Metal–Organic Framework-Derived CeO2/Gold Nanospheres in a Highly Sensitive Electrochemical Sensor for Uric Acid Quantification in Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods and Instrumentation
2.3. Synthesizing CeBTC MOF and Obtaining CeO2 NPs
2.4. Preparation of MOFdNC/AuNP-Modified CPE
2.5. Preparation of Milk Samples
3. Results and Discussion
3.1. Structural Analysis and Morphological Characterization
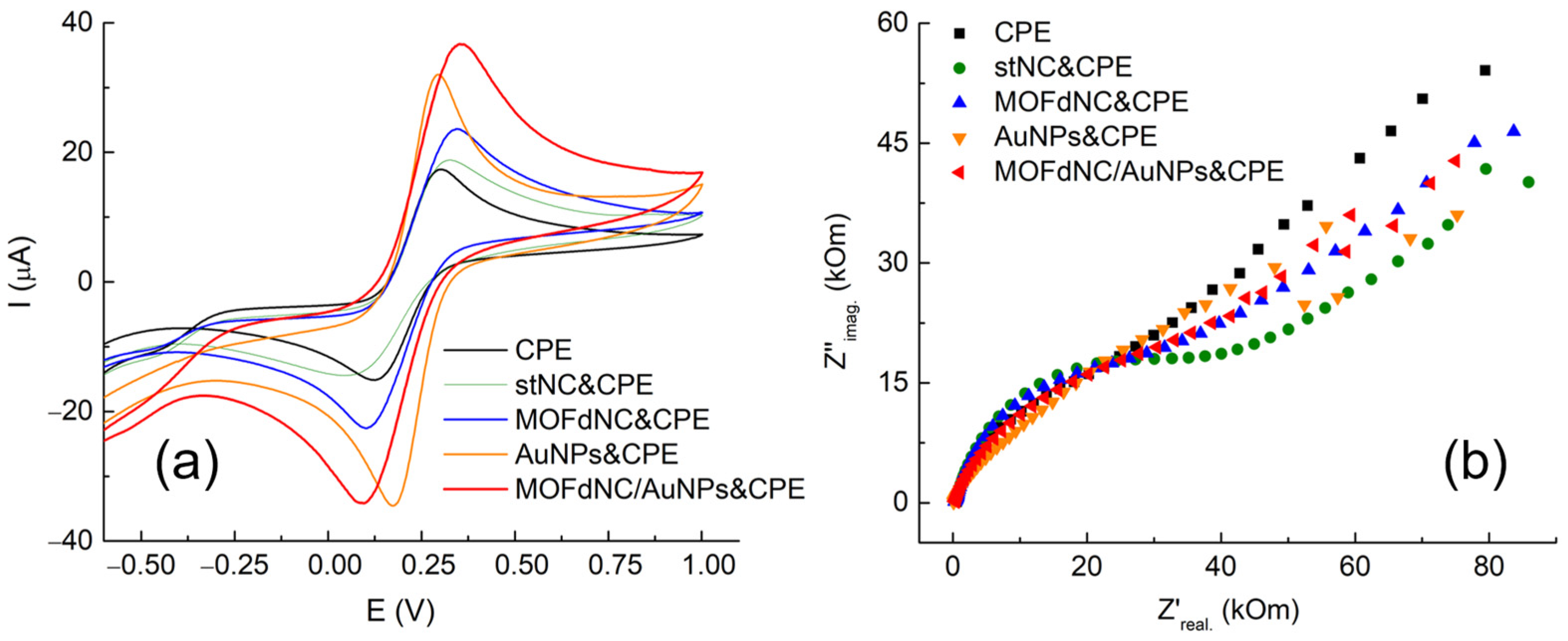
3.2. Determination of the Electrochemical Properties of the Selected Catalysts in the [Fe(CN)6]3−/4− Redox Probe
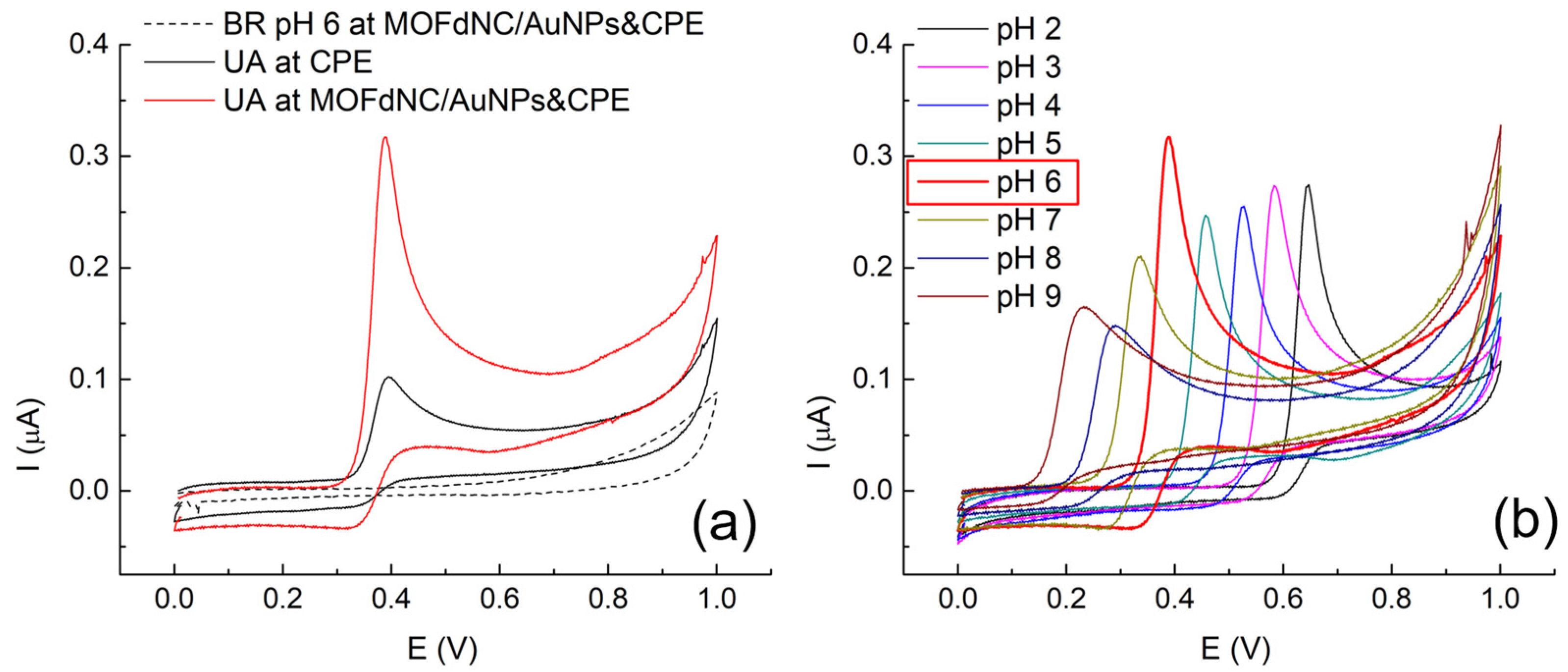
3.3. Electrochemical Behavior and Kinetics of UA over MOFdNC/AuNPs&CPE
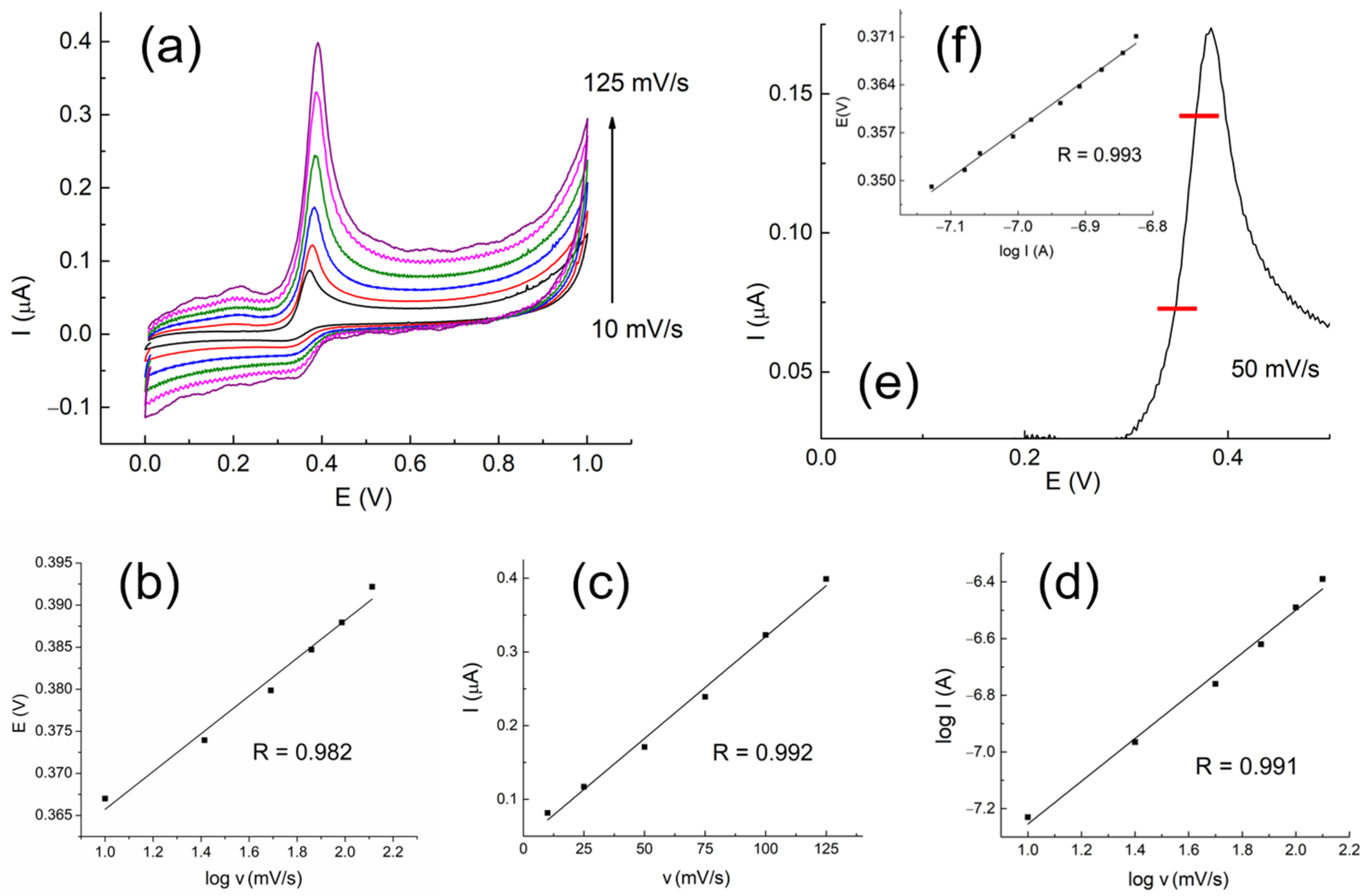
3.4. Effect of the Potential Scan Rate and Study of the Electrode Process
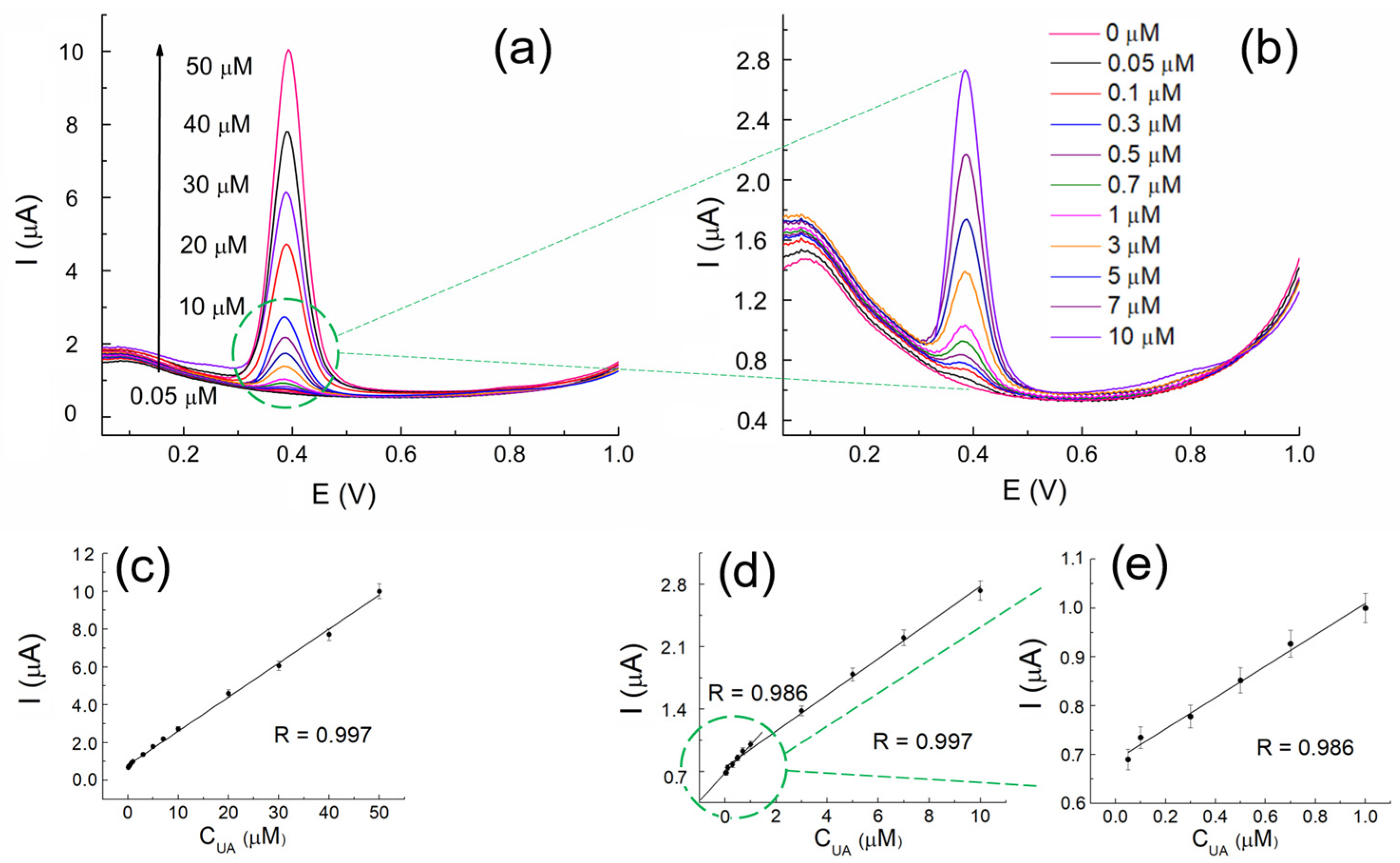
3.5. Quantification of UA at MOFdNC/AuNPs&CPE
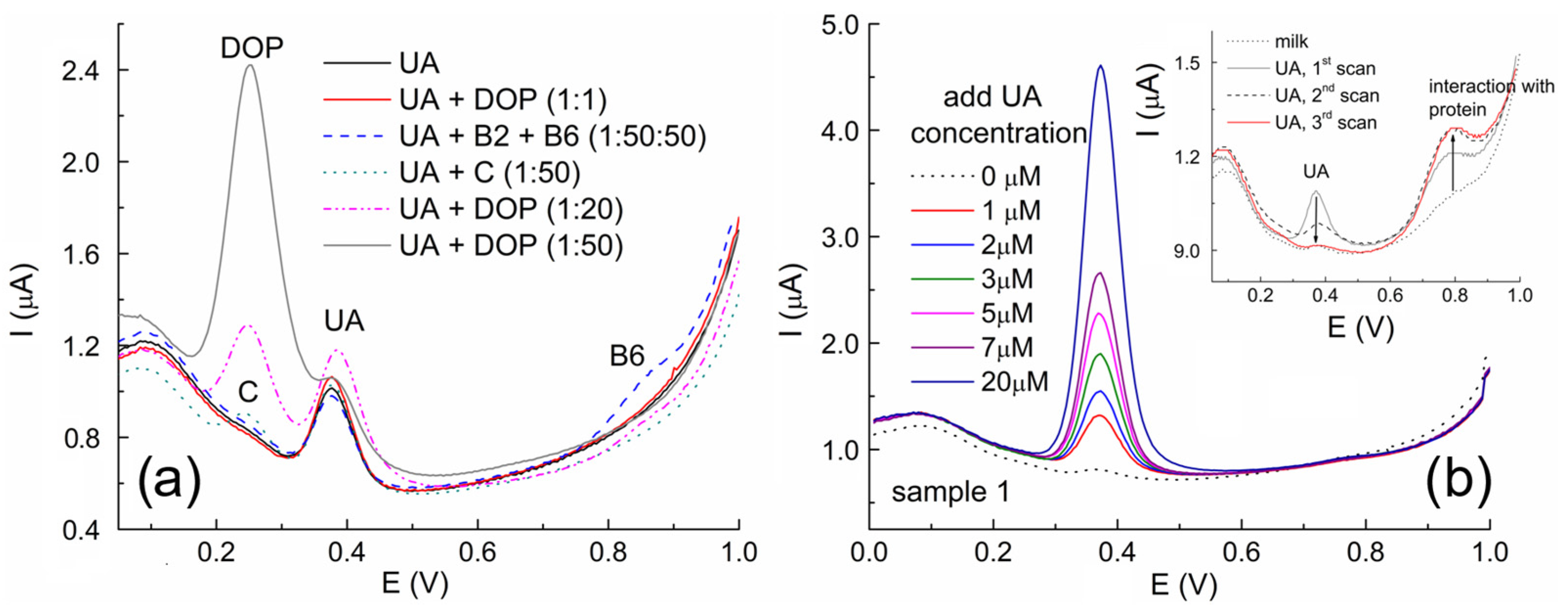
3.6. Interference Studies and Milk Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheele, K.W. Examen Chemicum Calculi Urinarii. Opuscula 1776, 2, 1776. [Google Scholar]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Jin, M. Uric Acid, Hyperuricemia and Vascular Diseases. Front. Biosci. 2012, 17, 656. [Google Scholar] [CrossRef] [PubMed]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological Functions and Pathogenic Potential of Uric Acid: A Review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Khamzina, E.; Bukharinova, M.; Stozhko, N. Uric Acid as a Marker of Milk Microbiological Spoilage. BIO Web Conf. 2023, 76, 02001. [Google Scholar] [CrossRef]
- Zuo, R.; Zhou, S.; Zuo, Y.; Deng, Y. Determination of Creatinine, Uric and Ascorbic Acid in Bovine Milk and Orange Juice by Hydrophilic Interaction HPLC. Food Chem. 2015, 182, 242–245. [Google Scholar] [CrossRef]
- Motshakeri, M.; Phillips, A.R.J.; Kilmartin, P.A. Application of Cyclic Voltammetry to Analyse Uric Acid and Reducing Agents in Commercial Milks. Food Chem. 2019, 293, 23–31. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, X.; Kong, J. Recent Progress on Uric Acid Detection: A Review. Crit. Rev. Anal. Chem. 2020, 50, 359–375. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in Enzyme-Free Electrochemical Sensors for Hydrogen Peroxide, Glucose, and Uric Acid. Microchim. Acta 2014, 181, 689–705. [Google Scholar] [CrossRef]
- Sun, M.; Cui, C.; Chen, H.; Wang, D.; Zhang, W.; Guo, W. Enzymatic and Non-Enzymatic Uric Acid Electrochemical Biosensors: A Review. ChemPlusChem 2023, 88, e202300262. [Google Scholar] [CrossRef] [PubMed]
- Knežević, S.; Ognjanović, M.; Nedić, N.; Mariano, J.F.M.L.; Milanović, Z.; Petković, B.; Antić, B.; Djurić, S.V.; Stanković, D. A Single Drop Histamine Sensor Based on AuNPs/MnO2 Modified Screen-Printed Electrode. Microchem. J. 2020, 155, 104778. [Google Scholar] [CrossRef]
- Stanković, D.M.; Ognjanović, M.; Fabián, M.; Avdin, V.V.; Manojlović, D.D.; Đurić, S.V.; Petković, B.B. CeO2-Doped—Domestic Carbon Material Decorated with MWCNT as an Efficient Green Sensing Platform for Electrooxidation of Dopamine. Surf. Interfaces 2021, 25, 101211. [Google Scholar] [CrossRef]
- Tharani, D.S.; Sivasubramanian, R. CeO2 Nanocubes-Based Electrochemical Sensor for the Selective and Simultaneous Determination of Dopamine in the Presence of Uric Acid and Ascorbic Acid. J. Chem. Sci. 2023, 135, 93. [Google Scholar] [CrossRef]
- Hashemzaei, Z.; Saravani, H.; Sharifitabar, M.; Shahbakhsh, M. Combustion Synthesis of Sponge-like CeO2 Powder for Selective Determination of Uric Acid in Biological Fluids. J. Part. Sci. Technol. 2021, 7, 73–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Chen, Y.; Deng, D.; He, H.; Lei, Y.; Luo, L. ZnO-CeO2 Hollow Nanospheres for Selective Determination of Dopamine and Uric Acid. Molecules 2024, 29, 1786. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C.; Murugan, R.; Ravi, G. An Ultrasensitive Electrochemical Sensor for Simultaneous Determination of Xanthine, Hypoxanthine and Uric Acid Based on Co Doped CeO2 Nanoparticles. Mater. Sci. Eng. C 2016, 65, 278–286. [Google Scholar] [CrossRef]
- Temerk, Y.; Ibrahim, H. A New Sensor Based on In Doped CeO2 Nanoparticles Modified Glassy Carbon Paste Electrode for Sensitive Determination of Uric Acid in Biological Fluids. Sens. Actuators B Chem. 2016, 224, 868–877. [Google Scholar] [CrossRef]
- Petrucci, R.; Bortolami, M.; Di Matteo, P.; Curulli, A. Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances. Nanomaterials 2022, 12, 959. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based Nanomaterials: Synthesis and Recent Progress in Electrochemical Sensor Applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef]
- Centeno, M.; Ramírez Reina, T.; Ivanova, S.; Laguna, O.; Odriozola, J. Au/CeO2 Catalysts: Structure and CO Oxidation Activity. Catalysts 2016, 6, 158. [Google Scholar] [CrossRef]
- Huang, P.; Chen, G.; Jiang, Z.; Jin, R.; Zhu, Y.; Sun, Y. Atomically Precise Au25 Superatoms Immobilized on CeO2 Nanorods for Styrene Oxidation. Nanoscale 2013, 5, 3668. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, N.; Yu, H.; Li, W.; Zhao, J.; Li, X.; Zhang, X. Fabrication of Strawberry-like Au@CeO2 Nanoparticles with Enhanced Catalytic Activity by Assembly of Block Copolymer Composite Micelles. RSC Adv. 2017, 7, 662–668. [Google Scholar] [CrossRef]
- Tang, H.; Ang Chen, Z.; Wu, M.; Li, S.; Ye, Z.; Zhi, M. Au-CeO2 Composite Aerogels with Tunable Au Nanoparticle Sizes as Plasmonic Photocatalysts for CO2 Reduction. J. Colloid Interface Sci. 2024, 653, 316–326. [Google Scholar] [CrossRef]
- Palanisamy, S. Simultaneous Electrochemical Detection of Dopamine and Uric Acid over Ceria Supported Three Dimensional Gold Nanoclusters. Mater. Res. Express 2014, 1, 045020. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef]
- Ma, J.; Fan, L.; Wang, X.; Li, L.; Zhang, Y.; Zhao, G.; Chai, B.; Gao, J. Defect Engineering on Metal-Organic Frameworks for Enhanced Photocatalytic Reduction of Cr(VI). Surf. Interfaces 2024, 54, 105174. [Google Scholar] [CrossRef]
- Lu, X.F.; Fang, Y.; Luan, D.; Lou, X.W.D. Metal–Organic Frameworks Derived Functional Materials for Electrochemical Energy Storage and Conversion: A Mini Review. Nano Lett. 2021, 21, 1555–1565. [Google Scholar] [CrossRef]
- Hammad, S.F.; Abdallah, I.A.; Bedair, A.; Abdelhameed, R.M.; Locatelli, M.; Mansour, F.R. Metal Organic Framework-Derived Carbon Nanomaterials and MOF Hybrids for Chemical Sensing. TrAC Trends Anal. Chem. 2024, 170, 117425. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Topkaya, S.N.; Gaffar, S.; Bahti, H.H.; Cetin, A.E. Synthesis and Characterization of Nanoceria for Electrochemical Sensing Applications. RSC Adv. 2021, 11, 16216–16235. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S. Morphology-Controllable Synthesis of Mesoporous CeO2 Nano- and Microstructures. Chem. Mater. 2005, 17, 4514–4522. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Wang, W.; Hu, B.; Wang, Z.; Yang, X.; Wang, Y.; Yang, L. Ce(III) Nanocomposites by Partial Thermal Decomposition of Ce-MOF for Effective Phosphate Adsorption in a Wide pH Range. Chem. Eng. J. 2020, 379, 122431. [Google Scholar] [CrossRef]
- Liu, X.-M.; Gao, W.-L.; Zhang, J. Facile Synthesis of Monodispersed CeO2 Nanostructures. J. Phys. Chem. Solids 2011, 72, 1472–1476. [Google Scholar] [CrossRef]
- Liu, Y.; Jie, W.; Liu, F.; Liu, Q.; Qiu, M.; Gong, X.; Hu, J.; Gong, L. Effect of Ce-BTC Precursor Morphology on CuO/CeO2 Catalysts for CO Preferential Oxidation in H2-Rich Gas. Solid State Sci. 2023, 139, 107182. [Google Scholar] [CrossRef]
- Hu, G.; Ma, Y.; Guo, Y.; Shao, S. Electrocatalytic Oxidation and Simultaneous Determination of Uric Acid and Ascorbic Acid on the Gold Nanoparticles-Modified Glassy Carbon Electrode. Electrochim. Acta 2008, 53, 6610–6615. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.; Zhan, D.; Liu, H.; Zhao, Q.; Kou, Y.; Shao, Y.; Li, M.; Zhuang, Q.; Zhu, Z. Selective Detection of Dopamine in the Presence of Ascorbic Acid and Uric Acid by a Carbon Nanotubes-Ionic Liquid Gel Modified Electrode. Talanta 2005, 66, 51–57. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and Sensitive Determination of Ascorbic Acid, Dopamine and Uric Acid via an Electrochemical Sensor Based on PVP-Graphene Composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Chelmea, L.; Badea, M.; Scarneciu, I.; Moga, M.A.; Dima, L.; Restani, P.; Murdaca, C.; Ciurescu, D.; Gaman, L.E. New Trends in Uric Acid Electroanalysis. Chemosensors 2023, 11, 341. [Google Scholar] [CrossRef]
- Knežević, S.; Ognjanović, M.; Stanković, V.; Zlatanova, M.; Nešić, A.; Gavrović-Jankulović, M.; Stanković, D. La(OH)3 Multi-Walled Carbon Nanotube/Carbon Paste-Based Sensing Approach for the Detection of Uric Acid—A Product of Environmentally Stressed Cells. Biosensors 2022, 12, 705. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; Aiello, G.; O’Riordan, A.; Torino, C.; Vilasi, A.; Inguanta, R. Electrochemical Detection of Uric Acid and Ascorbic Acid Using R-GO/NPs Based Sensors. Electrochim. Acta 2021, 388, 138652. [Google Scholar] [CrossRef]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A Highly Sensitive Uric Acid Electrochemical Biosensor Based on a Nano-Cube Cuprous Oxide/Ferrocene/Uricase Modified Glassy Carbon Electrode. Sci. Rep. 2020, 10, 10607. [Google Scholar] [CrossRef]
- Azizpour Moallem, Q.; Beitollahi, H. Electrochemical Sensor for Simultaneous Detection of Dopamine and Uric Acid Based on a Carbon Paste Electrode Modified with Nanostructured Cu-Based Metal-Organic Frameworks. Microchem. J. 2022, 177, 107261. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Zhang, Y.; Guo, R.; Hu, T. A Heterometallic Sensor Based on Ce@Zn-MOF for Electrochemical Recognition of Uric Acid. Microporous Mesoporous Mater. 2021, 322, 111126. [Google Scholar] [CrossRef]
- Motshakeri, M.; Travas-Sejdic, J.; Phillips, A.R.J.; Kilmartin, P.A. Rapid Electroanalysis of Uric Acid and Ascorbic Acid Using a Poly(3,4-Ethylenedioxythiophene)-Modified Sensor with Application to Milk. Electrochim. Acta 2018, 265, 184–193. [Google Scholar] [CrossRef]
- Jawad, M.A.; Dorie, J.; El Murr, N. Electrochemical Quantitative Analysis of Uric Acid in Milk. J. Food Sci. 1991, 56, 594–595. [Google Scholar] [CrossRef]
- Lindmark-Månsson, H.; Åkesson, B. Antioxidative Factors in Milk. Br. J. Nutr. 2000, 84, 103–110. [Google Scholar] [CrossRef]
- Garrel, D.; Verdy, M.; PetitClerc, C.; Martin, C.; Brulé, D.; Hamet, P. Milk- and Soy-Protein Ingestion: Acute Effect on Serum Uric Acid Concentration. Am. J. Clin. Nutr. 1991, 53, 665–669. [Google Scholar] [CrossRef]


| Electrochemical Sensor | Applied Technique | Linear Range (µM) | LOD (µM) | Ref. |
|---|---|---|---|---|
| CeO2 nanocubes/GCE | DPV | 10–700 | 4.3 | [14] |
| CPE/CeO2 sponge-lake porous | DPV | 0.25–10; 10–300 | 0.06 | [15] |
| ZnO-CeO2/GCE | DPV | 10–100 | 0.49 | [16] |
| Co-CeO2/GCE | SWV | 1–2200 | 0.12 | [17] |
| In–CeO2/GCPE | SWV | 0.079–148 | 0.0074 | [18] |
| ITO-rGO-AuNPs | LSV | 10–500 | 2.26 | [43] |
| PVP-GR/GCE | SDLSV | 0.04–100 | 0.02 | [38] |
| UOx/Fc/Cu2O/GCE Cu-BTC/CPE | DPV DPV | 0.1–1000 0.5–600 | 0.0596 0.2 | [44] [45] |
| Ce@Zn-MOF/GCE | DPV | 0–1.78 (0–300 ng/mL) * | 0.003 (0.51 ng) * | [46] |
| PEDOT/GCE | CV | 6–100 | 7 | [47] |
| MOFdNC/AuNPs&CPE | SWV | 0.05–1; 1–50 | 0.011 | This work |
| S1 (Working Solution) | S2 (Working Solution) | ||||
|---|---|---|---|---|---|
| Added (μM) | Found (μM) by SWV * | Recovery (%) | Added (μM) | Found (μM) by SWV * | Recovery (%) |
| 0 | 0.41 ± 0.03 | - | 0 | 0.44 ± 0.04 | - |
| 1 | 1.45 ± 0.06 | 102.8 | 5 | 5.63 ± 0.09 | 103.5 |
| 2 | 2.42 ± 0.04 | 100.4 | 10 | 10.33 ± 0.19 | 98.9 |
| 3 | 3.39 ± 0.04 | 99.4 | 15 | 16.80 ± 0.59 | 102.2 |
| 5 | 5.57 ± 0.07 | 102.9 | 20 | 20.54 ± 01.59 | 100.5 |
| 7 | 7.24 ± 0.18 | 98.2 | The final UA Concentration (μM) in Milk Samples | ||
| 20 | 20.23 ± 0.31 | 99.1 | SWV * | ||
| S1: 82 ± 6.23 | |||||
| S2: 88 ± 2.06 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ognjanović, M.; Marković, M.; Girman, V.; Nikolić, V.; Vranješ-Đurić, S.; Stanković, D.M.; Petković, B.B. Metal–Organic Framework-Derived CeO2/Gold Nanospheres in a Highly Sensitive Electrochemical Sensor for Uric Acid Quantification in Milk. Chemosensors 2024, 12, 231. https://doi.org/10.3390/chemosensors12110231
Ognjanović M, Marković M, Girman V, Nikolić V, Vranješ-Đurić S, Stanković DM, Petković BB. Metal–Organic Framework-Derived CeO2/Gold Nanospheres in a Highly Sensitive Electrochemical Sensor for Uric Acid Quantification in Milk. Chemosensors. 2024; 12(11):231. https://doi.org/10.3390/chemosensors12110231
Chicago/Turabian StyleOgnjanović, Miloš, Milena Marković, Vladimír Girman, Vladimir Nikolić, Sanja Vranješ-Đurić, Dalibor M. Stanković, and Branka B. Petković. 2024. "Metal–Organic Framework-Derived CeO2/Gold Nanospheres in a Highly Sensitive Electrochemical Sensor for Uric Acid Quantification in Milk" Chemosensors 12, no. 11: 231. https://doi.org/10.3390/chemosensors12110231
APA StyleOgnjanović, M., Marković, M., Girman, V., Nikolić, V., Vranješ-Đurić, S., Stanković, D. M., & Petković, B. B. (2024). Metal–Organic Framework-Derived CeO2/Gold Nanospheres in a Highly Sensitive Electrochemical Sensor for Uric Acid Quantification in Milk. Chemosensors, 12(11), 231. https://doi.org/10.3390/chemosensors12110231






