Synergistic Enhancement Effect of Ag/rGO as SERS Platform for Capture and Trace Detection of Fenvalerate Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
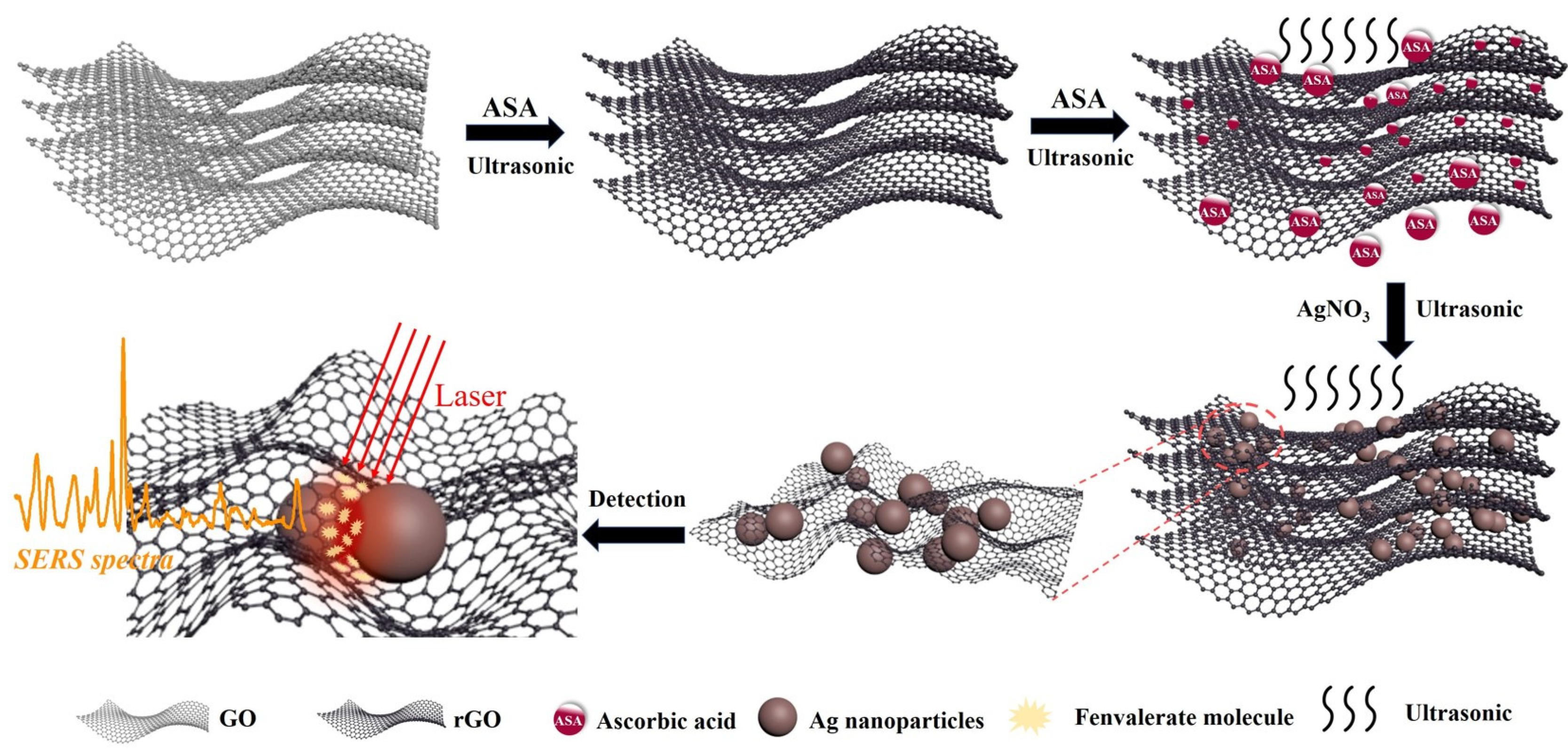
2.3. Fabrication of Ag/rGO Hybrid Material
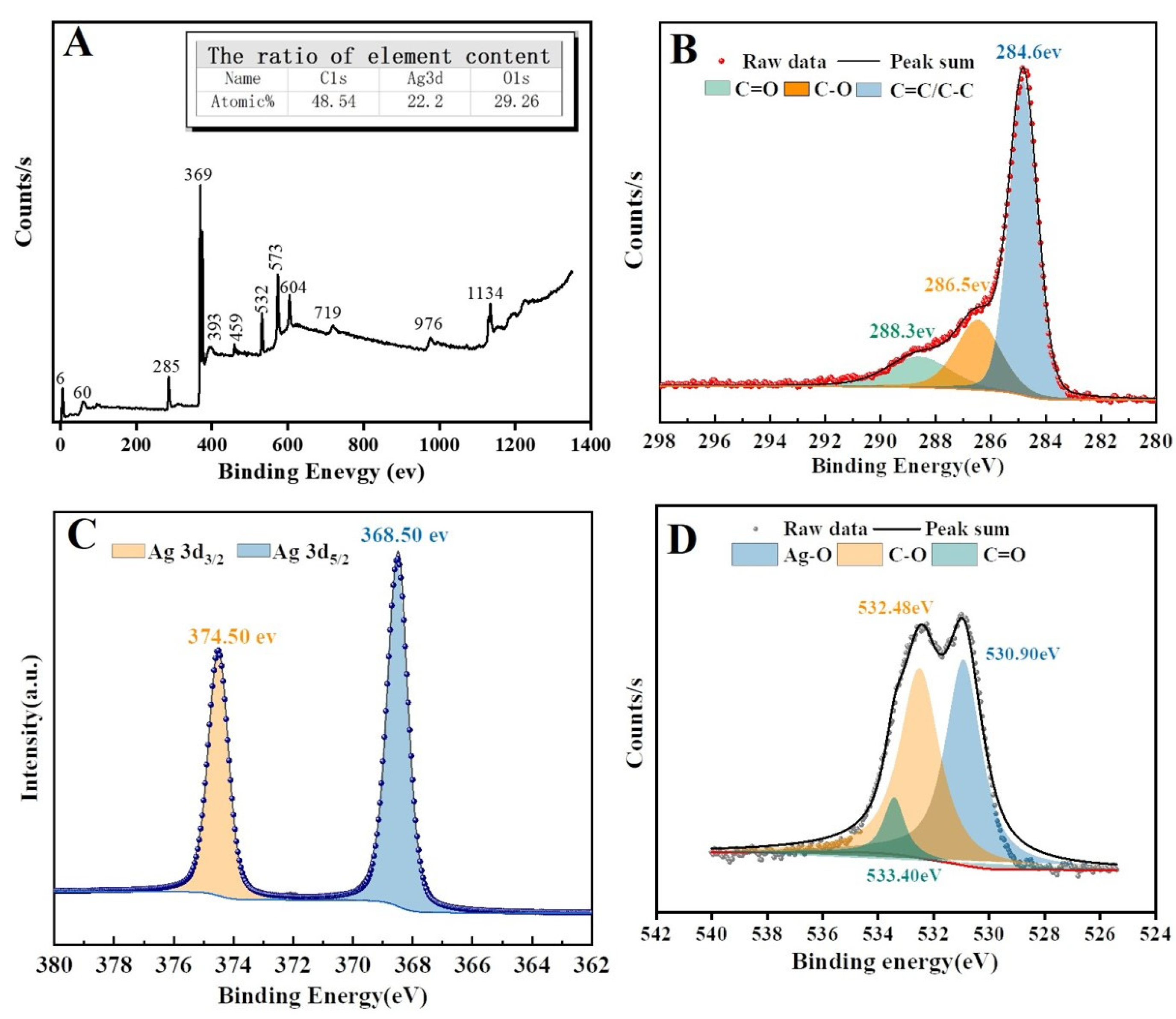
2.4. SEM, TEM, EDS, XPS, and Raman Analysis
2.5. FDTD Simulation of Ag/rGO Material
2.6. Vibrational Spectrum Calculation of Fenvalerate
2.7. Theoretical Modeling
2.8. Spectral Data Processing
3. Results and Discussion
3.1. Fabrication and Characterization of Ag/rGO
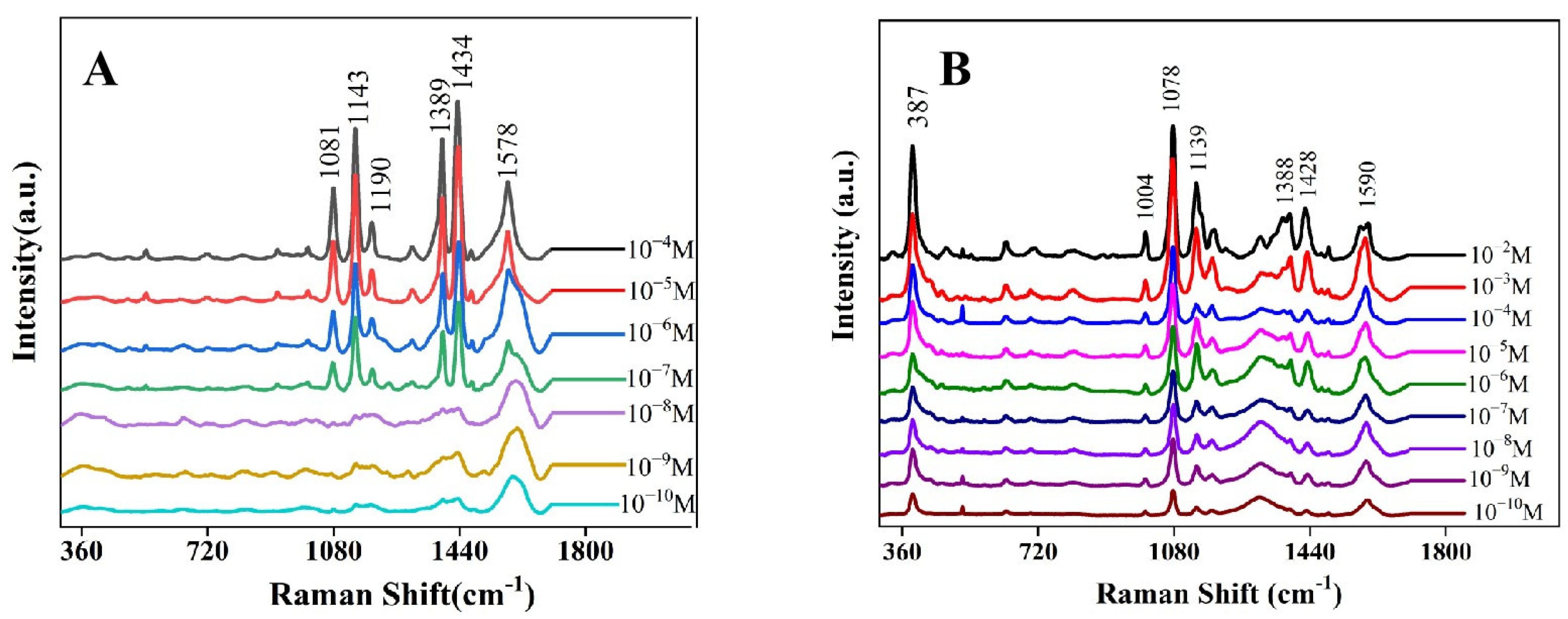
3.2. SERS Performance of Ag/rGO Composite Substrate
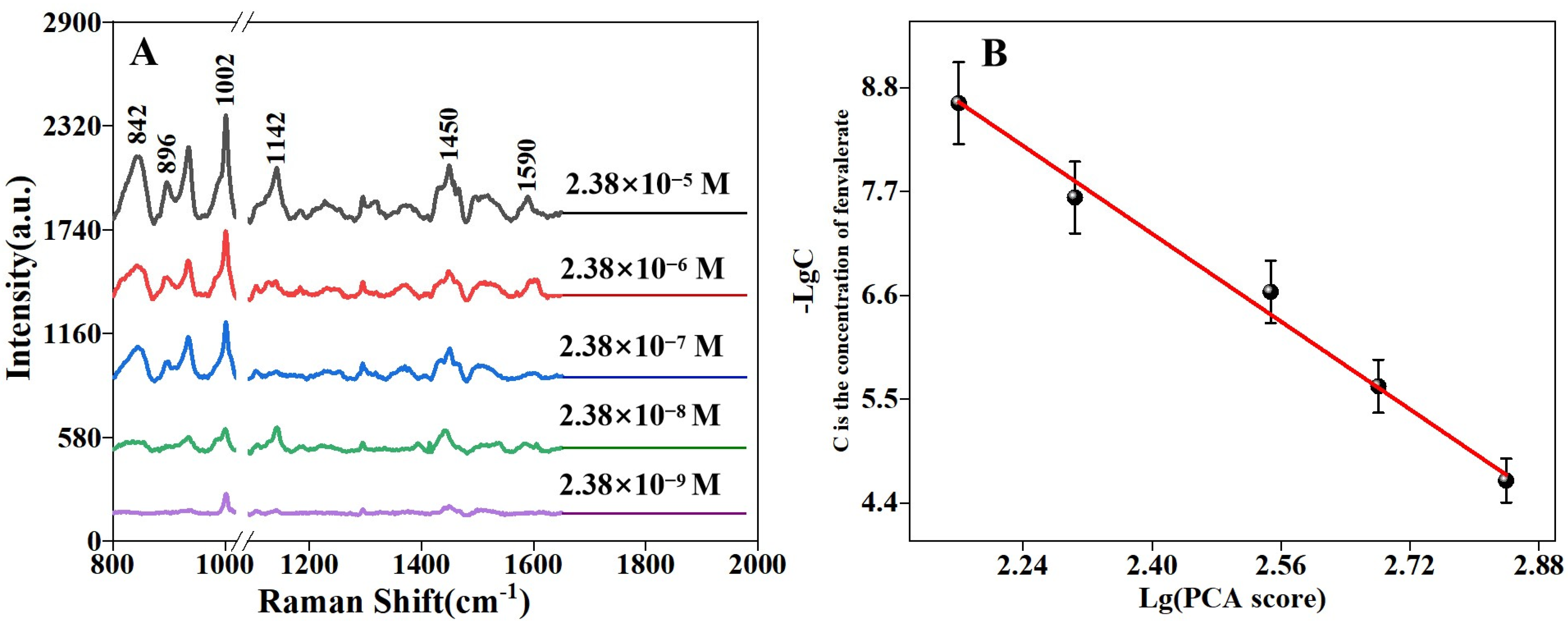
3.3. SERS Detection of Fenvalerate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, W.; Yu, L.; Li, X.; Chen, J.; Wang, X.; Deng, Z.; Xiao, Y. Hexafluoroisopropanol-based hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of pyrethroids in tea beverages and fruit juices. Food Chem. 2019, 274, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Kajimura, K.; Yamasaki, K.; Sawabe, Y.; Nomura, C.; Taguchi, S.; Obana, H. Simple and Rapid Determination of Cypermethrin and Fenvalerate Residues in Kampo Products by Gas Chromatography-Mass Spectrometry with Negative Chemical Ionization. J. Health Sci. 2009, 55, 777–782. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Liu, C.; Zhou, Z.; Wang, P. Recent advances in rapid detection of pesticide residues. Chin. J. Pestic. Sci. 2019, 21, 852–864. [Google Scholar] [CrossRef]
- Wang, M.; Kang, H.; Xu, D.; Wang, C.; Liu, S.; Hu, X. Label-free impedimetric immunosensor for sensitive detection of fenvalerate in tea. Food Chem. 2013, 141, 84–90. [Google Scholar] [CrossRef]
- Thiare, D.D.; Coly, A.; Sarr, D.; Khonte, A.; Diop, A.; Gaye-Seye, M.D.; Delattre, F.; Tine, A.; Aaron, J.-J. Determination of the fenvalerate insecticide in natural waters by a photochemically-induced fluorescence method. Maced. J. Chem. Chem. Eng. 2015, 34, 245–254. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, Y.; Zhang, J.; Qiao, Y.; Wang, Q.; Che, G. Fabrication of pollutant-resistance SERS imprinted sensors based on SiO2@TiO2@Ag composites for selective detection of pyrethroids in water. J. Phys. Chem. Solids 2020, 138, 109254. [Google Scholar] [CrossRef]
- Valley, N.; Greeneltch, N.; Van Duyne, R.P.; Schatz, G.C. A Look at the Origin and Magnitude of the Chemical Contribution to the Enhancement Mechanism of Surface-Enhanced Raman Spectroscopy (SERS): Theory and Experiment. J. Phys. Chem. Lett. 2013, 4, 2599–2604. [Google Scholar] [CrossRef]
- Schatz, G.C.; Young, M.A.; Van Duyne, R.P. Electromagnetic mechanism of SERS. Surf.-Enhanc. Raman Scatt. 2006, 103, 19–45. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, J.; Wang, X.; Song, G.; Lu, F.; You, L.; Li, J. Ag nanoparticles decorated Ag@ZrO2 composite nanospheres as highly active SERS substrates for quantitative detection of hexavalent chromium in waste water. J. Mol. Liq. 2020, 319, 114158. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, B.; Huang, J. Research progress on preparation of high active substrate base LSPR effect. Appl. Chem. Ind. 2020, 49, 709–714. [Google Scholar]
- Li, Y.; Yu, L.; Li, J.; Wang, L.; Lu, R. Polyamide@Ag coralloid nanoarrays with 3D high -density hot spots for ultrasensitive SERS sensing. Chem. Eng. J. 2020, 397, 125434. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Kim, N.-J.; Kim, H.; Yi, G.-C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of Chemical Enhancement Mechanism in Non-plasmonic Surface Enhanced Raman Spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Cui, Q.; Li, S.; Tian, Y.; Ma, H.; Wang, C.; Zhang, M.; Chen, M.; Li, G.; Liu, X. Double profound enhancements of Cu2O nano-octahedrons connected by intertwined Ag nanovines for elevating SERS activity toward ultrasensitive pesticide detection. Opt. Express 2022, 30, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liang, P.; Yu, Z.; Xia, J.; Ni, D.; Wang, D.; Zhou, Y.; Cao, Y.; Chen, J.; Chen, J.; et al. Self-assembled “bridge” substance for organochlorine pesticides detection in solution based on Surface Enhanced Raman Scattering. J. Hazard. Mater. 2020, 382, 121023. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.-W.; Pu, H.; Wei, Q. Polymer multilayers enabled stable and flexible Au@Ag nanoparticle array for nondestructive SERS detection of pesticide residues. Talanta 2021, 223, 121782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hao, R.; Wang, Z.; Zhang, H.; Hao, Y.; Zhang, C.; Liu, Y. Green synthesis of multi-dimensional plasmonic coupling structures: Graphene oxide gapped gold nanostars for highly intensified surface enhanced Raman scattering. Chem. Eng. J. 2018, 349, 581–587. [Google Scholar] [CrossRef]
- Ling, X.; Moura, L.G.; Pimenta, M.A.; Zhang, J. Charge-Transfer Mechanism in Graphene-Enhanced Raman Scattering. J. Phys. Chem. C 2012, 116, 25112–25118. [Google Scholar] [CrossRef]
- He, Q.; Han, Y.; Huang, Y.; Gao, J.; Gao, Y.; Han, L.; Zhang, Y. Reusable dual-enhancement SERS sensor based on graphene and hybrid nanostructures for ultrasensitive lead (II) detection. Sens. Actuators B-Chem. 2021, 341, 130031. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Zhao, B.-C.; Hao, R.; Wang, Z.; Hao, Y.-W.; Zhao, B.; Liu, Y.-Q. Graphene oxide-highly anisotropic noble metal hybrid systems for intensified surface enhanced Raman scattering and direct capture and sensitive discrimination in PCBs monitoring. J. Hazard. Mater. 2020, 385, 121510. [Google Scholar] [CrossRef]
- Biasotto, G.; Chiad, A.; Novara, C.; Fontana, M.; Armandi, M.; Zaghete, M.A.; Giorgis, F.; Rivolo, P. Graphenic Aerogels Decorated with Ag Nanoparticles as 3D SERS Substrates for Biosensing. Part. Part. Syst. Charact. 2020, 37, 2000095. [Google Scholar] [CrossRef]
- Zheng, H.; Ni, D.; Yu, Z.; Liang, P. Preparation of SERS-active substrates based on graphene oxide/silver nanocomposites for rapid zdetection of L-Theanine. Food Chem. 2017, 217, 511–516. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical-method for solving the local density functional for polyatomic-molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Zemlyanov, D.Y.; Savinova, E.; Scheybal, A.; Doblhofer, K.; Schlogl, R. XPS observation of OH groups incorporated in an Ag(111) electrode. Surf. Sci. 1998, 418, 441–456. [Google Scholar] [CrossRef]
- Feng, L.; Wang, W.; Li, X.; Chen, T. Spontaneous Growth of 3D Silver Mesoflowers on Poly(4-vinylpyridine) Brushes-Grafted-Graphene Oxide Films and Facile Creation of Nanoporosities over their Surface. Chem.-A Eur. J. 2019, 25, 16377–16381. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, F.; Aziz, M.H.; Fakhar-e-Alam, M.; Atif, M.; Fatima, M.; Ahmad, R.; Hanif, A.; Anwar, S.; Zafar, F.; Abbas, G.; et al. An In Vitro Study of the Photodynamic Effectiveness of GO-Ag Nanocomposites against Human Breast Cancer Cells. Nanomaterials 2017, 7, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liang, P.; Yu, Z.; Huang, J.; Ni, D.; Shu, H.; Dong, Q.-m. The effect of solvent environment toward optimization of SERS sensors for pesticides detection from chemical enhancement aspects. Sens. Actuators B-Chem. 2018, 256, 721–728. [Google Scholar] [CrossRef]
- Shi, M.; Zheng, J.; Liu, C.; Tan, G.; Qing, Z.; Yang, S.; Yang, J.; Tan, Y.; Yang, R. SERS assay of telomerase activity at single-cell level and colon cancer tissues via quadratic signal amplification. Biosens. Bioelectron. 2016, 77, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Zhu, H.-P.; Liu, G.-K.; Wu, D.-Y.; Ren, B.; Tian, Z.-Q. When the Signal Is Not from the Original Molecule To Be Detected: Chemical Transformation of para-Aminothiophenol on Ag during the SERS Measurement. J. Am. Chem. Soc. 2010, 132, 9244–9246. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, R.; Hu, X.; Zhang, L.; Dong, S. Templated assembly of gold nanoparticles into microscale tubules and their application in surface-enhanced Raman scattering. J. Phys. Chem. B 2006, 110, 14179–14185. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.W.; Zhou, Y.G.; Li, X.W.; Ji, L.; Lu, T.H.; Gu, R.A. Surface-enhanced Raman scattering of 4-aminothiophenol in assemblies of nanosized particles and the macroscopic surface of silver. Langmuir 2003, 19, 632–636. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wang, Z.; Wang, Y.; Dai, J.; Gao, L.; Wei, M.; Yan, Y.; Li, C. A polydopamine-based molecularly imprinted polymer on nanoparticles of type SiO2@rGO@Ag for the detection of lambda-cyhalothrin via SERS. Microchim. Acta 2018, 185, 193. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.-L.; Li, J.-G.; Sun, X.; Sakka, Y. Photocatalytic growth of Ag nanocrystals on hydrothermally synthesized multiphasic TiO2/reduced graphene oxide (rGO) nanocomposites and their SERS performance. Appl. Surf. Sci. 2017, 423, 1–12. [Google Scholar] [CrossRef]
- Zhang, C.; Lia, C.; Yu, J.; Jiang, S.; Xu, S.; Yang, C.; Liu, Y.J.; Gao, X.; Liu, A.; Man, B. SERS activated platform with three-dimensional hot spots and tunable nanometer gap. Sens. Actuators B-Chem. 2018, 258, 163–171. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Ye, J.; Xia, J.; Zhou, Y.; Huang, J.; Ni, D.; Tang, L.; Jin, S.; Yu, Z. Detection of systemic pesticide residues in tea products at trace level based on SERS and verified by GC-MS. Anal. Bioanal. Chem. 2019, 411, 7187–7196. [Google Scholar] [CrossRef] [PubMed]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- He, J.; Song, G.; Wang, X.; Zhou, L.; Li, J. Multifunctional magnetic Fe3O4/GO/Ag composite microspheres for SERS detection and catalytic degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2022, 893, 162226. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, C.; Zhang, Z. Graphene oxide embedded sandwich nanostructures for enhanced Raman readout and their applications in pesticide monitoring. Nanoscale 2013, 5, 3773–3779. [Google Scholar] [CrossRef]
- Qu, L.-L.; Liu, Y.-Y.; Liu, M.-K.; Yang, G.-H.; Li, D.-W.; Li, H.-T. Highly reproducible Ag NPs/CNT-intercalated GO membranes for enrichment and SERS detection of antibiotics. ACS Appl. Mater. Interfaces 2016, 8, 28180–28186. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.L.; Zhen, S.J.; Huang, C.Z. One-pot green synthesis of graphene oxide/gold nanocomposites as SERS substrates for malachite green detection. Analyst 2013, 138, 3075–3081. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Qin, C.; Yu, Z.; Sun, B.; Ni, D.; Zhang, D.; Liang, P. Synergistic Enhancement Effect of Ag/rGO as SERS Platform for Capture and Trace Detection of Fenvalerate Molecules. Chemosensors 2024, 12, 82. https://doi.org/10.3390/chemosensors12050082
Yu M, Qin C, Yu Z, Sun B, Ni D, Zhang D, Liang P. Synergistic Enhancement Effect of Ag/rGO as SERS Platform for Capture and Trace Detection of Fenvalerate Molecules. Chemosensors. 2024; 12(5):82. https://doi.org/10.3390/chemosensors12050082
Chicago/Turabian StyleYu, Minghui, Chongyang Qin, Zhi Yu, Biao Sun, Dejiang Ni, De Zhang, and Pei Liang. 2024. "Synergistic Enhancement Effect of Ag/rGO as SERS Platform for Capture and Trace Detection of Fenvalerate Molecules" Chemosensors 12, no. 5: 82. https://doi.org/10.3390/chemosensors12050082
APA StyleYu, M., Qin, C., Yu, Z., Sun, B., Ni, D., Zhang, D., & Liang, P. (2024). Synergistic Enhancement Effect of Ag/rGO as SERS Platform for Capture and Trace Detection of Fenvalerate Molecules. Chemosensors, 12(5), 82. https://doi.org/10.3390/chemosensors12050082





