Abstract
With the development of two-dimensional materials such as graphene, transition metal dichalcogenides (TMDs), MXenes and MBenes, these materials have received extensive attention from scholars in the field of gas sensing due to their unique and superior properties. Based on first-principles calculations, the adsorption energy, charge transfer, density of states and deformation charge density of CrB, an MBene successfully synthesized under laboratory conditions, were investigated for the adsorption of the decomposition components (CF4, C3F6 and COF2) of an insulating medium, C4F7N. The calculation results revealed strong chemisorption with an adsorption energy as high as −3.336 eV between CrB and COF2, as well as physical adsorption with CF4 and C3F6. However, the excessive interaction strength makes it difficult for COF2 molecules to escape from the binding of the CrB substrate, making CrB more suitable as an adsorbent to remove COF2 gas. Compared with COF2 and CF4, CrB has appropriate adsorption energy and charge transfer for C3F6 adsorption, and its theoretical recovery performance is acceptable, indicating its potential as a sensor for detecting C3F6.
1. Introduction
With the increasing emphasis on the sustainable development of the ecological environment by the international community, the use and emission of SF6, a strong greenhouse gas used for insulation in the electric power industry, have been subject to more restrictions [1,2]. To alleviate the exacerbation of greenhouse effects caused by the insulation gases used in the electric power industry, C4F7N, a new environmentally friendly gas with great application potential, was first synthesized in 2014 and has subsequently been used in power equipment in various countries around the world. The insulation strength of C4F7N is 2.2 times that of SF6, its potential greenhouse effect is 1/11 that of SF6, and its atmospheric lifetime is 30 years (compared to 3200 years for SF6) [3,4,5,6]. However, due to the high liquefaction temperature of C4F7N (−4 °C), it needs to be used in combination with CO2, N2 and other gases to meet the operational requirements of equipment in some cold regions [7,8,9,10]. Overall, gas-insulated equipment in long-term operation inevitably encounters unstable factors such as metal burrs or particles that cause electric field distortions, which cannot be detected in a timely manner and may result in partial discharge or overheating of the equipment [11]. Under ionization caused by discharge, the C4F7N mixture gas decomposes into a series of by-products, such as CF4, C2F6, C3F6 and COF2. Among them, according to Ye et al., the CF4 concentration produced by the decomposition of the mixed gas has a strong linear relationship with the discharge intensity and time for partial discharge [12], and COF2 has a good linear relationship with the temperature of overheating faults inside the equipment [2]. According to Li and Wu’s research, C4F7N decomposition can occur at low temperatures (100~200 °C) catalyzed by the materials inside the equipment (such as EPDM rubber seals, copper, aluminum, etc.), making C3F6 a characterization of early overheating faults [1,13]. By qualitatively and quantitatively detecting these characteristic gases, it is possible to eliminate early equipment failures in a timely manner, prevent the further deterioration of failures and ensure the stable operation of the power system.
Resistive gas sensors based on nanomaterials have the advantages of high sensitivity, low energy consumption and flexible deployment strategies and have been widely used in industries such as medicine, military and environmental monitoring, making them an indispensable and efficient way to obtain spatial gas information [14,15,16,17,18]. In order to pursue even better performance, scholars have been constantly exploring nanomaterials assembled in gas sensors, such as metal oxides, carbon nanotubes, phosphorus materials and transition metal dichalcogenides. In recent years, MXene, a new two-dimensional material with a large specific surface area, high carrier mobility and abundant surface functional groups (obtained via etching MAX), has sparked a research wave in related industries [19,20,21]. In the field of sensor research, this material can produce a certain response to many gases at room temperature. For fluorocarbon gases, Wu et al. used Ti3C2Tx as a dopant material and formed a heterojunction with SnO2, achieving high sensitivity detection of C4F7N at room temperature [22]. However, this material is prone to oxidation and structural collapse in an oxygen-containing environment, resulting in the attenuation or even disappearance of certain properties. In contrast, another similar compound, MBenes (obtained via etching MAB, a compound composed of an early transition metal (M), metals from groups 13 and 14 of the periodic table (A) and boron (B)), not only possesses the excellent properties of MXenes, such as sensing materials, but also exhibits enhanced structural stability and antioxidation performance [23,24,25]. Guo et al. evaluated the antioxidation properties of 14 MBenes, including CrB, using the limiting potential (UL) and redox potential (UR) and found that the selected materials have a superior surface oxidation suppression ability, which is related to the covalent properties of the metal–boron bonds in MBene [26]. To date, research on this type of material has been focused on synthesizing and etching. Zhang et al. successfully prepared Cr2AlB2 and CrB using high-temperature evaporation and acid etching methods and found that the obtained few-layer CrB demonstrates superior stability [25]. Wang’s team and Alameda’s team also prepared Ti2InB2 (TiB) and Mo2AlB2 (MoB) using similar methods [27]. Considering the potential application of MBene in the sensing field, it is necessary to carry out relevant theoretical calculations to explore the application possibilities of such materials in related industries, providing a reference for the application of these materials while serving modern production.
In this study, the sensing and capturing effects of CrB on the decomposition components of C4F7N mixed gas were analyzed using first-principles calculations. In the optimized system structure, CrB exhibited a strong interaction with COF2 compared to CF4 and C3F6. Molecular dynamics simulations and theoretical recovery time calculations revealed that COF2 can be stably adsorbed on the substrate surface over a temperature range of 300 K to 700 K, indicating that CrB can be used as an adsorbent to remove COF2. In contrast, C3F6 can be quickly desorbed, suggesting that CrB has the potential to be used as a gas sensor for C3F6. These results not only expand the application fields of CrB but also provide research ideas for the decomposition components of C4F7N and the sensing of related fluorocarbon gases.
2. Methods
The first-principles calculations presented in this work were carried out using the Vienna Ab initio Simulation Package (VASP). The ion–electron interaction was described using the Projector Augmented Wave (PAW) basis set [28,29]. The exchange–correlation functional approximation method used the Perdew–Burke–Ernzerhof (PBE) function, which is based on the generalized gradient approximation (GGA) in density functional theory [30]. To avoid interlayer interactions, a 20 Å vacuum layer was added between adjacent Cr layers. For structural optimization, the cutoff energy and self-consistent convergence threshold for system energy optimization were set to 520 eV and 10−5 eV, respectively. The block Davidson iterative method was used to handle interatomic forces, with a force value of less than 0.02 eV/Å as the convergence criterion. The Grimme DFT-D3 dispersion correction program was introduced to handle the weak van der Waals forces [31]. The initial structure was set as a 4 × 4 × 1 supercell consisting of the minimum unit cell, and the geometry optimization and electronic structure calculations were performed using a 4 × 4 × 1 Monkhorst–Pack grid for k-points. Bader charge analysis conducted with the Henkelman code using a near-grid algorithm refine-edge method was used to evaluate the charge exchange behavior between the gas molecule and the 2D CrB substrate [32,33,34]. For the optimized system structure, the adsorption energy (Eads) was used to describe the interaction strength between the gas molecule and CrB, defined as the following formula:
Eads = Egas/CrB − Egas − ECrB
3. Results and Discussion
3.1. Intrinsic Geometric Structure
Figure 1 shows the energy-minimum structures of CrB and various gas molecules. It can be seen from Figure 1a that CrB is composed of two layers of Cr atoms at the top and two layers of B atoms in the middle when viewed from the side, and when viewed from the top, it is composed of alternating chains of Cr and B atoms. Considering the symmetry of its structure, the initial interaction positions of the C4F7N decomposition components were chosen to be above the Cr atom at the center of the structure and the B atom in the adjacent row. The orientation of the gas molecules was also selected based on the symmetry of the structure. As shown in Figure 1b, the structure of C3F6 is highly asymmetric, so this paper only considered two orientations of the plane composed of the C=C-C bonds in C3F6, perpendicular and parallel to CrB. For CF4 with a highly symmetrical tetrahedral structure, three placement positions, with one C-F bond pointing downward, two C-F bonds pointing downward and three C-F bonds pointing downward, were taken into account. For COF2 with four atoms in the same plane, the orientation was selected such that the molecular plane was perpendicular to CrB (with the C-O bond pointing downward and two C-F bonds pointing downward) or parallel to CrB.
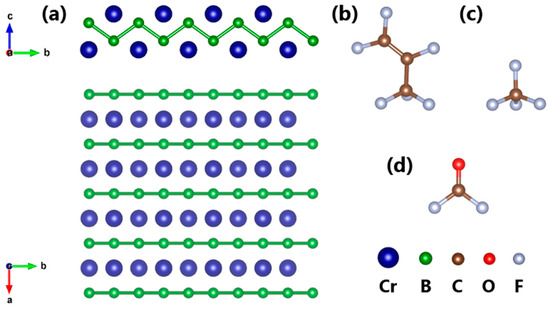
Figure 1.
Optimized structures of (a) CrB, (b) C3F6, (c) CF4 and (d) COF2.
3.2. Optimization of Adsorption Systems
Figure 2 shows the different adsorption configurations of COF2 on the CrB surface and their corresponding lowest energy structures, along with the corresponding Eads. For different adsorption sites on the CrB surface, it can be found that, under the same initial orientation, the distance between the gas molecules and the substrate in the adsorption system above the B atom increases significantly, indicating a weaker interaction between the gas molecules and CrB in that position, unable to form effective adsorption. Specifically, the Eads of COF2 after structural optimization above the B atom are all smaller than those above the Cr atom. Among them, the difference in adsorption energy for the C=O orientation is −0.2 eV, that for the C-F orientation is −0.1 eV, and the largest difference up to −2 eV can be found in the parallel orientation. This phenomenon indicates the highly active characteristic of the Cr atom in adsorbing gas molecules. For the orientation of COF2, when the gas molecule is placed perpendicular to the CrB plane, the interaction between the analyte only changes the relative distance of the molecules, and the structural parameters do not change significantly. However, when the atomic plane is parallel to CrB, besides the significant molecular displacement, the structural parameters of the entire system also change significantly, including bond breaking and the bond formation of gas molecules and the distortion of the substrate structure. Taking Figure 2d as an example, under the strong interaction, gas molecules originally more than 2 Å away from the plane are completely trapped in CrB and cause C-F bond breaking. The Cr-O distances are reduced from 2.436 Å to 2.091 Å and 2.133 Å, and the Cr-F distance is reduced from 2.341 Å to 1.819 Å. This suggests that a chemical bond may be formed between them. For the CF4 adsorption system shown in Figure 3, the interaction between the analyte is much weaker than those of the COF2 system. From the molecular structure of CF4 shown in Figure 1c, it can be seen that the molecule has an absolutely symmetrical molecular and electronic structure, which provides extremely high stability and makes it difficult for electron extraction or injection to occur in this gas molecule. From the relative distance analysis, all adsorption systems after structural optimization show the phenomenon in which the gas molecule is far away from the substrate, and neither the molecule nor the substrate undergoes a change in structural parameters, indicating a weak interaction between the analytes. Moreover, it can be seen that, with the increase in C-F bonds, the average Eads also increases, which may be related to the strong electron affinity of F atoms. The maximum Eads appears in the adsorption system with two C-F bonds facing the Cr atom, which is −0.160 eV. The Cr-F distance increases from 2.403 Å to 2.436 Å, and the two C-F bonds are slightly elongated from 1.344 Å to 1.360 Å under the influence of the interaction. Compared with CF4 and COF2, the interaction strength between C3F6 and CrB is at an intermediate level. With the bondage of the CrB structure, C3F6 is not captured and decomposed like COF2, nor does it move away from the substrate surface like CF4, but it undergoes some slight deformation near the initial position. Similar to the adsorption results in COF2, the Cr atom in CrB can make a stronger interaction with the C3F6 molecule. For the system with C=C-C parallel to CrB, the Eads (−0.353 eV) generated in the vicinity of the Cr atom is 0.08 eV greater than the result (−0.274 eV) in the vicinity of the B atom, and for the adsorption result with C=C-C perpendicular to CrB, this value increases to 0.1 eV.
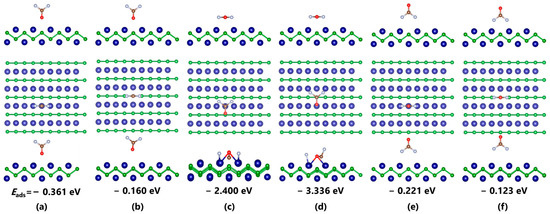
Figure 2.
Optimized adsorption systems of COF2–CrB with different initial orientations. (a,b) O-oriented configurations; (c,d) Parallel configurations; (e,f) F-oriented configurations.
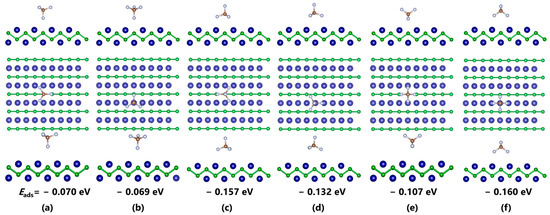
Figure 3.
Optimized adsorption systems of CF4–CrB with different initial orientations. (a,b) Single C-F configurations; (c,d) Three C-F configurations; (e,f) Two C-F configurations.
3.3. Electronic Structure Analysis
The phenomena of bond breaking, bond formation and displacement in the structure optimization are attributed to the hybridization of electron orbitals and other interactions between the atoms. To further explore the underlying mechanism of CrB adsorption on various gas molecules, this section focuses on the changes in the electronic structure in the most stable configurations after structure optimization, as shown in Figure 2d, Figure 3f and Figure 4d. Figure 5a shows the total and partial density of states (TDOS and PDOS) of the COF2–CrB adsorption system. The DOS near the Fermi level undergoes a significant change, and the peak intensity between −0.5 and 0.5 eV is significantly weakened due to the introduction of COF2, indicating the increased difficulty of electron transfer from the valence band to the conduction band and a lower electrical conductivity of CrB. From the PDOS, it can be seen that the F 2p and Cr 3d orbitals have a significant overlap in the range of 1 to −6 eV, and there is also obvious hybridization between the O 2p orbitals and Cr 3d orbitals at energy levels such as 0, −3.4 and −4.5 eV, indicating strong interactions between these atoms and a high possibility of forming new chemical bonds, which also explains the enormous adsorption energy and structural changes in the COF2–CrB system. Similarly, for the other two systems, the TDOS peak intensity near the Fermi level is also weakened, and new peaks appear at lower energy levels, indicating that the electrical conductivity of the system also decreases after gas molecule adsorption. Based on the PDOS, it can be seen that the hybridization degree between the F atoms in the two gases and the Cr atoms on the CrB surface is relatively weak. The F 2p orbital of CF4 and the Cr 3d orbital have hybridization between −6.6 eV and −9.6 eV, and for C3F6, the atomic orbitals have hybridization between −7 eV and −9.6 eV, as well as at −5 eV, indicating that the interaction between CrB and C3F6 is slightly stronger than that between CrB and CF4, which is consistent with the Eads results of the two systems.
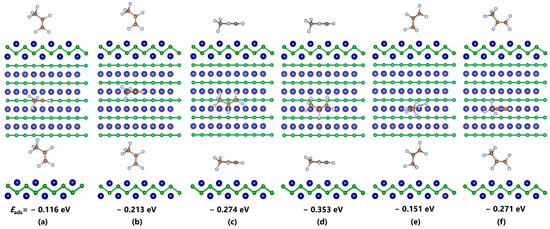
Figure 4.
Optimized adsorption systems of C3F6–CrB with different initial orientations. (a–f) Perpendicular configurations; (c,d) Parallel configurations.
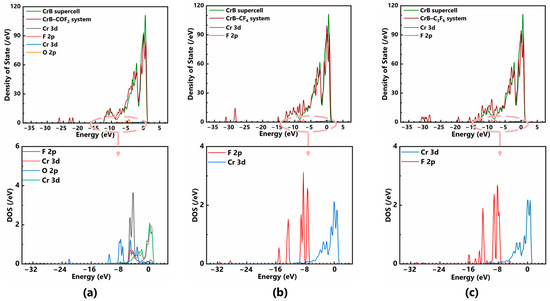
Figure 5.
TDOS and PDOS of (a) COF2–CrB system, (b) CF4–CrB system, and (c) C3F6–CrB system.
Apart from DOS, the deformation charge density (DCD) of each adsorption system is shown in Figure 6. The green and yellow regions indicate a decrease and increase in the charge density, respectively. As shown in Figure 6a, most regions surrounding COF2 exhibit an increase in the charge density, indicating that COF2 acts as an electron acceptor. Based on Bader charge analysis, the C atom loses 0.781 e. The rest of the atoms gain a total of 2.258 e, and the COF2 gas molecule gains −1.477 e during the adsorption process, which is consistent with the electron gain/loss distribution in the DCD. The DCD of the 2D CrB shows that the decrease in the electron density mainly concentrates on the Cr atom, and there is no significant change in the charge density near the B atom, indicating that the gas molecule mainly gains electrons from the Cr atom, which is consistent with the property of metal atoms tending to lose electrons easily. For the CF4–CrB system in Figure 6b, the change in the charge density is weaker, mainly involving electron exchanges among atoms inside the CF4 molecule. According to the Bader charge calculation, the C atom in CF4 loses 2.318 e, and the F atom gains 2.383 e, indicating that the gas molecule only draws 0.066 e from the CrB substrate. The DCD also shows that the yellow region surrounds the F atom, and the adjacent blue region overlaps with the C atom, indicating that the C atom acts as an electron donor to the F atom. For the C3F6–CrB system in Figure 6c, the charge exchange behavior is stronger than that of the CF4 system but weaker than that of the COF2 system. The DCD shows that, besides the charge exchange within the C3F6 molecule, there is also a blue region near the C atom with the minimum distance from the CrB substrate, indicating a certain degree of charge exchange between the gas molecule and the substrate. Based on Bader charge analysis, the C atoms in C3F6 lose 0.969 e, 0.785 e and 1.623 e, respectively, and the F atoms gain a total of 3.512 e. In other words, the gas molecule gains 0.134 e from the CrB substrate, and the main source of electrons is from the Cr atom. In order to give a distinct reference to verify the performance of CrB-based C4F7N decomposition gas sensors, a comparison with other theoretical studies is summarized in Table 1, as given below. It can be found that recent research about the theoretical calculation of C4F7N decomposition gas sensing has mainly been focused on CF4, COF2 and CF3CN, which confirms the significance and urgency to develop sensors for these gas species. In the results of these studies, it always seems difficult to give a stable adsorption to the CF4 molecule, and the adsorption energies are kept at a low level that ranges from −0.160 eV (this work) to −0.360 eV (Co-BN). This phenomenon can be attributed to the highly symmetric molecular structure and electronic structure, which leads to the increased difficulty of injecting or withdrawing an electron from this molecule, as the small Qt in all the previous works shows. Regarding the interactions in the COF2-involved systems, the results of Eads (from −0.320 eV to −3.336 eV) increased sharply compared to the results in the CF4 systems, which may be due to the highly active functional group C=O in COF2. In general, compared with other nanomaterials, CrB exhibited the strongest interaction strength with the COF2 molecule and moderate interaction strength with CF4 and C3F6, ensuring the promising potential of CrB to be employed as a high-performance C4F7N decomposition gas sensor.
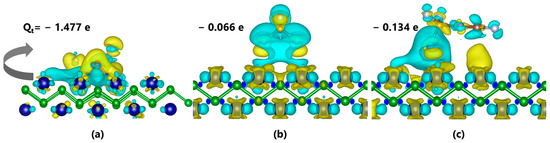
Figure 6.
Deformation charge density (DCD) of (a) C3F6–CrB system, (b) CF4–CrB system and (c) COF2–CrB system.

Table 1.
C4F7N decomposition sensing performance comparison of CrB and other nanomaterials.
For gas sensing applications, in addition to judging the strength of the interaction through adsorption energy, the stability of adsorption is also important. Therefore, the Andersen thermostat was used to analyze the thermal stability of each adsorption system in the NVT ensemble. The temperature was maintained at 300 K (i.e., room temperature), the time step was 1 fs, and the total simulation time was 105 fs. The collision probability that describes an average number of collisions per atom and time step was set as 0.5. The CrB layer was set to be flexible, and the gas molecules were not set to be rigid to well exhibit their structural variations during the interaction process. From Figure 7a, it can be seen that the C3F6 molecule can stably adsorb on the surface of CrB at room temperature, as the nearest distance between the F atom and Cr atom was shortened from 2.383 Å to 2.211 Å, and the total system energy was maintained at around −539 eV. Similarly, after 10 ps at room temperature, COF2 could also remain within the interaction range of the CrB surface with the distances between Cr-O atoms and Cr-F atoms changing from 2.091 Å and 1.819 Å to 1.996 Å and 1.879 Å, respectively. The total system energy fluctuated around −516 eV. Regarding the CF4 system, the distance between the molecule and the CrB substrate increased significantly within the time set, which varied from 2.496 Å to 2.964 Å, accompanied by a gradual decrease in the total system energy, indicating that CF4 cannot maintain effective adsorption on the CrB surface, which is directly related to its weaker interaction and smaller Eads. Therefore, it can be concluded that CrB has enough interaction strength with C3F6 and COF2 and can stably adsorb these two gases at room temperature. In addition, in practical applications, good recovery performance can prevent the poisoning of the sensor and improve the service life of the sensor. The recovery performance of the gas sensor is usually measured by the recovery time τ, which is calculated as follows [40]:
where F is the apparent frequency factor and defined as 1012 s−1 in this study [41,42,43], and kB and T are the Boltzmann constant (eV·K−1) and operating temperature (K), respectively. Ea represents the desorption energy barrier of the gas molecule, which is defined the same as the adsorption energy value. As indicated in the study of Cui et al., the desorption process can be considered the inverse process of adsorption, so it is reasonable to assume the value of Eads to be the desorption energy barrier (Ea) of the desorption process [42]. According to Equation (2), it can be obtained that, for COF2, desorption at room temperature requires a time as long as 1.063 × 1044 s, and even at 700 K, desorption still takes 1.027 × 1012 s, indicating a high risk of sensor poisoning when using a CrB-based sensor to detect COF2. In contrast, the desorption of C3F6 at room temperature only requires 8.413 × 10−7 s, indicating a fast theoretical recovery process. It should be noted that the desorption and adsorption behaviors are a process of dynamic equilibrium and happen at the same time. Therefore, the recovery time at the macro level is the result of the combined action of adsorption and desorption, and the accurate measurement of the recovery time in the experiment is almost impossible, as there are countless gas molecules undergoing simultaneous adsorption and desorption processes. However, this parameter can to some extent reflect the recovery difficulty because it is related to the value of the adsorption energy (Ea), which reflects the interaction strength between the gas molecules and the sensing surface. Based on this, it can be inferred that CrB possesses the potential to be used as an adsorbent to scavenge COF2 or works as the functional material in C3F6 sensing.
τ = F−1e−Ea/kBT
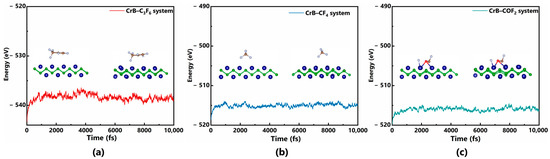
Figure 7.
Molecular dynamics calculation of (a) C3F6–CrB system, (b) CF4–CrB system and (c) COF2–CrB system.
4. Conclusions
In summary, this study computed the interactions between CrB and CF4, C3F6 and COF2 and evaluated the potential of CrB-based devices as sensors or adsorbents by analyzing parameters such as geometric structure variations, adsorption energy, charge transfer and DOS during the adsorption process. The results show that the adsorption strengths of the three gases on CrB decrease in the order of COF2 > C3F6 > CF4, with the corresponding most stable structures having adsorption energies of −3.336 eV, −0.353 eV and −0.157 eV, respectively. By analyzing DOS and DCD, it was found that all gas molecules act as electron acceptors, and in the adsorption of COF2 on CrB, there are bond-breaking behaviors and subsequent bonding behaviors between atoms and 2D substrates, which belong to strong chemical adsorption. However, only slight changes in molecular displacement and bond length were observed for C3F6 and CF4, indicating their physical adsorption on the CrB surface. This result suggests that the adsorption of C3F6 with a moderate charge transfer and acceptable desorption property endows CrB with the potential to be used as a C3F6 sensor. Regarding COF2, the huge adsorption energy allows the CrB surface to stably bind to the gas molecule, giving it the potential to be used as an adsorbent for COF2 scavenging.
Author Contributions
Methodology, R.Z., Z.N. and D.W.; Software, P.W., Z.N. and Y.Z.; Investigation, X.T., D.W., R.Z. and Y.Z.; Data Curation, P.W., Z.N. and X.T.; Writing—Original Draft Preparation, P.W.; Writing—Review and Editing, P.W., R.Z., D.W. and Y.Z.; Supervision, X.T. and Z.N.; Funding Acquisition, Z.N. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the science and technology project of China Southern Power Grid (Grant number YNKJXM20222118, YNKJXM20222131, YNKJXM20222043).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
I would like to express my great appreciation to my advisor Peng Wu and Yifan Zhang and all my fellow from my research group.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, X.; Wu, P.; Cheng, L.; Liang, S. Compatibility and Interaction Mechanism between EPDM Rubber and a SF6 Alternative Gas-C4F7N/CO2/O2. ACS Omega 2021, 6, 13293–13299. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhang, X.; Xie, C.; Sun, X.; Wu, P.; Xiao, S.; Tang, J.; Li, Y. Effect of Oxygen and Temperature on Thermal Decomposition Characteristics of C4F7N/CO2/O2 Gas Mixture for MV Equipment. IEEE Access 2020, 8, 221004–221012. [Google Scholar] [CrossRef]
- Fang, X.; Hu, X.; Janssens-Maenhout, G.; Wu, J.; Han, J.; Su, S.; Zhang, J.; Hu, J. Sulfur hexafluoride (SF6) emission estimates for China: An inventory for 1990-2010 and a projection to 2020. Environ. Sci. Technol. 2013, 47, 3848–3855. [Google Scholar] [CrossRef] [PubMed]
- Rabie, M.; Franck, C.M. Assessment of Eco-friendly Gases for Electrical Insulation to Replace the Most Potent Industrial Greenhouse Gas SF6. Environ. Sci. Technol. 2018, 52, 369–380. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z.; Li, Y.; Xiao, H.; Li, Y.; Tang, J.; Xiao, S. Abatement of SF6 in the presence of NH3 by dielectric barrier discharge plasma. J. Hazard. Mater. 2018, 360, 341–348. [Google Scholar] [CrossRef]
- Huang, L.; Shen, Y.; Dong, W.; Zhang, R.; Zhang, J.; Hou, H. A novel method to decompose two potent greenhouse gases: Photoreduction of SF6 and SF5CF3 in the presence of propene. J. Hazard. Mater. 2008, 151, 323–330. [Google Scholar] [CrossRef]
- Zhang, B.; Li, C.; Xiong, J.; Zhang, Z.; Li, X.; Deng, Y. Decomposition characteristics of C4F7N/CO2 mixture under AC discharge breakdown. AIP Adv. 2019, 9, 115212. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Yang, T.; Deng, Y.; Li, X.; Murphy, A.B. Thermal decomposition characteristics and kinetic analysis of C4F7N/CO2 gas mixture. J. Phys. D 2020, 53, 055502. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, K.; Cui, H.; Zhang, F.; Yu, J.; Tao, L.-Q.; Li, X.; Chen, X. High sensitivity gas sensor to detect SF6 decomposition components based on monolayer antimonide phosphorus. Chem. Phys. Lett. 2020, 756, 137868. [Google Scholar] [CrossRef]
- Xiao, S.; Gao, B.; Pang, X.; Zhang, X.; Li, Y.; Tian, S.; Tang, J.; Luo, Y. The sensitivity of C4F7N to electric field and its influence to environment-friendly insulating gas mixture C4F7N/CO2. J. Phys. D 2021, 54, 055501. [Google Scholar] [CrossRef]
- Gui, Y.; Zhang, X.; Lv, P.; Wang, S.; Tang, C.; Zhou, Q. Ni-CNT Chemical Sensor for SF6 Decomposition Components Detection: A Combined Experimental and Theoretical Study. Sensors 2018, 18, 3493. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhang, X.; Li, Y.; Yao, Y.; Xiao, S.; Zhang, X.; Xie, C.; Shao, X.; Sun, X. Effect of O2 on AC Partial Discharge and Decomposition Behavior of C4F7N/CO2/O2 Gas Mixture. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1440–1448. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Li, Y.; Chen, D.; Cui, Z.; Liu, W.; Tang, J. Interaction Mechanism between the C4F7N-CO2 Gas Mixture and the EPDM Seal Ring. ACS Omega 2020, 5, 5911–5920. [Google Scholar] [CrossRef]
- Cao, P.; Cai, Y.; Pawar, D.; Navale, S.T.; Rao, C.N.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; et al. Down to ppb level NO2 detection by ZnO/rGO heterojunction based chemiresistive sensors. Chem. Eng. J. 2020, 401, 125491. [Google Scholar] [CrossRef]
- Jung, G.; Shin, W.; Hong, S.; Jeong, Y.; Park, J.; Kim, D.; Bae, J.-H.; Park, B.-G.; Lee, J.-H. Comparison of the characteristics of semiconductor gas sensors with different transducers fabricated on the same substrate. Sens. Actuators B Chem. 2021, 335, 129661. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H. Pristine and Cu decorated hexagonal InN monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study. J. Hazard. Mater. 2019, 363, 346–357. [Google Scholar] [CrossRef]
- Kumar, V.; Azhikodan, D.; Roy, D.R. 2D Sb2C3 monolayer: A promising material for the recyclable gas sensor for environmentally toxic nitrogen-containing gases (NCGs). J. Hazard. Mater. 2021, 405, 124168. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Xiao, S.; Chen, J.; Tang, J.; Chen, D.; Zhang, X. SnO2 nanoparticles based highly sensitive gas sensor for detection of C4F7N: A new eco-friendly gas insulating medium. J. Hazard. Mater. 2022, 422, 126882. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hermawan, A.; Asakura, Y.; Hasegawa, T.; Kumagai, H.; Kato, H.; Kakihana, M.; Zhu, J.; Yin, S. SnO-SnO2 modified two-dimensional MXene Ti3C2Tx for acetone gas sensor working at room temperature. J. Mater. Sci. Technol. 2021, 73, 128–138. [Google Scholar] [CrossRef]
- Wu, M.; An, Y.; Yang, R.; Tao, Z.; Xia, Q.; Hu, Q.; Li, M.; Chen, K.; Zhang, Z.; Huang, Q.; et al. V2CTx and Ti3C2Tx MXenes Nanosheets for Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 6257–6268. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, L.; Wang, J.; Liu, F.; He, J.; Liu, A.; Lv, S.; You, R.; Yan, X.; Sun, P.; et al. Flexible resistive NO2 gas sensor of three-dimensional crumpled MXene Ti3C2Tx/ZnO spheres for room temperature application. Sens. Actuators B Chem. 2021, 326, 128828. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Xiao, S.; Chen, D.; Chen, J.; Tang, J.; Zhang, X. Room-Temperature Detection of Perfluoroisobutyronitrile with SnO2/Ti3C2Tx Gas Sensors. ACS Appl. Mater. Interfaces 2022, 14, 48200–48211. [Google Scholar] [CrossRef] [PubMed]
- Alameda, L.T.; Holder, C.F.; Fenton, J.L.; Schaak, R.E. Partial Etching of Al from MoAlB Single Crystals To Expose Catalytically Active Basal Planes for the Hydrogen Evolution Reaction. Chem. Mater. 2017, 29, 8953–8957. [Google Scholar] [CrossRef]
- Alameda, L.T.; Moradifar, P.; Metzger, Z.P.; Alem, N.; Schaak, R.E. Topochemical Deintercalation of Al from MoAlB: Stepwise Etching Pathway, Layered Intergrowth Structures, and Two-Dimensional MBene. J. Am. Chem. Soc. 2018, 140, 8833–8840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiang, H.; Dai, F.-z.; Zhang, Z.; Zhou, Y. First demonstration of possible two-dimensional MBene CrB derived from MAB phase Cr2AlB2. J. Mater. Sci. Technol. 2018, 34, 2022–2026. [Google Scholar] [CrossRef]
- Guo, X.; Lin, S.; Gu, J.; Zhang, S.; Chen, Z.; Huang, S. Establishing a Theoretical Landscape for Identifying Basal Plane Active 2D Metal Borides (MBenes) toward Nitrogen Electroreduction. Adv. Funct. Mater. 2020, 31, 2008056. [Google Scholar] [CrossRef]
- Wang, J.; Ye, T.N.; Gong, Y.; Wu, J.; Miao, N.; Tada, T.; Hosono, H. Discovery of hexagonal ternary phase Ti2InB2 and its evolution to layered boride TiB. Nat. Commun. 2019, 10, 2284. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pang, J.; Zhang, J.; Wei, G.; Wei, S.; Yan, J.; Jin, S. Exploring monolayer Janus MoSSe as potential gas sensor for Cl2, H2S and SO2. Comput. Theor. Chem. 2022, 1211, 113665. [Google Scholar] [CrossRef]
- Garg, P.; Choudhuri, I.; Pathak, B. Stanene based gas sensors: Effect of spin-orbit coupling. Phys. Chem. Chem. Phys. 2017, 19, 31325–31334. [Google Scholar] [CrossRef] [PubMed]
- Nisar, J.; Topalian, Z.; De Sarkar, A.; Osterlund, L.; Ahuja, R. TiO2-based gas sensor: A possible application to SO2. ACS Appl. Mater. Interfaces 2013, 5, 8516–8522. [Google Scholar] [CrossRef]
- Wang, X.; Gui, Y.; Xu, L.; Chen, X. Adsorption and gas sensing properties of CuO modified MoSe2 to C3F7CN decomposition products. Mater. Today Commun. 2021, 28, 102677. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, X.; Song, Y.; Zhang, X.; Tian, S.; Liu, L. Adsorption behaviour of CF4 and COF2 gas on the GaN monolayer doped with Pt catalytic: A first-principles study. Surf. Sci. 2022, 719, 122032. [Google Scholar] [CrossRef]
- Zhou, Q.; Zou, Y.; Wang, P.; Tong, J.; Xu, B.; Liu, Y. Comparative analysis of sensing characteristics of Rh-doped defect h-BN monolayer to C4F7N decomposition components under partial discharge. Mol. Phys. 2021, 119, e1976428. [Google Scholar] [CrossRef]
- Sun, H.; Tao, L.-Q.; Li, T.; Gao, X.; Wang, G.; Peng, Z.; Zhu, C.; Zou, S.; Gui, Y.; Xia, S.-Y.; et al. Sensing Characteristics of Toxic C4F7N Decomposition Products on Metallic- Nanoparticle Co-Doped BN Monolayer: A First Principles Study. IEEE Sens. J. 2021, 21, 13082–13089. [Google Scholar] [CrossRef]
- Sang, T.-Y.; Sun, H.; Hu, X.; Li, T.; Guo, L.-Y.; Peng, Z.; Wang, G.; Zhu, C.; Zou, S.; Zhang, X.; et al. Theoretical Exploration of C4F7N Decompositions on GeSe Monolayers for Gas Sensing Based on DFT Method. IEEE Sens. J. 2022, 22, 13915–13920. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A 2018, 124, 194. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, G.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Chen, D.; Cui, H.; Tang, J. Adsorption behaviour of SO2 and SOF2 gas on Rh-doped BNNT: A DFT study. Mol. Phys. 2019, 118, e1580394. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).