Applicability and Limitations of Fluorescence Intensity-Based Thermometry Using a Palette of Organelle Thermometers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
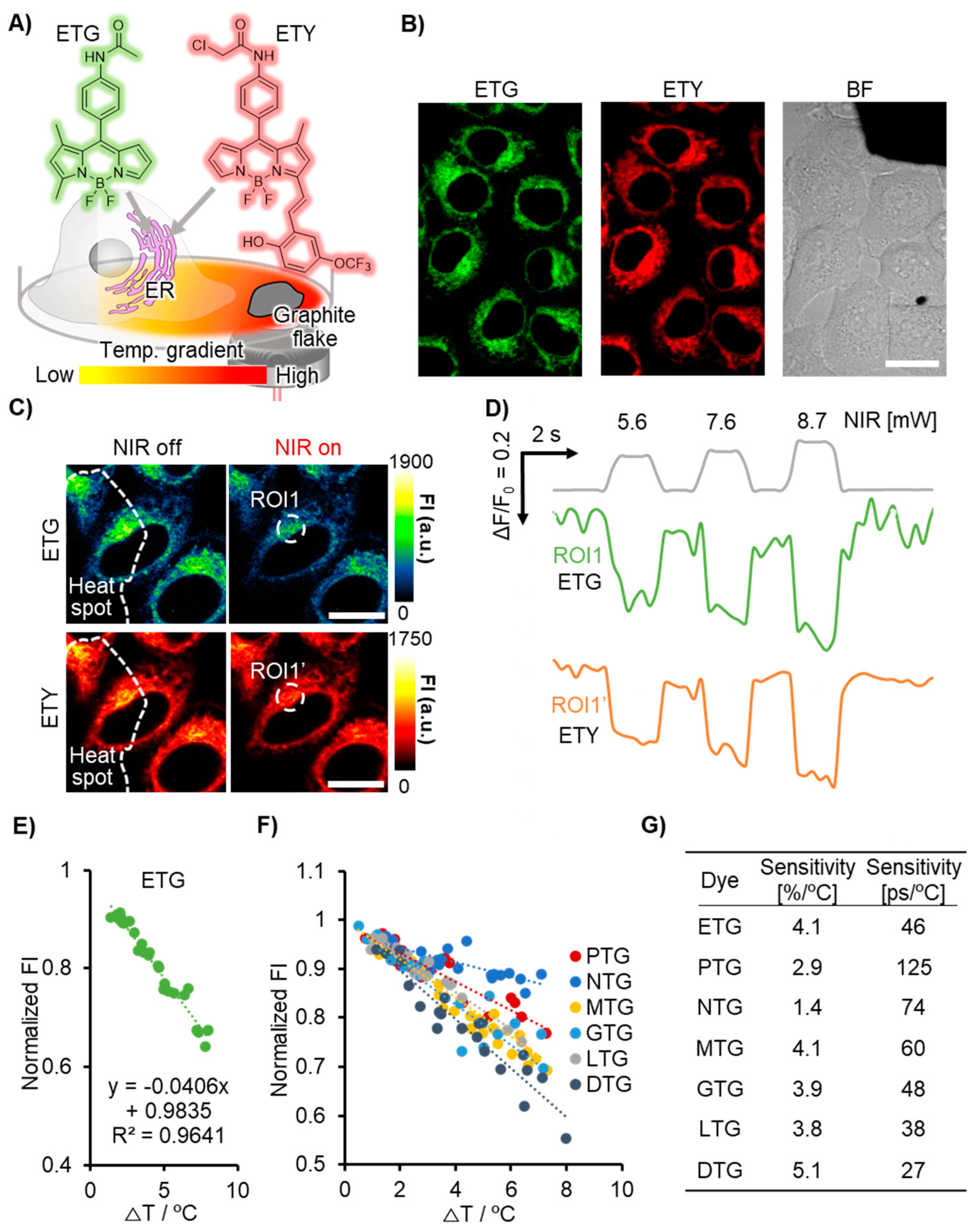
2.3. Evaluation of Temperature Sensitivity of OTGs through Photothermal Heating Using Graphite Flakes
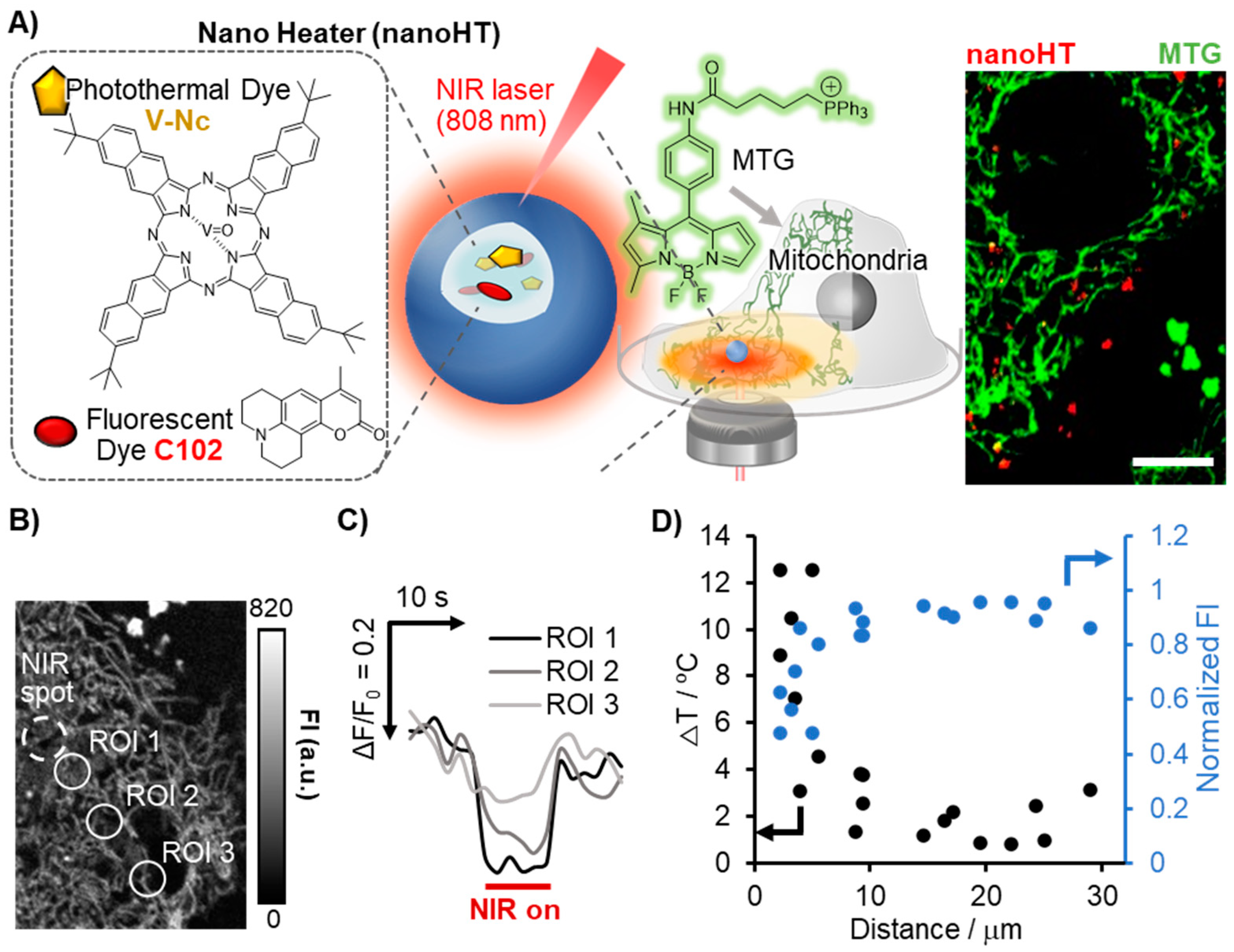
2.4. Visualization of Temperature Gradient Generated by Nano Heater (nanoHT) and Comparison of Accuracy between FI- and FLIM-Based Thermometry
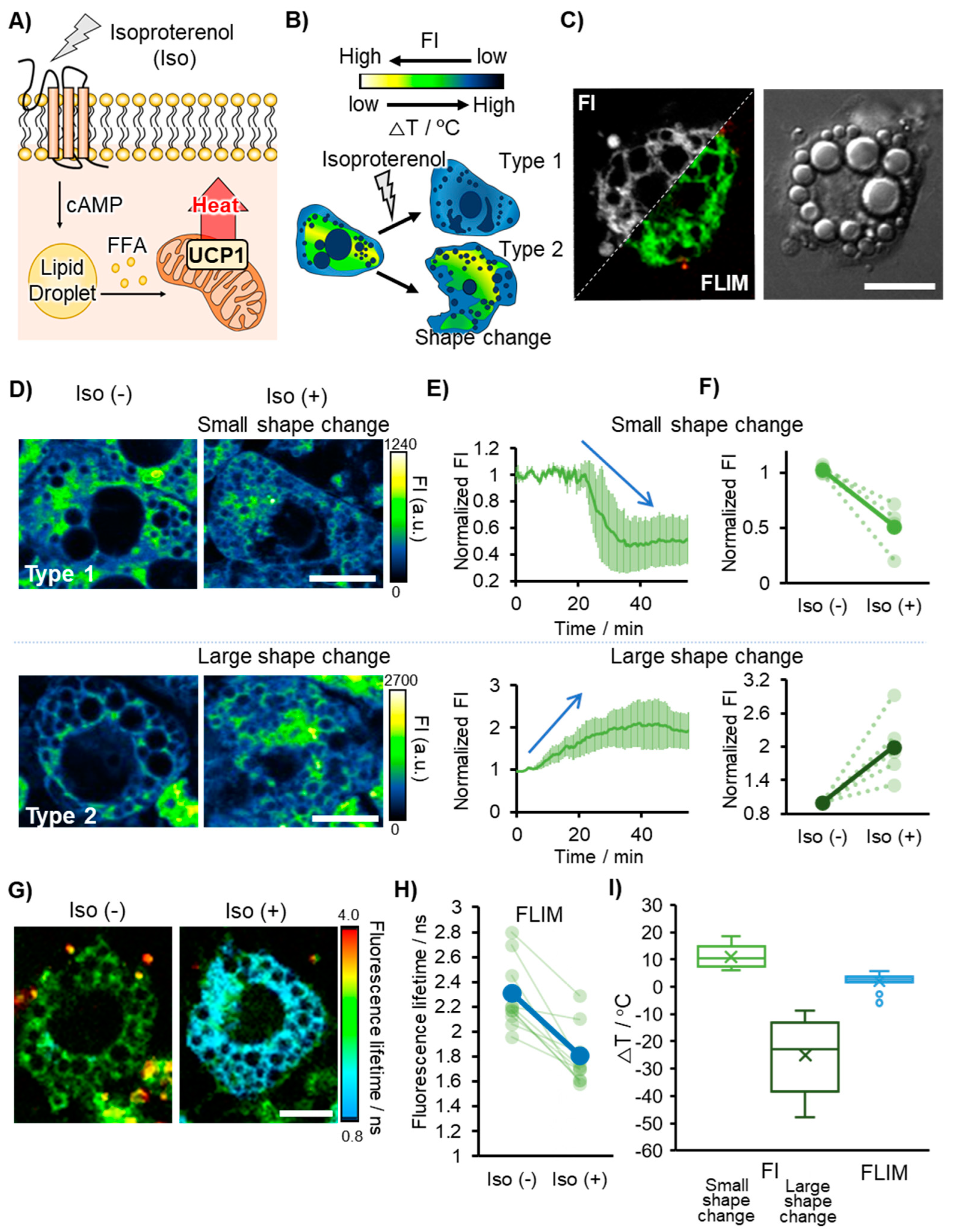
2.5. Visualization of Thermogenesis in Brown Adipocytes
2.6. Evaluation of the Diffusivity of Dyes in Each Organelle by Fluorescence Recovery after Photobleaching (FRAP)
3. Results and Discussion
3.1. Evaluation of FI-Based Temperature Sensitivity of OTGs
3.2. Visualization of Temperature Gradient Induced with a Nano Heater
3.3. Comparison between FI- and FLIM-Based Thermometry in Brown Adipocytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deguchi, T.; Itoh, M.; Urawa, H.; Matsumoto, T.; Nakayama, S.; Kawasaki, T.; Kitano, T.; Oda, S.; Mitani, H.; Takahashi, T.; et al. Infrared laser-mediated local gene induction in medaka, zebrafish and Arabidopsis thaliana. Dev. Growth Differ. 2009, 51, 769–775. [Google Scholar] [CrossRef]
- Harding, R.L.; Halevy, O.; Yahav, S.; Velleman, S.G. The effect of temperature on proliferation and differentiation of chicken skeletal muscle satellite cells isolated from different muscle types. Physiol. Rep. 2016, 4, e12770. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Kushimoto, S.; Yamanouchi, S.; Endo, T.; Sato, T.; Nomura, R.; Fujita, M.; Kudo, D.; Omura, T.; Miyagawa, N.; Sato, T. Body temperature abnormalities in non-neurological critically ill patients: A review of the literature. J. Intensive Care 2014, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Karnebogen, M.; Singer, D.; Kallerhoff, M.; Ringert, R.H. Microcalorimetric investigations on isolated tumorous and non-tumorous tissue samples. Thermochim. Acta 1993, 229, 147–155. [Google Scholar] [CrossRef]
- Okabe, K.; Sakaguchi, R.; Shi, B.; Kiyonaka, S. Intracellular thermometry with fluorescent sensors for thermal biology. Pflug. Arch. Eur. J. Physiol. 2018, 470, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Gu, N. Micro/nanoscale thermometry for cellular thermal sensing. Small 2016, 12, 4590–4610. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.M.; McWilliams, A.D.S.; Jiang, B.; Martí, A.A. Latest Trends in Temperature Sensing by Molecular Probes. ChemPhotoChem 2020, 4, 255–270. [Google Scholar] [CrossRef]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef]
- Gao, H.; Kam, C.; Chou, T.Y.; Wu, M.Y.; Zhao, X.; Chen, S. A simple yet effective AIE-based fluorescent nano-thermometer for temperature mapping in living cells using fluorescence lifetime imaging microscopy. Nanoscale Horiz. 2020, 5, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Liang, S.; Mei, M.; She, G.; Shi, W.; Mu, L. Single nanowire-based fluorescence lifetime thermometer for simultaneous measurement of intra- and extracellular temperature. Chem. Commun. 2023, 59, 4483–4486. [Google Scholar] [CrossRef] [PubMed]
- Chihara, T.; Umezawa, M.; Miyata, K.; Sekiyama, S.; Hosokawa, N.; Okubo, K.; Kamimura, M.; Soga, K. Biological Deep Temperature Imaging with Fluorescence Lifetime of Rare-Earth-Doped Ceramics Particles in the Second NIR Biological Window. Sci. Rep. 2019, 9, 12806. [Google Scholar] [CrossRef] [PubMed]
- Vyšniauskas, A.; Cornell, B.; Sherin, P.S.; Maleckaitė, K.; Kubánková, M.; Izquierdo, M.A.; Vu, T.T.; Volkova, Y.A.; Budynina, E.M.; Molteni, C.; et al. Cyclopropyl Substituents Transform the Viscosity-Sensitive BODIPY Molecular Rotor into a Temperature Sensor. ACS Sens. 2021, 6, 2158–2167. [Google Scholar] [CrossRef]
- Arai, S.; Lee, S.C.; Zhai, D.; Suzuki, M.; Chang, Y.T. A molecular fluorescent probe for targeted visualization of temperature at the endoplasmic reticulum. Sci. Rep. 2014, 4, 6701. [Google Scholar] [CrossRef]
- Liu, X.; Yamazaki, T.; Kwon, H.Y.; Arai, S.; Chang, Y.T. A palette of site-specific organelle fluorescent thermometers. Mater. Today Bio. 2022, 16, 100405. [Google Scholar] [CrossRef]
- Reed, B.C.; Lane, M.D. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 1980, 77, 285–289. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Miao, W.; Yu, C.; Hao, E.; Jiao, L. Functionalized BODIPYs as Fluorescent Molecular Rotors for Viscosity Detection. Front. Chem. 2019, 7, 825. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; Kuimova, M.K. A twisted tale: Measuring viscosity and temperature of microenvironments using molecular rotors. Int. Rev. Phys. Chem. 2018, 37, 259–285. [Google Scholar] [CrossRef]
- Liu, X.; Chi, W.; Qiao, Q.; Kokate, S.V.; Cabrera, E.P.; Xu, Z.; Liu, X.; Chang, Y.T. Molecular Mechanism of Viscosity Sensitivity in BODIPY Rotors and Application to Motion-Based Fluorescent Sensors. ACS Sens. 2020, 5, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular rotor measures viscosity of live cells via fluorescence lifetime imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chi, W.; de J Gómez-Infante, A.; Peña-Cabrera, E.; Liu, X.; Chang, Y.T. A Systematic Study on the Relationship between Viscosity Sensitivity and Temperature Dependency of BODIPY Rotors. Bull. Korean Chem. Soc. 2021, 42, 91–94. [Google Scholar] [CrossRef]
- Savchuk, O.A.; Carvajal, J.J.; Massons, J.; Aguiló, M.; Díaz, F. Determination of photothermal conversion efficiency of graphene and graphene oxide through an integrating sphere method. Carbon 2016, 103, 134–141. [Google Scholar] [CrossRef]
- Arata, H.F.; Löw, P.; Ishizuka, K.; Bergaud, C.; Kim, B.; Noji, H.; Fujita, H. Temperature distribution measurement on microfabricated thermodevice for single biomolecular observation using fluorescent dye. Sens. Actuators B Chem. 2006, 117, 339–345. [Google Scholar] [CrossRef]
- Nardo, L.; Lamperti, M.; Salerno, D.; Cassina, V.; Missana, N.; Bondani, M.; Tempestini, A.; Mantegazza, F. Effects of non-CpG site methylation on DNA thermal stability: A fluorescence study. Nucleic Acids Res. 2015, 43, 10722–10733. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Ferdinandus, M.; Suzuki, C.Q.; Vu, Y.; Harada, S.R.; Sarker, S.; Ishiwata, T.; Kitaguchi, S. Arai, Modulation of Local Cellular Activities using a Photothermal Dye-Based Subcellular-Sized Heat Spot. ACS Nano 2022, 16, 9004–9018. [Google Scholar] [CrossRef]
- Wilkening, A.; Rüb, C.; Sylvester, M.; Voos, W. Analysis of heat-induced protein aggregation in human mitochondria. J. Biol. Chem. 2018, 293, 11537–11552. [Google Scholar] [CrossRef]
- AShinde, B.; Song, A.; Wang, Q.A. Brown Adipose Tissue Heterogeneity, Energy Metabolism, and Beyond. Front. Endocrinol. 2021, 12, 651763. [Google Scholar] [CrossRef]
- Baffou, G.; Rigneault, H.; Marguet, D.; Jullien, L. A critique of methods for temperature imaging in single cells. Nat. Methods 2014, 11, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.J.; Hartley, R.C.; Cochemé, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.A.; Fink, B.D.; Gibbs, B.E.; Chheda, P.R.; Wu, M.; Sivitz, W.I.; Kerns, R.J. A Novel Triphenylphosphonium Carrier to Target Mitochondria without Uncoupling Oxidative Phosphorylation. J. Med. Chem. 2021, 64, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; He, Y.; Sharma, A.; Kim, J.; Pan, W.; Yang, Z.; Li, J.; Yan, W.; Liu, L.; Qu, J.; et al. Fluorescent Probes for Nanoscopic Imaging of Mitochondria. Chem 2019, 5, 1697–1726. [Google Scholar] [CrossRef]
- Meizoso-Huesca, A.; Pearce, L.; Barclay, C.J.; Launikonis, B.S. Ca2+ leak through ryanodine receptor 1 regulates thermogenesis in resting skeletal muscle. Proc. Natl. Acad. Sci. USA 2022, 119, e2119203119. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, T.; Liu, X.; Chang, Y.-T.; Arai, S. Applicability and Limitations of Fluorescence Intensity-Based Thermometry Using a Palette of Organelle Thermometers. Chemosensors 2023, 11, 375. https://doi.org/10.3390/chemosensors11070375
Yamazaki T, Liu X, Chang Y-T, Arai S. Applicability and Limitations of Fluorescence Intensity-Based Thermometry Using a Palette of Organelle Thermometers. Chemosensors. 2023; 11(7):375. https://doi.org/10.3390/chemosensors11070375
Chicago/Turabian StyleYamazaki, Takeru, Xiao Liu, Young-Tae Chang, and Satoshi Arai. 2023. "Applicability and Limitations of Fluorescence Intensity-Based Thermometry Using a Palette of Organelle Thermometers" Chemosensors 11, no. 7: 375. https://doi.org/10.3390/chemosensors11070375
APA StyleYamazaki, T., Liu, X., Chang, Y.-T., & Arai, S. (2023). Applicability and Limitations of Fluorescence Intensity-Based Thermometry Using a Palette of Organelle Thermometers. Chemosensors, 11(7), 375. https://doi.org/10.3390/chemosensors11070375






