The Art of Fluorescence Imaging with Chemical Sensors: The Next Decade 2012–2022
Abstract
1. Introduction
2. General Aspects and Imaging Systems
3. Biomedical Imaging and Tissue Engineering
3.1. Planar Optrodes
3.1.1. Oxygen Sensors
3.1.2. Sensors for pH, Carbon Dioxide, and Ions
3.1.3. Multiple Sensors
3.2. Nanoprobes
- -
- the capability for trace analysis
- -
- NIR excitation
- -
- a broad dynamic range
- -
- improved chemical robustness and photostability
3.2.1. Silica Nanoparticles
3.2.2. Polymer Nanoparticles
3.2.3. Inorganic Nanomaterials
Quantum Dots
Metal Nanoparticles
Nanocarbons
Photon Upconversion Nanosensors
4. Environmental Monitoring and Marine Research
4.1. Planar Optrodes
4.1.1. Oxygen Sensors
4.1.2. Sensors for pH
4.1.3. Multiple Sensor Systems
4.2. Micro- and Nanoparticles
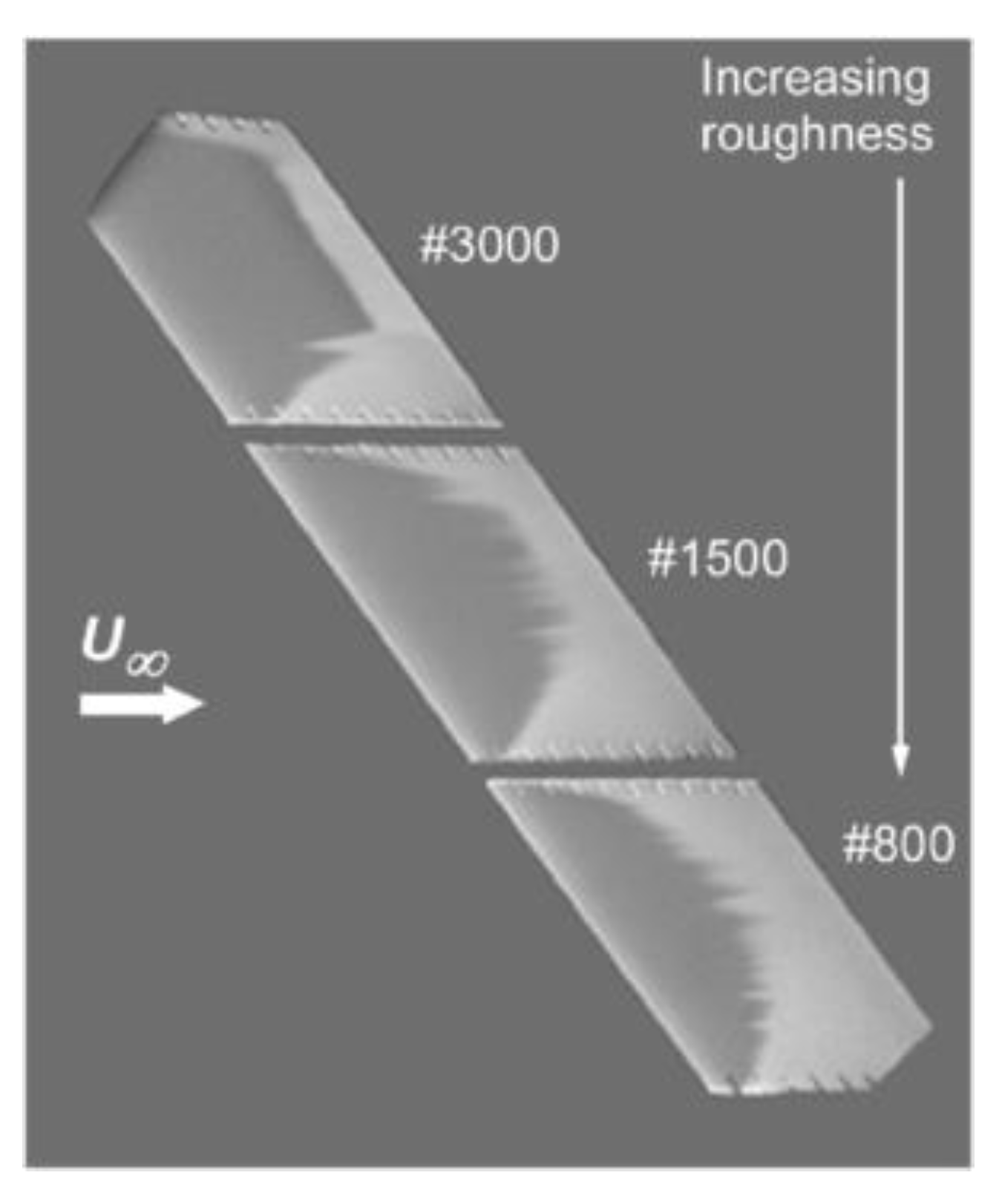
5. Aerodynamics
5.1. Pressure-Sensitive Paints
5.2. Temperature-Sensitive Paints
5.3. Dual Paints
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Schäferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. Engl. 2012, 51, 3532–3554. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Tóth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- He, J.; Li, C.; Ding, L.; Huang, Y.; Yin, X.; Zhang, J.; Zhang, J.; Yao, C.; Liang, M.; Pirraco, R.P.; et al. Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatfigurement. Adv. Mater. 2019, 31, 1902409. [Google Scholar] [CrossRef]
- Schäferling, M.; Resch-Genger, U. Luminescent nanoparticles for chemical sensing and Imaging. In Reviews in Fluorescence 2016; Geddes, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–109. [Google Scholar] [CrossRef]
- Koren, K.; Zieger, S.E. Optode Based Chemical Imaging—Possibilities, Challenges, and New Avenues in Multidimensional Optical Sensing. ACS Sens. 2021, 6, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.; Henriksen-Lacey, M.; Renero-Lecuna, C.; Liz-Marzán, L.M. Challenges for optical nanothermometry in biological environments. Chem. Soc. Rev. 2022, 51, 4223–4242. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.A.; Nagl, S. Microfluidic platforms employing integrated fluorescent or luminescent chemical sensors: A review of methods, scope and applications. Methods Appl. Fluoresc. 2015, 3, 034003. [Google Scholar] [CrossRef] [PubMed]
- Schäferling, M.; Dürkop, A. Intrinsically Referenced Fluorimetric Sensing and Detection Schemes: Methods, Advantages and Applications. In Standardization and Quality Assurance in Fluorescence Measurement; Resch-Genger, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 373–413. [Google Scholar]
- Bambot, S.B.; Lakowicz, J.R.; Rao, G. Potential applications of lifetime-based, phase-modulation fluorimetry in bioprocess and clinical monitoring. Trends Biotechnol. 1995, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Moßhammer, M.; Scholz, V.V.; Borisov, S.M.; Holst, G.; Kühl, M. Luminescence Lifetime Imaging of Chemical Sensors: A Comparison between Time-Domain and Frequency-Domain Based Camera Systems. Anal. Chem. 2019, 91, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.J.; Fischer, L.H.; Wolfbeis, O.S.; Schäferling, M. Referenced luminescent sensing and imaging with digital color cameras: A comparative study. Sens. Actuators B 2013, 177, 500–506. [Google Scholar] [CrossRef]
- Hakonen, A.; Beves, J.E.; Strömberg, N. Digital Colour Tone for Fluorescence Sensing: A Direct Comparison of Intensity, Ratiometric and Hue Based Quantification. Analyst 2014, 139, 3524–3527. [Google Scholar] [CrossRef]
- Zieger, S.E.; Mosshammer, M.; Kuhl, M.; Koren, K. Hyperspectral Luminescence Imaging in Combination with Signal Deconvolution Enables Reliable Multi-Indicator-Based Chemical Sensing. ACS Sens. 2021, 6, 183–191. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Papkovsky, D.B.; Dmitriev, R.I. Biological detection by optical oxygen sensing. Chem. Soc. Rev. 2013, 42, 8700–8732. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, R.I.; Papkovsky, D.B. Intracellular probes for imaging oxygen concentration: How good are they? Methods Appl. Fluoresc. 2015, 3, 034001. [Google Scholar] [CrossRef] [PubMed]
- Schäferling, M. Nanoparticle-based luminescent probes for intracellular sensing and imaging of pH. WIREs Nanomed. Nanobiotechnol. 2016, 8, 378–413. [Google Scholar] [CrossRef] [PubMed]
- Lagadic-Gossmann, D.; Huc, L.; Lecureur, V. Alterations of Intracellular pH Homeostasis in Apoptosis: Origins and Roles. Cell Death Diff. 2004, 11, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.A.; Fine, R.E.; Johnson, R.J.; Levesque, C.A.; Rathbun, W.H.; Seetoo, K.F.; Smith, S.J.; Strohmeier, G.; Volicer, L.; Delva, L.; et al. Non-age Related Differences in Thrombin Responses by Platelets from Male Patients with Advanced Alzheimer′s Disease. Biochem. Biophys. Res. Commun. 1993, 194, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular pH. Nat. Rev. Mol. Cell. Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Miksa, M.; Komura, H.; Wu, R.; Shah, K.G.; Wang, P. A Novel Method to Determine the Engulfment of Apoptotic Cells by Macrophages Using pHrodo Succinimidyl Ester. J. Immunol. Methods 2009, 342, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lakadamyali, M.; Rust, M.J.; Babcock, H.P.; Zhuang, X. Visualizing Infection of Individual Influenza Viruses. Proc. Natl. Acad. Sci. USA 2003, 100, 9280–9285. [Google Scholar] [CrossRef] [PubMed]
- Adie, E.J.; Kalinka, S.; Smith, L.; Francis, M.J.; Marenghi, A.; Cooper, M.E.; Briggs, M.; Michael, N.P.; Milligan, G.; Game, S. A pH-Sensitive Fluor, CypHer 5, Used to Monitor Agonist-Induced G Protein-Coupled Receptor Internalization in Live Cells. BioTechniques 2002, 33, 1152–1156. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical pH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Babilas, P.; Liebsch, G.; Schacht, V.; Klimant, I.; Wolfbeis, O.S.; Szeimies, R.-M.; Abels, C. In Vivo Phosphorescence Imaging of pO2 Using Planar Oxygen Sensors. Microcirculation 2005, 12, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Meier, R.J.; Wolfbeis, O.S.; Landthaler, M.; Szeimies, R.-M.; Babilas, P. 2D Luminescence Imaging of pH in Vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Boss, M.K.; Palmer, G.; Dewhirst, M. Imaging the Hypoxic Tumor Microenvironment in Preclinical Models. In Hypoxia and Cancer. Cancer Drug Discovery and Development; Melillo, G., Ed.; Springer: New York, NY, USA, 2014; pp. 157–178. [Google Scholar] [CrossRef]
- Borisov, S.M.; Fischer, R.; Saf, R.; Klimant, I. Exceptional Oxygen Sensing Properties of New Blue Light-Excitable Highly Luminescent Europium(III) and Gadolinium(III) Complexes. Adv. Funct. Mater. 2014, 24, 6548–6560. [Google Scholar] [CrossRef]
- Meier, J.K.; Prantl, L.; Müller, S.; Moralis, A.; Liebsch, G.; Gosau, M. Simple, Fast and Reliable Perfusion Monitoring of Microvascular Flaps. Clin. Hemorheol. Microcirc. 2012, 50, 13–24. [Google Scholar] [CrossRef]
- Hofmann, J.; Meier, R.J.; Mahnke, A.; Schatz, V.; Brackmann, F.; Trollmann, R.; Bogdan, C.; Liebsch, G.; Wang, X.D.; Wolfbeis, O.S.; et al. Ratiometric Luminescence 2D In Vivo Imaging and Monitoring of Mouse Skin Oxygenation. Methods Appl. Fluoresc. 2013, 1, 045002. [Google Scholar] [CrossRef]
- Jennewein, J.; Matuszak, J.; Walter, S.; Felmy, B.; Gendera, K.; Schatz, V.; Nowottny, M.; Liebsch, G.; Hensel, M.; Hardt, W.D.; et al. Low-Oxygen Tensions Found in Salmonella-Infected Gut Tissue Boost Salmonella Replication in Macrophages by Impairing Antimicrobial Activity and Augmenting Salmonella Virulence. Cell Microbiol. 2015, 17, 1833–1847. [Google Scholar] [CrossRef]
- Jenkins, J.; Dmitriev, R.I.; Morten, K.; McDermott, K.W.; Papkovsky, D.B. Oxygen-Sensing Scaffolds for 3-Dimensional Cell and Tissue Culture. Acta Biomater. 2015, 16, 126–135. [Google Scholar] [CrossRef]
- Khosravi, Y.; Kandukuri, R.D.P.; Palmer, S.R.; Gloag, E.S.; Borisov, S.M.; Starke, E.M.; Ward, M.T.; Kumar, P.; de Beer, D.; Stoodle, A.C.P. Use of an Oxygen Planar Optode to Assess the Effect of High Velocity Microsprays on Oxygen Penetration in a Human Dental Biofilms In-Vitro. BMC Oral Health 2020, 20, 230. [Google Scholar] [CrossRef]
- Westphal, I.; Jedelhauser, C.; Liebsch, G.; Wilhelmi, A.; Aszodi, A.; Schieker, M. Oxygen Mapping: Probing a Novel Seeding Strategy for Bone Tissue Engineering. Biotechnol. Bioeng. 2017, 114, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Wolff, P.; Heimann, L.; Liebsch, G.; Meier, R.J.; Gutbrod, M.; van Griensven, M.; Balmayor, E.R. Oxygen-Distribution within 3-D Collagen I Hydrogels for Bone Tissue Engineering. Mat. Sci. Eng. C 2019, 95, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, H.; Nava, M.M.; Leonardi, G.; Mohebbi-Kalhori, D.; Sebastiano, R.; Samimi, A.; Raimondi, M.T. An Experimental-Numerical Investigation on the Effects of Macroporous Scaffold Geometry on Cell Culture Parameters. Int. J. Artif. Organs. 2017, 40, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Peniche Silva, C.J.; Liebsch, G.; Meier, R.J.; Gutbrod, M.S.; Balmayor, E.R.; van Griensven, M. A New Non-Invasive Technique for Measuring 3D-Oxygen Gradients in Wells During Mammalian Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Deygas, M.; Gadet, R.; Gillet, G.; Rimokh, R.; Gonzalo, P.; Mikaelian, I. Redox Regulation of EGFR Steers Migration of Hypoxic Mammary Cells towards Oxygen. Nat. Commun. 2018, 9, 4545. [Google Scholar] [CrossRef]
- Dargaville, R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectronics. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.J.; Simbürger, J.M.B.; Soukka, T.; Schäferling, M. Background-free referenced luminescence sensing and imaging of pH using upconverting phosphors and color camera read-out. Anal. Chem. 2014, 86, 5535–5540. [Google Scholar] [CrossRef]
- Dalfen, I.; Dmitriev, R.I.; Holst, G.; Klimant, I.; Borisov, S.M. Background-free fluorescence-decay-time sensing and imaging of pH with highly photostable diazaoxotriangulenium dyes. Anal. Chem. 2019, 91, 808–816. [Google Scholar] [CrossRef]
- Schreml, S.; Meier, R.J.; Weiß, K.T.; Cattani, J.; Flittner, D.; Gehmert, S.; Wolfbeis, O.S.; Landthaler, M.; Babilas, P. A sprayable luminescent pH sensor and its use for wound imagingin vivo. Exp. Dermatol. 2012, 21, 948–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Meier, R.J.; Schmittlein, C.; Schreml, S.; Schäferling, M.; Wolfbeis, O.S. A water-sprayable, thermogelating and biocompatible polymer host for use in fluorescent chemical sensing and imaging of oxygen, pH values and temperature. Sens. Actuators B 2015, 221, 37–44. [Google Scholar] [CrossRef]
- Schreml, S.; Zeller, V.; Meier, R.J.; Korting, H.C.; Behm, B.; Landthaler, M.; Babilas, P. Impact of Age and Body Site on Adult Female Skin Surface pH. Dermatology 2012, 224, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M.; Boyce, M.W.; Whitman, N.A.; Kromhout, B.P.; Lockett, M.R. A pH-Sensing Optode for Mapping Spatiotemporal Gradients in 3D Paper-Based Cell Cultures. Anal. Chem. 2018, 90, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical pH Sensor Covering the Range from pH 0–14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, C.; Breininger, J.; Klimant, I.; Borisov, S.M. Near-Infrared Fluorescent Aza-BODIPY Dyes for Sensing and Imaging of pH from the Neutral to Highly Alkaline Range. Analyst 2019, 144, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, D.; Russegger, A.; Klimant, I.; Borisov, S.M. Green to Red Emitting BODIPY Dyes for Fluorescent Sensing and Imaging of Carbon Dioxide. Sens. Actuators B 2020, 304, 127312. [Google Scholar] [CrossRef]
- Aigner, D.; Ungerbock, B.; Mayr, T.; Saf, R.; Klimant, I.; Borisov, S.M. Fluorescent Materials for pH Sensing and Imaging Based on Novel 1,4-Diketopyrrolo-[3,4-c]pyrrole Dyes. J. Mater. Chem. C 2013, 1, 5685–5693. [Google Scholar] [CrossRef]
- Schutting, S.; Borisov, S.M.; Klimant, I. Diketo-Pyrrolo-Pyrrole Dyes as New Colorimetric and Fluorescent pH Indicators for Optical Carbon Dioxide Sensors. Anal. Chem. 2013, 85, 3271–3279. [Google Scholar] [CrossRef]
- Schutting, S.; Klimant, I.; de Beer, D.; Borisov, S.M. New Highly Fluorescent pH Indicator for Ratiometric RGB Imaging of pCO2. Methods Appl. Fluoresc. 2014, 2, 024001. [Google Scholar] [CrossRef]
- Pedersen, L.L.; Dechesne, A.; Smets, B.F. A Nitrate Sensitive Planar Optode; Performance and Interferences. Talanta 2015, 144, 933–937. [Google Scholar] [CrossRef]
- Kumar, R.S.; Ashok Kumar, S.K.; Vijayakrishna, K.; Sivaramakrishna, A.; Paira, P.; Brahmmananada Rao, C.V.S.; Sivaraman, N.; Sahoo, S.K. Bipyridine Bisphosphonate-Based Fluorescent Optical Sensor and Optode for Selective Detection of Zn2+ Ions and its Applications. N. J. Chem. 2018, 42, 8494–8502. [Google Scholar] [CrossRef]
- Stich, M.I.J.; Schäferling, M.; Wolfbeis, O.S. Multicolor Fluorescent and Permeation-Selective Microbeads Enable Simultaneous Sensing of pH, Oxygen, and Temperature. Adv. Mater. 2009, 21, 2216–2220. [Google Scholar] [CrossRef]
- Borisov, S.M.; Seifner, R.; Klimant, I. A Novel Planar Optical Sensor for Simultaneous Monitoring of Oxygen, Carbon Dioxide, pH and Temperature. Anal. Bioanal. Chem. 2011, 400, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Meier, R.J.; Kirschbaum, M.; Kong, S.C.; Gehmert, S.; Felthaus, O.; Küchler, S.; Sharpe, J.R.; Wöltje, K.; Weiß, K.T.; et al. Luminescent Dual Sensors Reveal Extracellular pH-Gradients and Hypoxia on Chronic Wounds That Disrupt Epidermal Repair. Theranostics 2014, 4, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Auerswald, S.; Schreml, S.; Meier, R.J.; Blancke Soares, A.; Niyazi, M.; Marschner, S.; Belka, C.; Canis, M.; Haubner, F. Wound Monitoring of pH and Oxygen in Patients after Radiation Therapy. Radiat. Oncol. 2019, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, X.; Yang, X.; Shi, H. Functionalized Silica Nanoparticles: A Platform for Fluorescence Imaging at the Cell and Small Animal Levels. Acc. Chem. Res. 2013, 46, 1367–1376. [Google Scholar] [CrossRef]
- Du, X.; Li, N.; Chen, O.; Wu, Z.; Zhai, J.; Xie, X. Perspective on Fluorescence Cell Imaging with Ionophore-Based Ion-Selective Nanooptodes. Biomicrofluidics 2022, 16, 031301. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, S.; Genovese, D.; Juris, R.; Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Luminescent Silica Nanoparticles: Extending the Frontiers of Brightness. Angew. Chem. Int. Ed. 2011, 50, 4056–4066. [Google Scholar] [CrossRef]
- Chatterjee, S.; Li, X.-S.; Liang, F.; Yang, Y.-W. Design of Multifunctional Fluorescent Hybrid Materials Based on SiO2 Materials and Core–Shell Fe3O4@SiO2 Nanoparticles for Metal Ion Sensing. Small 2019, 15, 1904569. [Google Scholar] [CrossRef]
- Parra, M.; Gil, S.; Gaviña, P.; Costero, A.M. Mesoporous Silica Nanoparticles in Chemical Detection: From Small Species to Large Bio-Molecules. Sensors 2022, 22, 261. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chen, H.A.; Hung, Y.; Chien, F.C.; Chen, P.; Mou, C.Y. Surface Charge Effect in Intracellular Localization of Mesoporous Silica Nanoparticles as Probed by Fluorescent Ratiometric pH Imaging. RSC Adv. 2012, 2, 968–973. [Google Scholar] [CrossRef]
- Tsou, C.J.; Chu, C.-Y.; Hung, Y.; Mou, C.Y. A Broad Range Fluorescent pH Sensor Based on Hollow Mesoporous Silica Nanoparticles, Utilising the Surface Curvature Effect. J. Mater. Chem. B 2013, 1, 5557–5563. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, J.; Xu, W.; Wang, L.; Su, D.; Teoh, C.L.; Chang, Y.T. Silica Nanoparticle-Enhanced Fluorescent Sensor Array for Heavy Metal Ions Detection in Colloid Solution. Anal. Chem. 2018, 90, 1628–1634. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Z.; Qiao, M.; Peng, H.; Ding, L.; Fang, Y. Mesoporous Silica Nanoparticles-Based Fluorescent Mini Sensor Array with Dual Emission for Discrimination of Biothiols. Colloids Surf. A 2020, 606, 125433. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, P.; Zou, L.; Zhao, M.; Jiang, J.; Liu, S.; Zhang, K.Y.; Huang, W.; Zhao, Q. Using Ultrafast Responsive Phosphorescent Nanoprobe to Visualize Elevated Peroxynitrite In Vitro and In Vivo via Ratiometric and Time-Resolved Photoluminescence Imaging. Adv. Healthc. Mater. 2018, 7, 1800309. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.A.; Hoyer, M.; Philbert, M.A.; Kopelman, R. Optical Nanosensors for Chemical Analysis Inside Single Living Cells. 1. Fabrication, Characterization, and Methods for Intracellular Delivery of PEBBLE Sensors. Anal. Chem. 1999, 71, 4831–4836. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Zhang, C.; Dong, X.; Yao, S.Q. Recent Advances in Polymeric Nanoparticles for Enhanced Fluorescence and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2021, 60, 17797–17809. [Google Scholar] [CrossRef]
- Canfarotta, F.; Whitcombe, M.J.; Piletsky, S.A. Polymeric Nanoparticles for Optical Sensing. Biotechnol. Adv. 2013, 31, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Tavernaro, I.; Genger, C.; Welker, P.; Hübner, O.; Resch-Genger, U. Multicolor Polystyrene Nanosensors for the Monitoring of Acidic, Neutral, and Basic pH Values and Cellular Uptake Studies. Anal. Chem. 2022, 94, 9656–9664. [Google Scholar] [CrossRef]
- Aigner, D.; Dmitriev, R.I.; Borisov, S.M.; Papkovsky, D.B.; Klimant, I. pH-Sensitive Perylene Bisimide Probes for Live Cell Fluorescence Lifetime Imaging. J. Mater. Chem. B 2014, 2, 6792–6801. [Google Scholar] [CrossRef] [PubMed]
- Kondrashina, A.V.; Dmitriev, R.I.; Borisov, S.M.; Klimant, I.; O’Brien, I.; Nolan, Y.M.; Zhdanov, A.V.; Papkovsky, D.B. A Phosphorescent Nanoparticle-Based Probe for Sensing and Imaging of (Intra)Cellular Oxygen in Multiple Detection Modalities. Adv. Funct. Mater. 2012, 22, 4931–4939. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Zhdanov, A.V.; Nolan, Y.M.; Papkovsky, D.B. Imaging of Neurosphere Oxygenation with Phosphorescent Probes. Biomaterials 2013, 34, 9307–9317. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Borisov, S.M.; Düssmann, H.; Sun, S.; Müller, B.J.; Prehn, J.; Baklaushev, V.P.; Klimant, I.; Papkovsky, D.B. Versatile Conjugated Polymer Nanoparticles for High-Resolution O2 Imaging in Cells and 3D Tissue Models. ACS Nano 2015, 9, 5275–5288. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Borisov, S.M.; Kondrashina, A.V.; Pakan, J.M.P.; Anilkumar, U.; Prehn, J.H.M.; Zhdanov, A.V.; McDermott, K.W.; Klimant, I.; Papkovsky, D.B. Imaging Oxygen in Neural Cell and Tissue Models by Means of Anionic Cell-Permeable Phosphorescent Nanoparticles. Cell. Mol. Life Sci. 2015, 72, 367–381. [Google Scholar] [CrossRef]
- Bao, Y.; De Keersmaecker, H.; Corneillie, S.; Yu, F.; Mizuno, H.; Zhang, G.; Hofkens, J.; Mendrek, B.; Kowalczuk, A.; Smet, M. Tunable Ratiometric Fluorescence Sensing of Intracellular pH by Aggregation-Induced Emission-Active Hyperbranched Polymer Nanoparticles. Chem. Mater. 2015, 27, 3450–3455. [Google Scholar] [CrossRef]
- Müller, B.J.; Zhdanov, A.V.; Borisov, S.M.; Foley, T.; Okkelman, I.A.; Tsytsarev, V.; Tang, Q.; Erzurumlu, R.S.; Chen, Y.; Zhang, H.; et al. Nanoparticle-Based Fluoroionophore for Analysis of Potassium Ion Dynamics in 3D Tissue Models and In Vivo. Adv. Funct. Mater. 2018, 28, 1704598. [Google Scholar] [CrossRef]
- Lee, C.H.; Folz, J.; Zhang, W.; Jo, J.; Tan, J.W.Y.; Wang, X.; Kopelman, R. Ion-Selective Nanosensor for Photoacoustic and Fluorescence Imaging of Potassium. Anal. Chem. 2017, 89, 7943–7949. [Google Scholar] [CrossRef]
- Sahari, A.; Ruckh, T.T.; Hutchings, R.; Clark, H.A. Development of an Optical Nanosensor Incorporating a pH-Sensitive Quencher Dye for Potassium Imaging. Anal. Chem. 2015, 87, 10684–10687. [Google Scholar] [CrossRef]
- Yang, C.; Qin, Y.; Jiang, D.; Chen, H.-Y. Continuous Fluorescence Imaging of Intracellular Calcium by Use of Ion-Selective Nanospheres with Adjustable Spectra. ACS Appl. Mater. Interfaces 2016, 8, 19892–19898. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Boeneman Gemmill, K.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing Nanoparticles with Biological Molecules: Developing Chemistries that Facilitate Nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Alivisatos, P. The Use of Nanocrystals in Biological Detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Nie, S. Chemical Analysis and Cellular Imaging with quantum Dots. Analyst 2004, 129, 672–677. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Willner, I. Optical Molecular Sensing with Semiconductor Quantum Dots (QDs). Chem. Soc. Rev. 2012, 41, 4067–4085. [Google Scholar] [CrossRef]
- Lesiak, A.; Drzozga, K.; Cabaj, J.; Banski, M.; Malecha, K.; Podhorodecki, A. Optical Sensors Based on II-VI Quantum Dots. Nanomaterials 2019, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Orte, A.; Alvarez-Pez, J.M.; Ruedas-Rama, M.J. Fluorescence Lifetime Imaging Microscopy for the Detection of Intracellular pH with Quantum Dot Nanosensors. ACS Nano 2013, 7, 6387–6395. [Google Scholar] [CrossRef]
- Marín, M.J.; Galindo, F.; Thomas, P.; Russell, D.A. Localized Intracellular pH Measurement Using a Ratiometric Photoinduced Electron-Transfer-Based Nanosensor. Angew. Chem. Int. Ed. 2012, 51, 9657–9661. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chen, P.C.; Wang, C.W.; Lin, Y.S.; Ou, C.M.; Ho, L.C.; Chang, H.T. One-Pot Synthesis of Fluorescent BSA–Ce/Au Nanoclusters as Ratiometric pH Probes. Chem. Commun. 2014, 50, 8571–8574. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, Mechanisms, and Application of Carbon Quantum Dots in Sensors: A Review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, T.; He, P.; Shi, Y.; Li, Y.; Li, X.; Fan, L.; Yang, S. Carbon Quantum Dots: An Emerging Material for Optoelectronic Applications. J. Mater. Chem. C 2019, 7, 6820–6835. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.; Ma, H. A Tunable Ratiometric pH Sensor Based on Carbon Nanodots for the Quantitative Measurement of the Intracellular pH of Whole Cells. Angew. Chem. Int. Ed. 2012, 51, 6432–6435. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X.A. Carbon Dots with Continuously Tunable Full-Color Emission and their Application in Ratiometric pH sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Kong, B.; Zhu, A.; Ding, C.; Zhao, X.; Li, B.; Tian, Y. Carbon Dot-Based Inorganic–Organic Nanosystem for Two-Photon Imaging and Biosensing of pH Variation in Living Cells and Tissues. Adv. Mater. 2012, 24, 5844–5848. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-P.; Zhuang, Y.-F.; Lin, J.W.; Chen, J.H.; Han, Z.-Z. Synthesis of N,S Co-Doped Carbon Dots for Fluorescence Detection of Fe3+ Ions and Intracellular pH Imaging. Chin. J. Anal. Chem. 2022, 50, 100151. [Google Scholar] [CrossRef]
- Iverson, N.M.; Barone, P.W.; Shandell, M.; Trudel, L.J.; Sen, S.; Sen, F.; Ivanov, V.; Atolia, E.; Farias, E.; McNicholas, T.P.; et al. In vivo Biosensing via Tissue-Localizable Near-Infrared-Fluorescent Single-Walled Carbon Nanotubes. Nat. Nanotechn. 2013, 8, 873–880. [Google Scholar] [CrossRef]
- Del Bonis-O’Donnell, J.T.; Page, R.H.; Beyene, A.G.; Tindall, E.G.; McFarlane, I.R.; Landry, M.P. Dual Near-Infrared Two-Photon Microscopy for Deep-Tissue Dopamine Nanosensor Imaging. Adv. Funct. Mater. 2017, 27, 1702112. [Google Scholar] [CrossRef]
- Beyene, A.G.; Delevich, K.; Del Bonis-O’Donnell, J.T.; Piekarski, D.J.; Lin, W.C.; Wren Thomas, A.; Yang, S.J.; Kosillo, P.; Yang, D.; Prounis, G.S.; et al. Imaging Striatal Dopamine Release Using a Nongenetically Encoded Near Infrared Fluorescent Catecholamine Nanosensor. Sci. Adv. 2019, 5, eaaw3108. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, W.; Yang, T.; Yi, T.; Li, F. Upconversion Luminescence Imaging of Cells and Small Animals. Nat. Protoc. 2013, 8, 2033–2044. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Yang, T.; Gao, Y.; Yang, Y.; Hu, H.; Li, F. Water-Soluble NaYF4:Yb/Er Upconversion Nanophosphors: Synthesis, Characteristics and Application in Bioimaging. Inorg. Chem. Commun. 2010, 13, 92–394. [Google Scholar] [CrossRef]
- Hao, S.; Chen, G.; Yang, C. Sensing Using Rare-Earth-Doped Upconversion Nanoparticles. Theranostics 2013, 3, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Resch-Genger, U.; Schäferling, M. Surface Modifications for Photon-Upconversion-Based Energy-Transfer Nanoprobes. Langmuir 2019, 35, 5093–5113. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, D.; Ju, Q.; Wang, F. Photon Upconversion in Core–Shell Nanoparticles. Chem. Soc. Rev. 2015, 44, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Mahata, M.K.; Bae, H.; Lee, K.T. Upconversion Luminescence Sensitized pH Nanoprobes. Molecules 2017, 22, 2064. [Google Scholar] [CrossRef] [PubMed]
- Arppe, R.; Näreoja, T.; Nylund, S.; Mattsson, L.; Koho, S.; Rosenholm, J.M.; Soukka, T.; Schäferling, M. Photon Upconversion Sensitized Nanoprobes for Sensing and Imaging of pH. Nanoscale 2014, 6, 6837–6843. [Google Scholar] [CrossRef] [PubMed]
- Nareoja, T.; Deguchi, T.; Christ, S.; Peltomaa, R.; Prabhakar, N.; Fazeli, E.; Perala, N.; Rosenholm, J.M.; Arppe, R.; Soukka, T.; et al. Ratiometric Sensing and Imaging of Intracellular pH Using Polyethylenimine-Coated Photon Upconversion Nanoprobes. Anal. Chem. 2017, 89, 1501–1508. [Google Scholar] [CrossRef]
- Tsai, E.S.; Himmelstoß, S.F.; Wiesholler, L.M.; Hirsch, T.; Hall, E.A.H. Upconversion Nanoparticles for Sensing pH. Analyst 2019, 144, 5547–5557. [Google Scholar] [CrossRef]
- Tsai, E.S.; Joud, F.; Wiesholler, L.M.; Hirsch, T.; Hall, E.A.H. Upconversion Nanoparticles as Intracellular pH Messengers. Anal. Bioanal. Chem. 2020, 412, 6567–6581. [Google Scholar] [CrossRef]
- Radunz, S.; Andresen, E.; Wurth, C.; Koerdt, A.; Tschiche, H.R.; Resch-Genger, U. Simple Self-Referenced Luminescent pH Sensors Based on Upconversion Nanocrystals and pH-Sensitive Fluorescent BODIPY Dyes. Anal. Chem. 2019, 91, 7756–7764. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Bu, W.; Bu, J.; Sun, Y.; Du, J.; Shi, J. Ultrasensitive Nanosensors Based on Upconversion Nanoparticles for Selective Hypoxia Imaging in Vivo upon Near-Infrared Excitation. J. Am. Chem. Soc. 2014, 136, 9701–9709. [Google Scholar] [CrossRef]
- Lv, W.; Yang, T.; Yu, Q.; Zhao, Q.; Zhang, K.Y.; Liang, H.; Liu, S.; Lio, F.; Huang, W. A Phosphorescent Iridium(III) Complex-Modified Nanoprobe for Hypoxia Bioimaging via Time-Resolved Luminescence Microscopy. Adv. Sci. 2015, 2, 201500107. [Google Scholar] [CrossRef]
- Heydari, E.; Amir Ahmadi, J.; Ghazyani, N.; Bai, G.; Zare-Behtash, H.; Majles Ara, M.H. Dual-Mode Nanophotonic Upconversion Oxygen Sensors. Nanoscale 2022, 14, 13362–13372. [Google Scholar] [CrossRef]
- Zhao, L.; Peng, J.; Chen, M.; Liu, Y.; Yao, L.; Feng, W.; Li, F. Yolk–Shell Upconversion Nanocomposites for LRET Sensing of Cysteine/Homocysteine. ACS Appl. Mater. Interfaces 2014, 6, 11190–11197. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, A.; Jia, Q.; Zhai, X.; Liu, L.; Ma, L.; Zhou, J. Rationally Designed Upconversion Nanoprobe for Simultaneous Highly Sensitive Ratiometric Detection of Fuoride Ions and Fluorosis Theranostics. Chem. Sci. 2018, 9, 5242–5525. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, L.; Yang, T.; Yang, H.; Zhang, K.Y.; Zhao, X.; Lv, W.; Yu, Q.; Zhang, X.; Zhao, Q.; et al. Development of Upconversion Luminescent Probe for Ratiometric Sensing and Bioimaging of Hydrogen Sulfide. ACS Appl. Mater. Interfaces 2014, 6, 11013–11017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Peng, J.; Sun, L.; Li, F. High-Efficiency Upconversion Luminescent Sensing and Bioimaging of Hg(II) by Chromophoric Ruthenium Complex-Assembled Nanophosphors. ACS Nano 2011, 5, 8040–8048. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Wu, Y.-M.; Kong, X.-J.; Wu, S.; Yu, R.-Q.; Chu, X. Phospholipid-Modified Upconversion Nanoprobe for Ratiometric Fluorescence Detection and Imaging of Phospholipase D in Cell Lysate and in Living Cells. Anal. Chem. 2014, 86, 7119–7127. [Google Scholar] [CrossRef]
- Li, C.; Ding, S.; Yang, L.; Zhu, Q.; Chen, M.; Tsang, D.C.W.; Cai, G.; Feng, C.; Wang, Y.; Zhang, C. Planar optode: A two-dimensional imaging technique for studying spatial-temporal dynamics of solutes in sediment and soil. Earth Sci. Rev. 2019, 197, 102916. [Google Scholar] [CrossRef]
- Scholz, V.V.; Brodersen, K.E.; Kühl, M.; Koren, K. Resolving Chemical Gradients Around Seagrass Roots—A Review of Available Methods. Front. Mar. Sci. 2021, 8, 771382. [Google Scholar] [CrossRef]
- Clarke, J.S.; Achterberg, E.P.; Connelly, D.P.; Schuster, U.; Mowlem, M. Developments in Marine pCO2 Measurement Technology; Ttowards Sustained in situ Observations. TrAC 2017, 88, 53–61. [Google Scholar] [CrossRef]
- Hancke, K.; Sorell, B.K.; Lund-Hansen, L.C.; Larsen, M.; Hancke, T.; Glud, R.N. Effects of Temperature and Irradiance on a Benthic Microalgal Community: A Combined Two-Dimensional Oxygen and Fluorescence Imaging Approach. Limnol. Oceanogr. 2014, 59, 1599–1611. [Google Scholar] [CrossRef]
- Christel, W.; Zhu, K.; Hoefer, C.; Kreuzeder, A.; Santner, J.; Bruun, S.; Magid, J.; Jensen, L.S. Spatiotemporal Dynamics of Phosphorus Release, Oxygen Consumption and Greenhouse Gas Emissions after Localised Soil Amendment with Organic Fertilisers. Sci. Total Environ. 2016, 554–555, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ren, J.; Tang, H.; Xu, D.; Xie, X. Quantitative Imaging of Radial Oxygen Loss from Valisneria spiralis Roots with a Fluorescent Planar Optode. Sci. Total Environ. 2016, 569–570, 1232–1240. [Google Scholar] [CrossRef]
- He, Y.; Ding, N.; Yu, G.; Sunahara, G.I.; Lin, H.; Zhang, X.; Ullah, H.; Liu, J. High-Resolution Imaging of O2 Dynamics and Metal Solubilization in the Rhizosphere of the Hyperaccumulator Leersia hexandra Swartz. J. Hazard. Mater. 2023, 455, 131580. [Google Scholar] [CrossRef]
- Li, C.; Ding, S.; Ma, X.; Chen, M.; Zhong, Z.; Zhang, Y.; Ren, M.; Zhang, M.; Yang, L.; Rong, N.; et al. O2 Distribution and Dynamics in the Rhizosphere of Phragmites Australis, and Implications for Nutrient Removal in Sediments. Environ. Pollut. 2021, 287, 117193. [Google Scholar] [CrossRef]
- Nyer, S.C.; Volkenborn, N.; Aller, R.C.; Graffam, M.; Zhu, Q.; Price, R.E. Nitrogen Transformations in Constructed Wetlands: A Closer Look at Plant-Soil Interactions Using Chemical Imaging. Sci. Total Environ. 2022, 816, 151560. [Google Scholar] [CrossRef]
- Borer, B.; Tecon, R.; Or, D. Spatial Organization of Bacterial Populations in Response to Oxygen and Carbon Counter-gradients in Pore Networks. Nat. Commun. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed]
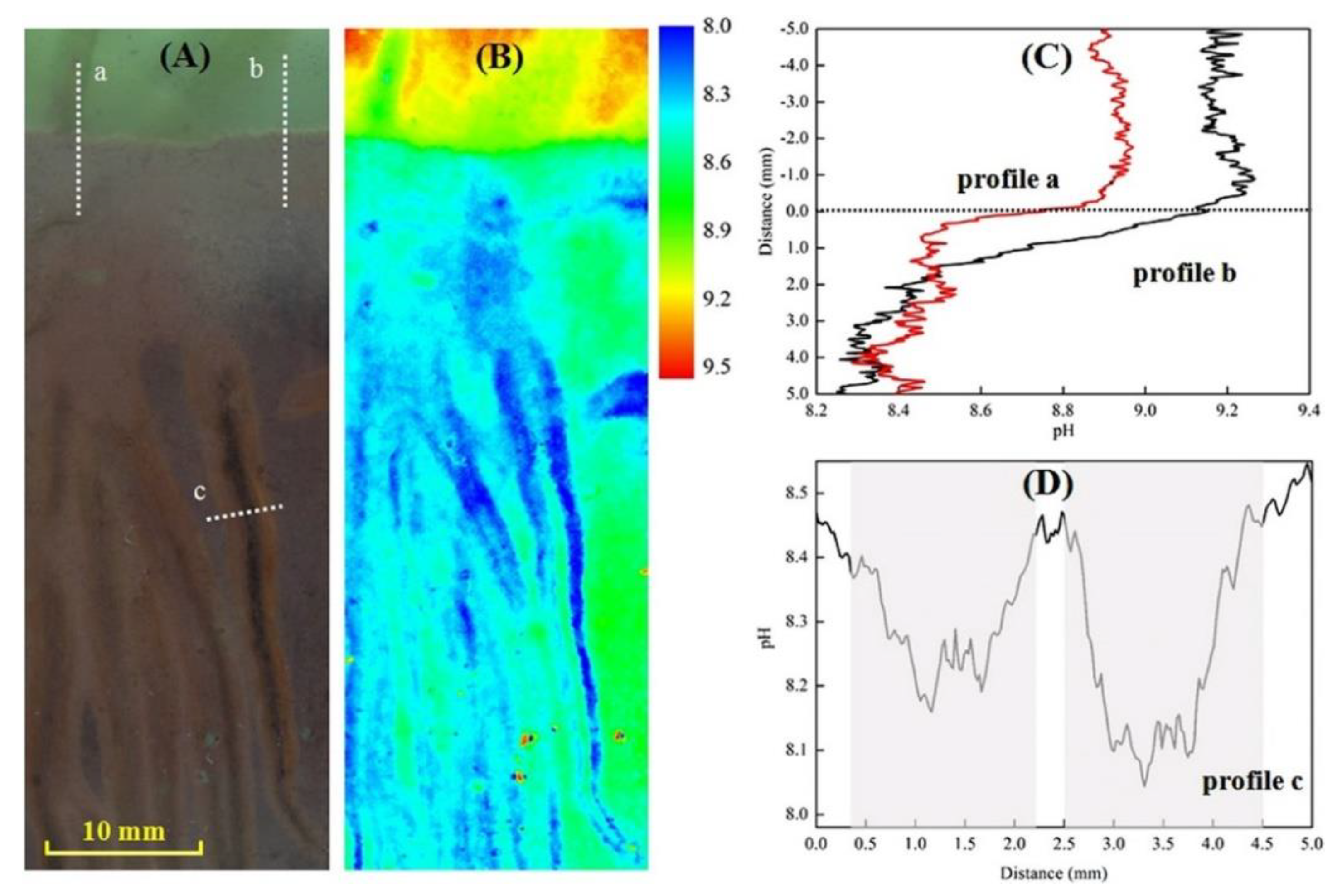
- Han, C.; Yao, L.; Xu, D.; Xi, X.; Zhang, C. High-Resolution Imaging of pH in Alkaline Sediments and Water Based on a New Rapid Response Fluorescent Planar Optode. Sci. Rep. 2016, 6, 26417. [Google Scholar] [CrossRef] [PubMed]
- Blossfeld, S.; Schreiber, C.M.; Liebsch, G.; Kuhn, A.J.; Hinsinger, P. Quantitative Imaging of Rhizosphere pH and CO2 Dynamics with Planar Optodes. Ann. Bot. 2013, 112, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yu, X.; Hao, Y. Design and Fabrication of a Ratiometric Planar Optode for Simultaneous Imaging of pH and Oxygen. Sensors 2017, 17, 1316. [Google Scholar] [CrossRef] [PubMed]
- Lenzewski, N.; Mueller, P.; Meier, R.J.; Liebsch, G.; Jensen, K.; Koop-Jakobsen, K. Dynamics of Oxygen and Carbon Dioxide in Rhizospheres of Lobelia dortmanna—A Planar Optode Study of Belowground Gas Exchange Between Plants and Sediment. N. Phytol. 2018, 218, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Koop-Jakobsen, K.; Mueller, P.; Meier, R.J.; Liebsch, G.; Jensen, K. Plant-Sediment Interactions in Salt Marshes—An Optode Imaging Study of O2, pH, and CO2 Gradients in the Rhizosphere. Front. Plant Sci. 2018, 9, 541. [Google Scholar] [CrossRef]
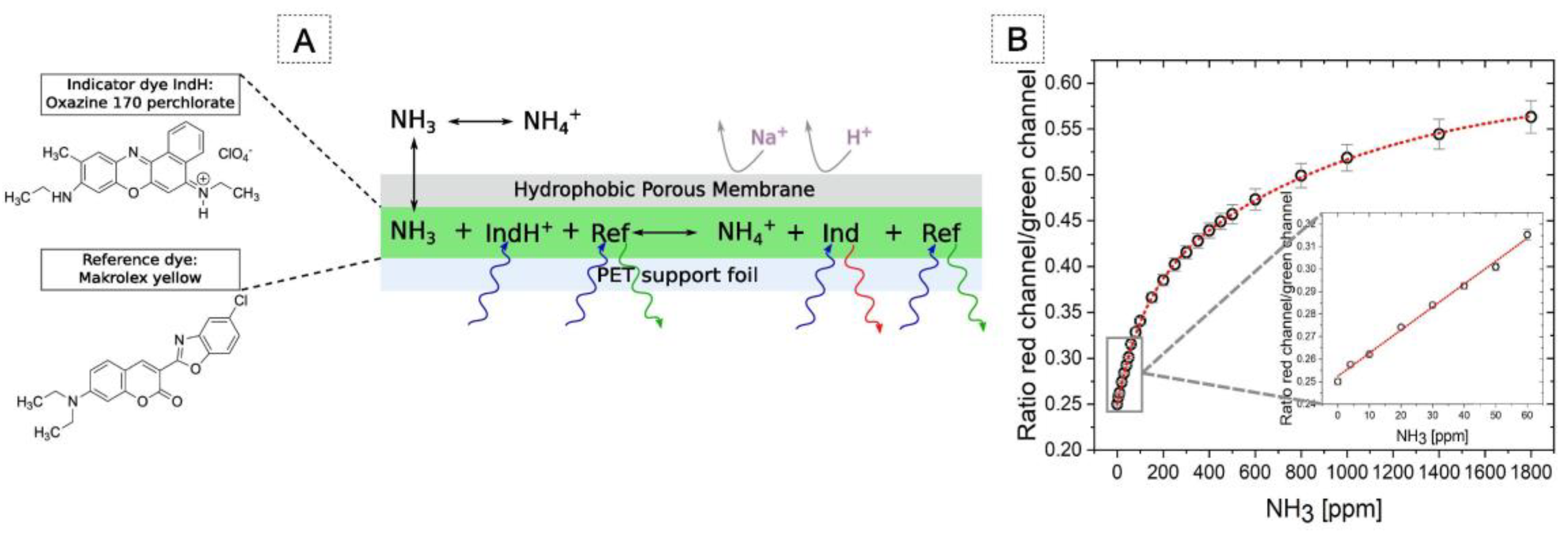
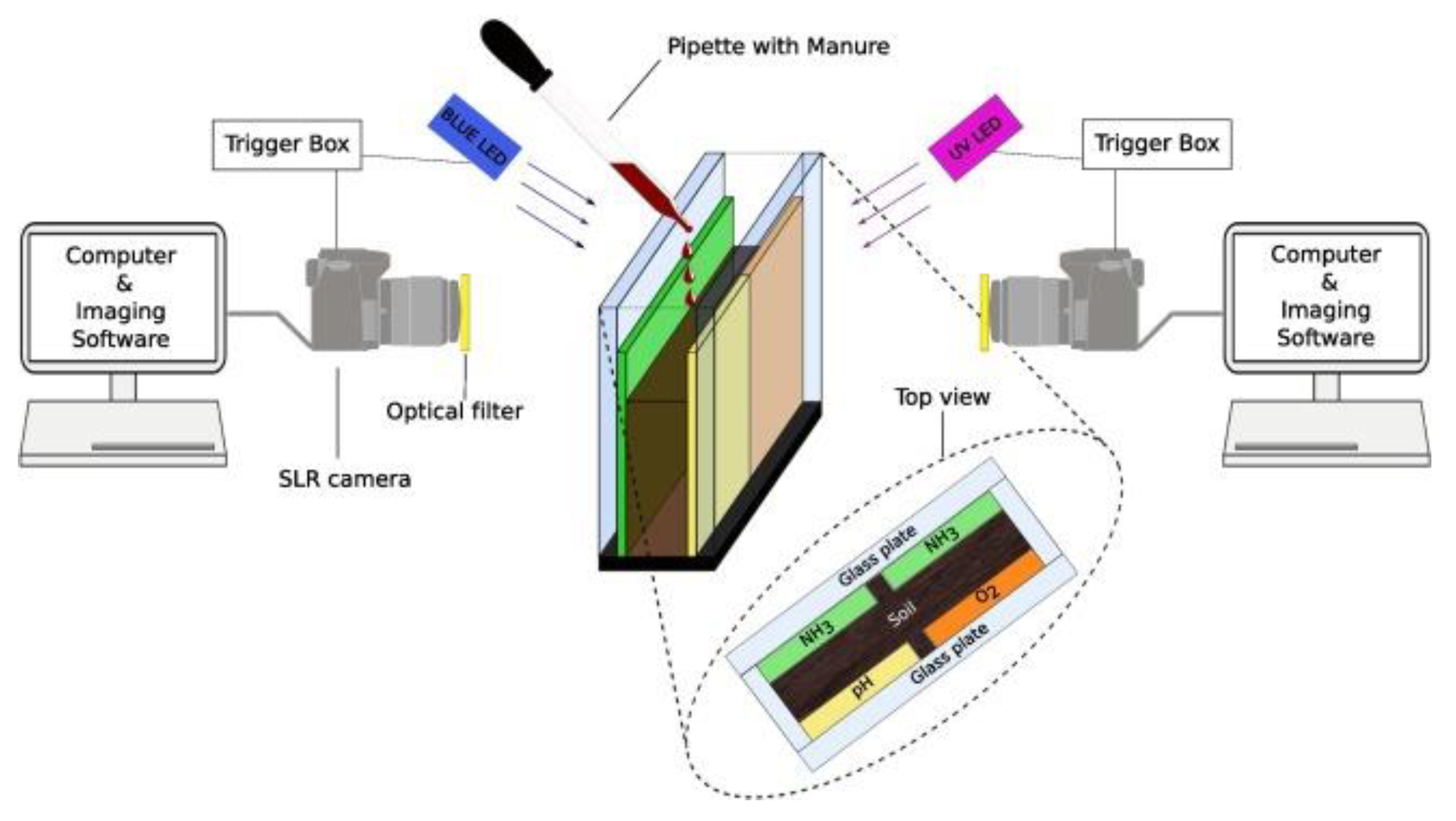
- Merl, T.; Koren, K. Visualizing NH3 Emission and the Local O2 and pH Microenvironment of Soil Upon Manure Application Using Optical Sensors. Environ. Int. 2020, 144, 106080. [Google Scholar] [CrossRef]
- Moßhammer, M.; Strobl, M.; Kühl, M.; Klimant, I.; Borisov, S.M.; Koren, K. Design and Application of Optical Sensor for Simultaneous Imaging of pH and Dissolved O2 with Low Cross Talk. ACS Sens. 2016, 1, 681–687. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Paegle, I.; Borisov, S.M.; Kjeldsen, K.U.; Røy, H.; Skibsted, J.; Koren, K. Optical Sensing of pH and O2 in the Evaluation of Bioactive Self Healing Cement. ACS Omega 2019, 4, 20237–20243. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Jakobsen, S.L.; Kühl, M. In-vivo Imaging of O2 Dynamics on Coral Surfaces Spray-Painted with Sensor Nanoparticles. Sens. Actuators B Chem. 2016, 237, 1095–1101. [Google Scholar] [CrossRef]
- Koren, K.; Brodersen, K.E.; Jakobsen, S.L.; Kühl, M. Optical Sensor Nanoparticles in Artificial Sediments–A New Tool to Visualize O2 Dynamics around the Rhizome and Roots of Seagrasses. Environ. Sci. Technol. 2015, 49, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
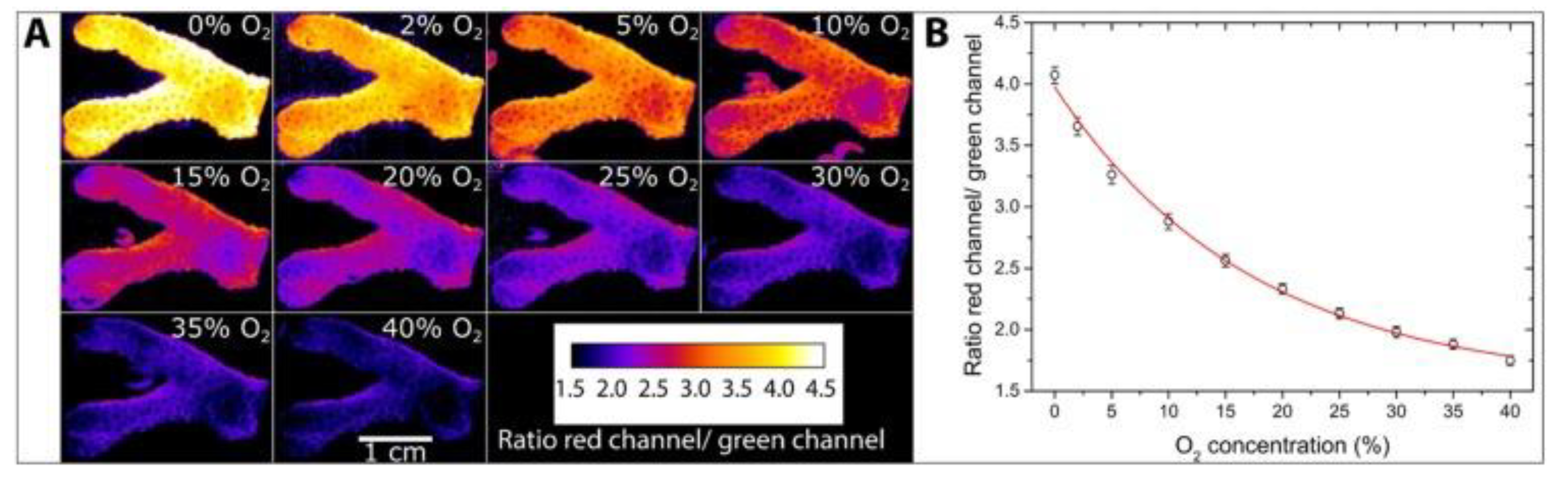
- Fabricius-Dyg, J.; Mistlberger, G.; Staal, M.; Borisov, S.M.; Klimant, I.; Kühl, M. Imaging of Surface O2 Dynamics in Corals with Magnetic Micro Optode Particles. Mar. Biol. 2012, 159, 1621–1631. [Google Scholar] [CrossRef]
- Liu, T.; Sullivan, J.P.; Asai, K.; Klein, C.; Egami, Y. Pressure and Temperature Sensitive Paints, 2nd ed.; Experiment Fluid Mechanics; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Puklin, E.; Carlson, B.; Gouin, S.; Costin, C.; Green, E.; Ponomarev, S.; Tanji, H.; Gouterman, M. Ideality of Pressure-Sensitive Paint. I. Platinum tetra(pentafluorophenyl) porphine in Fuoroacrylic Polymer. J. Appl. Polym. Sci. 2000, 77, 2795–2804. [Google Scholar] [CrossRef]
- Juliano, T.J.; Disotell, K.J.; Gregory, J.W.; Crafton, J.; Fonov, S. Motion-Deblurred, Fast-Response Pressure-Sensitive Paint on a Rotor in Forward Flight. Meas. Sci. Technol. 2012, 23, 045303/1–045303/11. [Google Scholar] [CrossRef]
- Kameda, M.; Seki, H.; Makoshi, T.; Amao, Y.; Nakakita, K. Fast-Response Pressure Sensor Based on a Dye-Adsorbed Silica Nanoparticle Film. Sens. Actuators B 2012, 171–172, 343–349. [Google Scholar] [CrossRef]
- Matsuda, Y.; Yamaguchi, H.; Niimi, T. Development of Pressure-Sensitive Channel Chip for Micro Gas Flows. J. Phys. Conf. Ser. 2012, 362, 012036/1–012036/6. [Google Scholar] [CrossRef]
- Sakaue, H.; Kodama, H.; Morita, K.; Ishikawa, H. Super-Hydrophobic Porous Pressure-Sensitive Paint for Global Unsteady Flow Measurements. Sens. Actuators B 2013, 185, 154–158. [Google Scholar] [CrossRef]
- Idris, A.C.; Saad, M.R.; Zare-Behtash, H.; Kontis, K. Luminescent Measurement Systems for the Investigation of a Scramjet Inlet-Isolator. Sensors 2014, 14, 6606–6632. [Google Scholar] [CrossRef]
- Obata, M.; Asato, R.; Mitsuo, K.; Hirohara, S. Radical Polymerization of Trifluoromethyl-Substituted Methyl Methacrylates and their Application for Use in Pressure-Sensitive Paint. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 963–972. [Google Scholar] [CrossRef]
- Obata, M.; Asato, R.; Hirohara, S.; Mitsuo, K. Effect of Polymer Matrix on the Performance of Pressure-Sensitive Paint Comprising 5,10,15,20-tetrakis(pentafluorophenyl)porphinato platinum(II) and poly(1,1,1,3,3,3-hexafluoroisopropyl-co-tert-butyl methacrylates). J. Appl. Polym. Sci. 2016, 133, 43316. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chen, Y.-H.; Wan, S.-A.; Wang, Y.-C. Quantitative Visualization of Asymmetric Gas Flow in Constricted Microchannels by Using Pressure-Sensitive Paint. J. Micromech. Microengin 2016, 26, 105002/1–105002/9. [Google Scholar] [CrossRef]
- Akram, M.; Mei, Z.; Shi, J.; Wen, J.; Khalid, H.; Jiang, J.; Tian, Y.; Tian, Y. Electrospun Nanofibers and Spin Coated Films Prepared from Side-Chain Copolymers with Chemically Bounded Platinum (II) porphyrin Moieties for Oxygen Sensing and Pressure Sensitive Paints. Talanta 2018, 188, 124–134. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, Y.; Jiang, J.; Pan, T.; Mei, Z.; Wen, J.; Yang, C.; Wang, Z.; Tian, Y. Multi-arm Polymers Prepared by Atom Transfer Rradical Polymerization (ATRP) and their Electrospun Films as Oxygen Sensors and Pressure Sensitive Paints. Eur. Polym. J. 2019, 112, 214–221. [Google Scholar] [CrossRef]
- Shi, J.; Wei, C.; Deng, M.; Liang, L.; Tian, Y. Synthesis of Fluorinated Copolymers and their Applications as Pressure-Responsive Materials. J. Appl. Polym. Sci. 2023, 140, e53969. [Google Scholar] [CrossRef]
- Pandey, A.; Gregory, J.W. Step Response Characteristics of Polymer/Ceramic Pressure-Sensitive Paint. Sensors 2015, 15, 22304–22324. [Google Scholar] [CrossRef]
- Ali, M.Y.; Pandey, A.; Gregory, J.W. Dynamic Mode Decomposition of Fast Pressure Sensitive Paint Data. Sensors 2016, 16, 862. [Google Scholar] [CrossRef]
- Peng, D.; Wang, S.; Liu, Y. Fast PSP Measurements of Wall-Pressure Fluctuation in Low-Speed Flows: Improvements Using Proper Orthogonal Decomposition. Exp. Fluids 2016, 57, 45. [Google Scholar] [CrossRef]
- Khojasteh, A.R.; Wang, S.F.; Peng, D.; Yavuzkurt, S.; Liu, Y. Structure Analysis of Adiabatic Film Cooling Effectiveness in the Near Field of a Single Inclined Jet: Measurement Using Fast-Response Pressure-Sensitive Paint. Int. J. Heat Mass Transf. 2017, 110, 629–642. [Google Scholar] [CrossRef]
- Wu, J.; Bitter, M.; Cai, G.; He, B.; Kaehler, C. Investigation on Aerodynamic Force Effect of Vacuum Plumes Using Pressure-Sensitive Paint Technique and CFD-DSMC Solution. Sci. China Technol. Sci. 2017, 60, 1058–1067. [Google Scholar] [CrossRef]
- Sugioka, Y.; Arakida, K.; Kasai, M.; Nonomura, T.; Asai, K.; Egami, Y.; Nakakita, K. Evaluation of the Characteristics and Coating Film Structure of Polymer/Ceramic Pressure-Sensitive Paint. Sensors 2018, 18, 4041. [Google Scholar] [CrossRef]
- Peng, D.; Gu, F.; Li, Y.; Liu, Y. A Novel Sprayable Fast-Responding Pressure-Sensitive Paint Based on Mesoporous Silicone Dioxide Particles. Sens. Actuators A 2018, 279, 390–398. [Google Scholar] [CrossRef]
- Egami, Y.; Konishi, S.; Sato, Y.; Matsuda, Y. Effects of Solvents for Luminophore on Dynamic and Static Characteristics of Sprayable Polymer/Ceramic Pressure-Sensitive Paint. Sens. Actuators A 2019, 286, 188–194. [Google Scholar] [CrossRef]
- Kasai, M.; Sasaki, D.; Nagata, T.; Nonomura, T.; Asai, K. Frequency Response of Pressure-Sensitive Paints under Low-Pressure Conditions. Sensors 2021, 21, 3187. [Google Scholar] [CrossRef]
- Egami, Y.; Yamazaki, Y.; Hori, N.; Sugioka, Y.; Nakakita, K. Investigation of Factors Causing Nonuniformity in Luminescence Lifetime of Fast-Responding Pressure-Sensitive Paints. Sensors 2021, 21, 6076. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Liang, L.; Liu, Y.; Peng, D. Simultaneous 3D Surface Profile and Pressure Measurement Using Phase-Shift Profilometry and Pressure-Sensitive Paint. Rev. Sci. Instrum. 2021, 92, 035107. [Google Scholar] [CrossRef]
- Kasai, M.; Suzuki, A.; Egami, Y.; Nonomura, T.; Asai, K. A Platinum-Based Fast-Response Pressure-Sensitive Paint Containing Hydrophobic Titanium Dioxide. Sens. Actuators A 2023, 350, 114140. [Google Scholar] [CrossRef]
- Matsuda, Y.; Uchida, K.; Egami, Y.; Yamaguchi, H.; Niimi, T. Polymer-Particle Pressure Sensitive Paint with High Photostability. Sensors 2016, 16, 550. [Google Scholar] [CrossRef]
- Peng, D.; Zhong, Z.; Gu, F.; Zhou, W.; Qi, F.; Liu, Y. Pressure-Sensitive Paint with Imprinted Pattern for Full-Field Endoscopic Measurement Using a Colour Camera. Sens. Actuators A 2019, 290, 28–35. [Google Scholar] [CrossRef]
- Peak, S.M.; Watkins, A.N. Addition of Silica-Coated Ag Nanoparticles to Enhance Luminescence Intensity of Pressure-Sensitive Paints. ACS Appl. Nano Mater. 2020, 3, 9813–9821. [Google Scholar] [CrossRef]
- Zhou, B.; Gu, F.; Liu, Y.; Peng, D. Signal Enhancement of Pressure-Sensitive Film Based on Localized Surface Plasmon Resonance. Sensors 2021, 21, 7627. [Google Scholar] [CrossRef]
- Yorita, D.; Klein, C.; Henne, U.; Ondrus, V.; Beifuss, U.; Hensch, A.-K.; Guntermann, P.; Quest, J. Application of Lifetime-based Pressure-Sensitive Paint Technique to Cryogenic Wind Tunnel Tests. In Proceedings of the 54th AIAA Aerospace Sciences Meeting, San Diego, CA, USA, 4–8 January 2016. [Google Scholar] [CrossRef]
- Yorita, D.; Klein, C.; Henne, U.; Ondrus, V.; Beifuss, U.; Hensch, A.-K.; Longo, R.; Guntermann, P.; Quest, J. Successful Application of Cryogenic Pressure Sensitive Paint Technique at ETW. In Proceedings of the 2018 AIAA Aerospace Sciences Meeting, Kissimmee, FL, USA, 8–12 January 2018. [Google Scholar] [CrossRef]
- Klein, C.; Yorita, D.; Henne, U.; Kleindienst, T.; Koch, S.; Ondrus, V. Unsteady Pressure Measurements by means of PSP in Cryogenic Conditions. In Proceedings of the AIAA SciTech 2020 Forum, Orlando, FL, USA, 6–10 January 2020. [Google Scholar] [CrossRef]
- Klein, C.; Yorita, D.; Henne, U.; Kleindienst, T.; Ondrus, V. Time-Resolved Pressure Measurements by Means of PSP in Cryogenic Conditions. In Proceedings of the AIAA SciTech 2022 Forum, San Diego, CA, USA, 3–7 January 2022. [Google Scholar] [CrossRef]
- Yang, L.; Erdem, E.; Zare-Behtash, H.; Kontis, K. Application of AA-PSP to Hypersonic Flows: The Double Ramp Model. Sens. Actuators B 2012, 161, 100–107. [Google Scholar] [CrossRef]
- Kameda, M.; Yoshida, M.; Sekiya, T.; Nakakita, K. Humidity Effects in the Response of a Porous Pressure-Sensitive Paint. Sens. Actuators B 2015, 208, 399–405. [Google Scholar] [CrossRef]
- Peng, D.; Chen, J.; Jiao, L.; Liu, Y. A Fast-Responding Semi-Transparent Pressure-Sensitive Paint Based on Through-Hole Anodized Aluminum Oxide Membrane. Sens. Actuators A 2018, 274, 10–18. [Google Scholar] [CrossRef]
- Sakaue, H.; Hayashi, T.; Ishikawa, H. Luminophore Application Study of Polymer-Ceramic Pressure-Sensitive Paint. Sensors 2013, 13, 7053–7064. [Google Scholar] [CrossRef]
- Hayashi, T.; Houpt, A.W.; Leonov, S.B.; Sakaue, H. Motion-Capturing Pressure-Sensitive Paint Method Under Transient Illumination by Plasma Source. J. Phys. D Appl. Phys. 2019, 52, 324005. [Google Scholar] [CrossRef]
- Egami, Y.; Hasegawa, A.; Matsuda, Y.; Ikami, T.; Nagai, H. Ruthenium-Based Fast-Responding Pressure-Sensitive Paint for Measuring Small Pressure Fluctuation in Low-Speed Flow Field. Meas. Sci. Technol. 2021, 32, 024003. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Lin, Y.-F.; Huang, Y.-X.; Chung, K.-M. Pressure-Sensitive Paint Measurements with Temperature Correction on the Wing of AGARD-B Under Transonic Flow Conditions. Meas. Sci. Technol. 2021, 32, 094001. [Google Scholar] [CrossRef]
- Quinn, M.K.; Kontis, K. Pressure-Sensitive Paint Measurements of Transient Shock Phenomena. Sensors 2013, 13, 4404–4427. [Google Scholar] [CrossRef]
- Sakaue, H.; Kuriki, T.; Miyazaki, T. A Temperature-Cancellation Method of Pressure-Sensitive Paint on Porous Anodic Alumina Using 1-Pyrenesulfonic Acid. J. Lumin. 2012, 132, 256–260. [Google Scholar] [CrossRef]
- Sakaue, H.; Morita, K.; Iijima, Y.; Sakamura, Y. Response Time Scales of Anodized-Aluminum Pressure-Sensitive Paints. Sens. Actuators A 2013, 199, 74–79. [Google Scholar] [CrossRef]
- Yomo, K.; Ikami, T.; Fujita, K.; Nagai, H. Investigation of Formulations on Pyrene-Based Anodized-Aluminum Pressure Sensitive Paints for Supersonic Phenomena. Sensors 2022, 22, 4430. [Google Scholar] [CrossRef]
- Banishev, A.A.; Dlott, D.D. Ultrafast Pressure-Sensitive Paint for Shock Compression Spectroscopy. J. Appl. Phys. 2014, 115, 203515/1–203515/9. [Google Scholar] [CrossRef]
- Pilch, M.; Ortyl, J.; Chachaj-Brekiesz, A.; Galek, M.; Popielarz, R. Europium-Based Luminescent Sensors for Mapping Pressure Distribution on Surfaces. Sens. Actuators B 2020, 305, 127409. [Google Scholar] [CrossRef]
- Cellini, F.; Peterson, S.D.; Porfiri, M. Highly Compressible Fluorescent Particles for Pressure Sensing in Liquids. Appl. Phys. Lett. 2017, 110, 221904. [Google Scholar] [CrossRef]
- Yang, D.; Li, J.; Ren, J.; Wang, Q.; Zhou, S.; Wang, Q.; Xie, Z.; Qu, X. Fast-Response Oxygen Sensitive Transparent Coating for Inner Pressure Ratiometric Optical Mapping. J. Mater. Chem. C 2021, 9, 3919–3927. [Google Scholar] [CrossRef]
- Strozik, M.; Majkut, M.; Dykas, S. Measuring System for Pressure Sensitive Paint (PSP) Calibration in the Range of Low Pressure Gains. Mod. Appl. Sci. 2015, 9, 116–122. [Google Scholar] [CrossRef][Green Version]
- Tagliabue, A.; Scharnowski, S.; Kähler, C.J. Surface Pressure Determination: A Comparison Between PIV-Based Methods and PSP Measurements. J. Vis. 2017, 20, 581–590. [Google Scholar] [CrossRef]
- Quinn, M.K. Binary Pressure-Sensitive Paint Measurements Using Miniaturised, Colour, Machine Vision Cameras. Meas. Sci. Technol. 2018, 29, 055107. [Google Scholar] [CrossRef]
- Park, J. Optical Glucose Sensor Using Pressure Sensitive Paint. Sensors 2021, 21, 4474. [Google Scholar] [CrossRef]
- Lenzi, T.; Picchi, A.; Becchi, R.; Andreini, A.; Facchini, B. Swirling Main Flow Effects on Flm Cooling: Time Resolved Adiabatic Effectiveness Measurements in a Gas Turbine Combustor Model. Int. J. Heat Mass Transf. 2023, 200, 123554. [Google Scholar] [CrossRef]
- Kolodner, P.; Tyson, A. Microscope Fluorescent Imaging of Surface Temperature Profiles with 0.01 °C Resolution. Appl. Phys. Lett. 1982, 40, 782–784. [Google Scholar] [CrossRef]
- Kolodner, P.; Tyson, A. Remote Thermal Imaging with 0.7 Micron Spatial Resolution Using Temperature Dependent Fluorescent Thin Films. Appl. Phys. Lett. 1983, 42, 117–119. [Google Scholar] [CrossRef]
- Kolodner, P.; Tyson, A. Non-Contact Surface Temperature Measurement During Reactive-Ion Etching Using Fluorescent Polymer Films. Appl. Phys. Lett. 1983, 42, 749–751. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y. Thermo-Sensitive Luminescence of Lanthanide Complexes, Clusters, Coordination Polymers and Metal–Organic Frameworks with Organic Photosensitizers. J. Mater. Chem. C 2019, 7, 7494–7511. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Tanaka, S. Patternable Temperature Sensitive Paint using Eu(TTA)3 for the Micro Thermal Imaging. J. Phys. Conf. Ser. 2013, 476, 012073. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Li, C.-A.; Wang, H.-Y.; Liou, T.-M. The Application of Temperature-Sensitive Paints for Surface and Fluid Temperature Measurements in Both Thermal Developing and Fully Developed Regions of a Microchannel. J. Micromech. Microeng. 2013, 23, 037001. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Esashi, M.; Tanaka, S. High Spatial, Temporal and Temperature Resolution Thermal Imaging Method Using Eu(TTA)3 Temperature Sensitive Paint. J. Micromech. Microeng. 2013, 23, 114015. [Google Scholar] [CrossRef]
- Liu, T.; Chen, T.; Salazar, D.M.; Miozzi, M. Skin Friction and Surface Optical Flow in Viscous Flows. Phys. Fluids 2022, 34, 067101. [Google Scholar] [CrossRef]
- Egami, Y.; Matsuda, Y.; Yamaguchi, H.; Niimi, T. Property Changes of Temperature-Sensitive Paint Immobilized in Acrylic Polymer Matrices. Sens. Actuators B 2014, 195, 677–681. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, X.; Jin, B.; Shi, J.; Zhi-tian, L.; Xi-Gao, J. Effect of Polymer Matrix on Temperature Sensitivity of Temperature Sensitive Paints. Chin. J. Polym. Sci. 2015, 33, 1351–1358. [Google Scholar] [CrossRef]
- Ondrus, V.; Meier, R.J.; Klein, C.; Henne, U.; Schäferling, M.; Beifuss, U. Europium 1,3-Di(thienyl)propane-1,3-Diones with Outstanding Properties for Temperature Sensing. Sens. Actuators A 2015, 233, 434–441. [Google Scholar] [CrossRef]
- Costantini, M.; Hein, S.; Henne, U.; Klein, C.; Koch, S.; Schojda, L.; Ondrus, V.; Schröder, W. Pressure Gradient and Nonadiabatic Surface Effects on Boundary Layer Transition. AIAA J. 2016, 54, 3465–3480. [Google Scholar] [CrossRef]
- Risius, S.; Costantini, M.; Koch, S.; Hein, S.; Klein, C. Unit Reynolds Number, Mach Number and Pressure Gradient Effects on Laminar–Turbulent Transition in Two-Dimensional Boundary Layers. Exp. Fluids 2018, 59, 86. [Google Scholar] [CrossRef]
- Costantini, M.; Fuchs, C.; Henne, U.; Klein, C.; Ondrus, V.; Bruse, M.; Löhr, M.; Jacobs, M. A Reliable Experimental Methodology for the Study of Wind-Turbine Rotor Blade Aerodynamics. J. Phys. Conf. Ser. 2019, 1222, 012001. [Google Scholar] [CrossRef]
- Ikami, T.; Fujita, K.; Nagai, H.; Yorita, D. Measurement of Boundary Layer Transition on Oscillating Airfoil Using cntTSP in Low-Speed Wind Tunnel. Meas. Sci. Technol. 2021, 32, 075301. [Google Scholar] [CrossRef]
- Costantini, M.; Fuchs, C.; Henne, U.; Klein, C.; Ondrus, V.; Bruse, M.; Löhr, M.; Jacobs, M. Experimental Analysis of the Performance of a Wind-Turbine Airfoil Using Temperature-Sensitive Paint. AIAA J. 2021, 59, 4449–4464. [Google Scholar] [CrossRef]
- Miozzi, M.; Capone, A.; Costantini, M.; Fratto, L.; Klein, C.; Di Felice, F. Skin Friction and Coherent Structures Within a Laminar Separation Bubble. Exp. Fluids 2019, 60, 13. [Google Scholar] [CrossRef]
- Costantini, M.; Henne, U.; Klein, C.; Miozzi, M. Skin-Friction-Based Identification of the Critical Lines in a Transonic, High Reynolds Number Flow via Temperature-Sensitive Paint. Sensors 2021, 21, 5106. [Google Scholar] [CrossRef]
- Capone, A.; Klein, C.; Di Felice, F.; Beifuss, U.; Miozzi, M. Fast-Response Underwater TSP Investigation of Subcritical Instabilities of a Cylinder in Crossflow. Exp. Fluids 2015, 56, 196. [Google Scholar] [CrossRef]
- Lemarechal, J.; Klein, C.; Puckert, D.K.; Rist, U. Application of the Temperature-Sensitive Paint Method for Quantitative Measurements in Water. Meas. Sci. Technol. 2021, 32, 105301. [Google Scholar] [CrossRef]
- Ozawa, H. Experimental Study of Unsteady Aerothermodynamic Phenomena on Shock-Tube Wall Using Fast-Response Temperature-Sensitive Paints. Phys. Fluids 2016, 28, 046103/1–046103/15. [Google Scholar] [CrossRef]
- Ozawa, H.; Laurence, S.J.; Schramm, J.M.; Wagner, A.; Hannemann, K. Fast-Response Temperature-Sensitive-Paint Measurements on a Hypersonic Transition Cone. Exp. Fluids 2015, 56, 1853. [Google Scholar] [CrossRef]
- Ozawa, H.; Laurence, S.J. Experimental Investigation of the Shock-Induced Flow Over a Wall-Mounted Cylinder. J. Fluid Mech. 2018, 849, 1009–1042. [Google Scholar] [CrossRef]
- Bitter, M.; Hilfer, M.; Schubert, T.; Klein, C.; Niehuis, R. An Ultra-Fast TSP on a CNT Heating Layer for Unsteady Temperature and Heat Flux Measurements in Subsonic Flows. Sensors 2022, 22, 657. [Google Scholar] [CrossRef]
- Goodman, K.Z.; Lipford, W.E.; Watkins, A.N. Boundary-Layer Detection at Cryogenic Conditions Using Temperature Sensitive Paint Coupled with a Carbon Nanotube Heating Layer. Sensors 2016, 16, 2062. [Google Scholar] [CrossRef]
- Klein, C.; Henne, U.; Costantini, M.; Ondrus, V.; Beifuss, U.; Zhai, J.; Quest, J. Development of a Highly Sensitive Temperature-Sensitive Paint for Measurements Under Cryogenic Temperature (100–160 K) Conditions. In Proceedings of the 54th AIAA Aerospace Sciences Meeting, San Diego, CA, USA, 4–8 January 2016. [Google Scholar] [CrossRef]
- Egami, Y.; Fey, U.; Klein, C.; Quest, J.; Ondrus, V.; Beifuss, U. Development of New Two-Component Temperature-Sensitive Paint (TSP) for Cryogenic Testing. Meas. Sci. Technol. 2012, 23, 115301. [Google Scholar] [CrossRef]
- Watkins, A.N.; Goodman, K.; Peak, S. Transition Detection at Cryogenic Temperatures Using a Carbon-Based Resistive Heating Layer Coupled with Temperature Sensitive Paint. In Proceedings of the AIAA Scitech 2019 Forum, San Diego, CA, USA, 7–11 January 2019. [Google Scholar] [CrossRef]
- Claucherty, S.; Sakaue, H. Temperature Characterization of an Optical-Chemical Tunable-Peak Sensor Using CdSe/ZnS Quantum-Dots Applied on Anodized-Aluminum for Surface Temperature Measurement. Sens. Actuators B 2017, 251, 958–962. [Google Scholar] [CrossRef]
- Claucherty, S.; Sakaue, H. An Optical-Chemical Sensor Using Rhodamine B on Anodized-Aluminum for Surface Temperature Measurement from 150 to 500 K. Sens. Actuators B 2017, 240, 956–961. [Google Scholar] [CrossRef]
- Claucherty, S.; Sakaue, H. Phenol-Formaldehyde Resin for Optical-Chemical Temperature Sensing. Sensors 2018, 18, 1756. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kitagawa, Y.; Nakanishi, T.; Fushimi, K.; Hasegawa, Y. Ligand-Assisted Back Energy Transfer in Luminescent TbIII Complexes for Thermosensing Properties. Chem. Eur. J. 2018, 24, 17719. [Google Scholar] [CrossRef]
- Hirai, Y.; Mallette, A.; Nishio, Y.; Patterson, W.; Hasegawa, Y.; Sakaue, H. Visualization of Icing of Supercooled Water Using Tb(III)-Based Temperature-Sensitive Paint. Sens. Actuators A 2019, 285, 599–602. [Google Scholar] [CrossRef]
- Iijima, Y.; Sakaue, H. Platinum Porphyrin and Luminescent Polymer for Two-Colour Pressure- and Temperature-Sensing Probes. Sens. Actuators A 2012, 184, 128–133. [Google Scholar] [CrossRef]
- Kameya, T.; Matsuda, Y.; Egami, Y.; Yamaguchi, H.; Niimi, T. Dual Luminescent Arrays Sensor Fabricated by Inkjet-Printing of Pressure- and Temperature-Sensitive Paints. Sens. Actuators B 2014, 190, 70–77. [Google Scholar] [CrossRef]
- Klein, C.; Henne, U.; Sachs, W.; Hock, S.; Falk, N.; Beifuss, U.; Ondrus, V.; Schaber, S. Pressure Measurement on Rotating Propeller Blades by Means of the Pressure-Sensitive Paint Lifetime Method. In Proceedings of the 51st AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Dallas, TX, USA, 7–10 January 2013. [Google Scholar]
- Fischer, L.H.; Karakus, C.; Meier, R.J.; Risch, N.; Wolfbeis, O.S.; Holder, E.; Schäferling, M. Referenced Dual Pressure- and Temperature-Sensitive Paint for Digital Colour Camera Read Out. Chem. Eur. J. 2012, 18, 15706–15713. [Google Scholar] [CrossRef]
- Peng, D.; Liu, Y. A Grid-Pattern PSP/TSP System for Simultaneous Pressure and Temperature Measurements. Sens. Actuators B 2016, 222, 141–150. [Google Scholar] [CrossRef]
- Moon, K.-J.; Mori, H.; Furukawa, M. Simultaneous Measurement Method of Pressure and Temperature Using Dual-Layer PSP/TSP with Lifetime-Based Method. Meas. Sci. Technol. 2018, 29, 125301. [Google Scholar] [CrossRef]
- Disotell, K.J.; Peng, D.; Juliano, T.J.; Gregory, J.W.; Crafton, J.W.; Komerath, N.M. Single-Shot Ttemperature- and Pressure-Sensitive Paint Measurements on an Unsteady Helicopter Blade. Exp. Fluids 2014, 55, 1671. [Google Scholar] [CrossRef]
- Gu, Q.-S.; Li, T.; Liu, T.; Yu, G.; Mao, G.-J.; Xu, F.; Li, C.-Y. Recent Advances in Design Strategies and Imaging Applications of Fluorescent Probes for ATP. Chemosensors 2023, 11, 417. [Google Scholar] [CrossRef]
- Sun, X.; Zhai, W.; Fossey, J.S.; James, T.D. Boronic Acids for Fluorescence Imaging of Carbohydrates. Chem. Commun. 2016, 52, 3456–3469. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R.; Zhao, X.; Ma, Y.; Ren, L.; Ren, Y.; Chen, G.; Ye, D.; Wu, J.; Hu, X.; et al. Reversible Recognition-Based Boronic Acid Probes for Glucose Detection in Live Cells and Zebrafish. J. Am. Chem. Soc. 2023, 145, 8408–8416. [Google Scholar] [CrossRef]
- Lin, V.S.; Dickinson, B.C.; Chang, C.J. Boronate-Based Fluorescent Probes: Imaging Hydrogen Peroxide in Living Systems. Methods Enzymol. 2013, 526, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.F.; Garcia, F.J.; Onak, C.S.; Carroll, K.S.; Chang, C.J. Chemical Approaches to Discovery and Study of Sources and Targets of Hydrogen Peroxide Redox Signaling Through NADPH Oxidase Proteins. Ann. Rev. Biochem. 2015, 84, 765–790. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, L.; Fu, L.; Hou, J.; Wang, L.; Sun, M.; Wang, X.; Chen, L. Molecular Fluorescent Probes for Imaging and Evaluation of Peroxynitrite Fluctuations in Lving Cells and In Vivo Under Hypoxic Stress. Sens. Actuators B 2022, 370, 132410. [Google Scholar] [CrossRef]
- Yan, Z.; Tang, Z.; Wang, X.; Zheng, Z.; Tian, Z.; Geng, X.; Li, Y.; Jiang, H. A Novel Golgi-Targetable Fluorescent Probe for Imaging Peroxynitrite in Golgi Stress and Sepsis-Iinduced Acute Lung Injury. Sens. Actuators B 2022, 369, 132352. [Google Scholar] [CrossRef]
- Niu, P.; Zhu, J.; Wie, L.; Liu, X. Application of Fluorescent Probes in Reactive Oxygen Species Disease Model. Crit. Rev. Anal. Chem. 2022, 11, 2080495. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Borisov, S.M.; Papkovsky, D.B.; Dmitriev, R.I. Sulforhodamine Nanothermometer for Multiparametric Fluorescence Lifetime Imaging Microscopy. Anal. Chem. 2016, 88, 10566–10572. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Sakaguchi, R.; Shi, B.; Kiyonaka, S. Intracellular Thermometry with Fluorescent Sensors for Thermal Biology. Pflugers Arch. Eur. J. Physiol. 2018, 470, 717–731. [Google Scholar] [CrossRef]
- Duan, N.; Yang, S. Research Progress on. Multifunctional Fluorescent Probes for Biological Imaging, Food and Environmental Detection. Crit. Rev. Anal. Chem. 2022, 18, 1–43. [Google Scholar] [CrossRef]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal–Organic Frameworks as Sensory Materials and Imaging Agents. Inorg. Chem. 2014, 53, 1916–1924. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.-P.; Chao, H.-Y.; Yuan, D.; Tan, T.T.Y.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-Dimensional Metal–Organic Framework with Wide Channels and Responsive Turn-On Fluorescence for the Chemical Sensing of Volatile Organic Compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Voort, P.V.D. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tissot, A.; Serre, C. Recent Progress on MOF-Based Optical Sensors for VOC Sensing. Chem. Sci. 2022, 13, 13978–14000. [Google Scholar] [CrossRef] [PubMed]
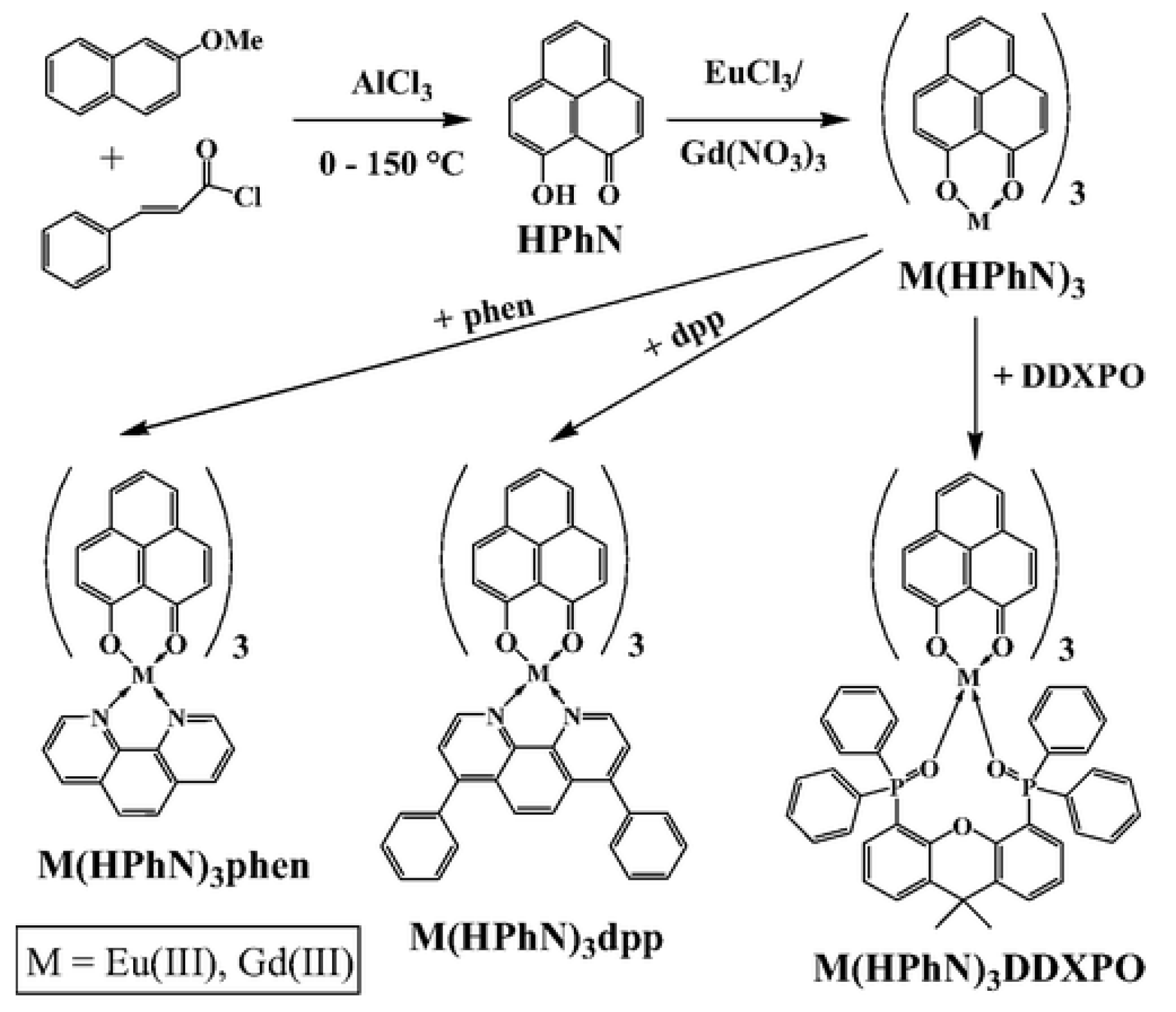

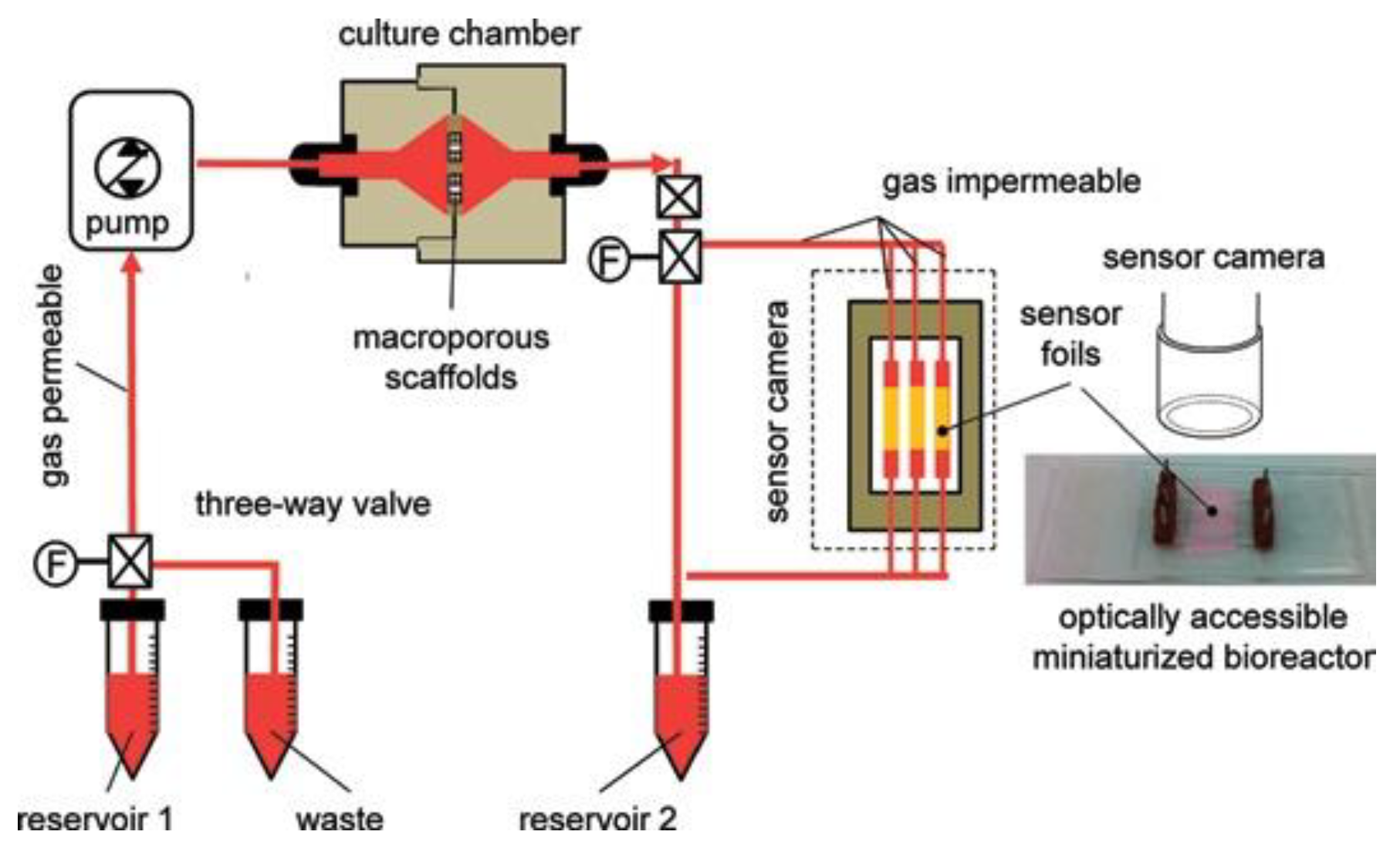
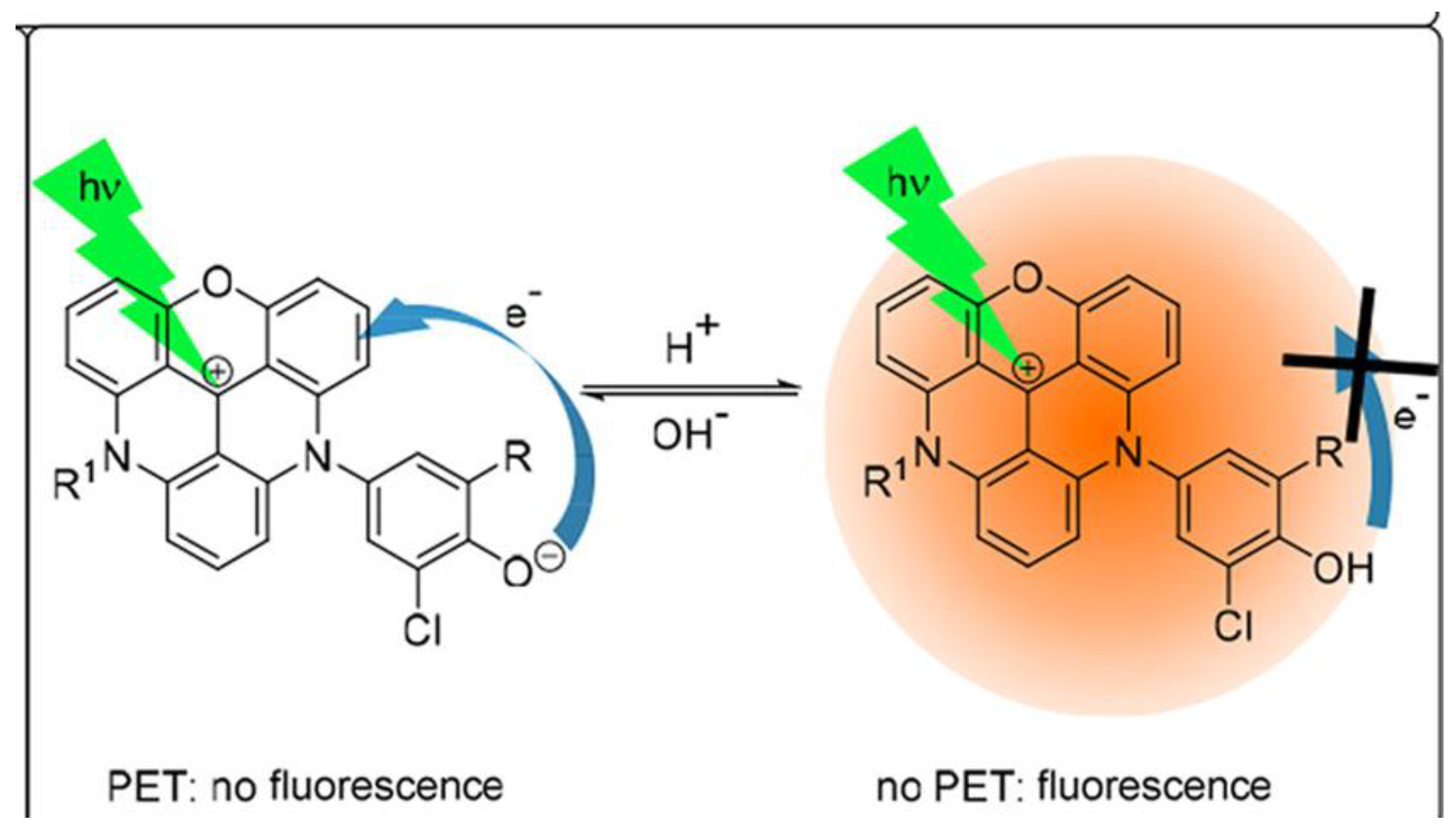
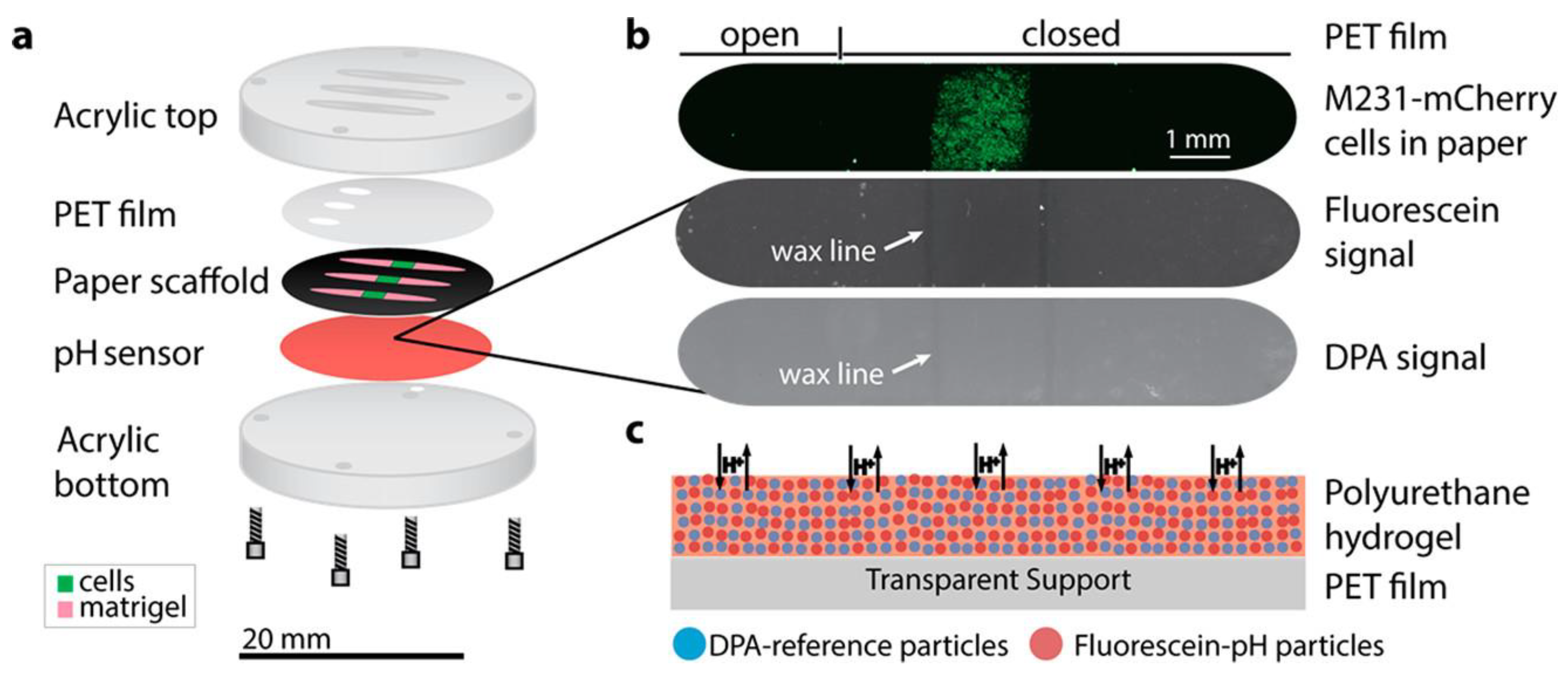
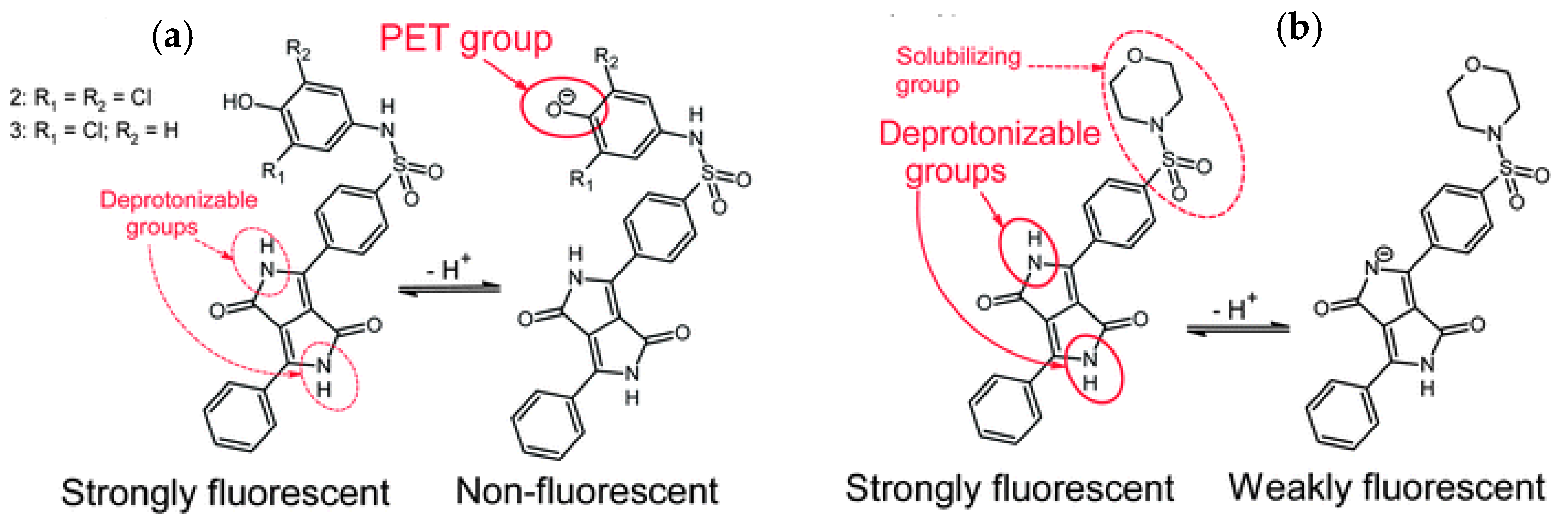
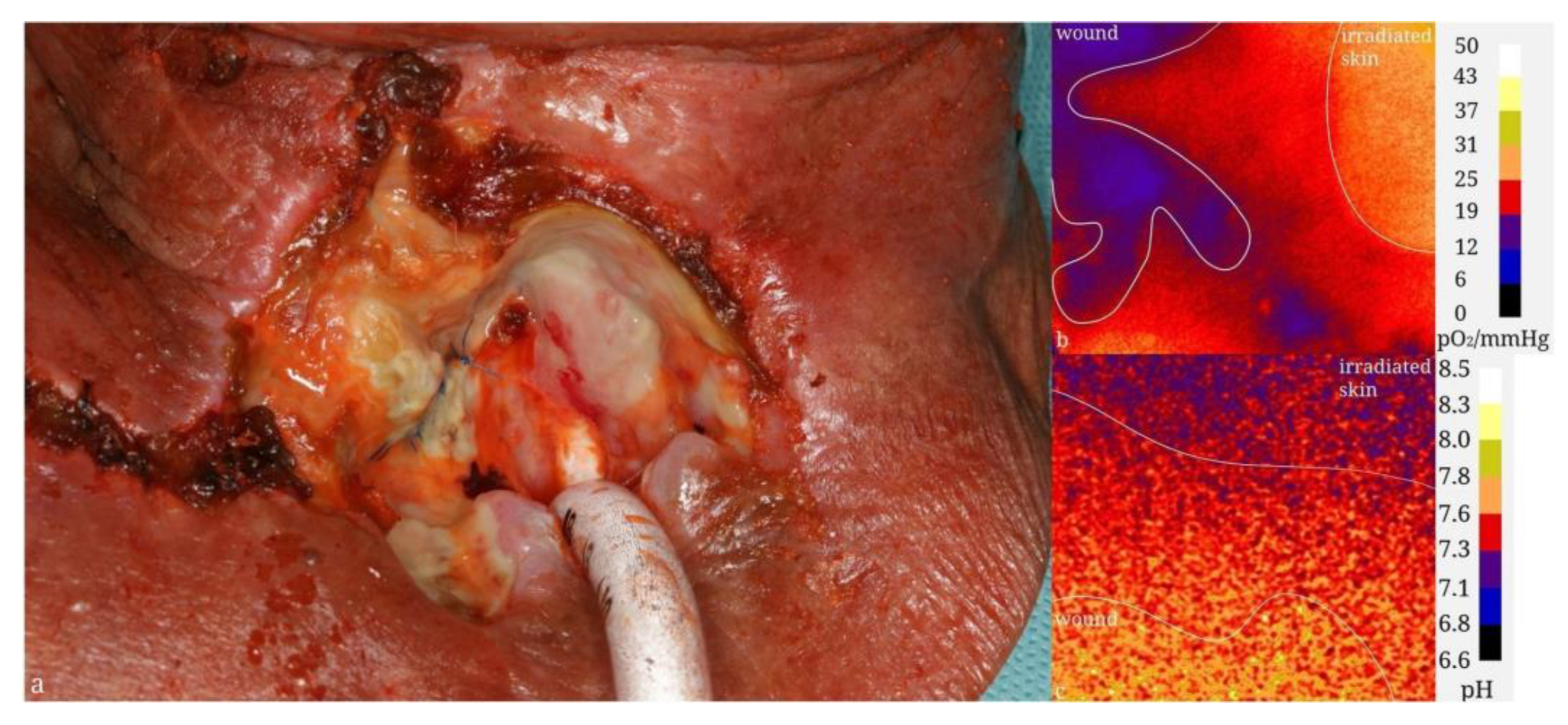
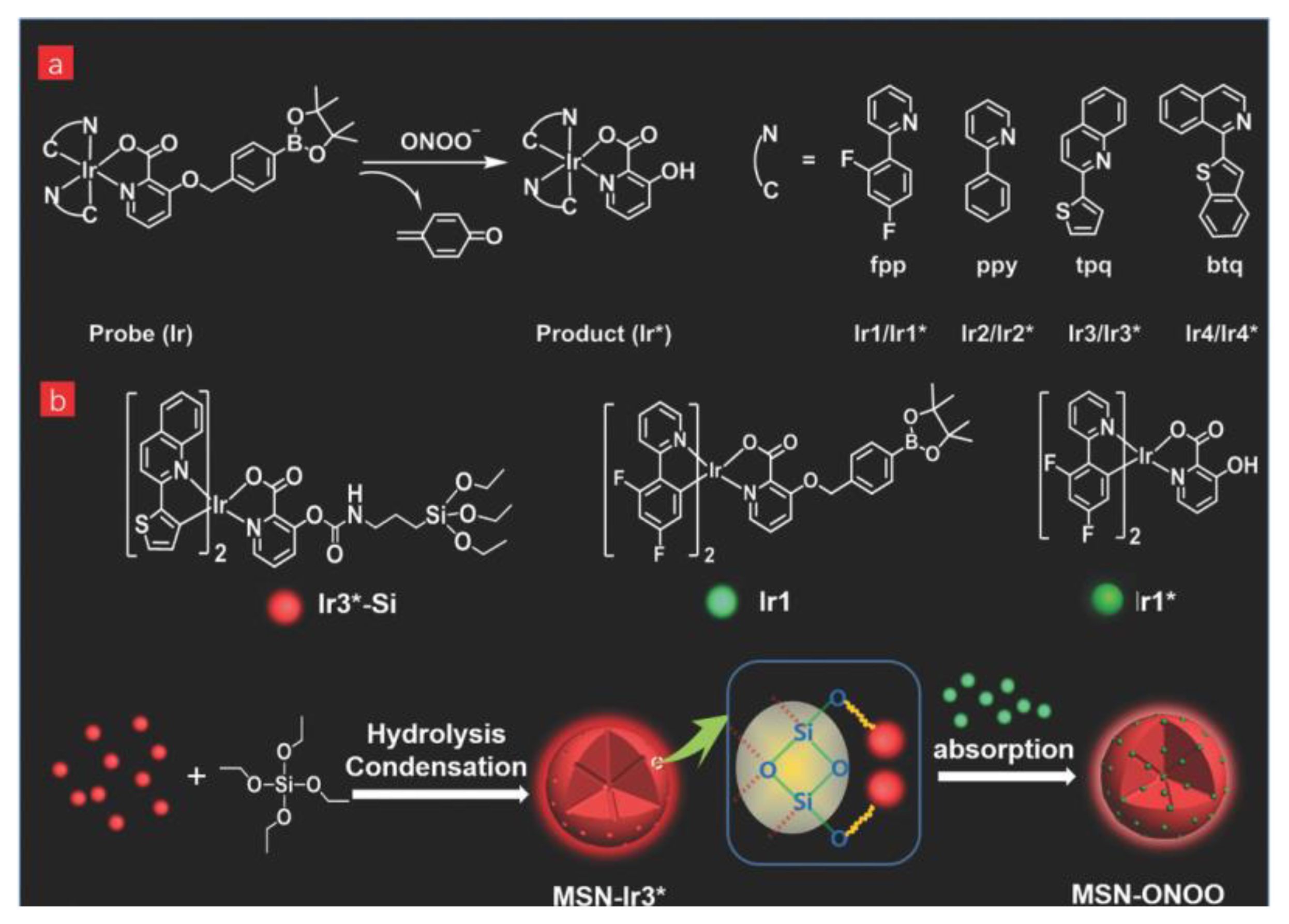
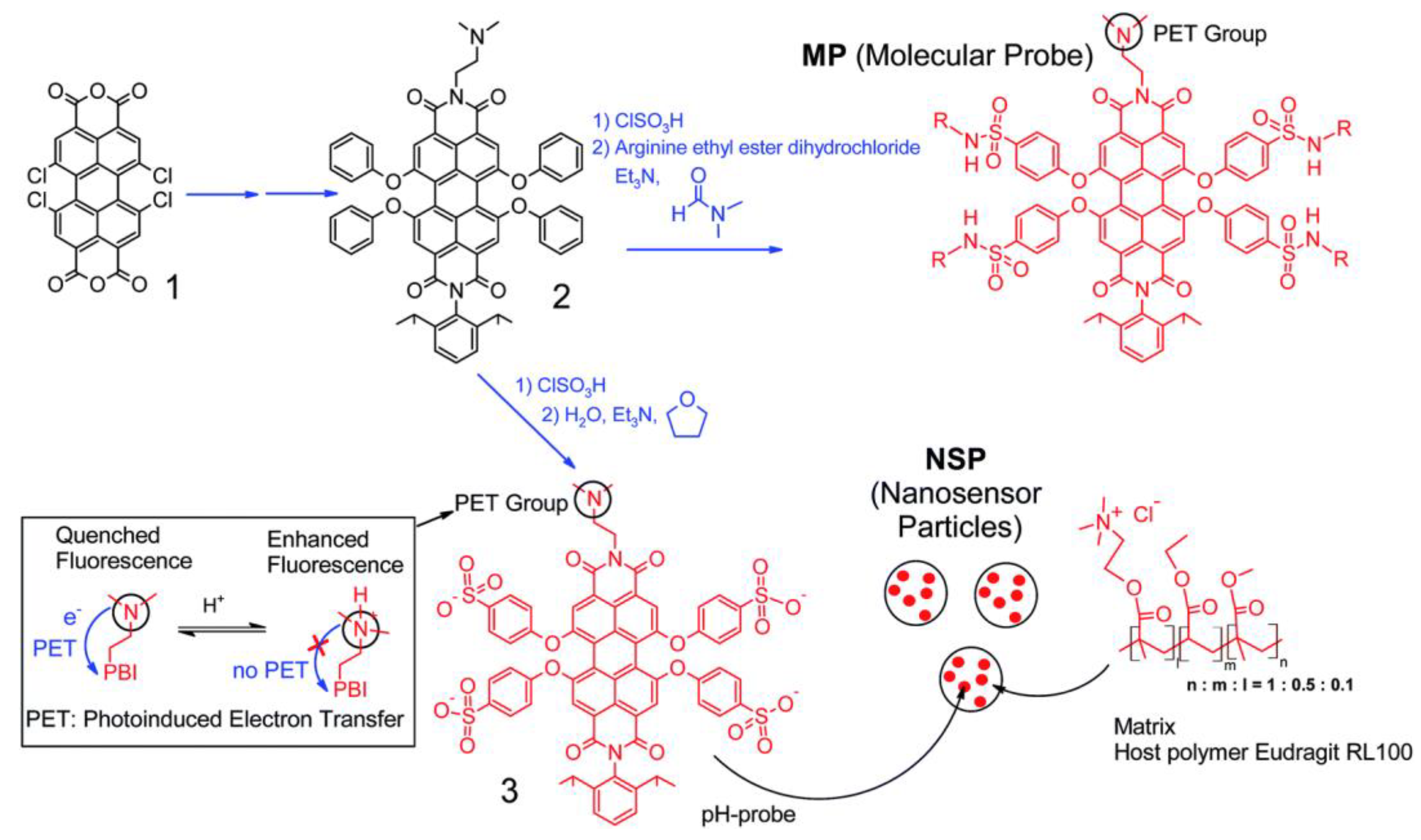
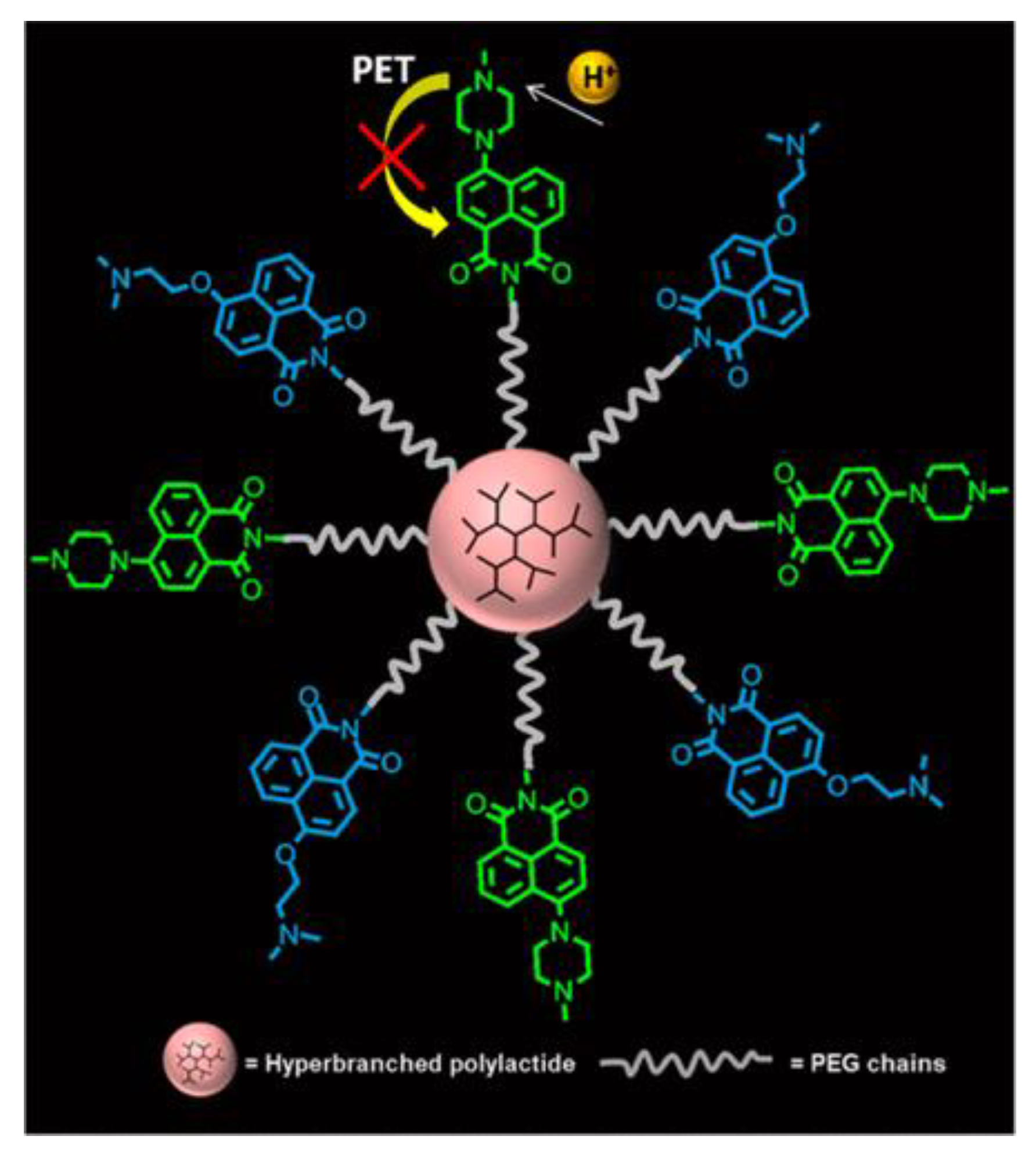
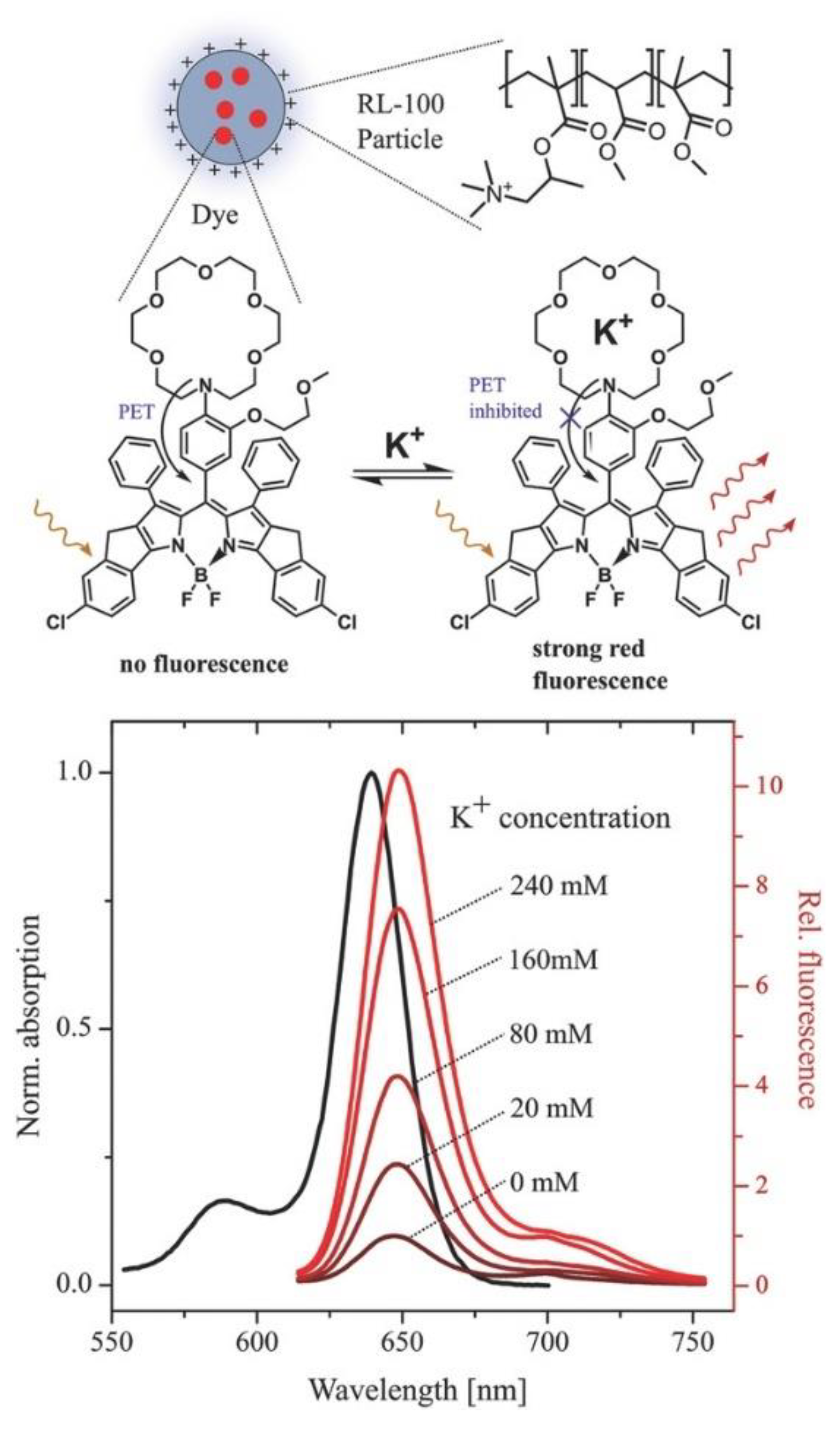
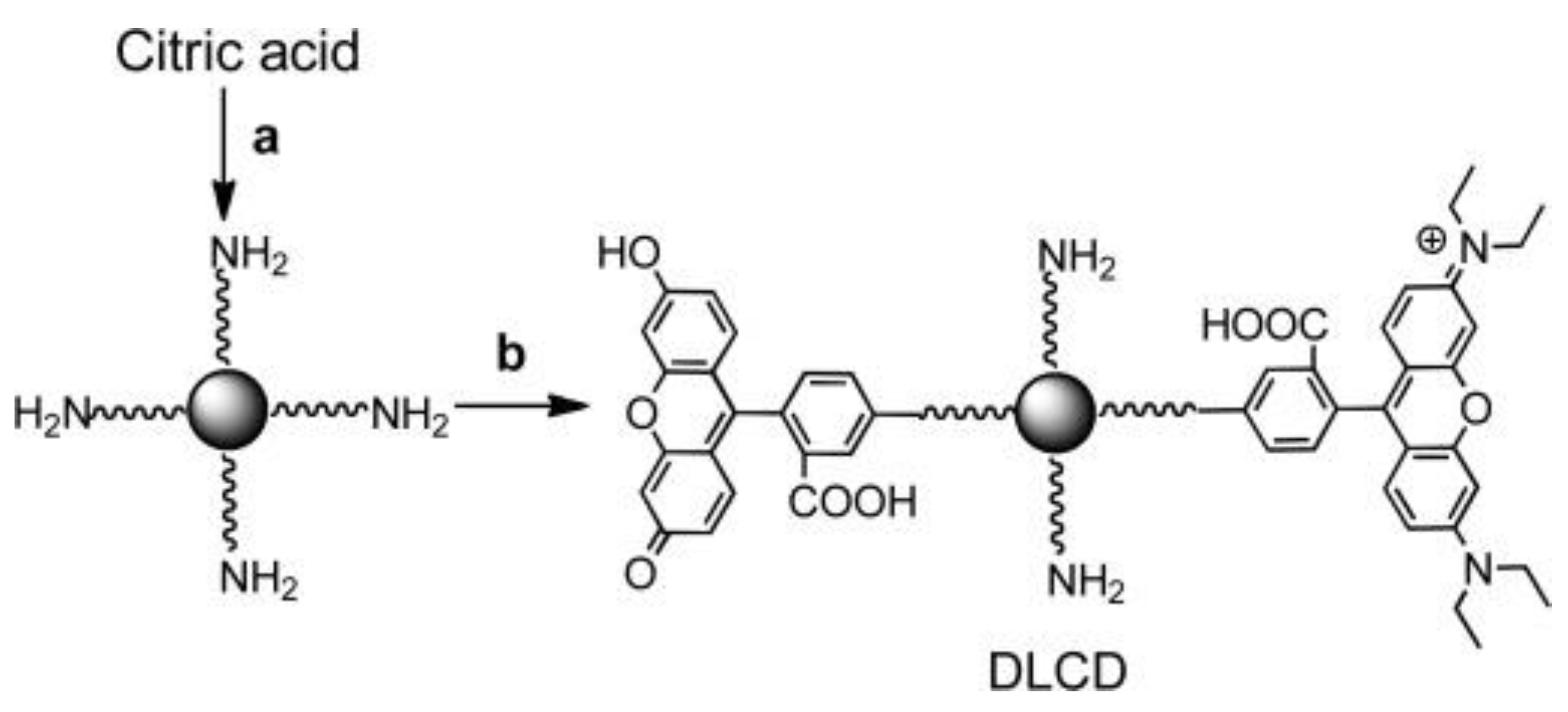
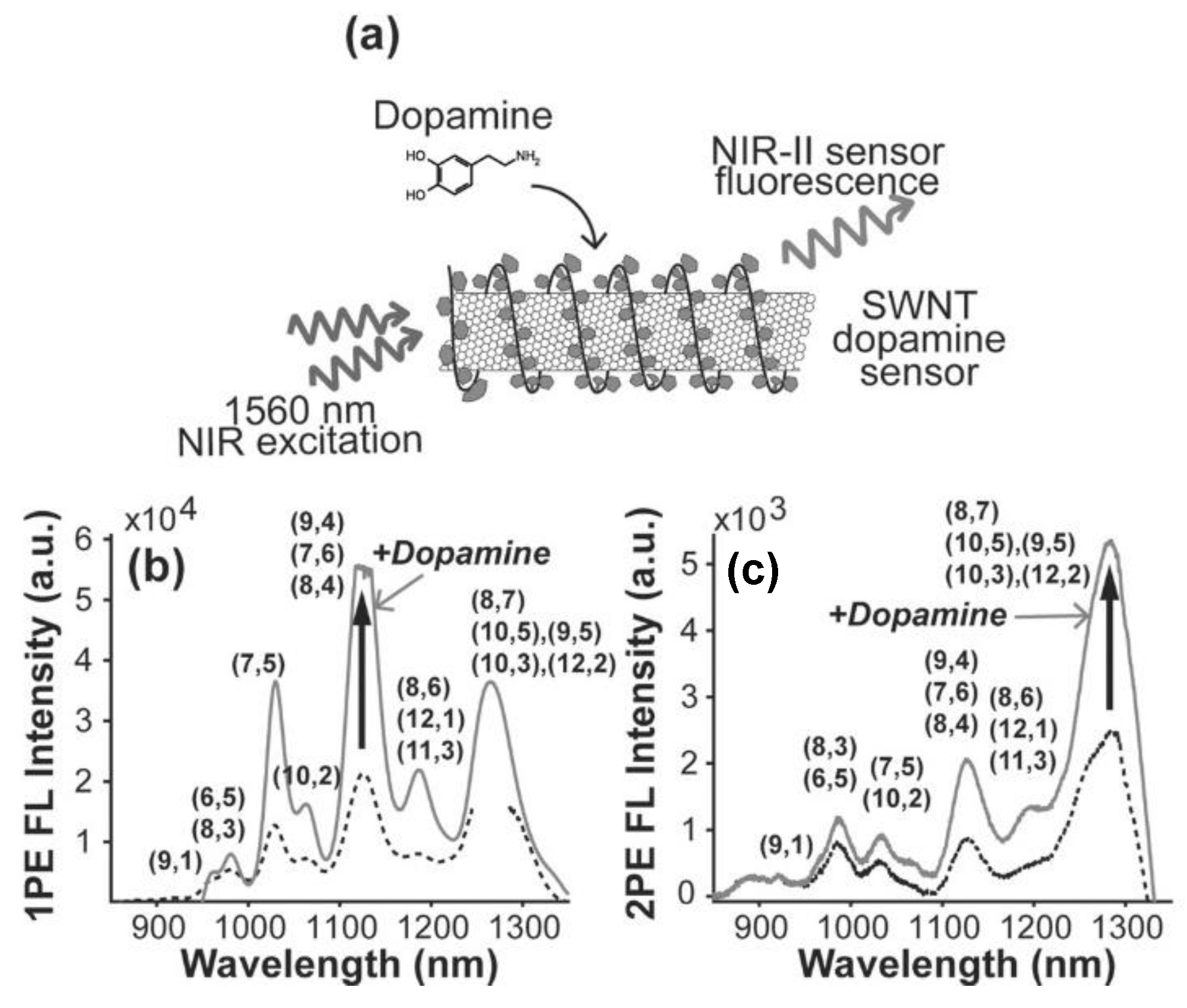
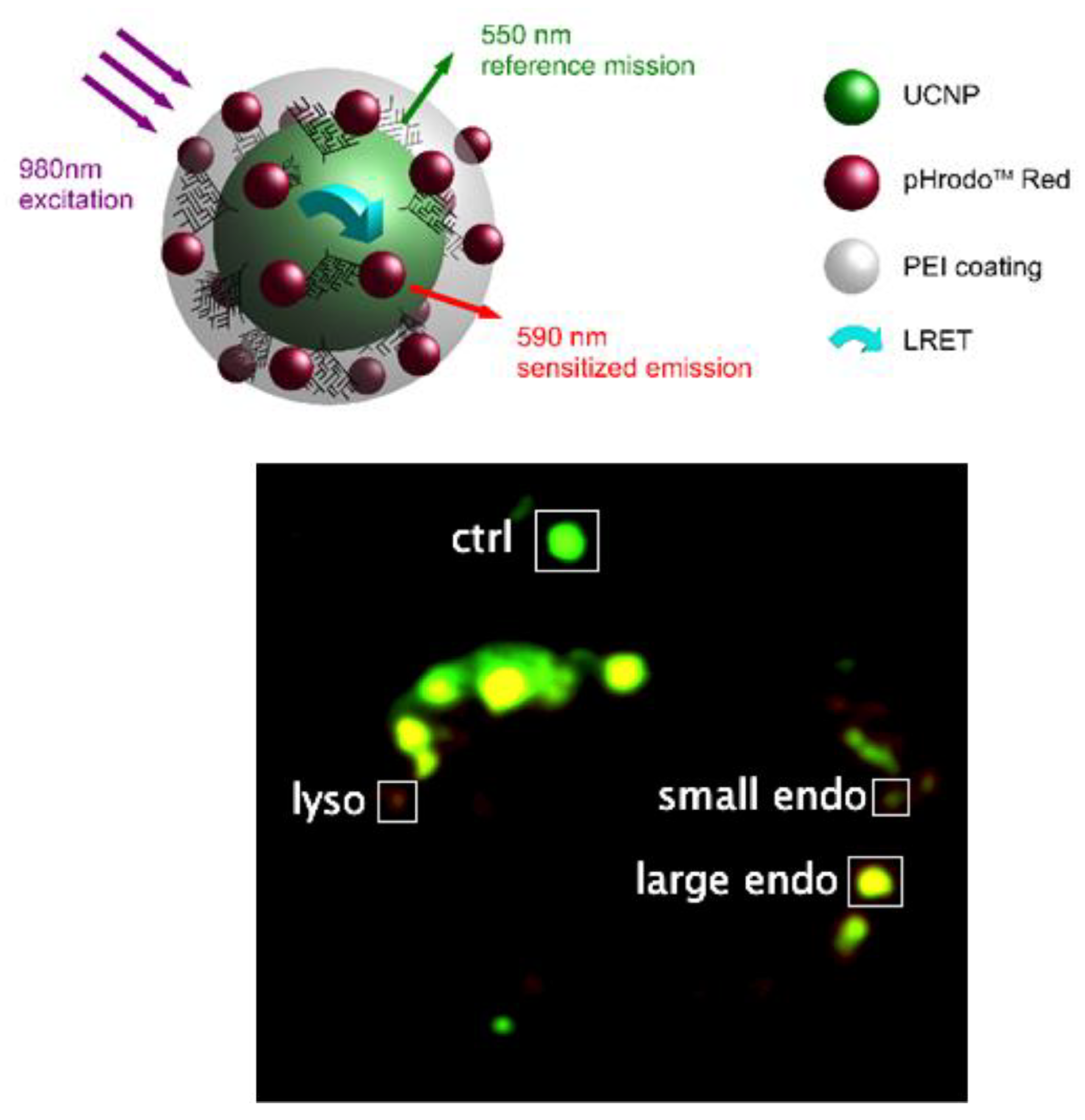
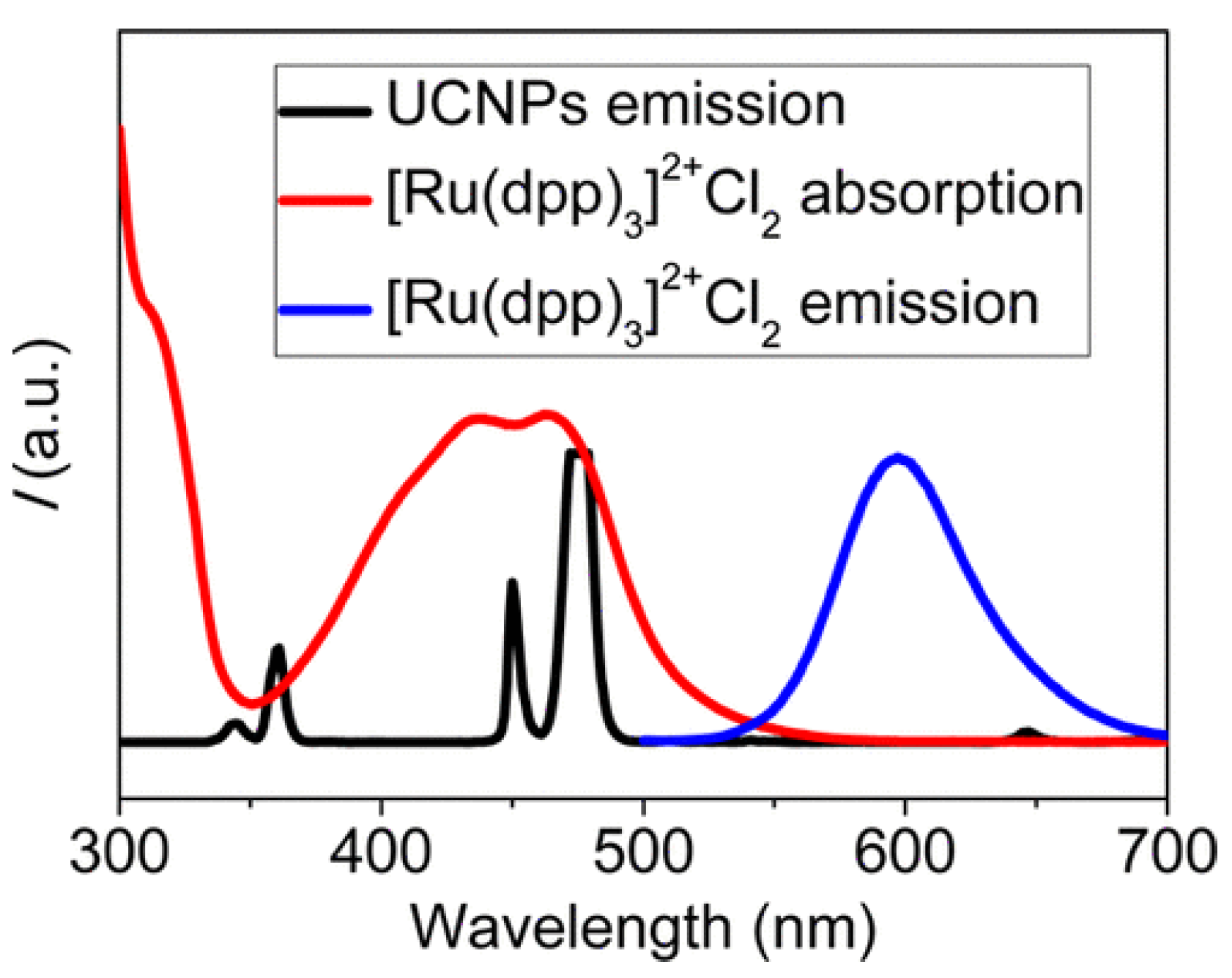
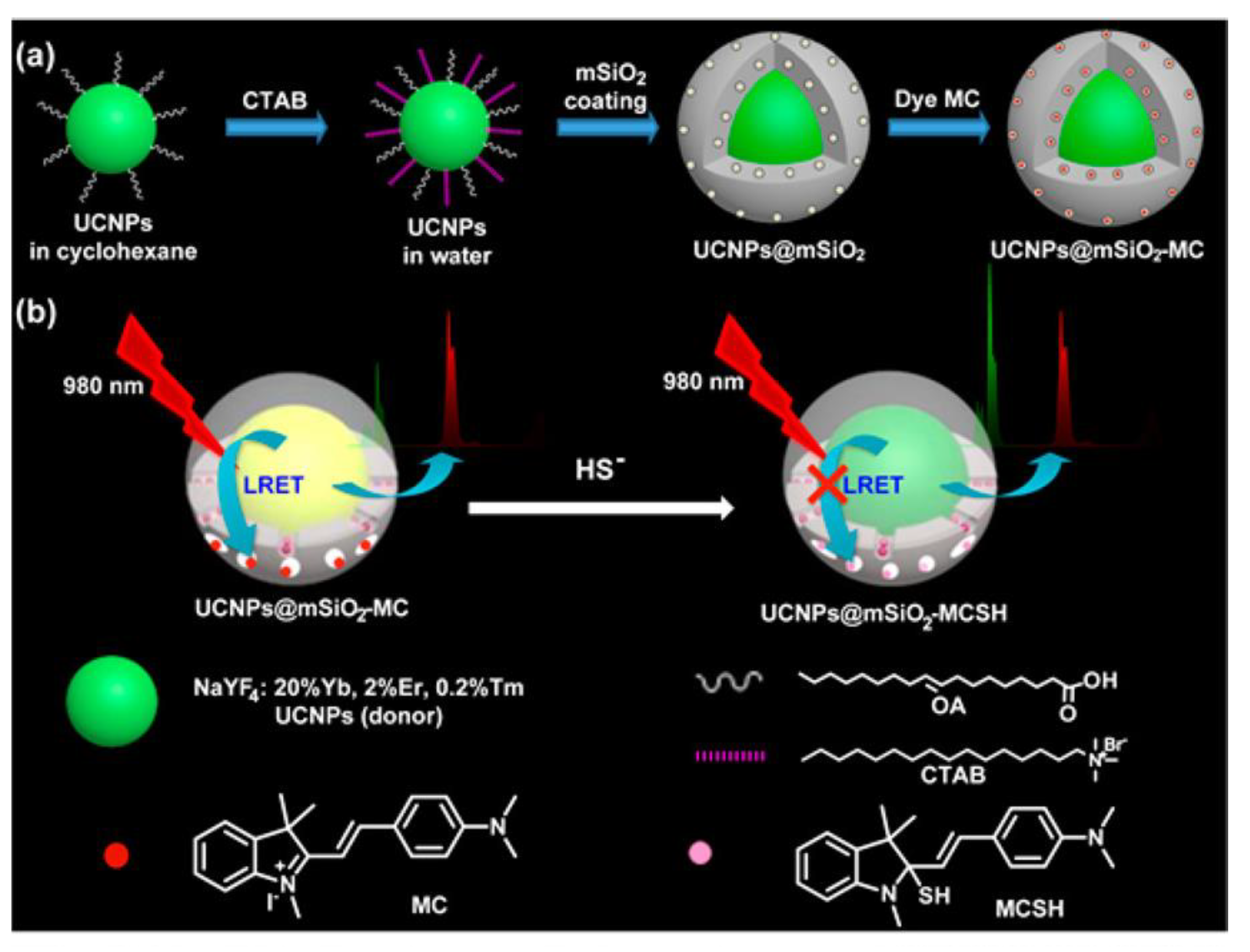
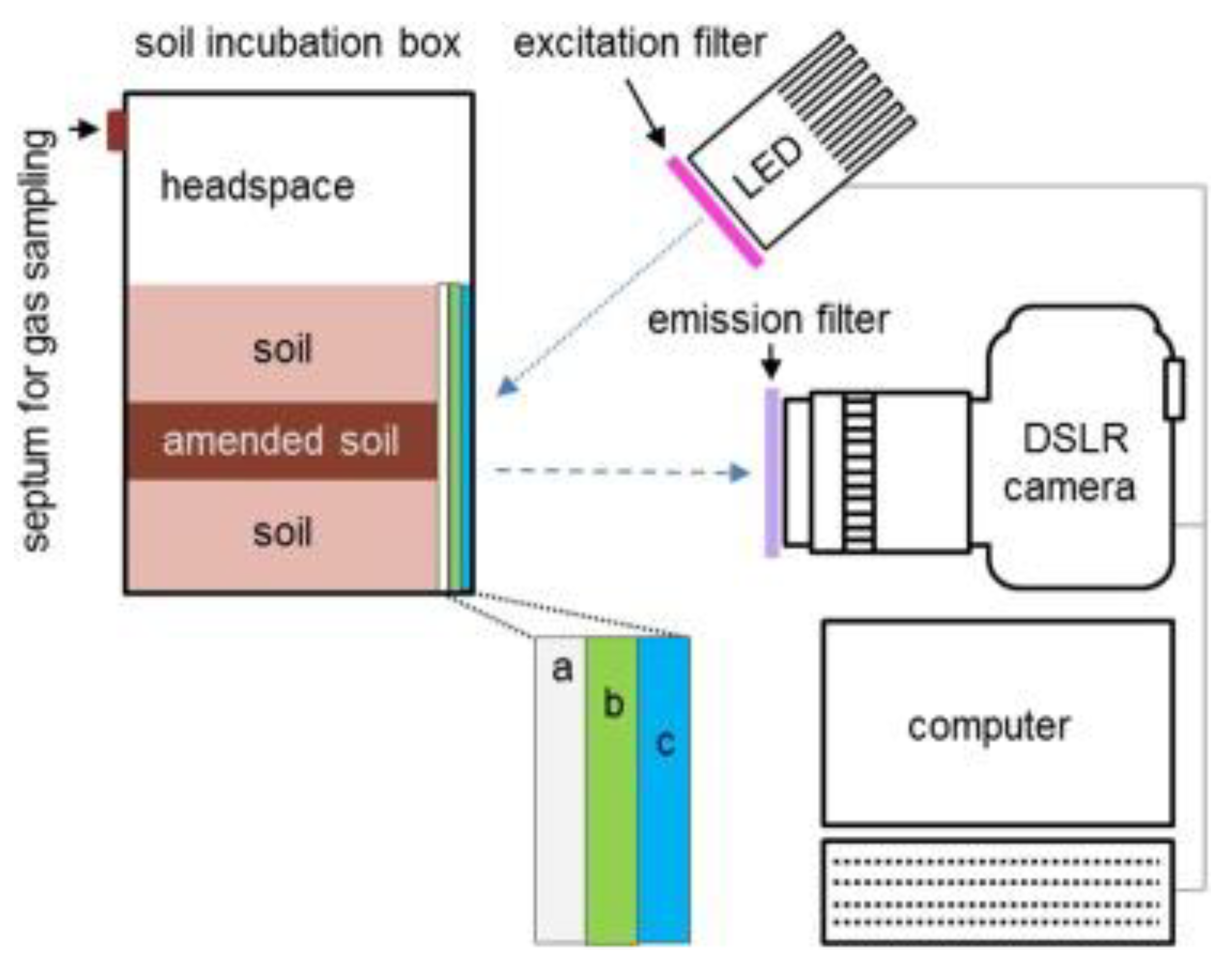

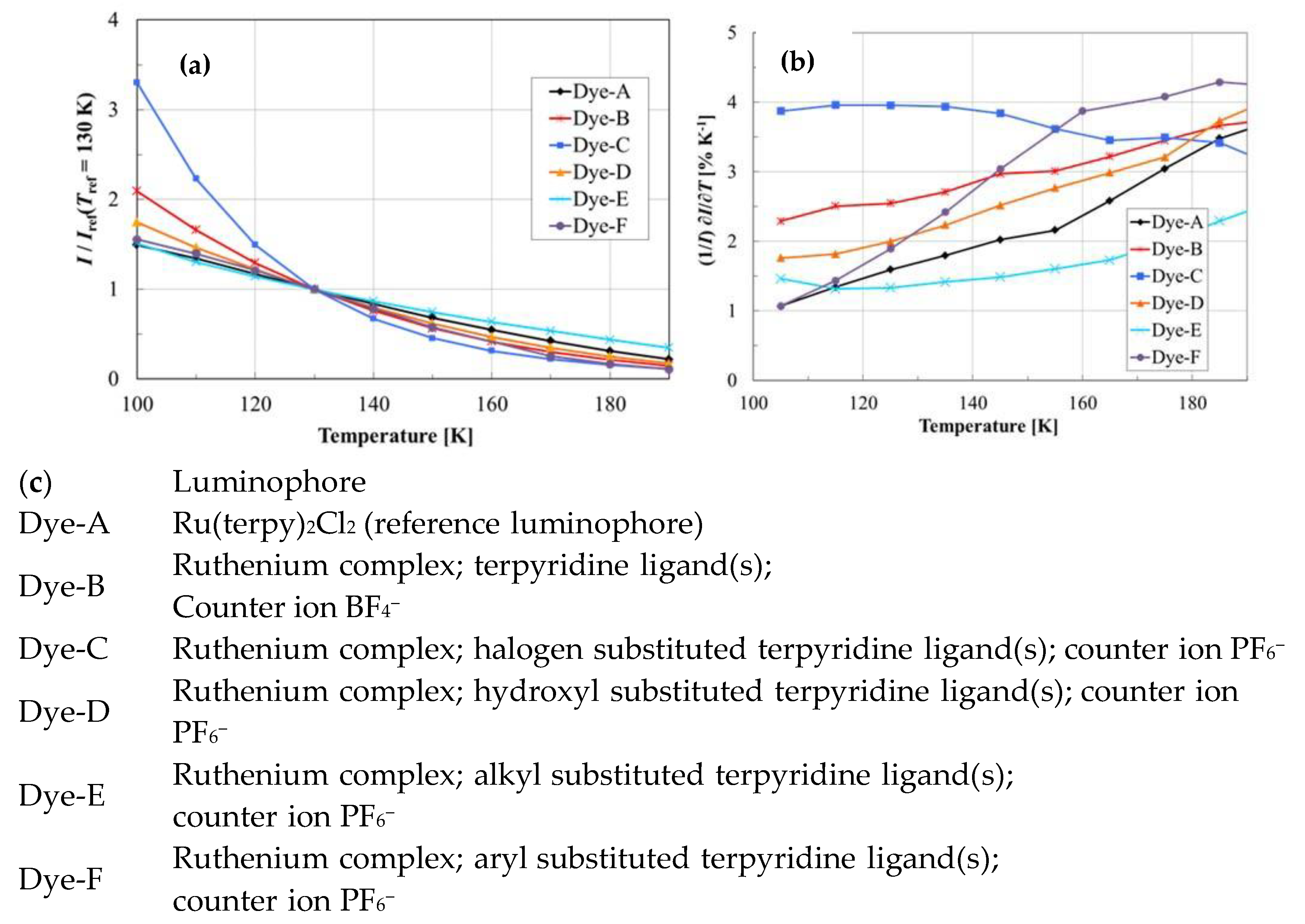

| Material | Composition | Luminescence Properties | Sensing Mechanism |
|---|---|---|---|
| Polymer NPs | PS, PMMA, PAM, cationic hydrogel | - | Fluorescent probes incorporated or attached to surface |
| Silica NPs | SiO2 | - | Fluorescent probes incorporated or attached to surface |
| Quantum dots | CdSe/CdS CdSe/ZnS | Intrinsic size-dependent fluorescence, high photostability | Surface modification with fluorescent probes |
| Nanocarbon | C | Intrinsic tunable fluorescence, size- and excitation-wavelength-dependent | Surface modification with fluorescent probes |
| UCNPs | NaYF4:Yb3+,Er3+ NaYF4:Yb3+,Tm3+ | Sensitized emission of Yb3+ or Er3+ | Surface modification with fluorescent probes, energy transfer |
| Dye | λex [nm] | λem [nm] | Binding Matrix | Application | Refs. |
|---|---|---|---|---|---|
| Pyrene sulfonic acid | 350 | 395, 455–465 | AA | Fast PSP | [187,188,189] |
| Rhodamine 6G | 527 | 580 | PMMA | Fast PSP | [190] |
| EuTTA complexes | 336–342 | 613–616 | Copolymer BA-DEGDA Silicone elastomer | Anaerobic PSP | [191] |
| Nile Red | 460 | 570 | PDMS | PSP in liquid | [192] |
| Ru-Silane | 365 | 630 | SiCD, silicone | Fast PSP | [193] |
| Sensitive Dye | λex [nm] | λem [nm] | Reference | Binder | Application | Refs. |
|---|---|---|---|---|---|---|
| Eu-hydroxyphenalenone | 460 | 615 | Coumarin | PS | Trace oxygen sensing | [30] |
| Gd-hydroxyphenalenone | 460 | 540, 600 | Coumarin | PS | Trace oxygen sensing | [30] |
| PtTFPP | 392, 507, 540 | 650 | NCCPN | Not specified | In vivo imaging of skin oxygenation | [32] |
| PtTFPP | FLIM | PS scaffold | Cell culture O2 gradients | [34] | ||
| PtTFPP | Macrolex Fluorescent Yellow | PS | O2 dynamics in soil, rhizosphere, cement | [129,130,142] | ||
| PtOEP | 382, 536 | 647 | Coumarin | PET | Dental biofilm | [35] |
| PtOEP | Quantum Dot | PS | O2 dynamics in seawater | [137] | ||
| Ir(CS)2 acac | 445, 475 | 564 | FLIM | PS | Benthic microalgal community | [128] |
| Eu(HPhN)3dpp | 400–470 | 617 | Bu3Coum | PS | O2 dynamics in microbial mats | [141] |
| Sensitive Dye | λex [nm] | λem [nm] | Reference | Binder | Application/Dynamic Range | Ref. |
|---|---|---|---|---|---|---|
| Neutral Red/UCNP | 980 | 550, 660 | UCNP emission | PU | Human serum pH 6–7.5 | [42] |
| Diazaoxatriangulenium | 560 | 590 | FLIM | PU | pH 4–7 | [43] |
| Flourescein/Aminoethylcellulose * | 507 | 542 | Ru(dpp)3/PAN * | Traumasept wound gel | Wound imaging, pH 4–9 | [44] |
| Fluorescein | Diphenylanthracene | PU | pH gradient in tumor models | [47] | ||
| Phenolic BODIPY series | 495 | 530 | PU/test stripe | Sensor array pH 1–13 | [48] | |
| Aza-BODIPY series | 506–667 | 516–680 | Lumogen Red/Fluorescent pigment green | EC | CO2 sensor, LOD 0.009 hPa | [50] |
| Aza-Bodipy | 694 | 720 | Bu3Coum | PU | pH 8–11 in cement | [142] |
| 1,4-Diketopyrrolo-[3,4-c]pyrroles | 509, 543/575, 606 | 580 | Macrolex Fluorescence Yellow | PU | Microfluidic system, pH 6–9 | [51] |
| 496–550 | 564–587 | Dual emission | EC | CO2 sensor LOD 0.07 kPa | [52] | |
| 501, 572 | 524, 605 | Dual emission | EC | CO2 sensor | [53] | |
| Chlorophenyliminopropenyl-anailine | 550 | 590 | Macrolex Fluorescence Yellow | Sediment dynamics | [135] | |
| 5-Hexadecanoyl-aminofluorescein | 495 | 520 | Quantum Dot | PU | pH 6–8, dynamics in seawater | [137] |
| Oxazine 170 perchlorate | 624 | 645 | Macrolex Fluorescence Yellow | PU/fluoropolymer | NH3 sensor in soil, LOD 2.11 ppm | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäferling, M.; Ondrus, V. The Art of Fluorescence Imaging with Chemical Sensors: The Next Decade 2012–2022. Chemosensors 2024, 12, 31. https://doi.org/10.3390/chemosensors12030031
Schäferling M, Ondrus V. The Art of Fluorescence Imaging with Chemical Sensors: The Next Decade 2012–2022. Chemosensors. 2024; 12(3):31. https://doi.org/10.3390/chemosensors12030031
Chicago/Turabian StyleSchäferling, Michael, and Vladimir Ondrus. 2024. "The Art of Fluorescence Imaging with Chemical Sensors: The Next Decade 2012–2022" Chemosensors 12, no. 3: 31. https://doi.org/10.3390/chemosensors12030031
APA StyleSchäferling, M., & Ondrus, V. (2024). The Art of Fluorescence Imaging with Chemical Sensors: The Next Decade 2012–2022. Chemosensors, 12(3), 31. https://doi.org/10.3390/chemosensors12030031








