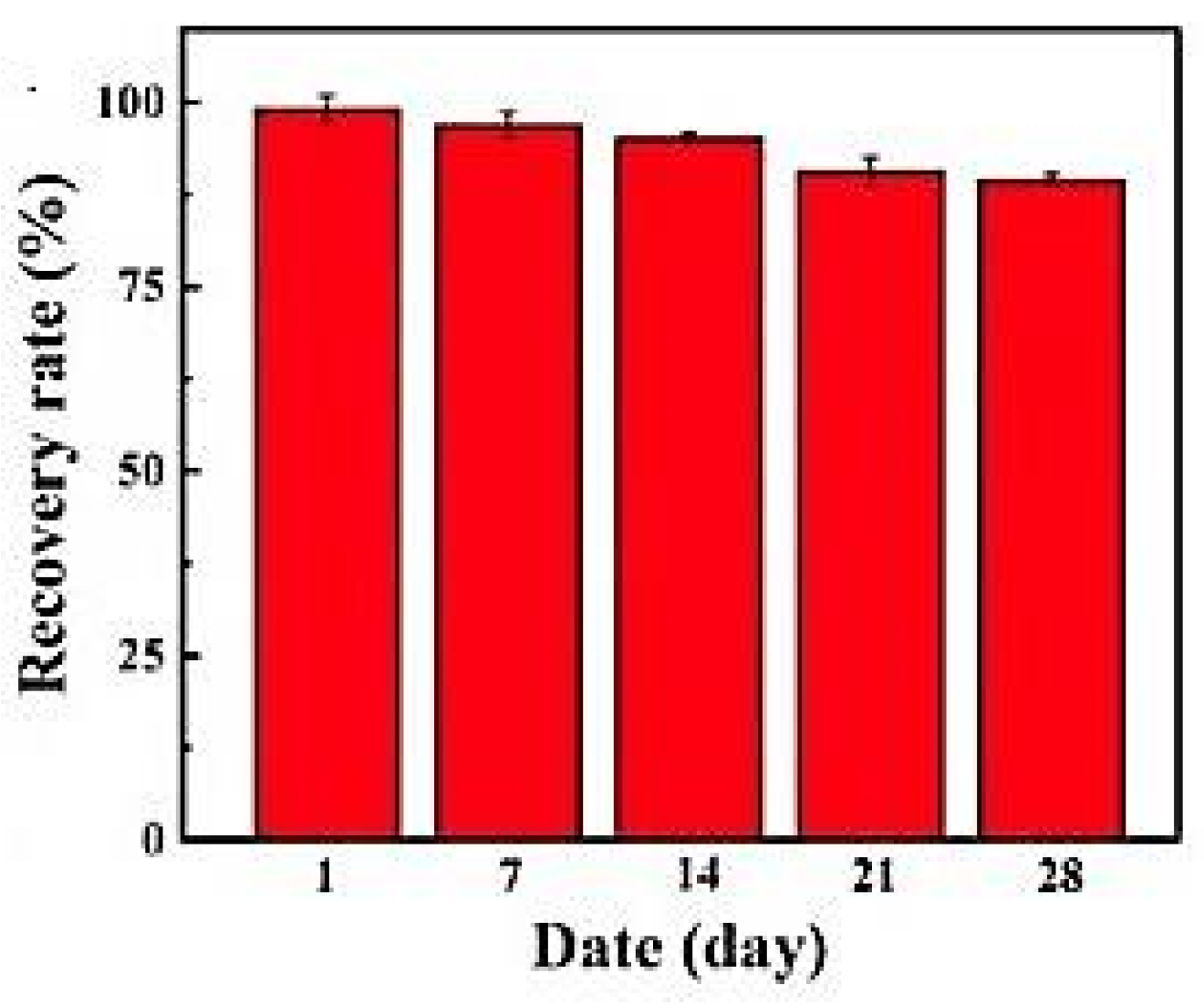
Y. Zhang et al. [120], using an electrochemical biosensor based on carboxylated polystyrene nanospheres, an aptamer, and horseradish peroxidase assembled on a carbon nanofiber/carbon felt support for the determination of aflatoxin B1, found that after four weeks, recovery rates were reduced by 10.4% (Figure 11).
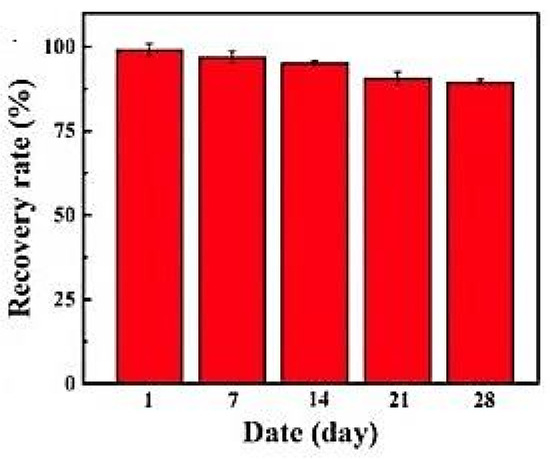
Figure 11.
Stability of biosensor as a function of the time, from [120], MDPI 2022.
Zhao et al., building an evanescent optical fiber aptasensor, checked the reproducibility in 35 cycles that included the sequential injections of the blank (copy DNA only), 0.5% sodium dodecyl sulfate (regeneration buffer at 1.9 pH value), and toxin sample (1 pM ZEN and copy DNA). The developed sensor was stable for use, undergoing proper regeneration for up to 28 cycles [121].
An aptasensor based on an anti-AFB1 aptamer with a methylene blue redox tag present at the 3′-end immobilized on the surface of a gold electrode allowed for the determination of AFB1 in 20 times diluted beer and in 50 times diluted white wine, the stability determined being one week after storage at 4 °C in phosphate buffer solution pH 7.5, containing MgCl2 [122].
An aptasensor relying on electrochemical impedance spectroscopy as a transduction mode for the quantitation of FB1, AFB1, ZEN, and OTA present in the same corn flour sample was tested at 0, 7, 14, 21, and 28 days, respectively. The biosensing chip’s analytical responses to FB1, AFB1, ZEN, and OTA after 28 days were 87.2%, 92.4%, 90.5%, and 92.7%, respectively, of their original levels [123].
Song and collaborators [124] highlighted the dependence of the stability of biosensors on the nature of the interface (the layer between the sensing elements and the electrode’s surface), stressing the role of noble metal particles, along with carbon-based materials and polymers. Nanomaterials can mimic the activity of biocatalysts and can substitute bioenzymes, enhancing stability. Nanomaterials based on noble metals and alloys, as well as bimetallic nanocrystals, are endowed with high catalytic activities.
Given the variety of biorecognition elements, support materials, transducers, different immobilization methods, etc., it is very difficult to compare the performances of biosensors with each other, including regarding stability. In addition, the stability of a biosensor depends on storage conditions (dry or wet), the composition of the atmosphere (air or nitrogen), pH, the composition of the buffer, and the influence of matrix components [52]. The stability of the biosensor strongly influences the sensitivity; this is determined, along with the chemical stability of the biomolecules, by the transducer and the electronics of the device (amplification, signal processing) [125].
The response time of a biosensor differs from biosensor to biosensor and is defined as the time in which at least 90% of the value of the signal transmitted to the transducer is reached. The response time is influenced by many parameters related to the analyte, substrate, biological recognition system, hydrodynamic conditions, pH, temperature, etc. [52].
A novel sensitive graphene field effect transistor-aptamer sensor for OTA showed a response time as low as 10 s and proved more than 30 times faster when compared to other reported aptamer-based techniques for mycotoxin analysis [60].
Using a poly(2-hydroxyethyl methacrylate-co-aminoethyl methacrylate) hydrogel and an amperometric bioassay for Ochratoxin A based on NFO4 immobilized synthetic peptide for biorecognition, the maximum inhibition was obtained at 10 μg L−1 and the response time was less than one minute (50 s) [64].
An acetylcholinesterase-based conductometric biosensor allowed for the determination of aflatoxin B1 in 5 min, highlighted by the authors as much lower when compared to HPLC, ELISA, or TLC assay time (20, 40, and 60 min, respectively) [73].
Biosensors based on optical planar waveguides operating as polarization interferometers and imparting high sensitivity in the sub-ppt level had the analytical signal reported as a time-dependent phase shift, stabilized after 300 s [82]. Microfluidic paper-based analytical tools relying on specific aptamer-gold nanoparticles nanoconjugates allow for on-site assay of food toxins in less than one minute [126].
5. Analytical Performances of Biosensors Applied to Mycotoxins Quantitation
Table 2 shows the main performances of some biosensors used in the determination of mycotoxins in the last 5 years [49,60,62,68,73,82,85,87,120,121,122,123,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170].


Table 2.
Analytical performances at mycotoxin assay in various food matrixes by biosensors with different transduction modes [49,60,62,68,73,82,85,87,120,121,122,123,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170].
Table 2.
Analytical performances at mycotoxin assay in various food matrixes by biosensors with different transduction modes [49,60,62,68,73,82,85,87,120,121,122,123,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170].
| No. | Mycotoxin | Matrix | Detection/Bioelement | LOD | RSD% | Linearity | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | AFB1 | milk | colorimetric/aptamer | up to10 nM in spiked samples | 1 pM–1 µM | [126] | |
| 2. | OTA, AFB1 | peanut, barley | colorimetric/aptasensor | 4.7–8.4 ng mL−1 | 5–250 ng mL−1 (AFB1) 0.5–80 ng mL−1 (OTA) | [127] | |
| 3. | AFB1 | rice, flour, seed, maize, pistachio | colorimetric/aptasensor | 0–25 nM | [128] | ||
| 4. | AFB1 | spiked cattle feed samples | colorimetric/aptasensor | 0.88 µg mL−1 (at 1:10 AuNP:aptamer ratio) | 0–10 µg mL−1 | [129] | |
| 5. | AFB1 | maize flour | colorimetric/aptasensors developed through in silico maturation and computational simulation approaches | 0.1 and 0.5 ng mL−1 | 0.1–50 and 0.5–50 ng mL−1 | [130] | |
| 6. | AFB1 | foodstuffs | colorimetric and fluorescence/aptasensor | 7.32 ng mL−1 (colorimetric) 1.48 ng mL−1 (fluorescence) | <13.3 | 10–320 ng mL−1 3–320 ng mL−1 | [131] |
| 7. | AFB1 FB1 | whole grain samples (wheat and maize) | light reflectance spectroscopy/immunosensor | 0.05 ng mL−1 (aflatoxin B1) 1.0 ng mL−1 (fumonisin B1) | 0.1–5.0 ng mL−1 2.0-50 ng mL−1 | [132] | |
| 8. | AFB1 | optical planar waveguide operating as a polarization interferometer/aptasensor | 0.7 pg mL−1 | mean standard deviation of phase shift as ± 0.5 rad | 0.01–100 ng mL−1 | [133] | |
| 9. | ZEN | planar waveguide operating as a polarization interferometer/immunosensor | 0.01 ng mL−1 | 0.01–1000 ng mL−1 | [85] | ||
| 10. | AFB1 OTA | optical planar waveguide operating as polarization interferometer/immunosensor | 2 pg mL−1 | 0.01–1111.11 ng mL−1 | [82] | ||
| 11. | OTA | wheat and corn | fluorescence/aptamer | 2.28 nM | 6.08 | 10–5000 nM | [134] |
| 12. | AFB1 | rice sample extract | fluorescence/aptamer | 0.05 nM | 0.05–5 nM | [135] | |
| 13. | AFM1 | milk powder | fluorescence/aptamer | 0.05 μg kg−1 | 0.2–10 μg kg−1 | [136] | |
| 14. | FB1, OTA | maize | fluorescence/aptamer | 0.019 pg mL−1 (FB1) 0.015 pg mL−1 (OTA) | 0.0001–0.5 ng mL−1 | [137] | |
| 15. | ZEN | cereal samples (maize, wheat, rye flour) | fluorescence/aflatoxin-smartphone-based molecularly imprinted polymer | 1 µg mL−1 | 1–10 µg mL−1 | [138] | |
| 16. | ZEN | spiked maize samples | fluorescence/immunosensor | 20 pg mL−1 | 3.0-9.0 | 0.05–0.5 ng mL−1 as dynamic range | [139] |
| 17. | T2 toxin | barley sample | fluorescence/immunosensor | 0.1 ng mL−1 | 9.42–15.73 | 1–100 ng mL−1 | [140] |
| 18. | PAT | apple juice samples | fluorescence/immunosensor | 8.0 μg L−1 | 0–150 μg L−1 | [141] | |
| 19. | OTA | coffee, tea, and corn | fluorescence/aptasensor | 8 pg mL−1 | 0.01–35 ng mL−1 | [142] | |
| 20. | AFB1 | diluted beer and corn flour | fluorescence/aptasensor | 61 pM | 0.2–31.2 nM | [143] | |
| 21. | OTA | grain samples | luminescence/immunosensor | 0.045 μg L−1 | <4.2 | 0.1–63 μg L−1 | [144] |
| 22. | AFM1, AFB1 | peanuts and pure milk | fluorescence/aptasensor | 6.24 pg mL−1 (AFM1) 9.0 pg mL−1 (AFB1) | 1.8–8.6 | 0.01–200 ng mL−1 (AFM1) 0.01–150 ng mL−1 (AFB1) | [145] |
| 23. | OTA, PAT | apple juice samples | fluorescence/aptasensor | 0.06 ng mL−1 (OTA) 0.09 ng mL−1 (PAT) | 4.3-8.5 (OTA) 4.5–7.8 (PAT) | 0.10–50 ng L−1 (OTA and PAT) | [146] |
| 24. | AFB1, FB1, OTA, ZEN | three infant foods | chemiluminescence optical fiber/aptasensor | 0.032 pg mL−1 0.015 pg mL−1 0.432 pg mL−1 and 0.275 pg mL−1, respectively | <7.2 | 0.3–2 × 104 pg mL−1 0.3–3.2 × 104 pg mL−1 2.5–6 × 104 pg mL−1 2.0–3.5 × 104 pg mL−1 respectively | [87] |
| 25. | OTA | wine, beer | electrochemiluminescence/aptamer | 0.012 nM | 2.25–8.16 | 0.05–5 nM | [147] |
| 26. | OTA | electrochemiluminescence aptasensor | 0.03 ng mL−1 | 0.1–320 ng mL-1 | [148] | ||
| 27. | OTA, STG, ZEN, DON | aqueous solutions | bioluminescence/Photobacte rium phosphoreum cells immobilized in poly(vinyl alcohol) cryogel | 0.017–56 mg L−1, 0.010–33 mg mL−1, 0.009–14 mg L−1, 0.026–177 mg L−1, respectively | [149] | ||
| 28. | ZEN | corn flour extract | nanoscale affinity double layer evanescent wave optical-fiber/aptasensor | 2.31 fM | 1 fM–100 pM | [121] | |
| 29. | OTA | coffee samples | surface plasmon resonance/monoclonal ochratoxin A antibodies | 5.7 ng mL−1 for chitosan and 3.8 ng mL−1 for carboxymethyl chitosan as nanomatrix substrates | coefficient of variance in coffee 16% | 0–50 ng mL−1 | [150] |
| 30. | AFB1, OTA, ZEN, DON | corn and wheat | surface plasmon resonance/mycotoxin antigens | 0.59 ng mL−1, 1.27 ng mL−1, 7.07 ng mL−1 and 3.26 ng mL−1 respectively | 2.52 to 9.8 | 0.99–21.92 ng mL−1 1.98–28.22 ng mL−1 10.37–103.31 ng mL−1 5.31–99.37 ng mL−1 respectively | [151] |
| 31. | AFB1 | milk and groundnut samples | photo-electrochemical/aptasensor | 1 pg mL−1 | 4.5 inter-assay | 0.005–50 ng mL−1 | [152] |
| 32 | OTA | red wine samples | electrochemical-graphene field-effect transistors/aptasensor | 1.4 pM | 6 pM–100 pM | [60] | |
| 33 | AFB1 | amperometry at MWCNTs-modified Pt transducers/enzyme sensor | 0.5 ng mL−1 | 6.1% | 1–225 ng mL−1 | [62] | |
| 34. | AFM1 | spiked milk samples | chronoamperometry at SWCNTs-modified silver transducers/immunosensor | 0.0259 µg L−1 | 0.01–1 µg L−1 | [153] | |
| 35. | OTA | paddy rice-spiked samples | current-voltage measurements at electrospun cellulose acetate-doped 3D-graphene nanofibre transducers/aptasensor | 156 fg mL−1 | 1 fg mL−1–1 ng mL−1 | [154] | |
| 36. | AFB1 | rice milk | cyclic voltammetry at functionalized gold screen-printed transducers/imunosensor (anti-AFB1 antibodies) | 50 fg mL−1 | 50 fg mL−1–5 ng mL−1 | [155] | |
| 37. | OTA | wheat | alternating current voltammetry at modified gold transducers/aptasensor | 3.3 pg mL−1 | 3.9–5.2 | 0.01–10 ng mL−1 | [156] |
| 38. | AFB1, OTA | corn and wheat | alternating current voltammetry at modified gold transducers/aptasensor | 4.3 pg mL−1 13.3 pg mL−1 for AFB1 and OTA | 3.2 (hairpin DNA-based aptasensor) and 6.5 (single stranded DNA-based aptasensor) | 10–3000 pg mL−1 30–10,000 pg mL−1 for AFB1 and OTA | [157] |
| 39. | AFB1 | maize flour samples | differential pulse voltammetry at poly(aniline-anthranilic acid) modified graphite screen-printed transducers/aptamer | 0.086 ng mL−1 | 5–10 | 0.1–10 ng mL−1 | [158] |
| 40. | AFB1 | human blood plasma pasteurized cow milk | differential pulse voltammetry at graphene oxide nanosheets modified glassy carbon transducers/aptasensor | 0.07 nM | 2.9 | 0.5 nM–4 μM | [159] |
| 41. | PAT | patulin solution | differential pulse voltammetry at graphene oxide-gold nanocomposite transducer s/immunosensor | 5 µg L−1 | 10–200 µg L−1 | [160] | |
| 42. | AFB1, FuB1 | spiked corn samples | differential pulse voltammetry at indium tin oxide transducers/molecularly imprinted polymer | 0.313 and 0.322 pg mL−1 for AFB1 and FuB1 | 2.73 | 1 pg mL−1–500 ng mL−1 | [161] |
| 43. | AFB1, OTA | spiked wine samples | differential pulse voltammetry at phosphorene-gold nanocomposite transducers/aptasensor | 0.023 (AFB1), 0.039 ng mL−1 (OTA) | 6.9 | 0.05–10 ng mL−1 | [162] |
| 44. | ZEN | corn powder samples | differential pulse voltammetry at glassy carbon transducers/aptasensor | 3.1 × 10−12 mol L−1 | 10−11–10−6 mol L−1 | [163] | |
| 45. | DON | maize flour samples | differential pulse voltammetry at polyaniline-gold nanoparticles-graphite screen-printed transducers/aptasensor | 3.2 ng mL−1 | 5 | 5.0–30.0 ng mL−1 | [164] |
| 46. | ZEN | semen coicis real samples | differential pulse voltammetry at polyethyleneimine-functionalized multi-walled carbon nanotubes nanocomposite transducers/aptasensor | 1.0 × 10− 5 ng mL−1 | 1.37–1.61% | 5.0 × 10−5 to 50.0 ng mL−1 | [165] |
| 47. | AFB1 | wine and soy sauce | pulse voltammetry at gold thin-film transducers/aptasensor | 0.016 pg mL−1 | <3 | 0.1–10 pg mL−1 | [120] |
| 48. | AFB1 | beer, withe grape wine | square wave voltammetry at gold transducers/aptasensor | 2 nM | ˂4 | 2 nM–4 μM | [122] |
| 49. | ZEN | extract of maize grain | square wave voltammetry at gold transducers/aptasensor | 0.017 ng mL−1 | 0.01–1000 ng mL−1 | [166] | |
| 50. | OTA | red wine | square wave voltammetry at NiCo2S4 nanoparticle-dispersed MoS2 nanosheets transducers/aptasensor | 0.42 pg mL−1 | 0.5 pg mL−1–1 ng mL−1 | [167] | |
| 51. | FB1, AFB1, ZEN, OTA | spiked corn flour samples | impedance spectroscopy at gold nanoparticles—indium tin oxide transducers/aptasensor | 2.47, 3.19, 5.38, 4.87 ng mL−1 for FB1, AFB1, ZEN, OTA, respectively | 7.5, 8.2, 6.6 and 7.2 for FB1, AFB1, ZEN, OTA, respectively | 5–1000, 10–250, 10–1250, 10–1500 ng mL−1 for FB1, AFB1, ZEN, OTA, respectively | [123] |
| 52. | OTA | wine (red and withe), grape juices | impedance spectroscopy at gold nanoparticles-cysteamine-gold transducers/aptasensor | 0.030 ng mL−1 | 1.30–8.20 | 0.1–10 ng mL−1 | [168] |
| 53. | OTA | spiked beer samples | electrochemical impedance spectroscopy at pencil graphite transducers/impedimetric aptasensor | 0.1 ng mL−1 | 3.49–4.95 | 0.1–2.0 ng mL−1 | [169] |
| 54. | OTA | electrochemical impedance spectroscopy at 4-carboxyphenyl monolayer-gold transducers/immunosensor | 0.5 ng mL−1 | 5.8 | 1–20 ng mL−1 | [68] | |
| 55. | AFB1 | conductometric at interdigitated thin film transducers fabricated by gold vapor deposition /enzyme sensor based on acetylcholinesterase | 0.05 µg mL−1 | 7 | 0.25–1 mM | [73] | |
| 56. | T2-toxin, ZEN, FB1 semiquanti tative detection | saliva | mass sensitive/immunosensor | 6.1 ng mL−1, 3.6 ng mL−1, 2.4 ng mL−1 for T2, FB1 and ZEN (sensitivities reported as IC50 values in the multiplex analysis with the portable microfluidic device) | for each toxin coefficients of variation are all below 18 | 0.1–10 ng mL−1 | [49] |
| 57. | AFB1 | contaminated samples (peanut, pistachio, rice, and wheat) | quartz crystal microbalance sensor | 2.8 pg mL−1 | 3.67 | 0.05–75 ng mL−1 | [170] |
6. Comparative Overview and Future Perspective
The conventional analysis methods (chromatography, ELISA, and immunoassay techniques) have been largely applied to mycotoxin detection in food but suffer from a series of limitations, such as laborious steps concerning sample preparation (that may imply grinding, mixing, and homogenization), extraction, and clean up procedures. The use of solvents, the requirement for skilled personnel, the high analysis cost, and the difficulty of collecting representative samples given the heterogenous character represent other drawbacks [171]. The inconsistency linked to food analysis by these methods could be reduced by increasing sample size, the degree of crushing, and the number of aliquots analyzed [172]. Moreover, the use of organic solvents might lead to antibody denaturation in the case of ELISA and immunoaffinity analysis [173].
Hence, biosensors are classed among the novel approaches to minimize the aforementioned drawbacks of the traditional analysis. One important advantage of biosensors over other rapid screening strip tests is their recycling use. Even if their application has been reported as straightforward when compared to laborious conventional techniques, the use of biosensors as analytical tools may still involve sample cleanup [171].
Biosensors, in general, have proven to be useful analytical tools through the performances they achieve, depending on the nature of the bioreceptor, the nature of immobilization support, the transducer, the matrix, etc.
Optical detection based on optical fibers, fluorescence, optical waveguides, or colorimetry has experienced a special development due to the progress made in the field of optical measurements [82,86,87,174]. Fluorescence detection has been used for both immunosensors and aptamers [175]. Immunosensors with optical detection can guarantee good sensitivity and specificity. Thus, Wu et al. [176] analyzed FB1, ZEN, OTA, and AFB1 with an immunochromatographic nanosensor based on gold nanospheres, characterized by accurate, fast, and sensitive visual detection, obtaining LOD values of 3.27, 0.70, 0.10, and 0.06 ng mL−1, respectively. Fluorescence imparts a viable optical detection for mycotoxins [176,177] by using organic dyes (e.g., fluorescein, rhodamine, etc.) and other fluorescence sensing compounds, such as silica nanoparticles, organic polymers nanoparticles, Au, or Ag nanoparticles, etc. When determining AFB1 from peanut, corn, soy sauce, vegetable oil, or mouse feed with a fluorescence-based immunosensor, an LOD between 0.06 and 0.12 μg kg−1 was obtained [178], and when determining ZEN from beer and wine with an aptasensor, a LOD of 0.5 ng mL−1 was obtained [179].
The analytical signal can also be measured colorimetrically. If the recognition bioelement is an enzyme, the measurement is based on the enzymatic transformation of the chromogenic substrate into colored products, followed by optical analysis (naked eye, spectrophotometric, or colorimetric) [180]. The most used enzymes are horseradish peroxidase, acetylcholinesterase, G-quadruplex sequences, or deoxyribozymes. When determining AFB1 from peanut samples using G-quadruplex as a signal reporter, a very good sensitivity was obtained, namely an LOD of 1 pM. The mentioned advantages are low-cost, facility of detection (e.g., naked-eye detection), good selectivity, simple operation, wash-free, label-free format, and applicability to samples with complex matrices. The long incubation time, about 40 min, is mentioned as a disadvantage, which increases the analysis time [180,181]. Another disadvantage is the need for mycotoxin extraction from complex samples, with the possibility of interference with proteins, lipids, sugars, and salts [180]. For the simultaneous detection of AFB1, OTA, and ZEN, ELISA-based methods demonstrated high sensitivity and selectivity [182,183].
Gold nanoparticles used in colorimetric biosensors impart stability, sensitivity, accuracy, and repeatability. They can be employed as markers by coupling with antibodies or ligands through electrostatic adsorption and chemical bonds [125].
The shortcomings of optical sensors have been discussed, along with the possibilities of counteracting them. Colorimetry is considered as a non-expensive, portable, and useful technique. Nevertheless, it is limited to the assay of colored samples. Interferent species or matrix components imparting the same color can lead to erroneous results. Moreover, the technique is not highly sensitive, accurate wavelength bandwidth selection is necessary, and changes in source light intensity may lead to errors [184].
Fluorescent dyes suffer from limitations such as hydrophobicity and photobleaching, and the analytical response is highly pH-sensitive. Nanomaterials, such as quantum dots and carbon-based dots, served as a viable alternative to fluorescent dyes, exhibiting high quantum yield, very good photochemical stability, and adaptive photoluminescence [185,186,187]. Fluorescent lateral flow immunochromatographic assay technology integrates the convenient optical properties of fluorescent magnetic quantum beads and the inexpensive, portable, rapid, and versatile lateral flow immunochromatographic detection [187].
Hence, by integrating nanomaterials, fluorescence-based biosensors could hamper the drawbacks of diminished stability, increased background interference, and photobleaching, optimizing assay time and sensitivity. However, some shortcomings that may be encountered are interference from the environment and sample autofluorescence [125].
Also, elevated costs, laborious synthesis, and high heavy metals toxicity (Cd and Se) are some shortcomings that cannot be overlooked when working with quantum dots-based transducers. In recent years, carbonaceous quantum dots (carbon dots, graphene quantum dots, and graphene oxide quantum dots) proved biocompatible, inexpensive, and benefited from easy synthesis and low toxicity [187,188].
Chemiluminescent detection proved low detection limit and low background signal interference factors and employs a combination of biomolecules (recognition elements) to analyze target compounds by recording the analytical signal coming from the chemical probes; no external light source is required, and the technique benefits from sensitivity and broad calibration range. Biorecognition elements such as antibodies or aptamers can function as tracers in chemiluminescent biosensors [125]. Some drawbacks are limited antigen detection, a limited panel of tested toxins, low reagent stability, and elevated costs, and the assay is restricted to closed analytical systems.
Labeled surface-enhanced Raman spectroscopy is an indirect assay technology employed to detect and characterize biotoxins using organic molecules such as dyes endowed with high Raman activity in contact with the metallic surface instead of targeted analytes. Nevertheless, regarding high-quality surface-enhanced Raman spectroscopy, an active substrate is necessary, which should possess a large area of high-density hotspots, very good uniformity and signal reproducibility, and an elevated high enhancement factor [125].
Improvement of optical biosensors’ performances encompasses the following: the use of efficient enrichment and separation techniques applied to complex trace samples; the introduction of novel nano-materials with large specific area, enhanced conductivity, and magnetic separation capacity, enabling the use of multiple recognition elements, accelerating the electron transfer or facilitating separation steps; the use of novel recognition elements with remarkable affinity, molecular stability, long life, repeatable use, resulting in selectivity and versatility of the analytical tool; the quest for modern nanomaterials with enhanced activity, diminished cost and outstanding stability to enable signal amplification, to replace expensive and unstable natural enzymes; developing biosensing arrays for simultaneous microbial toxin detection; the integration of microelectronics and microfluidics technology for obtaining high-throughput, portable, miniaturized optical biosensors for real time detection; coupling biosensors with other detection techniques such as spectrographic or chromatographic approaches, to ensure accuracy; also, including wireless communication, using smartphone, “cloud” computing and data storage systems, to be integrated into optical biosensors, enables the achievement of high-frequency online analysis for long periods [187]. Smartphones may be embedded with an optical chemical or biosensing base, and different filters, lenses, diffraction gratings, and alternative light sources may be accommodated to enhance signal detection in colorimetric, fluorescent assays, chemi- or bioluminescence, as well as scattering-based techniques [184].
By considering the above-mentioned aspects, using modalities of development that promote the advantages and overcome the limitations, it has been concluded that optical biosensors can constitute a viable alternative for food control, pharmaceutical analysis, and environmental monitoring [187].
Various novel developments in optical biosensors have been reviewed by Singh et al. Surface plasmon resonance-based biosensors were considered as the most broadly used, versatile, and prone to a wide range of applications: food analysis, pharmacy, medical diagnosis, or military defense [77]. They can be applied with the facility; nevertheless, they require initial training and are prone to automation and portability, with biochips being regenerated. Results are provided in real time, with no washing steps and no incubation periods. Nevertheless, biosensors of this type are expensive and can require labeling or elevated antibody concentrations to enhance sensitivity [49].
The progress in nanotechnologies and novel materials is presently leading to miniaturization and portability [77].
In novel studies, biotin-labeled aptamers were bound to the surface of streptavidin-coated magnetic nanoparticles. The use of hybrid nanomaterials led to the reduction of sample preparation and treatment, improved performances, and, eventually, circumvented false positive and negative analytical responses [128].
When determining mycotoxins with the help of electrochemical biosensors, Farré et al. [189] note the following advantages: low cost, simplicity, sensitivity and selectivity, miniaturization, and portability.
Among the electrochemical biosensors, some authors consider amperometric biosensors as the most broadly used, characterizing them as more competitive than conductometric or potentiometric ones, being more sensitive, cheaper, and more available [54]. Graphene-coated electrodes represent effective electrochemical transducers. Chronoamperometry allows fast assay, and it is considered as appropriate for assessing the repeatability and stability of electrochemical biosensors [190]. Majer-Baranyi and collaborators [191] also emphasize that electrochemical biosensors are the most broadly used due to the selectivity and sensitivity of the determinations and the advantages imparted by low cost, miniaturization, simplicity, and portability. The introduction of screen-printed electrodes brings great advantages in that they can be developed starting from different materials: Au, Ag, magnetic nanoparticles, graphene, or other carbon-based nanotubes [192,193,194]. Electrochemical biosensors allow analysis in colored, turbid, or viscous media using small amounts of sample [193]. Carbon-based nanomaterials, especially graphene, have good conductivity, large surface area (which allows the fixation of biomolecules), good chemical stability, and low price [194]. SWCNTs achieved the increase in the surface area of the working electrodes (like Ag paste printed electrodes), enabling the attachment of more bioelements such as antibodies and promoting the electrode’s sensitivity [153].
Hence, nanomaterials are considered as appropriate carriers for biomolecules, such as enzymes, antibodies, aptamers, cells, and tissues. They have a high specific area and electro-conductivity and are prone to easy modification, enabling the increase in the number and variety of immobilized biorecognition elements, leading to the simultaneous detection of multiple analytes. Nanomaterials can preserve the activity of biomolecules and enhance their physiological stability [195]. Gong et al. [195] discussed comparatively the performances of different types of nanomaterials. Gold nanoparticles have proved to be excellent transducers for developing electrochemical biosensors. Nevertheless, noble metals are expensive, and there is a request to improve nano-biosensor stability. Graphene-based sensors using various biorecognition elements (antibodies or aptamers) had a stable response over a storage period of 60-90 days. Sensors based on carbon nanomaterials (carbon nanotubes, graphene, or their derivatives) showed a reproducible analytical response. To develop sensitive, versatile transducers, functionalized carbonaceous materials (MWCNTs, SWCNTs) were used in combination with metal (Au, Pd, Pt) or metal oxide-based nanoparticles (magnetite, indium tin oxide), integrating polymeric materials (polypyrrole, polyethylene imine or polyaniline) to promote electrochemical performance. The incorporation of materials to be used as modifiers should consider aspects such as the degree of toxicity or biocompatibility [195].
The analytical performances of biosensors in which the recognition bioelements are antibodies, antibody fragments, and antigens were analyzed comparatively. Such biosensors are called immunosensors and are characterized by very good analytical performances, LOD having values of 0.05 ng mL−1 (electrochemical impedance spectroscopy, AFB1), 0.5 pg mL−1 (differential pulse voltammetry, ZEN), 8.6 ppb (differential pulse voltammetry, DON) [193].
Aptasensors represent a category of biosensors that use DNA or RNA molecules as biological recognition elements [196]. Aptamers contain, in length, up to 80 nucleotides capable of recognizing different analytes [117]. The aptamers impart very good results in the determination of mycotoxins. Beheshti-Marnani et al. [159] obtained an LOD of 0.07 nM for the electrochemical determination of AFB1. Guo et al. [197] discussed comparatively the performances of immunosensors with those of aptamers in the detection of mycotoxins, showing that aptamers are more efficient. Although immunosensors are highly specific, the elevated costs and storage stability of antibodies hinder their applications, so aptasensors benefit from a broader range of target compounds. Aptamers used in biorecognition represent single-stranded DNA or RNA oligonucleotides that induce aptamer/target complex formation with excellent affinity and specificity through conformational change. Among the qualities of aptamers, the authors mention better stability (temperature-induced denaturation is reversible), they can be stored/transported at room temperature, and they can detect a wide range of target substances with lowered production costs and in a short time. Qian et al. [123] demonstrated that aptamers resulted in excellent selectivity, repeatability, long-term stability, great simplicity and portability, easy operation, and multiple mycotoxins recognition. By exploiting the stable 2D structure of hairpin DNA to provide binding sites for the aptamers, a highly specific, sensitive, and reliable electrochemical aptasensor was developed based on dual-ratiometric simultaneous detection of AFB1 and OTA. An anthraquinone-2-carboxylic acid-labeled complementary DNA, a ferrocene-labeled AFB1 aptamer, and a methylene blue-labeled OTA aptamer were used to develop the sensing interface. The oxidation current of anthraquinone gave the reference signal, whereas ferrocene and methylene blue resulted in simultaneous response signals. In the presence of AFB1 and OTA, the recognition step resulted in the dissociation of both ferrocene-labeled and methylene blue-labeled aptamers from the electrode, resulting in the diminution of the signals proper to ferrocene and methylene blue, while the oxidation current of anthraquinone remained unchanged. The ratios between the current intensities, ferrocene to anthraquinone and methylene blue to anthraquinone, were then exploited for quantification of AFB1 and OTA, respectively [157].
Biosensors based on electrochemical impedance spectroscopy as transduction mode [66], are appreciated for their sensitivity, selectivity, and portability [66,67,198]. Thus, Kesici and Erdem [198] achieved a detection limit for FUM of 3.69 ng mL−1 with an impedimetric biosensor. Modification of the electrode surface at which biorecognition takes place, increased sensitivity and electrocatalytic activities, and the analyte can diffuse more rapidly on the surface of the electrode: the graphite electrode modified with nanofibers developed by electrospinning technique was used for the determination of T2 toxin, by voltammetry and electrochemical impedance spectroscopy, minimizing interferences in complex polluted matrixes [199]. Apart from the advantages of impedimetric detection, such as low detection limit, high stability and response reproducibility, versatility, broad linear range, reduced power requirement, portability, and high resolution, some authors also mention several disadvantages, such as short shelf life, sensitivity toward the changes occurring in the environmental conditions (temperature), high susceptibility to cross-sensitivity [200].
The comparative assessment of the largely used voltammetric detection methods is revealed in differential pulse voltammetry; the difference between the two current intensities (before the application of the pulse and at the end of each pulse) is quantified, and this procedure leads to minimization of the capacitive current in favor of the Faradaic current. The background current is significantly reduced, and the low capacitive current improves sensitivity. The small potential step sizes give narrower peaks than in cyclic voltammetry, favoring discrimination of analytes possessing similar or very close oxidation potentials. As in the case of other potential step techniques, one shortcoming is the duration of the experiment, which is reported as being longer than in amperometry or ramping techniques such as cyclic voltammetry [201]. Given the low detection limits imparted and excellent sensitivity, the square-wave voltammetric technique is reported as prone to simultaneous multiple analyte biosensing [190]. Nevertheless, some authors consider that electrochemical sensors, mostly those using aptamers as bioelements, though simple, sensitive, fast, and cost-effective, still require signal amplification protocols for sensitivity increase. In a novel electrochemical assay, sensitivities of the order of pM were obtained using exonuclease I and branched hybridization chain reaction of the biorecognition element, promoting signal amplification for zearalenone differential pulse voltammetric detection [163].
Mass-sensitive microarray biosensors were characterized as very simple to use and prone to regeneration and reuse; by clearing the surface of the previous coating analyte, they allow multiple analyses. They necessitate small reagent and sample volumes, are relatively inexpensive, sensitive, and label-free, with results available in real time, and require no long incubation periods and no washing steps. Nevertheless, automated biosensors are costly (particularly in the case of non-portable devices), and in portable technology, one sample can be analyzed at a time [49].
The mentioned trends [123,125,191,202,203,204] for the improvement of detection with biosensors are related to the efficiency of biomolecules, the improvement of their fixation on the transducer, modifications brought to the surfaces or to the pH, the use of nanomaterials, the improvement of detection signals, the possibility of multi-analyte detection, miniaturization, portability. Aptamers, regardless of the detection modality, are considered extremely efficient and described as being superior to ELISA antibody technology, although the affinity of aptamers is considered, in general, weaker compared to that of antibodies [197]. ELISA [205] is considered by some authors like Matson [206] to be a biosensor. In some biosensors, this type of enzyme immuno-assay is integrated for key signaling [206].
The detection of mycotoxins based on a single analytical signal has proven, in some cases, due to matrix effects, to be false negative or false positive. For this reason, multimodal biosensors have appeared, which can generate different analytical signals, resulting in linearity increase and accuracy [125]. OTA was determined with a dual-readout electrochemical/visual (paper-based) aptasensor. The linearity ranges were 0.1–200 ng mL−1 (colorimetry) and 0.0001–200 ng mL−1 (electrochemistry) [207]. AFB1 was determined with a dual biosensor based on electrostatically mediated fluorescence resonance energy transfer and being exploited as a signal enhancement approach. In the presence of the analyte, the aptamer selectively binds to AFB1, leading to the aggregation of bare gold nanoparticles, promoted by the presence of sodium chloride. This eventually leads to a color change of gold nanoparticle solution from wine-red to purple, exploited in a colorimetric assay. Measured analytical signals were of colorimetric or fluorescent nature, obtaining linearity domains of 10–320 ng mL−1 and 3–320 ng mL−1, respectively [131]. The AFB1 photothermal detection based on Cu2-xSe-Au nanoparticles enhanced the linear range and sensitivity (LOD of 0.00842 μg/L) when compared to the simple visual analysis methods [208].
Very good selectivity and elevated sensitivity were obtained by integrating the features of photoelectrochemical transducers (semiconductors and semiconductor-based nano-heterostructures incorporating graphene oxide, metal or metal oxide-based nanoparticles) and the high-affinity recognition elements such as molecularly imprinted polymers. Sensitivities below 1 pg mL−1 were obtained in the case of aflatoxin B1 and ochratoxin. Analytical protocols based on ratiometric detection were used to minimize unstability factors that may originate from the light source [209].
Recently, a highly sensitive and selective electrochemiluminescent aptasensor relying on a self-enhanced luminophore enabled the detection of ochratoxin A. Polyethyleneimine functionalized multi-walled carbon nanotubes decorated with gold nanoparticles served as electrochemical transducers to promote electronic transfer and represented an adequate microenvironment for self-enhanced luminophore loading and electrochemiluminescent analytical signal enhancement. The electron transfer distance was significantly shortened, as black phosphorus quantum dots and oxidized tris (2,2′-bipyridyl) ruthenium(II) were bound within one nanoparticle, hence optimizing electrochemiluminescent efficiency to reach the “switch-on” state. Then, the luminophore was quenched using ferrocenes, which corresponded to the “switch-off” state of the sensor. Excellent analytical parameters were achieved, such as sensitivity (0.03 ppb detection limit), stability, and reproducibility [148].
A novel automated reaction equipment and nanozyme-amplified immunosorbent assay were developed for accurate and sensitive determination of AFB1. Highly effective signal probes were coupled with the automated immunoassay equipment. Polystyrene nanoparticles served as signal carriers for loading a robust in situ-synthesized platinum/palladium nanozyme. The horseradish peroxidase-labeled goat anti-mouse antibody present on the surface of the nanozyme led to the generation of a bimetallic nanozyme-bioenzyme hybrid material with analytical signal enhancement. The reported analytical parameters showed a broad linear range of 10–104 pg mL−1 and sensitivity, with a low detection limit of 5.52 pg mL−1, 65-fold lower than that obtained by ELISA [210].
Recently, wearable biosensors using various biorecognition elements have attracted increasing attention, employing accurate electrochemical and optical detection [211]. Considering the analytical performances of the biosensors, the simplicity, the possibilities of miniaturization and use of nanomaterials, of the in situ application, of increasing the stability, we think that these have become essential tools in the analysis of mycotoxins, alternatives to other much more expensive methods (e.g., HPLC-MS). Future trends imply quantifying more than one analytical parameter (using multimodal detection), employing nanomaterials whose optical and electrochemical features can be exploited with great facility, achieving sensitivity and fast response, applying automation, integrating hybrid materials, and applying signal amplification protocols for analytical response enhancement. Electrode materials with high surface area and incorporated modifiers should be environmentally friendly and are expected to achieve versatility by accommodating multiple bioelements for recognition.
Thus, a market of commercial biosensors has been developed, including for the determination of mycotoxins. The commercial products grow steadily. The market developed from 7.3 billion in 2013 to 18.6 billion in 2018, then to 26.8 billion in 2022, with an estimated worth of 34.3 billion in 2025 and 36.7 billion USD in 2026 [212,213,214,215].
Author Contributions
A.M.P.: Conceptualization, Validation, Writing—original draft, Writing—review and editing, Supervision. F.I.: Conceptualization, Validation, Writing—original draft. L.S.: Conceptualization, Supervision, Writing—original draft. E.M.: Supervision, Validation, Writing—review and editing. L.B.S.: Supervision, Visualization, Writing—review and editing. O.I.G.: Supervision, Visualization, Writing—review and editing. L.B.: Validation, Supervision, Writing—review and editing. A.I.S.: Conceptualization, Validation, Writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Dohlman, E. Mycotoxin hazards and regulations-impacts on food and animal feed crop trade. In International Trade and Food Safety Economic Theory and Case Studies; Buzby, J.C., Ed.; Agricultural Economic Report No. 828; United States Department of Agriculture: Washington, DC, USA, 2003; Chapter 6; pp. 97–108. Available online: https://www.ers.usda.gov/webdocs/publications/41603/15644_aer828_1_.pdf?v=0 (accessed on 4 February 2024).
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- da Silva, J.V.B.; de Oliveira, C.A.F.; Ramalho, L.N.Z. An overview of mycotoxins, their pathogenic effects, foods where they are found and their diagnostic biomarkers. Food Sci. Technol. 2022, 42, e48520. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius-Codex General Standard for Contaminants and Toxins in Food and Feed (Codex Stan 193-1995). Available online: https://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf (accessed on 8 March 2024).
- Commission Regulation (EU). 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance). Off. J. Eur. Union 2023, L119, 103–157. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 20 December 2023).
- US FDA—Chemical Contaminants, Metals, Natural Toxins & Pesticides Guidance Documents & Regulations, 2000–2010. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ChemicalContaminantsMetalsNaturalToxinsPesticides/default.htm (accessed on 15 December 2023).
- Ministry of Health and Family Welfare (Food Safety and Standards Authority of India). Notification, New Delhi, the 7th August, 2020. In The Gazette of India: Extraordinary, Part. III, Sec 4; Ministry of Health and Family Welfare: New Delhi, India, 2020; pp. 18–30. Available online: https://www.fssai.gov.in/upload/notifications/2020/08/5f3d09f97b78aGazette_Notification_Limit_Metal_19_08_2020.pdf (accessed on 29 November 2023).
- Stein, R.A.; Bulboacă, A.E. Mycotoxins. In Foodborne Diseases, 3rd ed.; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 407–446. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef]
- European Commission, Health & Consumer Protection Directorate-General, Scientific Commitee on Food, the 119th Pleanry Meeting, Opinion on Fusarium Toxins. 1999. Available online: https://food.ec.europa.eu/document/download/62e47971-e232-4e39-a9d7-2e3429eb5957_en?filename=cs_contaminants_catalogue_fusarium_out44_en.pdf (accessed on 14 May 2024).
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and Toxicological Effects of Patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Mycotoxins and human health. In Improving Public Health through Mycotoxin Control; Pitt, J.I., Wild, C.P., Baan, R.A., Gelderblom, W.C.A., Miller, J.D., Riley, R.T., Wu, F., Eds.; IARC Scientific Publication No. 158, E, 2012, Chapter 6; International Agency for Research on Cancer: Lyon, France, 2012; pp. 87–104. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Improving-Public-Health-Through-Mycotoxin-Control-2012 (accessed on 16 May 2024).
- Ahmed Adam, M.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (Review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Outcome of a Public Consultation on the Draft Risk Assessment of Aflatoxins in Food; EFSA Supporting Publication: Parma, Italy, 2020; p. EN-1798. 53p. [Google Scholar] [CrossRef]
- Mohebbi, A.; Nemati, M.; Mogaddam, M.R.A.; Farajzadeh, M.A.; Lotfipour, F. Dispersive micro-solid-phase extraction of aflatoxins from commercial soy milk samples using a green vitamin-based metal-organic framework as an efficient sorbent followed by high performance liquid chromatography-tandem mass spectrometry determination. J. Chromatogr. A 2022, 1673, 463099. [Google Scholar] [CrossRef]
- Shuib, N.S.; Saad, B. In-syringe dispersive micro-solid phase extraction method for the HPLC-fluorescence determination of aflatoxins in milk. Food Control 2022, 132, 108510. [Google Scholar] [CrossRef]
- Romero-Sánchez, I.; Ramírez-García, L.; Gracia-Lor, E.; Madrid-Albarrán, Y. Simultaneous determination of aflatoxins B1, B2, G1 and G2 in commercial rices using immunoaffinity column clean-up and HPLC-MS/MS. Food Chem. 2022, 395, 133611. [Google Scholar] [CrossRef]
- Maneeboon, T.; Chuaysrinule, C.; Mahakarnchanakul, W. Optimization and validation of dispersive liquid-liquid microextraction for simultaneous determination of aflatoxins B1, B2, G1, and G2 in Senna leaves and pods using HPLC-FLD with pre-column derivatization. Toxins 2023, 15, 277. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Hou, C.; Dai, Y.Q.; Chu, L.L.; Geng, S.W.; Zheng, S.L.; Kang, X.J. Determination of aflatoxin B1 by novel nanofiber-packed solid-phase extraction coupled with a high performance liquid chromatography-fluorescence detector. Anal. Methods 2023, 15, 472–481. [Google Scholar] [CrossRef]
- Noori, H.; Feizi, J.; Eshaghi, Z. Development of a nanoparticle-assisted fabric phase sorptive extraction technique coupled with high-performance liquid chromatography for sensitive determination of aflatoxins in food samples. Anal. Bioanal. Chem. Res. 2023, 10, 121–134. [Google Scholar] [CrossRef]
- Sartori, A.V.; de Moraes, M.H.P.; dos Santos, R.P.; Souza, Y.P.; Candido, F.S.; da Nobrega, A.W. Determination of aflatoxins M1, M2, B1, B2, G1, G2 and ochratoxin A in infant formulas from Brazil using a modified QuEChERS method and UHPLC-MS/MS. Food Anal. Methods 2023, 16, 841–849. [Google Scholar] [CrossRef]
- Medina, M.L.J.; Lafarga, T.; Frenich, A.G.; Romero-Gonzalez, R. Natural occurrence, legislation, and determination of aflatoxins using chromatographic methods in food: A review (from 2010 to 2019). Food Rev. Int. 2021, 37, 244–275. [Google Scholar] [CrossRef]
- Schincaglia, A.; Aspromonte, J.; Franchina, F.A.; Chenet, T.; Pasti, L.; Cavazzini, A.; Purcaro, G.; Beccaria, M. Current developments of analytical methodologies for Aflatoxins’ determination in food during the last decade (2013–2022), with a particular focus on nuts and nut products. Foods 2023, 12, 527. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Iordache, F.; Stanca, L.; Ionescu Petcu, A.; Purdoiu, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Comprehensive overview and critical perspective on the analytical techniques applied to aflatoxin determination—A review paper. Microchem. J. 2023, 191, 108770. [Google Scholar] [CrossRef]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P.; Pisoschi, A. Ascorbic acid determination by an amperometric ascorbate oxidase-based biosensor. Rev. Chim. 2010, 61, 339–344. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Negulescu, G.P. Ethanol determination by an amperometric bienzyme sensor based on a Clark-type transducer. J. Electroanal. Chem. 2012, 671, 85–91. [Google Scholar] [CrossRef]
- Pisoschi, A.M. Biosensors as bio-based materials in chemical analysis: A Review. J. Biobased Mater. Bioenergy 2013, 7, 19–38. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Q.; Hao, N.; Kun, W. Recent developments of photoelectrochemical biosensors for food analysis. J. Mater. Chem. B 2019, 7, 7283–7300. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Erkmen, C.; Uslu, B. Frontiers in electrochemical enzyme based biosensors for food and drug analysis. TRAC Trends Anal. Chem. 2020, 124, 115809. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, H.; Yin, J.; Guo, Q.; Zhang, Y.; Sun, X.; Guo, Y.; Yang, Q.; Zhang, Y.; Li, F.; et al. Recent advances and future prospects of aptamer-based biosensors in food safety analysis. Int. J. Electrochem. Sci. 2022, 17, 22019. [Google Scholar] [CrossRef]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-based electrochemical biosensors for food safety analysis. Biosensors 2022, 12, 1088. [Google Scholar] [CrossRef]
- Li, W.; Xiao, F.; Bai, X.; Xu, H. Magnetic nanoparticles for food hazard factors sensing: Synthesis, modification and application. J. Chem. Eng. 2023, 465, 142816. [Google Scholar] [CrossRef]
- Sadanandan, S.; Meenakshi, V.S.; Ramkumar, K.; Pillai, N.P.; Anuvinda, P.; Sreelekshmi, P.J.; Devika, V.; Ramanunni, K.; Sankar, R.J.; Sreejaya, M.M. Biorecognition elements appended gold nanoparticle biosensors for the detection of food-borne pathogens-A review. Food Control 2023, 148, 109510. [Google Scholar] [CrossRef]
- Tetyana, P.; Morgan Shumbula, P.; Njengele-Tetyana, Z. Biosensors: Design, development and applications. In Nanopores; Ameen, S., Akhtar, M.S., Shin, H.-S., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Morales, M.A.; Halpern, J.M. Guide to selecting a biorecognition element for biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef]
- Takeda, K.; Nakamura, N. Biosensors: Enzyme Sensors. In Encyclopedia of Sensors and Biosensors; Ikebukuro, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 297–381. [Google Scholar] [CrossRef]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and antibody-derived analytical biosensors. Essays Biochem. 2016, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Ma, J.; Li, D.; Wang, R. DNA-based biosensors for the biochemical analysis: A review. Biosensors 2022, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, K. Biosensors: Biomimetic sensors. In Encyclopedia of Sensors and Biosensors; Ikebukuro, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 393–404. [Google Scholar] [CrossRef]
- Cieplak, M.; Kutner, W. Artificial biosensors: How can molecular imprinting mimic biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Meira, D.I.; Barbosa, A.I.; Borges, J.; Reis, R.L.; Correlo, V.M.; Vaz, F. Recent advances in nanomaterial-based optical biosensors for food safety applications: Ochratoxin-A detection, as case study. Crit. Rev. Food Sci. Nutr. 2023, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, M.; Sichani, S.B.; Cornelis, P.; Wackers, G.; Wagner, P. The hot-wire concept: Towards a one-element thermal biosensor platform. Biosens. Bioelectron. 2021, 179, 113043. [Google Scholar] [CrossRef] [PubMed]
- Nolan, P.; Auer, S.; Spehar, A.; Oplatowska-Stachowiak, M.; Campbell, K. Evaluation of mass sensitive micro-array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 2021, 222, 121521. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Teo, A.J.T.; Li, K.H.H. Acoustic biosensors and microfluidic devices in the decennium: Principles and applications. Micromachines 2021, 13, 24. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-enabled biosensors: A review of fundamentals, design principles, materials, and applications. Biosensors 2023, 13, 40. [Google Scholar] [CrossRef]
- Thevenot, D.; Toth, K.; Durst, R.; Wilson, G. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Voeroes, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, M.; Abdi, M. Recent developments, characteristics, and potential applications of electrochemical biosensors. Anal. Sci. 2004, 20, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Rameil, S.; Schubert, P.; Grundmann, P.; Dietrich, R.; Märtlbauer, E. Use of 3-(4-hydroxyphenyl)propionic acid as electron donating compound in a potentiometric aflatoxin M1-immunosensor. Anal. Chim. Acta 2010, 661, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Stepurska, K.V.; Soldatkin, O.O.; Arkhypova, V.M.; Lagarde, F.; Jaffrezic-Renault, N.; Dzyadevych, S.V. Development of novel enzyme potentiometric biosensor based on pH-sensitive field effect transistors for aflatoxin B1 analysis in real samples. Talanta 2015, 144, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, T.; Kakkar, D.; Wadhwa, G.; Raj, B. Recent advances and progress in development of the field effect transistor biosensor: A review. J. Electron. Mater. 2019, 48, 7635–7646. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent advances in field effect transistor biosensors: Designing strategies and applications for sensitive assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, N.; Jaric, S.; Kireev, D.; Emelianov, A.V.; Orlov, A.V.; Gadjanski, I.; Nikitin, P.I.; Akinwande, D.; Bobrinetskiy, I. Real-time detection of ochratoxin A in wine through insight of aptamer conformation in conjunction with graphene field-effect transistor. Biosens. Bioelectron. 2022, 200, 113890. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Li, S.C.; Chen, J.H.; Cao, H.; Yao, D.S.; Liu, D.L. Amperometric biosensor for aflatoxin B1 based on aflatoxin-oxidase immobilized on multiwalled carbon nanotubes. Food Control 2011, 22, 43–49. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernández, S.; Bertolín, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Tria, S.A.; Lopez-Ferber, D.; Gonzalez, C.; Bazin, I.; Guiseppi-Elie, A. Microfabricated biosensor for the simultaneous amperometric and luminescence detection and monitoring of Ochratoxin A. Biosens. Bioelectron. 2016, 79, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Lazanas, A.C.; Prodomidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Radi, A.E.; Muñoz-Berbel, X.; Lates, V.; Marty, J.L. Label-free impedimetric immunosensor for sensitive detection of ochratoxin A. Biosens. Bioelectron. 2009, 24, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Malvano, F.; Albanese, D.; Crescitelli, A.; Pilloton, R.; Esposito, E. Impedimetric label-free immunosensor on disposable modified screen-printed electrodes for Ochratoxin A. Biosensors 2016, 6, 33. [Google Scholar] [CrossRef]
- Dzyadevych, S.; Jaffrezic-Renault, N. Conductometric biosensors. In Biological Identification; Paul Schaudies, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 153–193. [Google Scholar] [CrossRef]
- Adley, C.C.; Ryan, M.P. Conductometric biosensors for high throughput screening of pathogens in food. In High Throughput Screening for Food Safety Assessment Biosensor Technologies, Hyperspectral Imaging and Practical Applications; Bhunia, A.K., Kim Moon, S., Taitt, C.R.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 315–326. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Electrochemical biosensors for hormone analyses. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Burdak, O.S.; Sergeyeva, T.A.; Arkhypova, V.M.; Dzyadevycha, S.V.; Soldatkin, A.P. Acetylcholinesterase-based conductometric biosensor for determination of aflatoxin B1. Sens. Actuators B Chem. 2013, 188, 999–1003. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Stepurska, K.V.; Arkhypova, V.M.; Soldatkin, A.P.; El’skaya, A.V.; Lagarde, F.; Dzyadevych, S.V. Conductometric enzyme biosensor for patulin determination. Sens. Actuators B Chem. 2017, 239, 1010–1015. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.R.; Jagajjanani Rao, K. A review on label free biosensors. Biosens. Bioelectron. X 2022, 11, 100216. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical biosensors: A decade in review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, X. Optical ring resonators for biochemical and chemical sensing. Anal. Bioanal. Chem. 2011, 399, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sarkaleh, A.K.; Lahijani, B.V.; Saberkari, H.; Esmaeeli, A. Optical ring resonators: A platform for biological sensing applications. J. Med. Signals Sens. 2017, 7, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, M.; Yan, H. A portable optical fiber biosensor for the detection of zearalenone based on the localized surface plasmon resonance. Sens. Actuators B Chem. 2021, 336, 129752. [Google Scholar] [CrossRef]
- Campell, D.P. Interferometric biosensors. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer: New York, NY, USA, 2008; pp. 169–211. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Rubaye, A.G.; Al-Jawdah, A.M.; Tsargorodska, A.; Marty, J.-L.; Catanante, G.; Szekacs, A.; Takacs, E. Novel optical biosensing technologies for detection of mycotoxins. Opt. Laser Technol. 2019, 109, 212–221. [Google Scholar] [CrossRef]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, L.Y. Subtle application of electrical field-induced lossy mode resonance to enhance performance of optical planar waveguide biosensor. Biosensors 2021, 11, 86. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Jawdah, A.M.; Gémes, B.; Takács, E.; Székács, A. An optical planar waveguide-based immunosensors for determination of fusarium mycotoxin zearalenone. Toxins 2021, 13, 89. [Google Scholar] [CrossRef]
- Al-Jawdah, A.; Nabok, A.; Jarrah, R.; Holloway, A.; Tsargorodska, A.; Takacs, E.; Szekacs, A. Mycotoxin biosensor based on optical planar waveguide. Toxins 2018, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, S.; Li, D.; Yang, J.; Yang, L. Portable chemiluminescence optical fiber aptamer-based biosensors for analysis of multiple mycotoxins. Food Control 2023, 144, 109361. [Google Scholar] [CrossRef]
- Li, S.; Zhong, X.; Xu, Y.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Smartphone-based reading system integrated with phycocyanin-enhanced latex nanospheres immunoassay for on-site determination of aflatoxin b1 in foodstuffs. Food Chem. 2021, 360, 130019. [Google Scholar] [CrossRef] [PubMed]
- Kłos-Witkowska, A. Enzyme-based fluorescent biosensors and their environmental, clinical and industrial applications. Pol. J. Environ. Stud. 2015, 24, 19–25. [Google Scholar] [CrossRef]
- Selvin, P.R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Mol. Biol. 2000, 7, 730–734. [Google Scholar] [CrossRef]
- Das, A.P.; Kumar, P.S.; Swain, S. Recent advances in biosensor based endotoxin detection. Biosens. Bioelectron. 2014, 51, 62. [Google Scholar] [CrossRef]
- Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents. Sensors 2018, 18, 2357. [Google Scholar] [CrossRef]
- Tan, H.; Ma, L.; Guo, T.; Zhou, H.; Chen, L.; Zhang, Y.; Dai, H.; Yu, Y. A novel fluorescence aptasensor based on mesoporous silica nanoparticles for selective and sensitive detection of aflatoxin B1. Anal. Chim. Acta 2019, 1068, 87–95. [Google Scholar] [CrossRef]
- Ebanks, F.; Nasrallah, H.; Garant, T.M.; McConnell, E.M.; DeRosa, M.C. Colorimetric detection of aflatoxins B1 and M1 using aptamers and gold and silver nanoparticles. Adv. Agrochem. 2023, 2, 221–230. [Google Scholar] [CrossRef]
- Dies, H.; Siampani, M.; Escobedo, C.; Docoslis, A. Direct detection of toxic contaminants in minimally processed food products using dendritic surface-enhanced Raman scattering substrates. Sensors 2018, 18, 2726. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman spectroscopic methods in food safety: A review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Monošík, R.; Streďanský, M.; Šturdík, E. Biosensors—Classification, characterization and new trends. Acta Chim. Slovaca 2012, 5, 109–120. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Fogel, R.; Limson, J.; Seshia, A.A. Acoustic biosensors. Essays Biochem. 2016, 60, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Basinskiene, L.; Vidmantiene, D.; Bartkiene, E.; Kunigelis, V.; de Koe, W.J. Rapid acoustic screening of deoxynivalenol (DON) in grain. World Mycotoxin J. 2008, 1, 267–274. [Google Scholar] [CrossRef]
- Ramanathan, K.; Danielsson, B. Principles and applications of thermal biosensors. Biosens. Bioelectron. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Vasuki, S.; Varsha, V.; Mithra, R.; Dharshni, R.A.; Abinaya, S.; Dharshini, R.D.; Sivarajasekar, N. Thermal biosensors and their applications. Am. Int. J. Res. Sci. Technol. Eng. Math. 2019, 1, 262–264. Available online: http://iasir.net/AIJRSTEMpapers/IBCFT-2019.pdf (accessed on 12 January 2024).
- Bhardwaj, T. Review on immobilization techniques of biosensors. Int. J. Eng. Res. Technol. 2014, 3, 294–298. [Google Scholar]
- Liébana, S.; Drago, G.A. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Frutiger, A.; Tanno, A.; Hwu, S.; Tiefenauer, R.F.; Vörös, J.; Nakatsuka, N. Nonspecific binding fundamental concepts and consequences for biosensing applications. Chem. Rev. 2021, 121, 8095–8160. [Google Scholar] [CrossRef] [PubMed]
- Asal, M.; Özen, Ö.; Sahinler, M.; Baysal, H.T.; Polatoglu, I. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Layer-by-layer nano-assembly: A powerful tool for optical fiber sensing applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Vikulina, A.S. Layer-by-layer assemblies of biopolymers: Build-up, mechanical stability and molecular dynamics. Polymers 2020, 12, 1949. [Google Scholar] [CrossRef] [PubMed]
- Iost, R.M.; Crespilho, F.N. Layer-by-layer self-assembly and electrochemistry: Applications in biosensing and bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Gibbson, T.D. Biosensors: The stability problem. Analusis 1999, 27, 630–638. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Panjan, P.; Ohtonen, E.; Tervo, P.; Virtanen, V.; Sesay, A.M. Shelf life of enzymatic electrochemical sensors. Proc. Technol. 2017, 27, 306–308. [Google Scholar] [CrossRef]
- Puggioni, G.; Calia, G.; Arrigo, P.; Bacciu, A.; Bazzu, G.; Migheli, R.; Fancello, S.; Serra, P.A.; Rocchitta, G. Low-temperature storage improves the over-time stability of implantable glucose and lactate biosensors. Sensors 2019, 19, 422. [Google Scholar] [CrossRef] [PubMed]
- Alhamoud, Y.; Yang, D.; Kenston, S.S.F.; Liu, G.; Liu, L.; Zhou, H.; Ahmed, F.; Zhao, J. Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 2019, 141, 111418. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Wei, D.; Cao, M.; Wang, Z.; Wang, M. Biosensors based on core–shell nanoparticles for detecting mycotoxins in food: A review. Food Chem. 2023, 429, 136944. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Wang, E.; Tai, C.S.; Chiu, Y.C.; Li, C.W.; Lin, Y.R.; Lee, T.H.; Huang, C.W.; Chen, J.C.; Chen, W.L. Improving the reproducibility, accuracy, and stability of an electrochemical biosensor platform for point-of-care use. Biosens. Bioelectron. 2020, 155, 112111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, T.; Shen, Y.; Li, H. A high-performance self-supporting electrochemical biosensor to detect Aflatoxin B1. Biosensors 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, S.; Wei, Z.; Lou, X. Evanescent wave optical-fiber aptasensor for rapid detection of zearalenone in corn with unprecedented sensitivity. Biosensors 2022, 12, 438. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.P.; Zhao, Q. A signal-on electrochemical aptasensor for rapid detection of aflatoxin B1 based on competition with complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Y.; Cui, H.; Yang, H.; Hussain, M.; Wang, K.; Wei, J.; Long, L.; Ding, L.; Wang, C. Fabrication of a disposable aptasensing chip for simultaneous label-free detection of four common coexisting mycotoxins. Anal. Chim. Acta 2023, 1282, 341921. [Google Scholar] [CrossRef]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and methods of biosensor interfaces with stability. Front. Mater. 2021, 7, 583739. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, J.; Yang, C.; Jiang, J.; Zhang, Q.; Ping, J.; Li, P. Current trends in biosensors for biotoxins (mycotoxins, marine toxins, and bacterial food toxins): Principles, application, and perspective. TRAC Trends Anal. Chem. 2023, 165, 117144. [Google Scholar] [CrossRef]
- Kasoju, A.; Shrikrishna, N.S.; Shahdeo, D.; Khan, A.A.; Alanazi, A.M.; Gandhi, S. Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay. RSC Adv. 2020, 10, 11843–11850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef] [PubMed]
- Rafati, A.; Dorosti, N.; Gil, P. Smartphone-based technology for nanomolecular detection of aflatoxin B1 by aptamer-conjugated magnetic nanoparticles. World Mycotoxin J. 2022, 15, 159–169. [Google Scholar] [CrossRef]
- Contreras-Trigo, B.; Diaz-Garcia, V.; Oyarzun, P. A novel preanalytical strategy enabling application of a colorimetric nanoaptasensor for on-site detection of AFB1 in cattle feed. Sensors 2022, 22, 9280. [Google Scholar] [CrossRef] [PubMed]
- Mousivand, M.; Javan-Nikkhah, M.; Anfossi, L.; Di Nardo, F.; Salina, M.; Bagherzadeh, K. High performance aptasensing platform development through in silico aptamer engineering for aflatoxin B1 monitoring. Food Control 2023, 145, 109418. [Google Scholar] [CrossRef]
- Xiong, J.; He, S.; Qin, L.; Zhang, S.; Shan, W.; Jiang, H. Aptasensor-based assay for dual-readout determination of aflatoxin B1 in corn and wheat via an electrostatic force-mediated FRET strategy. Microchim. Acta 2023, 190, 80. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, V.; Koukouvinos, G.; Petrou, P.S.; Economou, A.; Dekker, J.; Harjanne, M.; Heimala, P.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Multiplexed mycotoxins determination employing white light reflectance spectroscopy and silicon chips with silicon oxide areas of different thickness. Biosens. Bioelectron. 2020, 153, 112035. [Google Scholar] [CrossRef]
- Al-Jawdah, A.; Nabok, A.; Abu-Ali, H.; Catanante, G.; Marty, J.-L.; Szekacs, A. Highly sensitive label-free in vitro detection of aflatoxin B1 in an aptamer assay using optical planar waveguide operating as a polarization interferometer. Anal. Bioanal. Chem. 2019, 411, 7717–7724. [Google Scholar] [CrossRef]
- Wang, C.; Tan, R.; Li, J. Exonuclease I-assisted fluorescent method for ochratoxin A detection using iron-doped porous carbon, nitrogen-doped graphene quantum dots, and double magnetic separation. Anal. Bioanal. Chem. 2019, 411, 2405–2414. [Google Scholar] [CrossRef]
- Ye, H.; Lu, Q.; Duan, N.; Wang, Z. GO-amplified fluorescence polarization assay for high-sensitivity detection of aflatoxin B1 with low dosage aptamer probe. Anal. Bioanal. Chem. 2019, 411, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wen, F.; Qiao, Q.; Zheng, N.; Saive, M.; Fauconnier, M.-L.; Wang, J. A novel graphene oxide-based aptasensor for amplified fluorescent detection of Aflatoxin M1 in milk powder. Sensors 2019, 19, 3840. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Khan, I.M.; Yan, L.; Khan, M.I.; Mohsin, A.; Duan, N.; Wu, S.; Wang, Z. Simultaneous detection of fumonisin B1 and ochratoxin a using dual-color, time-resolved luminescent nanoparticles (NaYF4: Ce, Tb and NH2-Eu/DPA@SiO2) as labels. Anal. Bioanal. Chem. 2019, 411, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.; Yarynka, D.; Dubey, L.; Dubey, I.; Piletska, E.; Linnik, R.; Antonyuk, M.; Ternovska, T.; Brovko, O.; Piletsky, S.; et al. Sensor based on molecularly imprinted polymer membranes and smartphone for detection of Fusarium contamination in cereals. Sensors 2020, 20, 4304. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Farka, Z.; Mickert, M.J.; Brandmeier, J.C.; Pastucha, M.; Hlaváček, A.; Martínez-Orts, M.; Canales, Á.; Skládal, P.; Benito-Peña, E.; et al. Competitive upconversion-linked immunoassay using peptide mimetics for the detection of the mycotoxin zearalenone. Biosens. Bioelectron. 2020, 170, 112683. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Fu, X.; Liu, H.; Ding, G.B.; Li, L.S.; Zhu, J.; Guo, Y. A competitive immunoassay based on engineered magnetic/fluorescent nanoparticles and biolayer interferometry-based assay for T-2 toxin determination. Microchim. Acta 2020, 187, 514. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, D.; Kim, M. Immunoliposome-based fluorometric patulin assay by using immunomagnetic nanoparticles. Microchim. Acta 2019, 186, 834. [Google Scholar] [CrossRef]
- Song, X.L.; Ding, Q.; Pu, Y.P.; Zhang, J.; Sun, R.L.; Yin, L.H.; Wei, W.; Liu, S.Q. Application of the dimeric G-quadruplex and toehold-mediated strand displacement reaction for fluorescence biosensing of ochratoxin A. Biosens. Bioelectron. 2021, 192, 113537. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, H.; Zhao, Q. A simple structure-switch aptasensor using label-free aptamer for fluorescence detection of aflatoxin B1. Molecules 2022, 27, 4257. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Zhang, H.; Zhu, R.; Meng, Z. Competitive ELISA based on pH-responsive persistent luminescence nanoparticles for autofluorescence-free biosensor determination of ochratoxin A in cereals. Anal. Bioanal. Chem. 2023, 415, 1877–1887. [Google Scholar] [CrossRef]
- Ge, G.; Wang, T.; Liu, Z.; Liu, X.; Li, T.; Chen, Y.; Fan, J.; Bukye, E.; Huang, X.; Song, L. A self-assembled DNA double-crossover-based fluorescent aptasensor for highly sensitivity and selectivity in the simultaneous detection of aflatoxin M1 and aflatoxin B1. Talanta 2023, 265, 124908. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Guo, H.; Li, K.; Zhang, Y.; Guo, H.; Wang, Z. Simultaneous detection of patulin and ochratoxin A based on enhanced dual-color AuNCs modified aptamers in apple juice. Talanta 2024, 266, 124949. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, C.; Xu, E.; Chen, J.; Xu, X.; Wei, W.; Liu, S. A simple and sensitive electrochemiluminescence aptasensor for determination of ochratoxin A based on a nicking endonuclease-powered DNA walking machine. Food Chem. 2019, 282, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Meng, X.; Zhang, Y.; Li, Z.; Zhou, Q.; Jing, X.; Sun, X.; Zhao, W. An “on-off-on” electrochemiluminescence aptasensor based on a self-enhanced luminophore for ochratoxin A detection. Anal. Bioanal. Chem. 2023, 415, 5833–5844. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Stepanov, N.; Maslova, O.; Akhundov, R.; Ismailov, A.; Efremenko, E. Immobilized luminescent bacteria for the detection of mycotoxins under discrete and flow-through conditions. Biosensors 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Rehmat, Z.; Mohammed, W.S.; Sadiq, M.B.; Somarapalli, M.; Kumar Anal, A. Ochratoxin A detection in coffee by competitive inhibition assay using chitosan-based surface plasmon resonance compact system. Colloids Surf. B Biointerfaces 2019, 174, 569–574. [Google Scholar] [CrossRef]
- Wei, T.; Ren, P.P.; Huang, L.L.; Ouyang, Z.C.; Wang, Z.Y.; Kong, X.F.; Li, T.J.; Yin, Y.L.; Wu, Y.N.; He, Q.H. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef]
- Asl, G.B.; Arvand, M.; Habibi, M.F. High affinity aptamers for ultra-sensitive detection of aflatoxin B1 in milk and groundnut samples with label-free photo-electrochemical aptasensor. Food Chem. 2022, 397, 133829. [Google Scholar] [CrossRef] [PubMed]
- Abera, B.D.; Falco, A.; Ibba, P.; Cantarella, G.; Petti, L.; Lugli, P. Development of flexible dispense-printed electrochemical immunosensor for Aflatoxin M1 detection in milk. Sensors 2019, 19, 3912. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M.; Mustapha, M. Electrospun cellulose acetate-doped 3D-graphene nanofibre for enhanced transduction of ochratoxin A determination. Bull. Mater. Sci. 2022, 45, 166. [Google Scholar] [CrossRef]
- Ben Abdallah, Z.; Grauby-Heywang, C.; Beven, L.; Cassagnere, S.; Moroté, F.; Maillard, E.; Sghaier, H.; Bouhacina, T.C. Development of an ultrasensitive label-free immunosensor for fungal aflatoxin B1 detection. Biochem. Eng. J. 2019, 150, 107262. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Shen, X.; Ma, S.; Liu, Y.; You, T. Ratiometric electrochemical aptasensor for ultrasensitive detection of Ochratoxin A based on a dual signal amplification strategy: Engineering the binding of methylene blue to DNA. Biosens. Bioelectron. 2020, 150, 111814. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Ma, S.; Wang, M.; You, T. Hairpin DNA assisted dual-ratiometric electrochemical aptasensor with high reliability and anti-interference ability for simultaneous detection of aflatoxin B1 and ochratoxin A. Biosens. Bioelectron. 2021, 174, 112654. [Google Scholar] [CrossRef]
- Selvolini, G.; Lettieri, M.; Tassoni, L.; Gastaldello, S.; Grillo, M.; Maran, C.; Marrazza, G. Electrochemical enzyme-linked oligonucleotide array for aflatoxin B1 detection. Talanta 2019, 203, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Beheshti-Marnani, A.; Hatefi-Mehrjardi, A.; Es’haghi, Z. A sensitive biosensing method for detecting of ultra-trace amounts of AFB1 based on “Aptamer/reduced graphene oxide” nano-bio interaction. Colloids Surf. B 2019, 175, 98–105. [Google Scholar] [CrossRef]
- Song, X.; Wang, D.; Kimb, M. Development of an immuno-electrochemical glass carbon electrode sensor based on graphene oxide/gold nanocomposite and antibody for the detection of patulin. Food Chem. 2021, 342, 128257. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A simple detection platform based on molecularly imprinted polymer for AFB1 and FuB1 mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, Q.; Liu, Y.; Qin, M.; Bikila, T.L.; Weng, X. A simple paper-based dual-analyte electrochemical nanobiosensor for mycotoxins using phosphorene-gold nanocomposites. IEEE Sens. J. 2022, 22, 19144–19151. [Google Scholar] [CrossRef]
- Liao, Z.; Guo, W.; Ning, G.; Wu, Y.; Wang, Y.; Ning, G. A sensitive electrochemical aptasensor for zearalenone detection based on target-triggered branched hybridization chain reaction and exonuclease I-assisted recycling. Anal. Bioanal. Chem. 2023, 415, 4911–4921. [Google Scholar] [CrossRef]
- Subak, H.; Selvolini, G.; Macchiagodena, M.; Ozkan-Ariksoysal, D.; Pagliai, M.; Procacci, P.; Marrazza, G. Mycotoxins aptasensing: From molecular docking to electrochemical detection of deoxynivalenol. Bioelectrochemistry 2020, 138, 107691. [Google Scholar] [CrossRef]
- Lai, H.; Ming, P.; Wu, M.; Wang, S.; Sun, D.; Zhai, H. An electrochemical aptasensor based on P-Ce-MOF@MWCNTs as signal amplification strategy for highly sensitive detection of zearalenone. Food Chem. 2023, 423, 136331. [Google Scholar] [CrossRef] [PubMed]
- Azri, F.A.; Eissa, S.; Zourob, M.; Chinnappan, R.; Sukor, R.; Yusof, N.A.; Raston, N.H.A.; Alhoshani, A.; Jinap, S. Electrochemical determination of zearalenone using a label-free competitive aptasensor. Microchim. Acta 2020, 187, 266. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yao, J.; Jiang, B.; Yuan, R.; Xiang, Y. NiCo2S4 nanoparticle-dispersed MoS2 nanosheets for catalytic and sensitive electrochemical aptamer sensing of ochratoxin A via cascaded amplifications. Sens. Actuators B Chem. 2022, 371, 132530. [Google Scholar] [CrossRef]
- Nan, M.; Bi, Y.; Xue, H.; Xue, S.; Long, H.; Pu, L.; Fu, G. Rapid determination of Ochratoxin A in grape and its commodities based on a label-free impedimetric aptasensor constructed by layer-by-layer self-assembly. Toxins 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, G.; Ben Aissa, S.; Nemčeková, K.; Catanante, G.; Raouafi, N.; Marty, J.L. Aptamer-modified pencil graphite electrodes for the impedimetric determination of ochratoxin A. Food Control 2020, 115, 107271. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Wu, X.; Pan, M.; Hu, N.; Wang, J.; Wang, S. Quartz crystal microbalance sensor based on covalent organic framework composite and molecularly imprinted polymer of poly(o-aminothiophenol) with gold nanoparticles for the determination of aflatoxin B1. Sens. Actuators B Chem. 2019, 291, 293–297. [Google Scholar] [CrossRef]
- Boshra, M.H.; El-Housseiny, G.S.; Farag, M.M.S.; Aboshanab, K.M. Innovative approaches for mycotoxin detection in various food categories. AMB Express 2024, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, T.B. Detecting mycotoxins in agricultural commodities. Mol. Biotechnol. 2003, 23, 61–71. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Huang, P.; Wei, F.; Ying, G.; Lu, J.; Zhou, L.; Kong, W. Regeneration and reuse of immunoafnity column for highly efficient clean-up and economic detection of ochratoxin A in malt and ginger. Toxins 2018, 10, 462. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Wang, F.; He, P. Recent advances in immunoassays and biosensors for mycotoxins detection in feedstuffs and foods. J. Anim. Sci. Biotechnol. 2021, 12, 108. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, R.; Catanante, G.; Sherazi, T.A.; Bhand, S.; Hayat, A.; Marty, J.L. Designed strategies for fluorescence-based biosensors for the detection of mycotoxins. Toxins 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Y.; Huang, H.; Chen, X.; Leng, Y.; Lai, W.; Hang, X.; Xiong, Y. Engineered gold nanoparticles as multicolor labels for simultaneous multi-mycotoxin detection on the immunochromatographic test strip nanosensor. Sens. Actuators B Chem. 2020, 316, 128107. [Google Scholar] [CrossRef]
- Altunbas, O.; Ozdas, A.; Yilmaz, M.D. Luminescent detection of Ochratoxin a using terbium chelated mesoporous silica nanoparticles. J. Hazard. Mater. 2020, 382, 121049. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, X.; Wang, D.; Zhang, Q.; Li, P.; Ding, X. Rapid on-site sensing aflatoxin B1 in food and feed via a chromatographic time-resolved fluoroimmunoassay. PLoS ONE 2015, 10, e0123266. [Google Scholar] [CrossRef]
- Yugender Goud, K.; Hayat, A.; Satyanarayana, M.; Sunil Kumar, V.; Catanante, G.; Vengatajalabathy Gobi, K.; Marty, J.L. Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim. Acta 2017, 184, 4401–4408. [Google Scholar] [CrossRef]
- Majdinasab, M.; Ben Aissa, S.; Marty, J.L. Advances in colorimetric strategies for mycotoxins detection: Toward rapid industrial monitoring. Toxins 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zeng, L.; Li, N.; Liu, C.; Chen, J. A wash-free and label-free colorimetric biosensor for naked-eye detection of aflatoxin B1 using G-quadruplex as the signal reporter. Food Chem. 2019, 298, 125034. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.; Zherdev, A.; Petrakova, A.; Sadykhov, E.; Koroleva, O.; Dzantiev, B. Rapid multiple immunoenzyme assay of mycotoxins. Toxins 2015, 7, 238–254. [Google Scholar] [CrossRef]
- Sheini, A. Colorimetric aggregation assay based on array of gold and silver nanoparticles for simultaneous analysis of aflatoxins, ochratoxin and zearalenone by using chemometric analysis and paper based analytical devices. Microchim. Acta 2020, 187, 167. [Google Scholar] [CrossRef]
- Kumari Shrestha, Y.; Krishna Shrestha, S. Fundamentals of colorimetry. In Advances in Colorimetry; Samanta, A.K., Ed.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Rene, W.; Lenoble, V.; Chioukh, M.; Branger, C. A turn-on fluorescent ion-imprinted polymer for selective and reliable optosensing of lead in real water samples. Sens. Actuators B Chem. 2020, 319, 128252. [Google Scholar] [CrossRef]
- Jo, E.-J.; Byun, J.-Y.; Mun, H.; Bang, D.; Son, J.H.; Lee, J.Y.; Lee, L.P.; Kim, M.-G. Single-step LRET aptasensor for rapid mycotoxin detection. Anal. Chem. 2018, 90, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ren, Y.; Li, Y.; Li, Y.; Long, F.; Zhu, A. Optical biosensors for microbial toxin detection: Recent advances and future trends. Microchem. J. 2023, 191, 108894. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Sun, C.; Zheng, M.; Zhang, J.; Liu, B.; Wang, Y.; Xie, Z.; Xu, N. Hybrids of carbon dots with subunit B of ricin toxin for enhanced immunomodulatory activity. J. Colloid Interface Sci. 2018, 523, 226–233. [Google Scholar] [CrossRef]
- Farré, M.; Kantiani, L.; Pérez, S.; Barceló, D. Sensors and biosensors in support of EU Directives. TRAC Trends Anal. Chem. 2009, 28, 170–185. [Google Scholar] [CrossRef]
- Kaur, M.; Gaba, J.; Singh, K.; Bhatia, Y.; Singh, A.; Singh, N. Recent advances in recognition receptors for electrochemical biosensing of mycotoxins—A review. Biosensors 2023, 13, 391. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Adányi, N.; Székács, A. Biosensors for Deoxynivalenol and Zearalenone determination in feed quality control. Toxins 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.-H.; Marty, J.L. Nano-aptasensing in mycotoxin analysis: Recent updates and progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Hianik, T. Electrochemical immuno-and aptasensors for mycotoxin determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef]
- Le, V.T.; Vasseghian, Y.; Dragoi, E.-N.; Moradi, M.; Khaneghah, A.M. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, Y.; Hu, X.; Zhang, J.; Chen, Q.; Chen, H. Recent progress in electrochemical nano-biosensors for detection of pesticides and mycotoxins in foods. Biosensors 2023, 13, 140. [Google Scholar] [CrossRef]
- Abd-Ellatief, R.; Abd-Ellatief, M.R. Electrochemical aptasensors: Current status and future perspectives. Diagnostics 2021, 11, 104. [Google Scholar] [CrossRef]
- Guo, X.D.; Wen, F.; Zheng, N.; Saive, M.; Fauconnier, M.L.; Wang, J.Q. Aptamer-based biosensor for detection of mycotoxins. Front. Chem. 2020, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Kesici, E.; Erdem, A. Impedimetric detection of Fumonisin B1 and its biointeraction with fsDNA. Int. J. Biol Macromol. 2019, 139, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Azizi-Lalabadi, M.; Motamedi, P.; Sadeghi, E. Electrochemical determination of T2 toxin by graphite/polyacrylonitrile nanofiber electrode. Food Sci. Nutr. 2021, 9, 1171–1179. [Google Scholar] [CrossRef]
- He, S.; Yuan, Y.; Nag, A.; Feng, S.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C.; Organ, D.R. A review on the use of impedimetric sensors for the inspection of food quality. Int. J. Environ. Res. Public Health 2020, 17, 5220. [Google Scholar] [CrossRef]
- Venton, B.J.; DiScenza, D.J. Voltammetry. In Electrochemistry for Bioanalysis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–50. [Google Scholar] [CrossRef]
- Meliana, C.; Liu, J.; Show, P.L.; Low, S.S. Biosensor in smart food traceability system for food safety and security. Bioengineered 2024, 15, 2310908. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.; Wang, J. Advanced biosensors for mycotoxin detection incorporating miniaturized meters. Biosens. Bioelectron. 2023, 224, 115077. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef] [PubMed]
- Geicu, O.I.; Bilteanu, L.; Stanca, L.; Ionescu Petcu, A.; Iordache, F.; Pisoschi, A.M.; Serban, A.I. Composition-based risk estimation of mycotoxins in dry dog foods. Foods 2023, 12, 110. [Google Scholar] [CrossRef]
- Matson, R.S. ELISA-based biosensors. Methods Mol. Biol. 2023, 2612, 225–238. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, H.; Zhu, M.; Wang, F.; Meng, H.; Feng, L. Electrochemical/visual dual-readout aptasensor for ochratoxin a detection integrated into a miniaturized paper-based analytical device. Biosens. Bioelectron. 2021, 180, 113146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, S.; Zhang, Y.; Wang, S.; Guo, J.; Wang, J. A highly sensitive photothermal immunochromatographic sensor for detection of aflatoxin B1 based on Cu2-xSe-Au nanoparticles. Food Chem. 2024, 401, 134065. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Pan, S.; Hong, F.; Lu, P.; Hu, X.; Jiang, F.; Wu, L.; Chen, Y. Bimetallic nanozyme-bioenzyme hybrid material-mediated ultrasensitive and automatic immunoassay for the detection of aflatoxin B1 in food. Biosens. Bioelectron. 2024, 248, 115992. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Amine, A. Biorecognition elements (Chapter 3). In Wearable Physical, Chemical and Biological Sensors; Morales-Narvaez, E., Dincer, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–70. [Google Scholar] [CrossRef]
- Oliveira, I.S.; da Silva Junior, A.G.; de Andrade, C.A.S.; Oliveira, M.D.L. Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr. Opin. Food Sci. 2019, 29, 64–79. [Google Scholar] [CrossRef]
- Sivasubramanian, S.P.; Vhanbatte, S.; Rajagopalan, S. Biosensor global market potential. Asian Tex. J. 2020, 29, 43–45. [Google Scholar]
- Bhide, A.; Ganguly, A.; Parupudi, T.; Ramasamy, M.; Muthukumar, S.; Prasad, S. Next-generation continuous metabolite sensing toward emerging sensor needs. ACS Omega 2021, 6, 6031–6040. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical biosensors and their applications for the detection of water pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).