Controlled Insertion of Silver Nanoparticles in LbL Nanostructures: Fine-Tuning the Sensing Units of an Impedimetric E-Tongue
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles Chemical Synthesis
2.2. Silver Nanoparticles Transmission Electron Microscopy and UV-Vis Absorption Analysis
2.3. Layer-by-Layer Technique and Deposition Parameters
2.3.1. Zeta Potential
2.3.2. Adsorption Kinetics
2.4. Microfluidic E-Tongue Setup
2.4.1. Analytes
2.4.2. Data Analysis
3. Results and Discussion
3.1. Synthesis of NPs
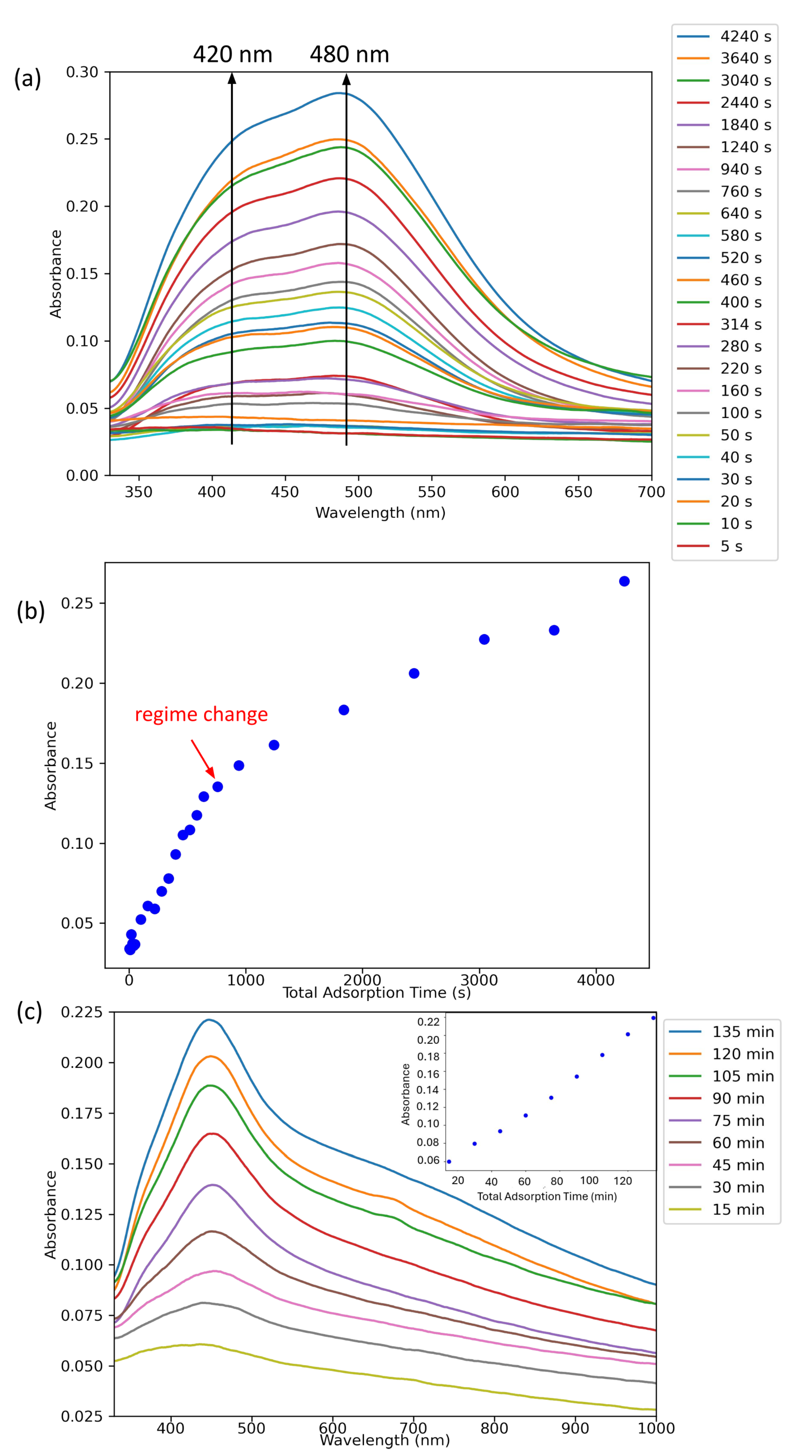
3.2. Silver NP Adsorption Kinetics
3.3. E-Tongue Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassie, A.T.; Bachheti, R.K.; Bachheti, A. Recent Advances in Nanoparticles for Environmental Monitoring and Sensing: An Overview. In Nanomaterials for Environmental and Agricultural Sectors; Bachheti, R.K., Bachheti, A., Husen, A., Eds.; Springer: Singapore, 2023; pp. 107–122. [Google Scholar] [CrossRef]
- Caroleo, F.; Magna, G.; Naitana, M.L.; Di Zazzo, L.; Martini, R.; Pizzoli, F.; Muduganti, M.; Lvova, L.; Mandoj, F.; Nardis, S.; et al. Advances in Optical Sensors for Persistent Organic Pollutant Environmental Monitoring. Sensors 2022, 22, 2649. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Dam, P.; Chakraborty, J.; Paret, M.L.; Katı, A.; Altuntas, S.; Sarkar, R.; Ghorai, S.; Gangopadhyay, D.; Mandal, A.K.; et al. Potential of nanobiosensor in sustainable agriculture: The state-of-art. Heliyon 2022, 8, e12207. [Google Scholar] [CrossRef] [PubMed]
- Chugh, B.; Poddar, D.; Singh, A.; Yadav, P.; Thakur, S.; Nguyen, T.A.; Rajendran, S. 6—Nanoparticles-based sensors for agricultural application. In Nanosensors for Smart Agriculture; Denizli, A., Nguyen, T.A., Rajendran, S., Yasin, G., Nadda, A.K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–146. [Google Scholar] [CrossRef]
- Nadporozhskaya, M.; Kovsh, N.; Paolesse, R.; Lvova, L. Recent Advances in Chemical Sensors for Soil Analysis: A Review. Chemosensors 2022, 10, 35. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Arshad, F.; Hassan, I.U.; Awan, T.; Salim, H.; Pedram, M.Z.; Ahmed, W.; Patel, V.; Karakoti, A.S.; Vinu, A. Nanomaterials-based sensors for the detection of COVID-19: A review. Bioeng. Transl. Med. 2022, 7, e10305. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Er, E.; Sánchez-Iglesias, A.; Silvestri, A.; Arnaiz, B.; Liz-Marzán, L.M.; Prato, M.; Criado, A. Metal Nanoparticles//MoS2 Surface-Enhanced Raman Scattering-Based Sandwich Immunoassay for α-Fetoprotein Detection. ACS Appl. Mater. Interfaces 2021, 13, 8823–8831. [Google Scholar] [CrossRef] [PubMed]
- Montes-García, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samorì, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sannegowda, L.K. Chapter 25—Metal nanoparticles for electrochemical sensing applications. In Handbook of Nanomaterials for Sensing Applications; Hussain, C.M., Kailasa, S.K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 589–629. [Google Scholar] [CrossRef]
- Białas, K.; Moschou, D.; Marken, F.; Estrela, P. Electrochemical sensors based on metal nanoparticles with biocatalytic activity. Microchim. Acta 2022, 189, 172. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Iwaki, L.E.O.; Soares, A.C.; Rodrigues, V.C.; Melendez, M.E.; Fregnani, J.H.T.G.; Reis, R.M.; Carvalho, A.L.; Corrêa, D.S.; Oliveira, O.N.J. Immunosensor for Pancreatic Cancer Based on Electrospun Nanofibers Coated with Carbon Nanotubes or Gold Nanoparticles. ACS Omega 2017, 2, 6975–6983. [Google Scholar] [CrossRef]
- Jensen, G.C.; Krause, C.E.; Sotzing, G.A.; Rusling, J.F. Inkjet-printed gold nanoparticle electrochemical arrays on plastic. Application to immunodetection of a cancer biomarker protein. Phys. Chem. Chem. Phys. 2011, 13, 4888–4894. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and their applications in early diagnosis of cancer. Sens. Bio Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A. Non-selective chemical sensors in analytical chemistry: From “electronic nose” to “electronic tongue”. Fresenius J. Anal. Chem. 1998, 361, 255–260. [Google Scholar] [CrossRef]
- Zniber, M.; Vahdatiyekta, P.; Huynh, T.P. Analysis of urine using electronic tongue towards non-invasive cancer diagnosis. Biosens. Bioelectron. 2023, 219, 114810. [Google Scholar] [CrossRef] [PubMed]
- Braz, D.C.; Neto, M.P.; Shimizu, F.M.; Sá, A.C.; Lima, R.S.; Gobbi, A.L.; Melendez, M.E.; Arantes, L.M.B.; Carvalho, A.L.; Paulovich, F.V.; et al. Using machine learning and an electronic tongue for discriminating saliva samples from oral cavity cancer patients and healthy individuals. Talanta 2022, 243, 123327. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.M.; Gaál, G.; Braunger, M.L.; Riul, A. Recent Developments on Devices Applied to Impedimetric Electronic Tongues; IOP Publishing: Bristol, UK, 2021. [Google Scholar] [CrossRef]
- Facure, M.H.; Braunger, M.L.; Mercante, L.A.; Paterno, L.G.; Riul, A., Jr. Electrical Impedance-Based Electronic Tongue In Encyclopedia of Sensors and Biosensors (First Edition), 1st ed.; Narayan, R., Ed.; Elsevier: Oxford, UK, 2023; pp. 567–590. [Google Scholar] [CrossRef]
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Riul, A.; dos Santos, D.S.; Wohnrath, K.; Di Tommazo, R.; Carvalho, A.C.P.L.F.; Fonseca, F.J.; Oliveira, O.N.; Taylor, D.M.; Mattoso, L.H.C. Artificial Taste Sensor: Efficient Combination of Sensors Made from Langmuir-Blodgett Films of Conducting Polymers and a Ruthenium Complex and Self-Assembled Films of an Azobenzene-Containing Polymer. Langmuir 2002, 18, 239–245. [Google Scholar] [CrossRef]
- Bonanni, A.; del Valle, M. Use of nanomaterials for impedimetric DNA sensors: A review. Anal. Chim. Acta 2010, 678, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, X.; Zhu, Z. Biomimetic sensors and biosensors for qualitative and quantitative analyses of five basic tastes. TrAC Trends Anal. Chem. 2017, 87, 58–70. [Google Scholar] [CrossRef]
- Toko, K.; Matsuno, T.; Yamafuji, K.; Hayashi, K.; Ikezaki, H.; Sato, K.; Toukubo, R.; Kawarai, S. Multichannel taste sensor using electric potential changes in lipid membranes. Biosens. Bioelectron. 1994, 9, 359–364. [Google Scholar] [CrossRef]
- Han, J.; Wang, B.; Bender, M.; Seehafer, K.; Bunz, U.H.F. Poly(p-phenyleneethynylene)-based tongues discriminate fruit juices. Analyst 2017, 142, 537–543. [Google Scholar] [CrossRef]
- Riul, A.; de Sousa, H.C.; Malmegrim, R.R.; dos Santos, D.S.; Carvalho, A.C.; Fonseca, F.J.; Oliveira, O.N.; Mattoso, L.H. Wine classification by taste sensors made from ultra-thin films and using neural networks. Sens. Actuators B Chem. 2004, 98, 77–82. [Google Scholar] [CrossRef]
- Ferreira, L.; Pinheiro, P.; Neto, N.B.; Reis, M. Buckypaper-Based Nanostructured Sensor for Port Wine Analysis. Sensors 2022, 22, 9732. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Scagion, V.P.; Pavinatto, A.; Sanfelice, R.C.; Mattoso, L.H.C.; Correa, D.S. Electronic Tongue Based on Nanostructured Hybrid Films of Gold Nanoparticles and Phthalocyanines for Milk Analysis. J. Nanomater. 2015, 2015, 890637. [Google Scholar] [CrossRef]
- Coatrini-Soares, A.; Coatrini-Soares, J.; Popolin Neto, M.; de Mello, S.S.; Pinto, D.D.S.C.; Carvalho, W.A.; Gilmore, M.S.; Piazzetta, M.H.O.; Gobbi, A.L.; de Mello Brandão, H.; et al. Microfluidic E-tongue to diagnose bovine mastitis with milk samples using Machine learning with Decision Tree models. Chem. Eng. J. 2023, 451, 138523. [Google Scholar] [CrossRef]
- Ferreira, E.J.; Pereira, R.C.T.; Delbem, A.C.B.; Oliveira Junior, O.N.d.; Mattoso, L.H.C. Random subspace method for analysing coffee with electronic tongue. Electron. Lett. 2007, 43, 1138–1140. [Google Scholar] [CrossRef]
- de Morais, T.C.B.; Rodrigues, D.R.; de Carvalho Polari Souto, U.T.; Lemos, S.G. A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chem. 2019, 273, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Polshin, E.; Rudnitskaya, A.; Kirsanov, D.; Legin, A.; Saison, D.; Delvaux, F.; Delvaux, F.R.; Nicolaï, B.M.; Lammertyn, J. Electronic tongue as a screening tool for rapid analysis of beer. Talanta 2010, 81, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Men, H.; Shi, Y.; Fu, S.; Jiao, Y.; Qiao, Y.; Liu, J. Mining Feature of Data Fusion in the Classification of Beer Flavor Information Using E-Tongue and E-Nose. Sensors 2017, 17, 1656. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, W.; Wang, Y.; Wang, J. Monitoring the fermentation, post-ripeness and storage processes of set yogurt using voltammetric electronic tongue. J. Food Eng. 2017, 203, 41–52. [Google Scholar] [CrossRef]
- Magro, C.; Moura, T.; Ribeiro, P.A.; Raposo, M.; Sério, S. Smart Sensing for Antibiotic Monitoring in Mineral and Surface Water: Development of an Electronic Tongue Device. Chem. Proc. 2021, 5, 58. [Google Scholar] [CrossRef]
- El Alami El Hassani, N.; Tahri, K.; Llobet, E.; Bouchikhi, B.; Errachid, A.; Zine, N.; El Bari, N. Emerging approach for analytical characterization and geographical classification of Moroccan and French honeys by means of a voltammetric electronic tongue. Food Chem. 2018, 243, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V.; Volkov, S.; Khaydukova, M.; Fedorov, A.; Lisitsyna, I.; Kirsanov, D.; Legin, A. Determination of three quality parameters in vegetable oils using potentiometric e-tongue. J. Food Compos. Anal. 2019, 75, 75–80. [Google Scholar] [CrossRef]
- Wójcik, S.; Ciepiela, F.; Jakubowska, M. Computer vision analysis of sample colors versus quadruple-disk iridium-platinum voltammetric e-tongue for recognition of natural honey adulteration. Measurement 2023, 209, 112514. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Torabi, F.; Faridbod, F.; Ghasemi, J.B.; González-Calabuig, A.; Del Valle, M. Voltammetric Electronic Tongue for the Simultaneous Determination of Three Benzodiazepines. Sensors 2019, 19, 5002. [Google Scholar] [CrossRef] [PubMed]
- Aramini, A.; Bianchini, G.; Lillini, S.; Bordignon, S.; Tomassetti, M.; Novelli, R.; Mattioli, S.; Lvova, L.; Paolesse, R.; Chierotti, M.R.; et al. Unexpected Salt/Cocrystal Polymorphism of the Ketoprofen–Lysine System: Discovery of a New Ketoprofen–l-Lysine Salt Polymorph with Different Physicochemical and Pharmacokinetic Properties. Pharmaceuticals 2021, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.; Psotta, C.; Cirovic, S.; Ohlsson, L.; Shleev, S. Electronic Tongue for Direct Assessment of SARS-CoV-2-Free and Infected Human Saliva—A Feasibility Study. Biosensors 2023, 13, 717. [Google Scholar] [CrossRef]
- Cruz, M.G.; Ferreira, N.S.; Gomes, M.T.S.; Botelho, M.J.; Costa, S.T.; Vale, C.; Rudnitskaya, A. Determination of paralytic shellfish toxins using potentiometric electronic tongue. Sens. Actuators B Chem. 2018, 263, 550–556. [Google Scholar] [CrossRef]
- Cetó, X.; del Valle, M. Electronic tongue applications for wastewater and soil analysis. iScience 2022, 25, 104304. [Google Scholar] [CrossRef]
- Singh, M.; del Valle, M. 25–Arsenic biosensors: Challenges and opportunities for high-throughput detection. In Handbook of Arsenic Toxicology (Second Edition), 2nd ed.; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2023; pp. 649–665. [Google Scholar] [CrossRef]
- Legin, E.; Zadorozhnaya, O.; Khaydukova, M.; Kirsanov, D.; Rybakin, V.; Zagrebin, A.; Ignatyeva, N.; Ashina, J.; Sarkar, S.; Mukherjee, S.; et al. Rapid Evaluation of Integral Quality and Safety of Surface and Waste Waters by a Multisensor System (Electronic Tongue). Sensors 2019, 19, 2019. [Google Scholar] [CrossRef]
- Iurgenson, N.; Wang, X.; Kong, L.; Sun, X.; Legin, A.; Wang, P.; Wan, H.; Kirsanov, D. Feasibility study of multisensor systems for the assessment of water pollution index induced by heavy metal contamination. Microchem. J. 2024, 197, 109762. [Google Scholar] [CrossRef]
- Lvova, L.; Jahatspanian, I.; Mattoso, L.H.; Correa, D.S.; Oleneva, E.; Legin, A.; Di Natale, C.; Paolesse, R. Potentiometric E-Tongue System for Geosmin/Isoborneol Presence Monitoring in Drinkable Water. Sensors 2020, 20, 821. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Hong, J.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210-211, 831–835. [Google Scholar] [CrossRef]
- Decher, G. Layer-by-Layer Assembly (Putting Molecules to Work). In Multilayer Thin Films; Wiley: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Bjonmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- PodraZka, M.; BAczyNska, E.; Kundys, M.; JeleN, P.S.; Witkowska Nery, E. Electronic Tongue—A Tool for All Tastes? Biosensors 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Mercante, L.A.; Andre, R.S.; Facure, M.H.M.; Fugikawa-Santos, L.; Correa, D.S. Design of a bioelectronic tongue for glucose monitoring using zinc oxide nanofibers and graphene derivatives. Sens. Actuators Rep. 2021, 3, 100050. [Google Scholar] [CrossRef]
- Magro, C.; Sardinha, M.; Ribeiro, P.A.; Raposo, M.; Sério, S. Magnetron Sputtering Thin Films as Tool to Detect Triclosan in Infant Formula Powder: Electronic Tongue Approach. Coatings 2021, 11, 336. [Google Scholar] [CrossRef]
- Fukushima, K.L.; Scagion, V.P.; Facure, M.H.M.; Pinheiro, A.C.M.; Correa, D.S.; Nunes, C.A.; Oliveira, J.E. Development of an Electronic Tongue Based on a Nanocomposite for Discriminating Flavor Enhancers and Commercial Salts. IEEE Sens. J. 2021, 21, 1250–1256. [Google Scholar] [CrossRef]
- Hensel, R.C.; Braunger, M.L.; Oliveira, B.; Shimizu, F.M.; Oliveira, O.N.; Hillenkamp, M.; Riul, A.; Rodrigues, V. Controlled Incorporation of Silver Nanoparticles into Layer-by-Layer Polymer Films for Reusable Electronic Tongues. ACS Appl. Nano Mater. 2021, 4, 14231–14240. [Google Scholar] [CrossRef]
- Wang, K.; Zhuang, H.; Bing, F.; Chen, D.; Feng, T.; Xu, Z. Evaluation of eight kinds of flavor enhancer of umami taste by an electronic tongue. Food Sci. Nutr. 2021, 9, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Q.; Shen, C.; Zhang, S.; Gan, Z.; Hu, R.; Zhao, J.; Ni, Y. Evaluation of monosodium glutamate, disodium inosinate and guanylate umami taste by an electronic tongue. J. Food Eng. 2013, 116, 627–632. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Chen, Y.P.; Liu, Z.; Jiang, S.; Chen, G.; Liu, Y. Exploring the relationships between perceived umami intensity, umami components and electronic tongue responses in food matrices. Food Chem. 2022, 368, 130849. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.R.; An, J.H.; Song, H.S.; Park, J.W.; Lee, S.H.; Kim, J.H.; Jang, J.; Park, T.H. Duplex Bioelectronic Tongue for Sensing Umami and Sweet Tastes Based on Human Taste Receptor Nanovesicles. ACS Nano 2016, 10, 7287–7296. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jung, J.W.; Kim, D.; Ahn, Y.J.; Hong, S.; Kwon, H.W. Discrimination of Umami Tastants Using Floating Electrode-Based Bioelectronic Tongue Mimicking Insect Taste Systems. ACS Nano 2015, 9, 11728–11736. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Elizarova, I.S.; Luckham, P.F. Layer-by-layer adsorption: Factors affecting the choice of substrates and polymers. Adv. Colloid Interface Sci. 2018, 262, 1–20. [Google Scholar] [CrossRef]
- Buron, C.C.; Filiâtre, C. Overshoots of adsorption kinetics during layer-by-layer polyelectrolyte film growth: Role of counterions. J. Colloid Interface Sci. 2014, 413, 147–153. [Google Scholar] [CrossRef]
- Kern, W. Cleaning solutions based on hydrogen peroxide for use in silicon semiconductor technology. RCA Rev. 1970, 31, 187–206. [Google Scholar]
- Braunger, M.L.; Fier, I.; Shimizu, F.M.; de Barros, A.; Rodrigues, V.; Riul, A. Influence of the Flow Rate in an Automated Microfluidic Electronic Tongue Tested for Sucralose Differentiation. Sensors 2020, 20, 6194. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Crumbliss, A.L.; Warwick, Z.S.; Graham, B.G. Thresholds for sodium salts in young and elderly human subjects: Correlation with molar conductivity of anion. Chem. Senses 1990, 15, 671–678. [Google Scholar] [CrossRef]
- Wu, J. Cluster Analysis and K-means Clustering: An Introduction. In Advances in K-Means Clustering: A Data Mining Thinking; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–16. [Google Scholar] [CrossRef]
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A.; Štajdohar, M.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. Available online: http://jmlr.org/papers/v14/demsar13a.html (accessed on 14 April 2024).
- University of Ljubljana. Orange, version 3.36.2; University of Ljubljana: Ljubljana, Serbia, 2022. Available online: https://orangedatamining.com (accessed on 14 April 2024).
- Garcia, M.A. Surface plasmons in metallic nanoparticles: Fundamentals and applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
- Hensel, R.C.; Moreira, M.; Riul, A.; Oliveira, O.N.; Rodrigues, V.; Hillenkamp, M. Monitoring the dispersion and agglomeration of silver nanoparticles in polymer thin films using localized surface plasmons and Ferrell plasmons. Appl. Phys. Lett. 2020, 116, 103105. [Google Scholar] [CrossRef]
- De Barros, A. CAPÍTULO 8—Filmes Automontados por Adsorção Física; Universidade Estadual de Campinas: Campinas, SP, Brazil, 2020. [Google Scholar]
- Introduction. In Spectrochemical Analysis by Atomic Absorption and Emission (2); Lajunen, L.H.J., Perämäki, P., Eds.; The Royal Society of Chemistry: London, UK, 2004; pp. 1–15. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.H.; Braunger, M.L.; de Barros, A.; Hensel, R.C.; Dalafini, J.G.; Mazali, I.O.; Corrêa, L.M.; Ugarte, D.; Riul Jr, A.; Rodrigues, V. Controlled Insertion of Silver Nanoparticles in LbL Nanostructures: Fine-Tuning the Sensing Units of an Impedimetric E-Tongue. Chemosensors 2024, 12, 87. https://doi.org/10.3390/chemosensors12060087
Gonçalves MH, Braunger ML, de Barros A, Hensel RC, Dalafini JG, Mazali IO, Corrêa LM, Ugarte D, Riul Jr A, Rodrigues V. Controlled Insertion of Silver Nanoparticles in LbL Nanostructures: Fine-Tuning the Sensing Units of an Impedimetric E-Tongue. Chemosensors. 2024; 12(6):87. https://doi.org/10.3390/chemosensors12060087
Chicago/Turabian StyleGonçalves, Maria Helena, Maria Luisa Braunger, Anerise de Barros, Rafael C. Hensel, Julianna G. Dalafini, Italo O. Mazali, Leonardo M. Corrêa, Daniel Ugarte, Antonio Riul Jr, and Varlei Rodrigues. 2024. "Controlled Insertion of Silver Nanoparticles in LbL Nanostructures: Fine-Tuning the Sensing Units of an Impedimetric E-Tongue" Chemosensors 12, no. 6: 87. https://doi.org/10.3390/chemosensors12060087
APA StyleGonçalves, M. H., Braunger, M. L., de Barros, A., Hensel, R. C., Dalafini, J. G., Mazali, I. O., Corrêa, L. M., Ugarte, D., Riul Jr, A., & Rodrigues, V. (2024). Controlled Insertion of Silver Nanoparticles in LbL Nanostructures: Fine-Tuning the Sensing Units of an Impedimetric E-Tongue. Chemosensors, 12(6), 87. https://doi.org/10.3390/chemosensors12060087





