Recent Advances in Metal Oxide Semiconductor Heterojunctions for the Detection of Volatile Organic Compounds
Abstract
1. Introduction
2. Types of Heterojunctions and Preparation Methods
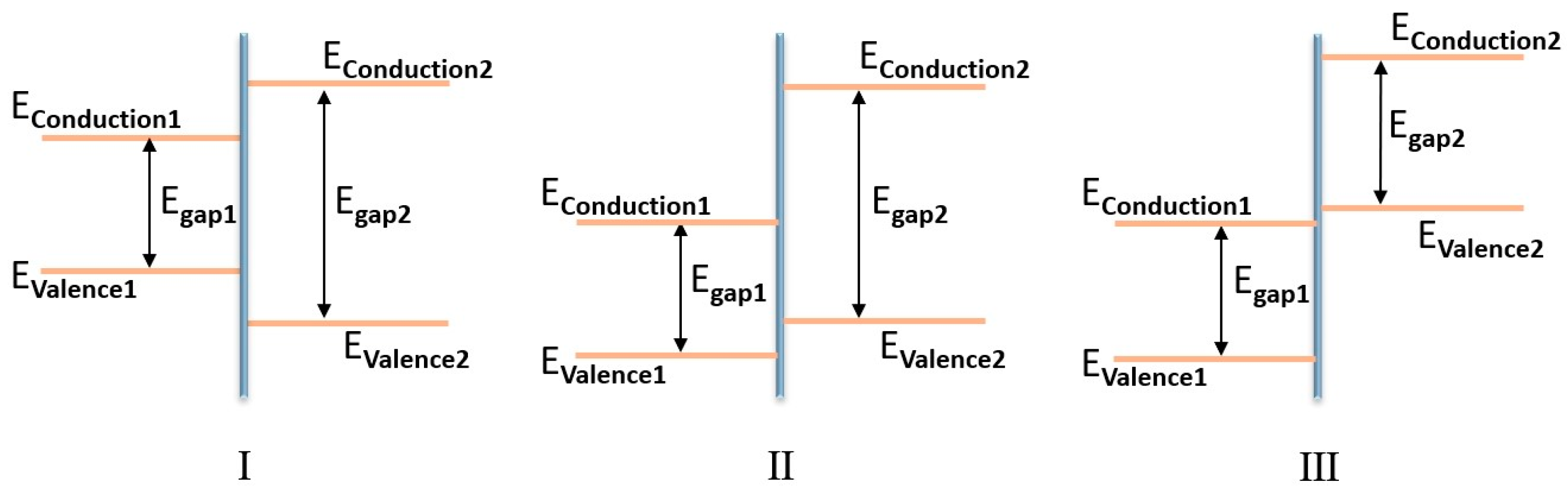
2.1. Types of Heterojunctions
2.2. Working Principle of Heterojunction Sensors
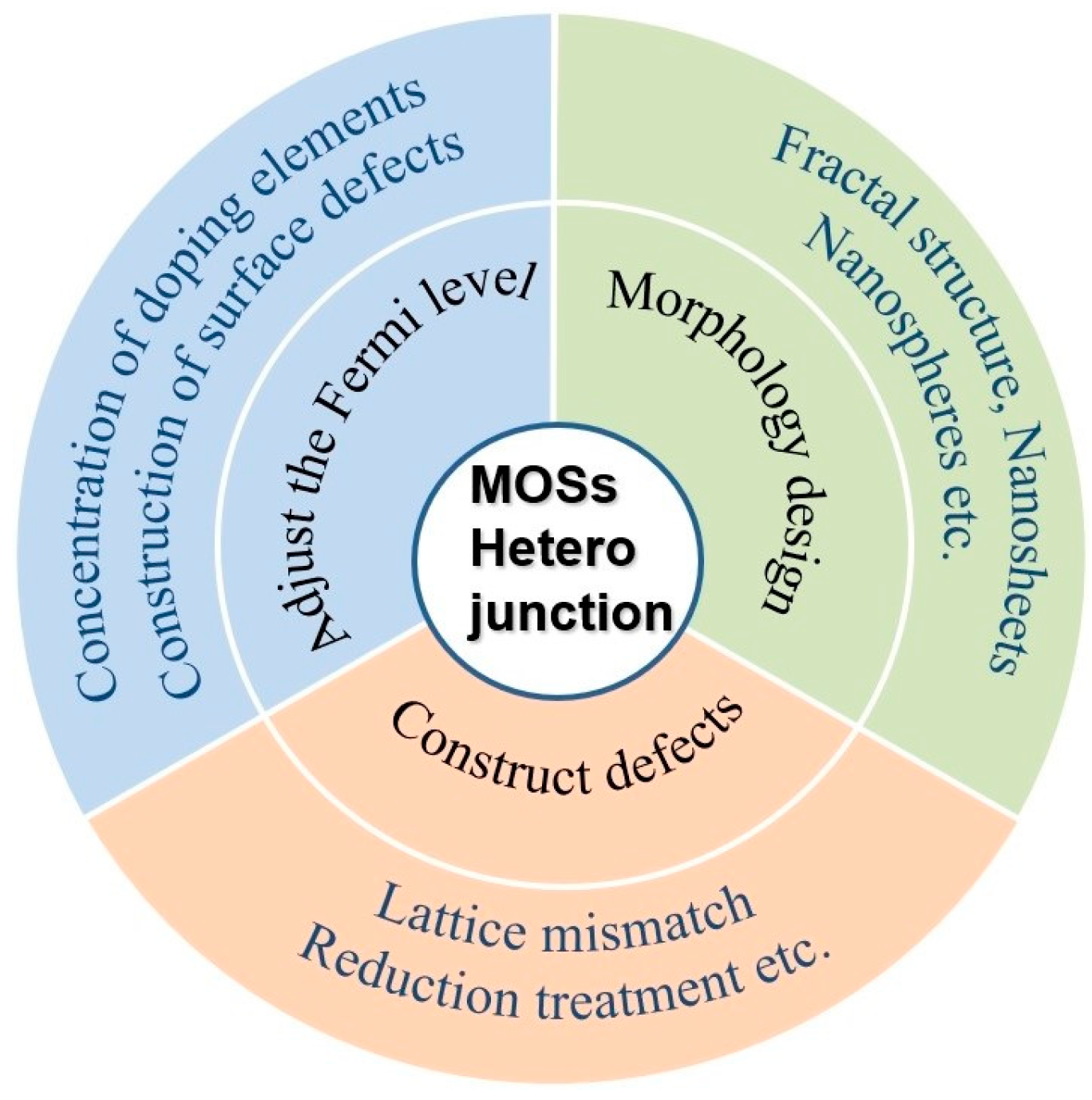
2.3. Strategies for Improving the VOC Sensing Performances of MOSs Heterojunctions
2.4. Preparation Methods
3. P-n Heterojunctions Sensors for VOC Detection
3.1. P-n Heterojunctions Sensors for Acetone Detection
3.2. P-n Heterojunctions Sensors for Ethanol Detection
4. N-n Heterojunction Sensors for VOC Detection
4.1. N-n Heterojunction Sensors for Acetone Detection
4.2. N-n Heterojunction Sensors for Ethanol Detection
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belluomo, I.; Boshier, P.R.; Myridakis, A.; Vadhwana, B.; Markar, S.R.; Spanel, P.; Hanna, G.B. Selected Ion Flow Tube Mass Spectrometry for Targeted Analysis of Volatile Organic Compounds in Human Breath. Nat. Protoc. 2021, 16, 3419–3438. [Google Scholar] [CrossRef]
- Nah, S.H.; Kim, J.B.; Chui, H.N.T.; Suh, Y.; Yang, S. Enhanced Colorimetric Detection of Volatile Organic Compounds Using a Dye-Incorporated Photonic Crystal-Based Sensor Array. Adv. Mater. 2024, 36, 2409297. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Maraloiu, V.-A.; Comini, E. Novel Christmas Branched like NiO/NiWO4/WO3 (p–p–n) Nanowire Heterostructures for Chemical Sensing. Adv. Funct. Mater. 2021, 31, 2104416. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, M.; Yang, Z.; Zhou, C.; Cui, D.; Haick, H.; Tang, N. AI-Driven Wearable Mask-Inspired Self-Healing Sensor Array for Detection and Identification of Volatile Organic Compounds. Adv. Funct. Mater. 2024, 34, 2309732. [Google Scholar] [CrossRef]
- Yang, X.Y.; Deng, Y.; Yang, H.T.; Liao, Y.Z.; Cheng, X.W.; Zou, Y.D.; Wu, L.M.; Deng, Y.H. Functionalization of Mesoporous Semiconductor Metal Oxides for Gas Sensing: Recent Advances and Emerging Challenges. Adv. Sci. 2023, 10, 2204810. [Google Scholar] [CrossRef]
- Kamathe, V.; Nagar, R. Designing Sensing Material with Fractal Geometry: A Gateway for Enhanced Ethanol Sensing under Ambient Conditions. Sens. Actuators B Chem. 2024, 410, 135642. [Google Scholar] [CrossRef]
- Khatib, M.; Haick, H. Sensors for Volatile Organic Compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.X.; Han, B.; Wang, M.Y.; Wang, Q.; Zhang, Y.N. Fiber Optic Volatile Organic Compound Gas Sensors: A Review. Coord. Chem. Rev. 2023, 493, 215297. [Google Scholar] [CrossRef]
- Gaur, S.; Singh, S.; Deb, J.; Bhutani, V.; Mondal, R.; Pareek, V.; Gupta, R. Site-Selective MoS2-Based Sensor for Detection and Discrimination of Triethylamine from Volatile Amines Using Kinetic Analysis and Machine Learning. Adv. Funct. Mater. 2024, 34, 2405232. [Google Scholar] [CrossRef]
- Singh, S.; Sajana, S.; Varma, P.; Sreelekha, G.; Adak, C.; Shukla, R.P.; Kamble, V.B. Metal Oxide-Based Gas Sensor Array for VOCs Determination in Complex Mixtures Using Machine Learning. Microchim. Acta 2024, 191, 196. [Google Scholar] [CrossRef]
- Kulkarni, S.; Ghosh, R. CuO-ZnO p-n Junctions for Accurate Prediction of Multiple Volatile Organic Compounds Aided by Machine Learning Algorithms. Anal. Chim. Acta 2023, 1253, 341084. [Google Scholar] [CrossRef]
- Khan, M.; Abid, K.; Ferlazzo, A.; Bressi, V.; Espro, C.; Hussain, M.; Foti, A.; Gucciardi, P.G.; Neri, G. A Sensitive and Selective Non-Enzymatic Dopamine Sensor Based on Nanostructured Co3O4-Fe2O3 Heterojunctions. Chemosensors 2023, 11, 379. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Xu, S.; Huang, M.; Wen, Y.; Shi, X.-R. DFT-Assisted Rational Design of CoMxP/CC (M = Fe, Mn, and Ni) as Efficient Electrocatalyst for Wide PH Range Hydrogen Evolution and Oxygen Evolution. Nano Res. 2022, 15, 8897–8907. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.R.; Hasani, A.; Hussain, T.; Ghanbari, H.; Fawzy, M.; Abnavi, A.; Ahmadi, R.; Kabir, F.; De Silva, T.; Rajapakse, R.K.N.D.; et al. Enhanced Sensitivity in Photovoltaic 2D MoS2/Te Heterojunction VOC Sensors. Small 2024. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Durairaj, A.; Saravanan, A.; Luong, J.H.T.; Bakandritsos, A.; Gedanken, A.; Zboril, R. The Innovative Design of Carbon Dots on Polymer Texture for Highly Selective Detection of Amino Compounds. Carbon 2024, 228, 119414. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.R.; Hasani, A.; Jaferzadeh, K.; Fawzy, M.; De Silva, T.; Abnavi, A.; Ahmadi, R.; Ghanbari, H.; Askar, A.; Kabir, F.; et al. Unique Photoactivated Time-Resolved Response in 2D GeS for Selective Detection of Volatile Organic Compounds. Adv. Sci. 2023, 10, 2205458. [Google Scholar] [CrossRef]
- Capman, N.S.S.; Zhen, X.V.; Nelson, J.T.; Chaganti, V.R.S.K.; Finc, R.C.; Lyden, M.J.; Williams, T.L.; Freking, M.; Sherwood, G.J.; Bühlmann, P.; et al. Machine Learning-Based Rapid Detection of Volatile Organic Compounds in a Graphene Electronic Nose. ACS Nano 2022, 16, 19567–19583. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, X.; Li, X.; Wang, A.; Zhang, M.; Chen, Y. Enhanced Acetone Sensing Properties of Pt@Al-Doped ZnO Core-Shell Nanoparticles. Sens. Actuators B Chem. 2021, 329, 129153. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Liu, T.; Yang, J.; Wang, S.; Li, Y.; Shao, D.; Feng, L.; Song, H. Facile Strategy to Synthesize Porous GO/ZnO Heterostructure for Enhanced Acetone Gas Sensing Properties. Sens. Actuators B Chem. 2022, 359, 131601. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Shingange, K.; Mhlongo, G.H. ZnO/ZnFe2O4 Heterostructure for Conductometric Acetone Gas Sensors. Sens. Actuators B Chem. 2023, 377, 133027. [Google Scholar] [CrossRef]
- Park, J.Y.; Kwak, Y.; Lim, H.-R.; Park, S.-W.; Lim, M.S.; Cho, H.-B.; Myung, N.V.; Choa, Y.-H. Tuning the Sensing Responses towards Room-Temperature Hypersensitive Methanol Gas Sensor Using Exfoliated Graphene-Enhanced ZnO Quantum Dot Nanostructures. J. Hazard. Mater. 2022, 438, 129412. [Google Scholar] [CrossRef]
- Zhao, L.J.; Sun, S.P.; Zhang, S.; Liu, Z.; Huang, B.Y.; Wang, N.; Zhang, J.W.; Li, X.G. UV-Activated Hollow ZnO@TiO2 Heterostructured Nanaospheres for Detecting Formaldehyde at Room Temperature. Sens. Actuators B-Chem. 2023, 394, 134306. [Google Scholar] [CrossRef]
- Amouzesh, S.P.; Khodadadi, A.A.; Mortazavi, Y.; Saris, S.; Asgari, M. MIL-100(Fe) /ZnO Nanocomposite Sensors: An Enhanced Ammonia Selectivity and Low Operating Temperature. Sens. Actuators B-Chem. 2024, 399, 134791. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Nakate, U.T.; Albargi, H.; Alsaiari, M.A.; Ahmed, F.; Alharthi, F.A.; Ali Alghamdi, A.; Al-Zaqri, N. Fabrication and Characterization of CuO Nanoplates Based Sensor Device for Ethanol Gas Sensing Application. Chem. Phys. Lett. 2021, 763, 138204. [Google Scholar] [CrossRef]
- Hermawan, A.; Zhang, B.; Taufik, A.; Asakura, Y.; Hasegawa, T.; Zhu, J.; Shi, P.; Yin, S. CuO Nanoparticles/Ti3C2Tx MXene Hybrid Nanocomposites for Detection of Toluene Gas. ACS Appl. Nano Mater. 2020, 3, 4755–4766. [Google Scholar] [CrossRef]
- Guo, M.M.; Luo, N.; Chen, Y.; Fan, Y.; Wang, X.H.; Xu, J.Q. Fast-Response MEMS Xylene Gas Sensor Based on CuO/WO3 Hierarchical Structure. J. Hazard. Mater. 2022, 429, 127471. [Google Scholar] [CrossRef]
- Wu, W.; Long, J.Y.; Guo, Y.J.; Zu, X.T.; Li, S.; Xiang, X. P-CuO/n-TiO2 Heterojunction Nanostructure-Based Surface Acoustic Wave Sensor with Strong Electric Loading Effect for Highly Sensitive H2 S Gas Sensing. Sens. Actuators B-Chem. 2023, 394, 134380. [Google Scholar] [CrossRef]
- Govind, A.; Bharathi, P.; Mohan, M.K.; Archana, J.; Harish, S.; Navaneethan, M. Highly Sensitive near Room Temperature Operable NO2 Gas-Sensor for Enhanced Selectivity via Nanoporous CuO@ZnO Heterostructures. J. Environ. Chem. Eng. 2023, 11, 110056. [Google Scholar] [CrossRef]
- Seekaew, Y.; Kamlue, S.; Wongchoosuk, C. Room-Temperature Ammonia Gas Sensor Based on Ti3C2Tx MXene/Graphene Oxide/CuO/ZnO Nanocomposite. ACS Appl. Nano Mater. 2023, 6, 9008–9020. [Google Scholar] [CrossRef]
- Su, C.; Li, M.Y.; Zhang, Y.F.; Liu, T.Q.; Ren, C.; Li, P.P.; Yin, X.Q.; Zhang, L.; Zhang, M.; Wu, W.W. Boosting Ethylene Glycol Sensing Performance with Dendritic Hierarchical CuO/Co3O4 Heterojunction Nanowire. ACS Appl. Nano Mater. 2023, 6, 19249–19256. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, D.Z.; Gao, Y.H.; Chen, F.J.; Wang, T.; Xia, H.; Sui, X.X.; Wang, Z.H. Fast-Response Hydrogen Sulfide Gas Sensor Based on Electrospinning Co3O4 Nanofibers-Modified CuO Nanoflowers: Experimental and DFT Calculation. Sens. Actuators B-Chem. 2023, 396, 134579. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Weber, I.C. A Strategy to Enhance Humidity Robustness of P-Type CuO Sensors for Breath Acetone Quantification. Small Sci. 2023, 3, 2200096. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Huang, D.; Wang, X.; Cai, L.; Chen, Y.; Wang, W.; Song, Y.; Han, G.; Zhen, B. A High-Performance Ethanol Gas Sensor Based on Ce-Doped SnO2 Nanomaterials Prepared by the Pechini Method. Mater. Sci. Semicond. Process 2022, 137, 106188. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Liu, W.; Dong, H.; Wang, D.; Liu, J.; Liu, Q.; Chen, X. MOF-Based Nanoscale Pt Catalyst Decorated SnO2 Porous Nanofibers for Acetone Gas Detection. J. Alloys Compd. 2022, 893, 162322. [Google Scholar] [CrossRef]
- Li, C.; Kim, K.; Fuchigami, T.; Asaka, T.; Kakimoto, K.; Masuda, Y. Acetone Gas Sensor Based on Nb2O5@SnO2 Hybrid Structure with High Selectivity and Ppt-Level Sensitivity. Sens. Actuators B Chem. 2023, 393, 134144. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Tan, C. Hollow Mesoporous SnO2/Zn2 SnO 4 Heterojunction and RGO Decoration for High-Performance Detection of Acetone. ACS Appl. Mater. Interfaces 2022, 14, 55249–55263. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, W.; Peng, C.; Wang, T.; Li, B.; Ma, H.; Su, Y.; Zhou, Z.; Yang, J.; Zeng, M. Construction, Application and Verification of a Novel Formaldehyde Gas Sensor System Based on Ni-Doped SnO2 Nanoparticles. IEEE Sens. J. 2021, 21, 11023–11030. [Google Scholar] [CrossRef]
- Zou, Y.H.; Chen, S.; Sun, J.; Liu, J.Q.; Che, Y.K.; Liu, X.H.; Zhang, J.; Yang, D.J. Highly Efficient Gas Sensor Using a Hollow SnO2 Microfiber for Triethylamine Detection. ACS Sens. 2017, 2, 897–902. [Google Scholar] [CrossRef]
- Gasso, S.; Sohal, M.K.; Mahajan, A. MXene Modulated SnO2 Gas Sensor for Ultra-Responsive Room-Temperature Detection of NO2. Sens. Actuators B-Chem. 2022, 357, 131427. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Z.H.; Zhao, H.C.; Wang, Y.J.; Li, J.; Zou, C. Two-Dimensional Black Phosphorus/Tin Oxide Heterojunctions for High-Performance Chemiresistive H2S Sensing. Anal. Chim. Acta 2023, 1245, 340825. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y.; Ren, C.; Chen, X.; Hu, J.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; et al. Controllable Synthesis of Crescent-Shaped Porous NiO Nanoplates for Conductometric Ethanol Gas Sensors. Sens. Actuators B Chem. 2019, 296, 126642. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, K.; Fei, T.; Gu, F.; Han, D. α-Fe2O3/NiO Heterojunction Nanorods with Enhanced Gas Sensing Performance for Acetone. Sens. Actuators B Chem. 2020, 318, 128191. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Ma, S.; Wang, Y.; Wang, P.; Zhang, G.; Gengzang, D.; Jiao, H.; Wang, M.; Chen, W. Multishelled NiO/NiCo2O4 Hollow Microspheres Derived from Bimetal-Organic Frameworks as High-Performance Sensing Material for Acetone Detection. J. Hazard. Mater. 2021, 415, 125662. [Google Scholar] [CrossRef]
- Li, C.; Choi, P.G.; Kim, K.; Masuda, Y. High Performance Acetone Gas Sensor Based on Ultrathin Porous NiO Nanosheet. Sens. Actuators B Chem. 2022, 367, 132143. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Cao, G.; Pan, G.; Yang, X.; Qiu, M.; Sun, C.; Shao, J.; Li, Z.; Zhang, H. Construction of Hollow NiO/ZnO p-n Heterostructure for Ultrahigh Performance Toluene Gas Sensor. Mater. Sci. Semicond. Process 2022, 141, 106435. [Google Scholar] [CrossRef]
- Nakate, U.T.; Yu, Y.T.; Park, S. High Performance Acetaldehyde Gas Sensor Based on P-n Heterojunction Interface of NiO Nanosheets and WO3 Nanorods. Sens. Actuators B Chem. 2021, 344, 130264. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Zhang, N.; Liu, C.; Zhou, J.; Liu, D.; Ruan, S. Self-Assembled Co3O4@WO3 Hollow Microspheres with Oxygen Vacancy Defects for Fast and Selective Detection of Toluene. Sens. Actuators B Chem. 2022, 351, 130931. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Sun, C.; Shao, J.; Li, Z.; Zhang, H.; Qiu, M.; Pan, G.; Yang, X. Construction of Co3O4/Fe3O4 Heterojunctions from Metal Organic Framework Derivatives for High Performance Toluene Sensor. Sens. Actuators B Chem. 2023, 375, 132863. [Google Scholar] [CrossRef]
- Yu, S.; Lin, H.; Liu, X.; Zhong, J.; Hu, L.; Lu, F.; Hu, Z.; Gao, W. Enhancing the Photoelectric Performance of TiO2-Based Heterojunction Using the Hydrothermal Decomposition of MIL-125 Strategy for Sensitive Detection of Bisphenol A. Sens. Actuators B Chem. 2024, 413, 135881. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, T.; Zhang, L.; Han, W.; Yang, J.; Wang, C.; Sun, Y.; Liu, F.; Sun, P.; Lu, G. Construction of Mesoporous In2O3-ZnO Hierarchical Structure Gas Sensor for Ethanol Detection. Sens. Actuators B Chem. 2023, 393, 134203. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Patel, M.; Kim, J. Self-Powered Transparent Photodetectors for Broadband Applications. Surf. Interfaces 2021, 23, 100934. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nanomicro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, H.; Yang, S.; Wei, L.; Han, Z.; Zhang, Y.; Pan, H. Preadsorption of O2 on the Exposed (001) Facets of ZnO Nanostructures for Enhanced Sensing of Gaseous Acetone. ACS Appl. Nano Mater. 2019, 2, 6144–6151. [Google Scholar] [CrossRef]
- Sun, B.T.; Ding, Y.L.; Wang, Q.; Song, P. Rational Design of 1D/2D Heterostructured ZnSnO3/ZnO/Ti3C2Tx MXene Nanocomposites for Enhanced Acetone Gas Sensing Performance. Sens. Actuators B-Chem. 2024, 409, 135541. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Z.; Chi, H.; Jiang, J.; Zhu, L.; Ye, Z. Ultrasensitive and Exclusive Chemiresistors with a ZIF-67-Derived Oxide Cage/Nanofiber Co3O4/In2O3 Heterostructure for Acetone Detection. ACS Appl. Mater. Interfaces 2024, 16, 9126–9136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.; Zhang, Q.; He, X.; Wang, X. Synthesis of MoO3 (1D) @SnO2 (2D) Core-Shell Heterostructures for Enhanced Ethanol Gas Sensing Performance. Sens. Actuators B Chem. 2023, 382, 133484. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhang, Q.B.; Mao, R.Z.; Duan, J.X.; Wang, H.Y.; Xu, G.G.; Wang, X.Z.; Xiong, Y.; Tian, J. Superior Ethanol Sensing Performance by In-Situ Construction of Porous Zn2SnO4/CdSnO3 Nanocubes n-n Heterostructure. Sens. Actuators B-Chem. 2024, 419, 136397. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, D.H.; Miao, T.T.; Liu, W.K.; Chen, J.; Cheng, B.; Qin, H.W.; Hu, J.F. Highly Sensitive P-SmFeO3/p-YFeO3 Planar-Electrode Sensor for Detection of Volatile Organic Compounds. Chemosensors 2023, 11, 187. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, N.; Wen, Y.; Liu, G.; Zhang, R.; Zhang, J.; Wang, F.; Liu, X.; Li, Q.; Tang, Z.; et al. Engineering Nanozymes Using DNA for Catalytic Regulation. ACS Appl. Mater. Interfaces 2019, 11, 1790–1799. [Google Scholar] [CrossRef]
- Xiao, Y.; Gong, W.; Zhao, M.; Zhang, M.; Lu, N. Surface-Engineered Prussian Blue Nanozymes as Artificial Receptors for Universal Pattern Recognition of Metal Ions and Proteins. Sens. Actuators B Chem. 2023, 390, 134006. [Google Scholar] [CrossRef]
- Cao, F.; Shi, X.-R.; Wang, P.; Zhao, W.; Huang, M.; Hu, J.; Xu, S.; Zhao, G. Multistage Interface Engineered Cobalt Polysulfides Core-Shell Nanostructures for Dual Energy Storage Devices and Hydrogen Evolution. Vacuum 2023, 216, 112461. [Google Scholar] [CrossRef]
- Huang, M.; Yao, H.; Cao, F.; Wang, P.; Shi, X.-R.; Zhang, M.; Xu, S. Structural Engineering Evoked Multifunctionality in Molybdate Nanosheets for Industrial Oxygen Evolution and Dual Energy Storage Devices Inspired by Multi-Method Calculations. J. Colloid. Interface Sci. 2024, 676, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Shi, X.-R.; He, X.; Zhang, X.; Cao, F.; Wang, P.; Sun, C.; Xu, S.; Zhang, M. Electronically Regulated FeOOH/c-NiMoO4 with Hierarchical Sandwich Structure as Efficient Electrode for Oxygen Evolution and Hybrid Supercapacitors. Electrochim. Acta 2022, 427, 140884. [Google Scholar] [CrossRef]
- Duan, Z.; Shi, X.-R.; Sun, C.; Lin, W.; Huang, S.; Zhang, X.; Huang, M.; Yang, Z.; Xu, S. Interface Engineered Hollow Co3O4@CoNi2S4 Nanostructure for High Efficiency Supercapacitor and Hydrogen Evolution. Electrochim. Acta 2022, 412, 140139. [Google Scholar] [CrossRef]
- Xu, S.R.; Wang, M.M.; Chen, C.P.; Feng, S.L. Sea Urchin-like SnO2/Alpha-Fe2O3 Heterostructural Microspheres for Enhanced Acetone Gas Sensing: Materials Preparation, Performance Evaluation, and Mechanism Investigation. Sens. Actuators B-Chem. 2023, 379, 133288. [Google Scholar] [CrossRef]
- Li, X.X.; Zhao, C.X.; Wang, Y.N.; Yuan, Z.Y. Precise Optical Fiber-Based Ammonia Sensor Using CdS Quantum Dots Decorated with ZnO at Heterointerface. Chemosensors 2024, 12, 169. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, N.; Zhang, J.; Yan, R.; Li, J.; Wang, L.; Wang, N.; Lv, M.; Zhang, M. Ultrasensitive Aptamer-Based Protein Assays Based on One-Dimensional Core-Shell Nanozymes. Biosens. Bioelectron. 2020, 150, 111881. [Google Scholar] [CrossRef]
- Guo, M.; Jin, Z.; Pan, J.; Xu, J.; Guo, L.; Yin, X.-B.; Lu, N.; Zhang, M. Construction of COFs@MoS2-Pd Hierarchical Tubular Heterostructures for Enhanced Catalytic Performance. Inorg. Chem. 2024, 63, 18263–18275. [Google Scholar] [CrossRef]
- Li, F.; Jing, J.; Li, J.; Li, S.; Ye, S.; Song, X.; Zhan, Z.; Zhang, Y. Fabrication of ZnO-SnO2 Heterojunction Inverse Opal Photonic Balls for Chemiresistive Acetone Sensing. Sens. Actuators B Chem. 2024, 400, 134887. [Google Scholar] [CrossRef]
- Shi, X.-R.; Wang, P.; Jing, C.; Wu, K.; Xu, S.; Klötzer, B. The Formation of O Vacancy on ZrO2/Pd and Its Effect on Methane Dry Reforming: Insights from DFT and Microkinetic Modeling. Appl. Surf. Sci. 2023, 619, 156679. [Google Scholar] [CrossRef]
- Meng, C.; Zhao, G.; Shi, X.-R.; Chen, P.; Liu, Y.; Lu, Y. Oxygen-Deficient Metal Oxides Supported Nano-Intermetallic InNi3C0.5 toward Efficient CO2 Hydrogenation to Methanol. Sci. Adv. 2021, 7, eabi6012. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Zhao, G.; Sun, W.; Liu, J.; Guan, C.; Yang, Y.; Shi, X.-R.; Lu, Y. Oxidative Coupling of Methane: Examining the Inactivity of the MnOx-Na2WO4/SiO2 Catalyst at Low Temperature. Angew. Chem. Int. Ed. 2022, 61, e202117201. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Singh, M.; Casotto, A.; Arachchige, H.M.M.M.; Sangaletti, L.; Comini, E. Quenching of Oxygen-Related Defects in Graphene Oxide Nanohybrid: Highly Selective Room-Temperature Ethanol Sensor. Appl. Phys. Rev. 2022, 9. [Google Scholar] [CrossRef]
- Abraham, N.; Aseena, S. Dielectric Studies of CuO-ZnO Heterojunction Nanocomposites Synthesized by Co-Precipitation Method. Mater. Today Proc. 2021, 43, 3698–3700. [Google Scholar] [CrossRef]
- Shruthi, J.; Jayababu, N.; Ghosal, P.; Ramana Reddy, M.V. Ultrasensitive Sensor Based on Y2O3-In2O3 Nanocomposites for the Detection of Methanol at Room Temperature. Ceram. Int. 2019, 45, 21497–21504. [Google Scholar] [CrossRef]
- Li, K.; Wu, Y.; Chen, M.; Rong, Q.; Zhu, Z.; Liu, Q.; Zhang, J. High Methanol Gas-Sensing Performance of Sm2O3/ZnO/SmFeO3 Microspheres Synthesized Via a Hydrothermal Method. Nanoscale Res. Lett. 2019, 14, 57. [Google Scholar] [CrossRef]
- Cao, E.; Wu, L.; Zhang, Y.; Sun, L.; Yu, Z.; Nie, Z. Hydrothermal Synthesis of Cubic-Rhombohedral-In2O3 Microspheres with Superior Acetone Sensing Performance. Appl. Surf. Sci. 2023, 613, 156045. [Google Scholar] [CrossRef]
- Yang, W.; Ou, Q.; Yan, X.; Liu, L.; Liu, S.; Chen, H.; Liu, Y. High Sensing Performance Toward Acetone Vapor Using TiO2 Flower-Like Nanomaterials. Nanoscale Res. Lett. 2022, 17, 82. [Google Scholar] [CrossRef]
- Qu, F.; Thomas, T.; Zhang, B.; Zhou, X.; Zhang, S.; Ruan, S.; Yang, M. Self-Sacrificing Templated Formation of Co3O4/ZnCo2O4 Composite Hollow Nanostructures for Highly Sensitive Detecting Acetone Vapor. Sens. Actuators B Chem. 2018, 273, 1202–1210. [Google Scholar] [CrossRef]
- Wang, X.; Cao, R.; Zhang, S.; Hou, P.; Han, R.; Shao, M.; Xu, X. Hierarchical Flowerlike Metal/Metal Oxide Nanostructures Derived from Layered Double Hydroxides for Catalysis and Gas Sensing. J. Mater. Chem. A Mater. 2017, 5, 23999–24010. [Google Scholar] [CrossRef]
- Nie, L.; Fan, G.; Wang, A.; Zhang, L.; Guan, J.; Han, N.; Chen, Y. Finely Dispersed and Highly Toluene Sensitive NiO/NiGa2O4 Heterostructures Prepared from Layered Double Hydroxides Precursors. Sens. Actuators B Chem. 2021, 345, 130412. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Neri, G. Acetone Sensing Behavior of P-SmFeO3/n-ZnO Nanocomposite Synthesized by Thermal Treatment Method. Sens. Actuators B Chem. 2020, 304, 127252. [Google Scholar] [CrossRef]
- Qin, Y.; Liang, Y.; Zhou, C.; Bai, Y. Homogeneous Heterophase Junction Based on Cubic/Orthomorphic SnS for Chemiresistive Room Temperature Trace-Ethanol Sensor. Sens. Actuators B Chem. 2024, 404, 135285. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhu, Y.; Wu, L.M.; You, B.; Zi, J. Fabrication of Robust Crystal Balls from the Electrospray of Soft Polymer Spheres/Silica Dispersion. Langmuir 2010, 26, 6604–6609. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, R.; Wang, C.; Wang, T.; Sun, Y.; Sun, P.; Lu, G. Flower-like ZnO-Co3O4 Heterojunction Composites for Enhanced Acetone Sensing. Sens. Actuators B Chem. 2023, 390, 133964. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Chen, Y.; Long, H. Regulation of O-Vacancy and Heterojunction Structure in MOF-Derived Fe2O3-Co3O4 Enhancing Acetone Sensing Performance. Sens. Actuators B Chem. 2024, 401, 135082. [Google Scholar] [CrossRef]
- Yang, L.; Qin, J.; Cui, S.; Liu, W. Silver Nanoparticle Functionalized Heterojunction NiO/SnO2 Nanotubes for Comprehensive Sensitization of Acetone Sensor. Sens. Actuators B Chem. 2024, 417, 136208. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, T.; Zhang, L.; Yang, J.; Han, W.; Sun, Y.; Liu, F.; Sun, P.; Zhang, H.; Lu, G. Separated Detection of Ethanol and Acetone Based on SnO2-ZnO Gas Sensor with Improved Humidity Tolerance. Sens. Actuators B Chem. 2023, 393, 134257. [Google Scholar] [CrossRef]
- Cheng, P.; Lv, L.; Wang, Y.; Zhang, B.; Zhang, Y.; Zhang, Y.; Lei, Z.; Xu, L. SnO2/ZnSnO3 Double-Shelled Hollow Microspheres Based High-Performance Acetone Gas Sensor. Sens. Actuators B Chem. 2021, 332, 129212. [Google Scholar] [CrossRef]
- Lee, J.E.; Lim, C.K.; Park, H.J.; Song, H.; Choi, S.-Y.; Lee, D.-S. ZnO–CuO Core-Hollow Cube Nanostructures for Highly Sensitive Acetone Gas Sensors at the Ppb Level. ACS Appl. Mater. Interfaces 2020, 12, 35688–35697. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Cheng, P.; Xu, L.; Dang, F.; Wang, T.; Lei, Z. In-Situ Generated TiO2/α-Fe2O3 Heterojunction Arrays for Batch Manufacturing of Conductometric Acetone Gas Sensors. Sens. Actuators B Chem. 2021, 340, 129926. [Google Scholar] [CrossRef]
- He, L.; Hu, J.; Yuan, Q.; Xia, Z.; Jin, L.; Gao, H.; Fan, L.; Chu, X.; Meng, F. Synthesis of Porous ZnFe2O4/SnO2 Core-Shell Spheres for High-Performance Acetone Gas Sensing. Sens. Actuators B Chem. 2023, 378, 133123. [Google Scholar] [CrossRef]
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous Detection of Dual Biomarkers Using Hierarchical MoS2 Nanostructuring and Nano-Signal Amplification-Based Electrochemical Aptasensor toward Accurate Diagnosis of Prostate Cancer. Biosens. Bioelectron. 2022, 197, 113797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, X.; Shen, Z.; Gu, Y.; He, L.; Zhang, M.; Lu, N. Single-Atom Fe Nanozymes Coupling with Atomic Clusters as Superior Oxidase Mimics for Ratiometric Fluorescence Detection. Chem. Eng. J. 2023, 469, 143923. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Cheng, Z.; Yang, J.; Li, Y. A Sensitive Acetone Sensor Based on WS2/WO3 Nanosheets with p-n Heterojunctions. ACS Appl. Nano Mater. 2022, 5, 12592–12599. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Ma, Q.; Che, Q.; Wang, J.; Wang, G.; Yang, P. ZIF-8 Derived Hexagonal-like α-Fe2O3/ZnO/Au Nanoplates with Tunable Surface Heterostructures for Superior Ethanol Gas-Sensing Performance. Appl. Surf. Sci. 2018, 439, 649–659. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, J.; Wang, Y.; Wang, T.; Wei, M.; Li, F.; Li, D.; Yang, Y.; Yu, H.; Dong, X. Polyoxometalates Electron Acceptor-Intercalated In2O3@SnO2 Nanofibers for Chemiresistive Ethanol Gas Sensors. Sens. Actuators B Chem. 2024, 410, 135728. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Hu, Z.; Li, T.; Chen, B.; Li, H.Y.; Liu, H. Hollow-Out Fe2O3-Loaded NiO Heterojunction Nanorods Enable Real-Time Exhaled Ethanol Monitoring under High Humidity. ACS Appl. Mater. Interfaces 2023, 15, 15707–15720. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Qiao, G.; Chen, X.; Wang, X.; Cui, H. Core-Double Shell ZnO@In2O3@ZnO Hollow Microspheres for Superior Ethanol Gas Sensing. Sens. Actuators B Chem. 2021, 341, 130002. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Zhang, Z.; Li, J.; Wang, H.; Xu, G.; Wang, X.; Tian, J. Synthesis of ZnO Nanosheets @In2O3 Hollow Micro-Rods Heterostructures for Enhanced Ethanol Gas Sensing Performance. Sens. Actuators B Chem. 2024, 404, 135271. [Google Scholar] [CrossRef]
- Kou, H.R.; Shao, T.T.; Dong, J.T.; Cheng, Y.Y.; Zhang, F.C.; Guo, J.M.; Liu, X.X.; Wang, X.Y. Ethanol Sensor Built on a SnO2/In2O3 Composite Generated from MOF. Sens. Actuators B-Chem. 2023, 396, 134628. [Google Scholar] [CrossRef]
- He, S.; Gui, Y.; Wang, Y.; Cao, L.; He, G.; Tang, C. CuO/TiO2 /MXene-Based Sensor and SMS-TENG Array Integrated Inspection Robots for Self-Powered Ethanol Detection and Alarm at Room Temperature. ACS Sens. 2024, 9, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.H.; Song, Y.; Jiang, G.J.; Wu, D.D.; Li, G.R. SnO2/ZnO/Ti3C2Tx MXene Nanocomposites for Highly Sensitive and Stable Ethanol Sensing at Low Temperature. Sens. Actuators B-Chem. 2024, 419, 136390. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Gao, S.W.; Sun, Y.N.; Xu, P.L.; Wu, S.H.; Kong, H.; Yang, G.Z.; Wei, G. The Combination of Two-Dimensional Nanomaterials with Metal Oxide Nanoparticles for Gas Sensors: A Review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef]
- Huang, S.; Shi, X.-R.; Sun, C.; Zhang, X.; Huang, M.; Liu, R.; Wang, H.; Xu, S. Template-Controlled in-Situ Growing of NiCo-MOF Nanosheets on Ni Foam with Mixed Linkers for High Performance Asymmetric Supercapacitors. Appl. Surf. Sci. 2022, 572, 151344. [Google Scholar] [CrossRef]
- Huang, S.; Shi, X.-R.; Sun, C.; Duan, Z.; Ma, P.; Xu, S. The Application of Metal–Organic Frameworks and Their Derivatives for Supercapacitors. Nanomaterials 2020, 10, 2268. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, S.; Wang, P.; Shao, X.; Sun, X.; Hu, J.; Shi, X.-R. Bimetallic FeCo Phosphide Nanoparticles Anchored on N-Doped Carbon Foam for Wide PH Hydrogen Evolution Reaction. J. Alloys Compd. 2023, 931, 167570. [Google Scholar] [CrossRef]
- Liu, R.; Shi, X.-R.; Wen, Y.; Shao, X.; Su, C.; Hu, J.; Xu, S. Trimetallic Synergistic Optimization of 0D NiCoFe-P QDs Anchoring on 2D Porous Carbon for Efficient Electrocatalysis and High-Energy Supercapacitor. J. Energy Chem. 2022, 74, 149–158. [Google Scholar] [CrossRef]
- Shao, X.; Xu, S.; Wang, P.; Wen, Y.; Sun, X.; Hong, M.; Wu, K.; Shi, X.-R. Carbon-Incorporated Bimetallic Phosphide Nanospheres Derived from MOFs as Superior Electrocatalysts for Hydrogen Evolution. Dalton Trans. 2022, 51, 14517–14525. [Google Scholar] [CrossRef]
- Ma, H.; Xu, S.; Wang, P.; Zhu, J.; Yang, C.; Zhang, S.; Shi, X.-R.; Yao, L. Synergistic Improvement the Zn Storage Performance of ZnMn2O4 Quantum Dots by Ni Doping and In-Situ Electrochemical Induction. Appl. Surf. Sci. 2024, 663, 160208. [Google Scholar] [CrossRef]
- Gonçalves, B.F.; Fernández, E.; Valverde, A.; Gaboardi, M.; Salazar, H.; Petrenko, V.; Porro, J.M.; Cavalcanti, L.P.; Urtiaga, K.; Esperança, J.M.S.S.; et al. Exploring the Compositional Space of a Metal–Organic Framework with Ionic Liquids to Develop Porous Ionic Conductors for Enhanced Signal and Selectivity in VOC Capacitive Sensors. J. Mater. Chem. A Mater. 2024, 12, 14595–14607. [Google Scholar] [CrossRef]
- Mollick, S.; Rai, S.; Frentzel-Beyme, L.; Kachwal, V.; Donà, L.; Schürmann, D.; Civalleri, B.; Henke, S.; Tan, J. Unlocking Diabetic Acetone Vapor Detection by A Portable Metal-Organic Framework-Based Turn-On Optical Sensor Device. Adv. Sci. 2024, 11, 2305070. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.L.H.; Kim, J.-Y.; Lee, J.-H.; Nguyen, L.H.T.; Dang, Y.T.; Bui, K.-B.T.; Pham, A.T.T.; Mirzaei, A.; Phan, T.B.; Kim, S.S. Preparation of N-ZnO/p-Co3O4 Heterojunctions from Zeolitic Imidazolate Frameworks (ZIF-8/ZIF-67) for Sensing Low Ethanol Concentrations. Sens. Actuators B Chem. 2021, 348, 130684. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.Y.; Liang, Q.F.; Zhang, J.N.; Liu, J.; Liu, Y.M.; Wang, C.L.; Gao, J.Z.; Lu, H.B. Nanoporous Co3O4-TiO2 Heterojunction Nanosheets for Ethanol Sensing. ACS Appl. Nano Mater. 2022, 5, 4779–4786. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Zheng, W.; Wang, H.; Li, H.-Y.; Yu, M.-H.; Chang, Z.; Bu, X.-H.; Liu, H. Facile Engineering of Metal–Organic Framework Derived SnO2-ZnO Composite Based Gas Sensor toward Superior Acetone Sensing Performance. Chem. Eng. J. 2023, 469, 143927. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, S.W.; Tang, P.G.; Feng, Y.J.; Li, D.Q. Ultra-Sensitive Ethanol Gas Sensors Based on Nanosheet-Assembled Hierarchical ZnO-In2O3 Heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef]
- Yan, W.J.; Chen, Y.L.; Zeng, X.M.; Wu, G.; Jiang, W.; Wei, D.; Ling, M.; Ng, K.W.; Qin, Y.X. Ultrasensitive Ethanol Sensor Based on Segregated ZnO-In2O3 Porous Nanosheets. Appl. Surf. Sci. 2021, 535, 147697. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhang, Z.K.; Yue, C.; Mu, Y.; Yang, Z.G.; Dastan, D.; Yin, X.T.; Ma, X.G. In-MIL-68 Derived In2O3/Fe2O3 Shuttle-like Structures with n-n Heterojunctions to Improve Ethanol Sensing Performance. Phys. Chem. Chem. Phys. 2024, 26, 4184–4193. [Google Scholar] [CrossRef]
- Ma, S.; Shen, L.Y.; Ma, S.H.; Wen, J.; Xu, J.Y. Emerging Zinc Stannate and Its Application in Volatile Organic Compounds Sensing. Coord. Chem. Rev. 2023, 490, 215217. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Li, T.; Li, Z.; Wu, W.; Zhou, C.; Sun, P.; Liu, F.; Yan, X.; Gao, Y.; et al. Highly Efficient Ethanol Gas Sensor Based on Hierarchical SnO2/Zn2SnO4 Porous Spheres. Sens. Actuators B Chem. 2019, 282, 339–346. [Google Scholar] [CrossRef]
- Ma, D.P.; Zhang, L.; Hu, J.T.; Fu, Z.W.; Luo, T.; Yang, D.; Fang, D.; Li, J.; Peng, J.B.; Wang, Y.W. Preparation and Gas-Sensitive Properties of Hollow Zn2SnO4/SnO2 Nano-Cubes. Inorg. Chem. Commun. 2022, 141, 109507. [Google Scholar] [CrossRef]
- Guo, R.; Wang, H.; Tian, R.; Shi, D.; Li, H.; Li, Y.; Liu, H. The Enhanced Ethanol Sensing Properties of CNT@ZnSnO3 Hollow Boxes Derived from Zn-MOF(ZIF-8). Ceram. Int. 2020, 46, 7065–7073. [Google Scholar] [CrossRef]
- Cao, H.; Hu, Z.; Wei, X.; Wang, H.; Tian, X.; Ding, S. Conductometric Ethanol Gas Sensor Based on a Bilayer Film Consisting of SnO2 Film and SnO2/ZnSnO3 Porous Film Prepared by Magnetron Sputtering. Sens. Actuators B Chem. 2023, 382, 133562. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Yang, X.W.; Bao, H.M.; Lei, B.; Chen, K.; Wei, Y.; Zhao, Q.; Zhang, H.W.; Cai, W.P. Vortex Engineering on Oxide Bowl-Coated Oxide/Gold Dual-Layer Array for Dual Electrical/Spectroscopic Monitoring of Volatile Organic Compounds. Adv. Funct. Mater. 2024, 34, 2402173. [Google Scholar] [CrossRef]
- Li, D.; Liu, G.; Zhang, Q.; Qu, M.; Fu, Y.Q.; Liu, Q.; Xie, J. Virtual Sensor Array Based on MXene for Selective Detections of VOCs. Sens. Actuators B Chem. 2021, 331, 129414. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Wang, P.; Wei, M.; Wang, S.; Shi, X.-R. High-Throughput Screening of Efficient Graphdiyne Supported Transition Metal Single Atom toward Water Electrolysis and Oxygen Reduction. J. Catal. 2024, 439, 115773. [Google Scholar] [CrossRef]
- Ferko, N.; Djeziri, M.A.; Al Sheikh, H.; Moubayed, N.; Bendahan, M.; El Rafei, M.; Seguin, J.L. Methodology for Estimating Ethanol Concentration with Artificial Intelligence in the Presence of Interfering Gases and Measurement Delay. Sens. Actuators B-Chem. 2024, 421, 136502. [Google Scholar] [CrossRef]
- Li, J.W.; Weng, H.D.; Yang, Q.Q.; Shen, J. Data-Driven Diagnosis Method of High-Pressure Hydrogen Leakage Based on Actual Driving Conditions and Probabilistic Neutral Network. Int. J. Hydrog. Energy 2024, 71, 411–421. [Google Scholar] [CrossRef]
- Shao, S.F.; Yan, L.W.; Zhang, L.; Zhang, J.; Li, Z.X.; Kim, H.W.; Kim, S.S. Utilizing Data Mining for the Synthesis of Functionalized Tungsten Oxide with Enhanced Oxygen Vacancies for Highly Sensitive Detection of Triethylamine. ACS Appl. Mater. Interfaces 2024, 16, 6098–6112. [Google Scholar] [CrossRef]
- Nakano-Baker, O.; Fong, H.; Shukla, S.; Lee, R.V.; Cai, L.; Godin, D.; Hennig, T.; Rath, S.; Novosselov, I.; Dogan, S.; et al. Data-Driven Design of a Multiplexed, Peptide-Sensitized Transistor to Detect Breath VOC Markers of COVID-19. Biosens. Bioelectron. 2023, 229, 115237. [Google Scholar] [CrossRef]











| Materials | Type | Concentration (ppm) | Operating Temperature (°C) | Response (Ra/Rg) | Detection Limit (ppm) | Response/Recovery Time (s) | Reference |
|---|---|---|---|---|---|---|---|
| Fe2O3-Co3O4 | p-n | 100 | 200 | 91.5 | N.A. | 20/21 | [86] |
| Co3O4/In2O3 | p-n I | 50 | 300 | 954 | 0.0188 | 4/148 | [55] |
| Ag-NiO/SnO2 | p-n II | 1 | 190 | 15.7 | 0.05 | 12/25 | [87] |
| SnO2-ZnO | n-n II | 100 | 250 | 0.2 | 35 | 3/333 | [88] |
| SnO2/ZnSnO3 | n-n I | 100 | 290 | 0.045 | 30 | 1/6 | [89] |
| ZnO-CuO | p-n II | 1 | 200 | 11.14 | 0.009 | N.A./N.A. | [90] |
| TiO2/a-Fe2O3 | n-n II | 100 | 225 | 21.9 | 0.036 | 13/10 | [91] |
| ZnFe2O4/SnO2 | n-n II | 100 | 210 | 120 | 0.1 | 30/197.2 | [92] |
| ZnSnO3/ZnO/Ti3C2Tx MXene | N.A. | 100 | 120 | 15.68 | 90 | 5 /12 | [54] |
| Materials | Type | Concentration (ppm) | Operating Temperature (°C) | Response (Ra/Rg) | Detection Limit (ppm) | Response/Recover Time (s) | Reference |
|---|---|---|---|---|---|---|---|
| Mesoporous In2O3-ZnO | n-n II | 100 | 225 | 35 | 0.2 | 4/90 | [50] |
| Core-double shell ZnO@In2O3 @ZnO | n-n | 100 | 200 | 453 | 1 | 20/190 | [99] |
| MoO3 nanorods @SnO2 nanosheets | N.A. | 100 | 200 | 48.64 | N.A. | 65/230 | [56] |
| In2O3@PW12@SnO2 | n-n | 100 | 320 | 22.6 | 0.0139 | 1/132 | [97] |
| Porous Zn2SnO4/CdSnO3 nanocubes | n-n II | 100 | 300 | 214.38 | 0.285 | 30/55 | [57] |
| Hollowed-out Fe2O3-loaded NiO heterojunction nanorods | p-n I | 10 | 150 | 51.2 | 0.5 | N.A./N.A. | [98] |
| ZnO nanosheets @In2O3 hollow microrods | n-n | 100 | 200 | 1 | 269.1 | 18/35 | [100] |
| In2O3/SnO2 | n-n II | 100 | 200 | N.A. | 49.16 | 32/40 | [101] |
| CuO/TiO2/MXene | N.A. | 1 | 30 | 0.95 | 0.3 | 16/13 | [102] |
| SnO2/ZnO/Ti3C2Tx MXene | N.A. | 100 | 120 | 121.1 | N.A. | 3/141 | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhang, H.; Yao, H.; Wang, P.; Zhu, M.; Shi, X.; Xu, S. Recent Advances in Metal Oxide Semiconductor Heterojunctions for the Detection of Volatile Organic Compounds. Chemosensors 2024, 12, 244. https://doi.org/10.3390/chemosensors12120244
Zhang S, Zhang H, Yao H, Wang P, Zhu M, Shi X, Xu S. Recent Advances in Metal Oxide Semiconductor Heterojunctions for the Detection of Volatile Organic Compounds. Chemosensors. 2024; 12(12):244. https://doi.org/10.3390/chemosensors12120244
Chicago/Turabian StyleZhang, Shengming, Heng Zhang, Haiyu Yao, Peijie Wang, Min Zhu, Xuerong Shi, and Shusheng Xu. 2024. "Recent Advances in Metal Oxide Semiconductor Heterojunctions for the Detection of Volatile Organic Compounds" Chemosensors 12, no. 12: 244. https://doi.org/10.3390/chemosensors12120244
APA StyleZhang, S., Zhang, H., Yao, H., Wang, P., Zhu, M., Shi, X., & Xu, S. (2024). Recent Advances in Metal Oxide Semiconductor Heterojunctions for the Detection of Volatile Organic Compounds. Chemosensors, 12(12), 244. https://doi.org/10.3390/chemosensors12120244






