Enhancing the Potentiometric H2 Sensing of Pr0.1Ce0.9O2−δ Using Fe2O3 Surface Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials Synthesis and Sensors Preparation
2.3. Characterization and Gas Sensing Measurements
2.4. Electrochemical Analysis Methods
3. Results
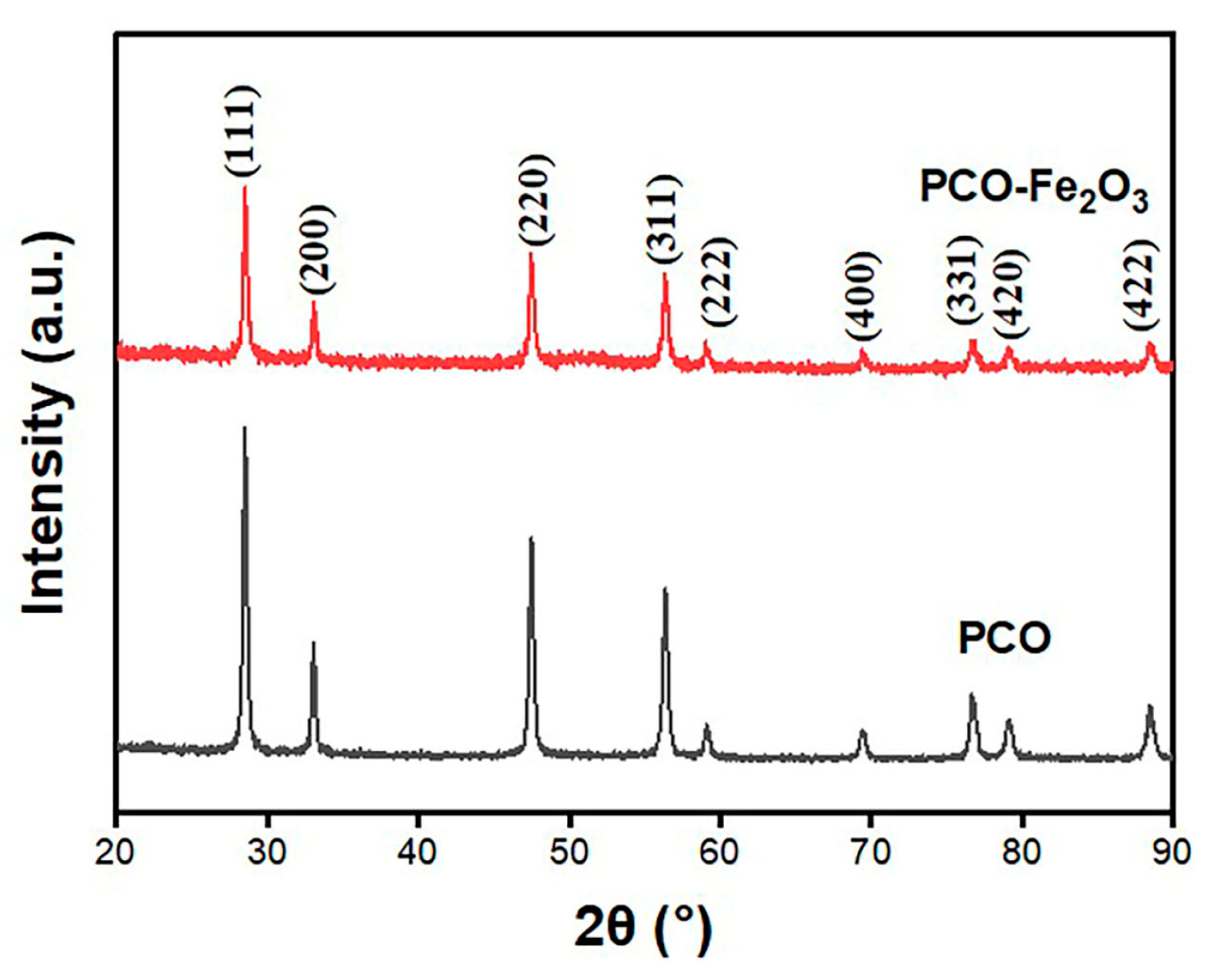
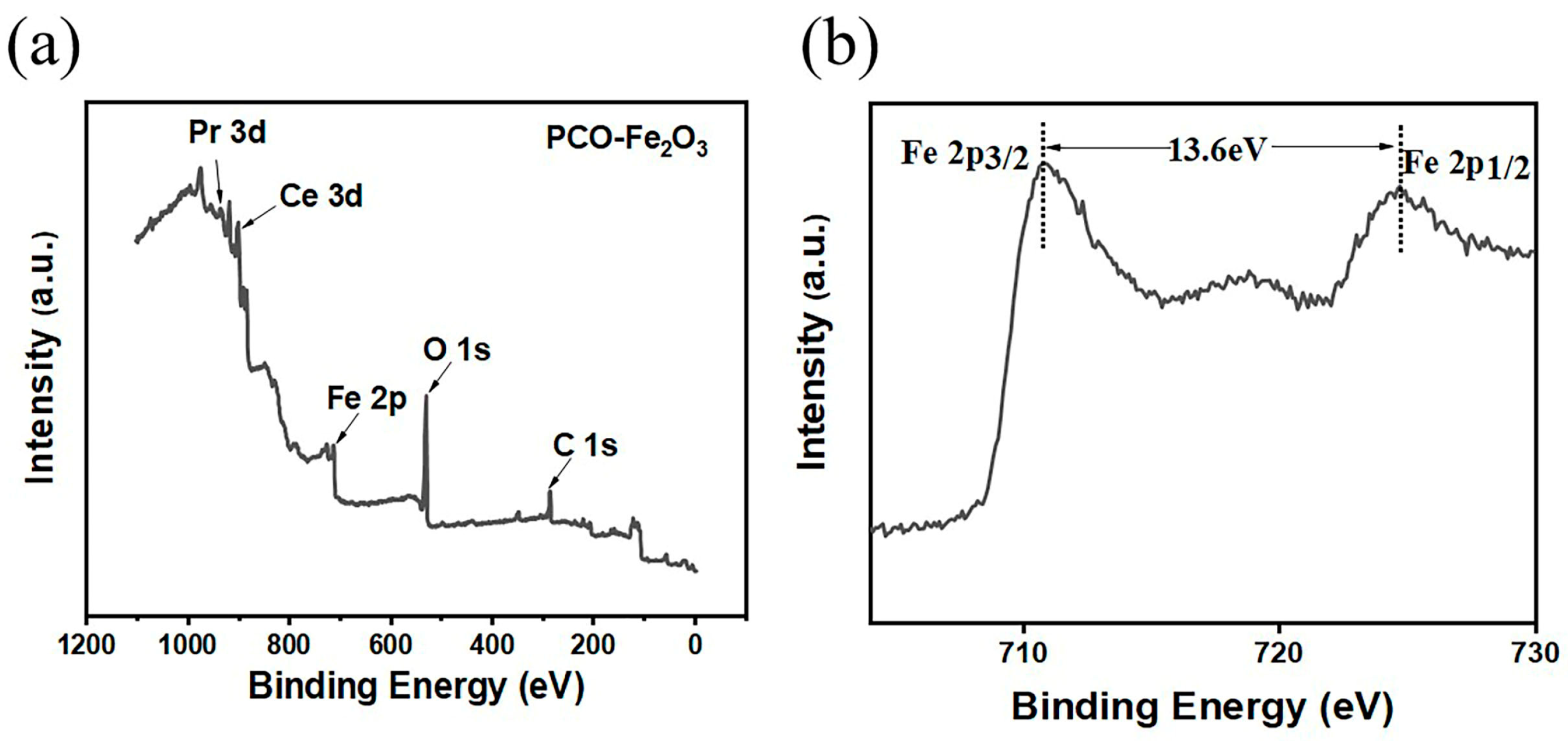
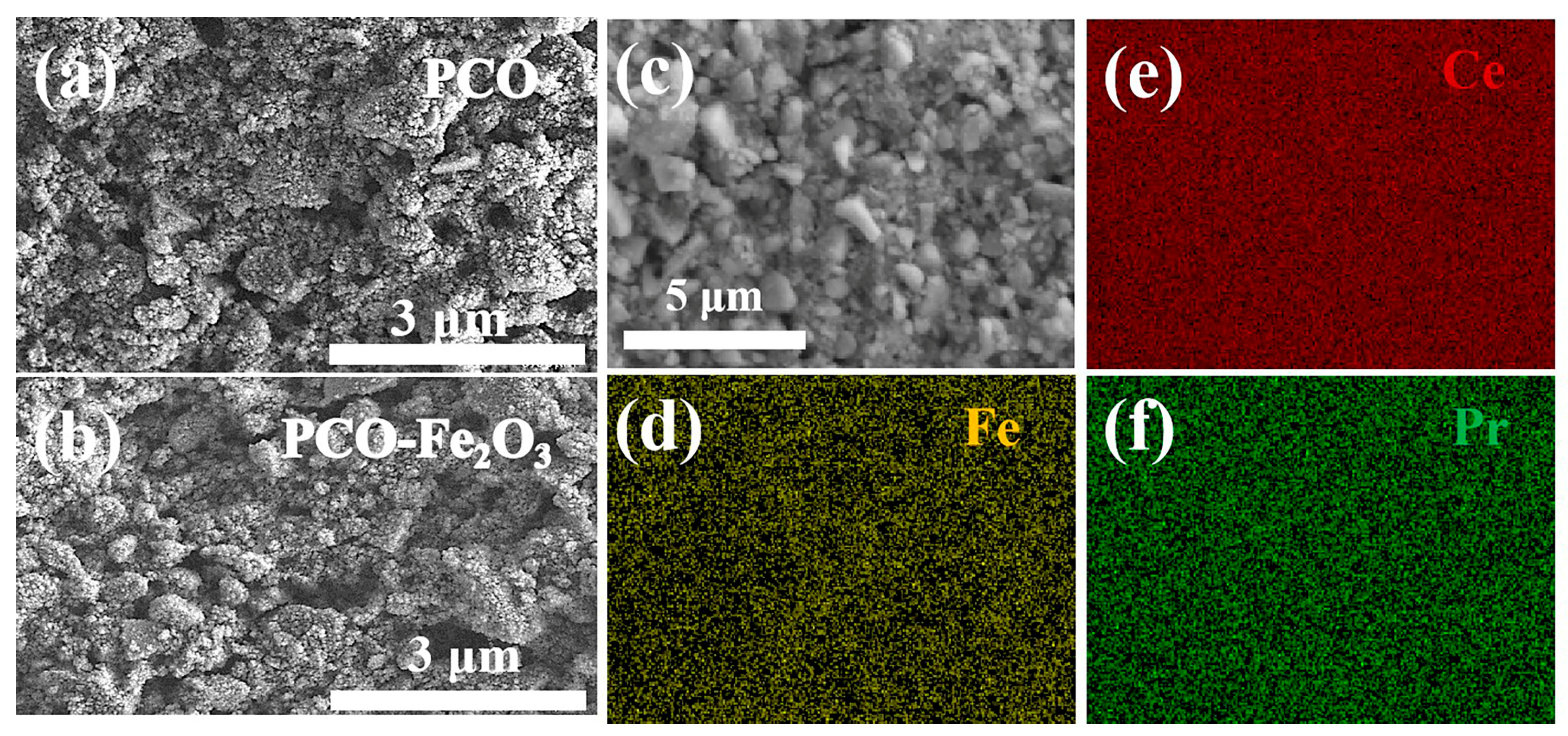
3.1. Characterization Results of Materials
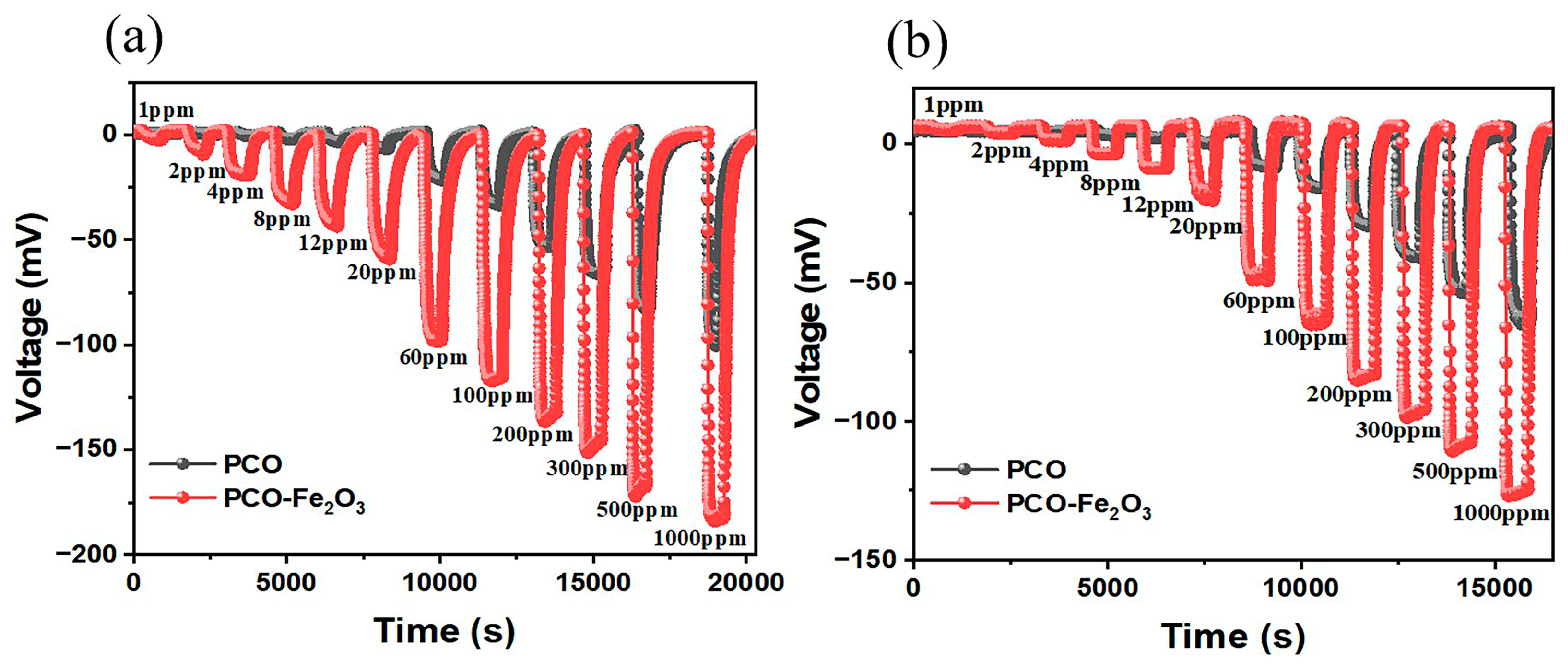
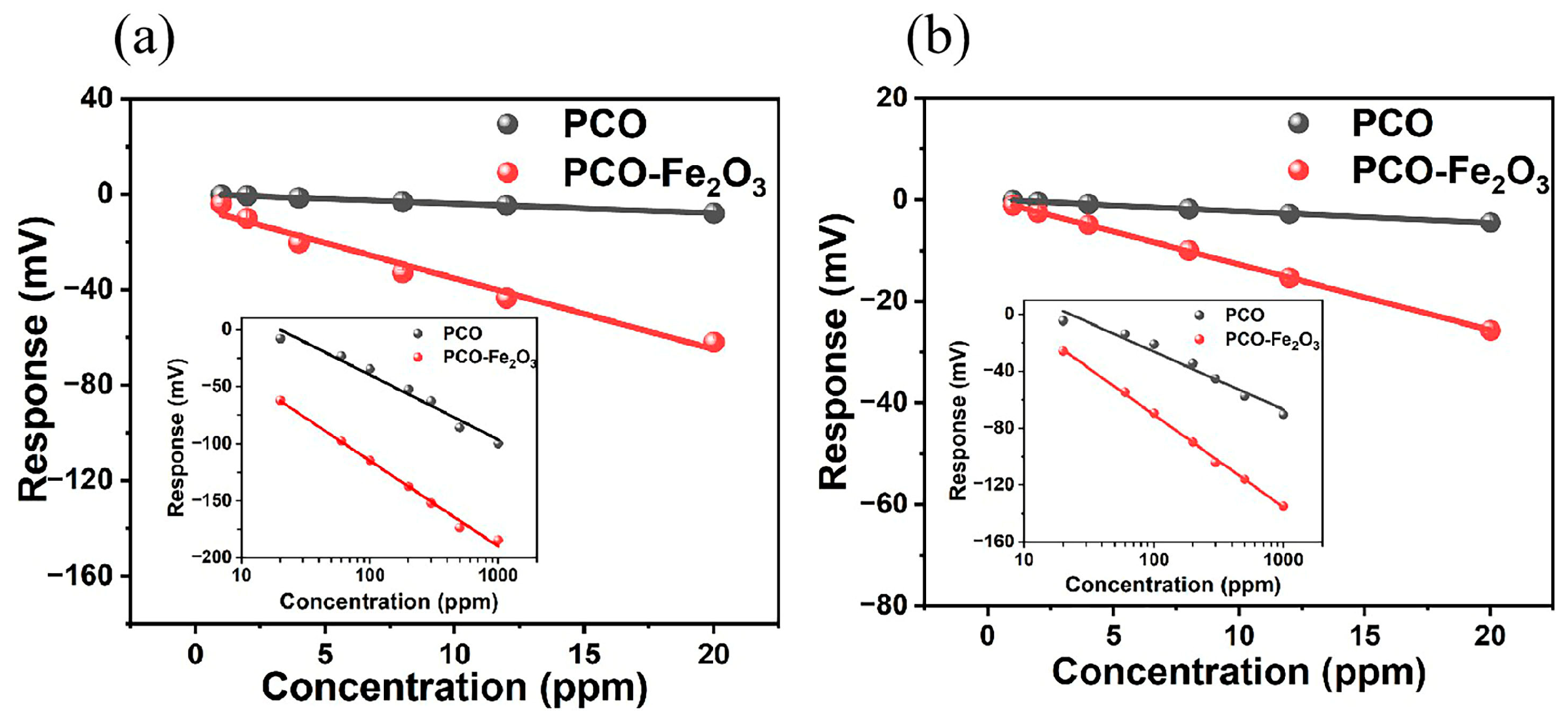
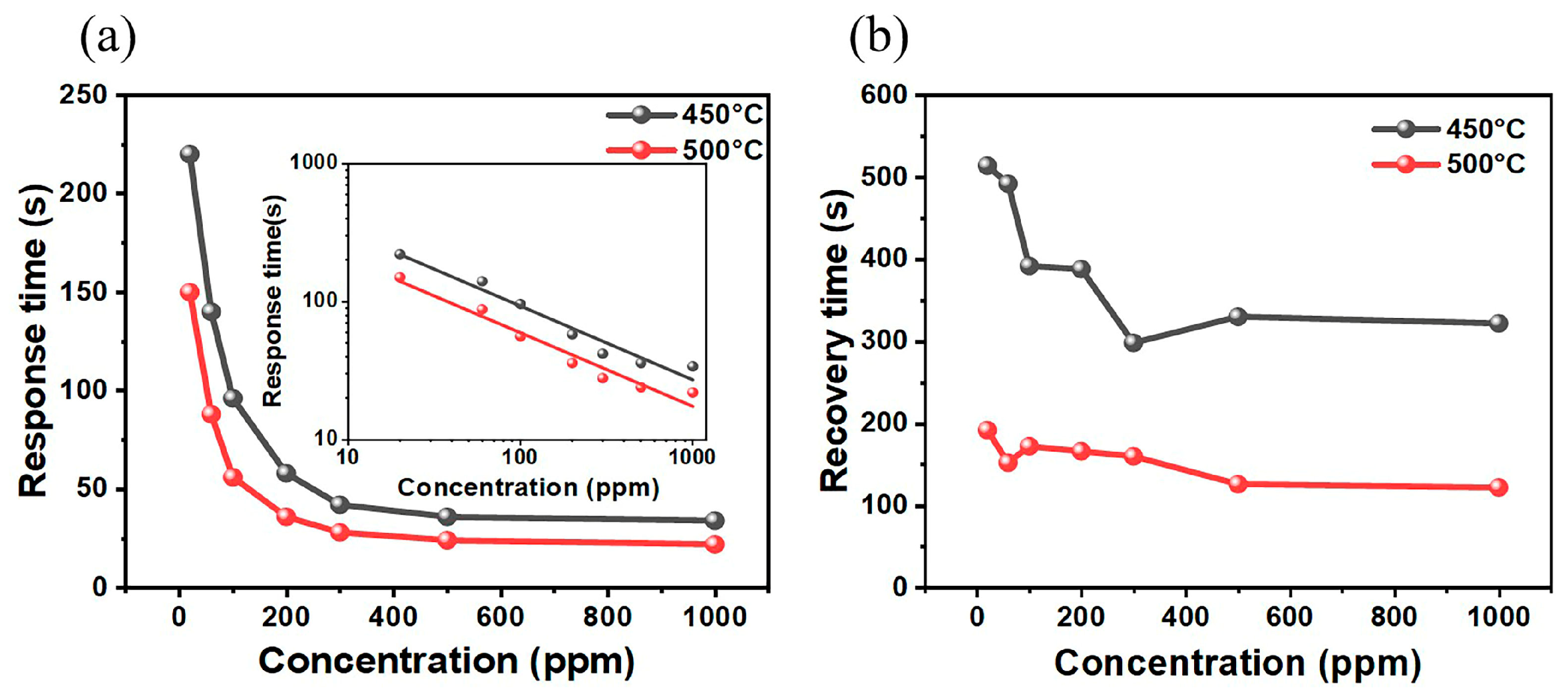
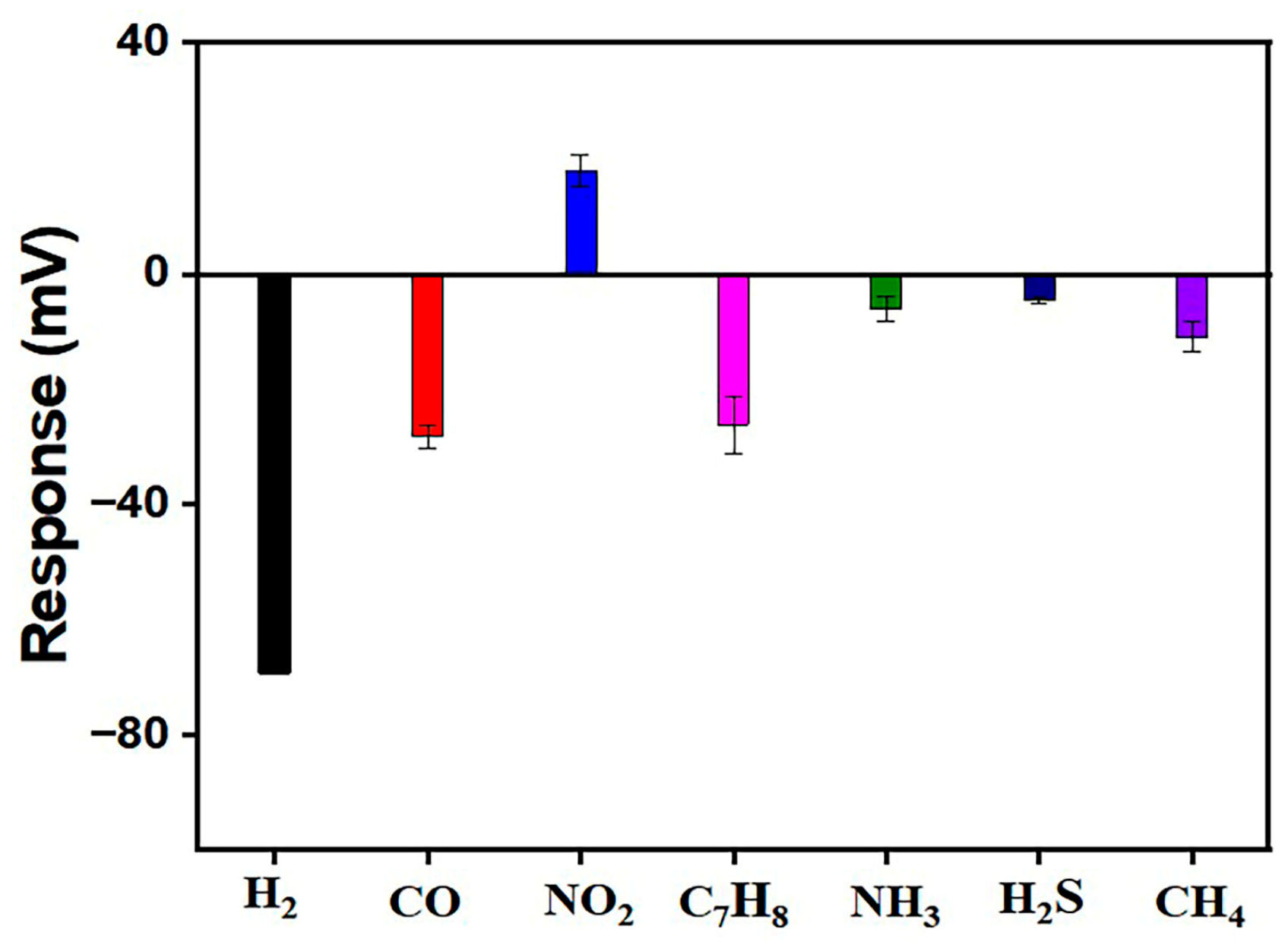
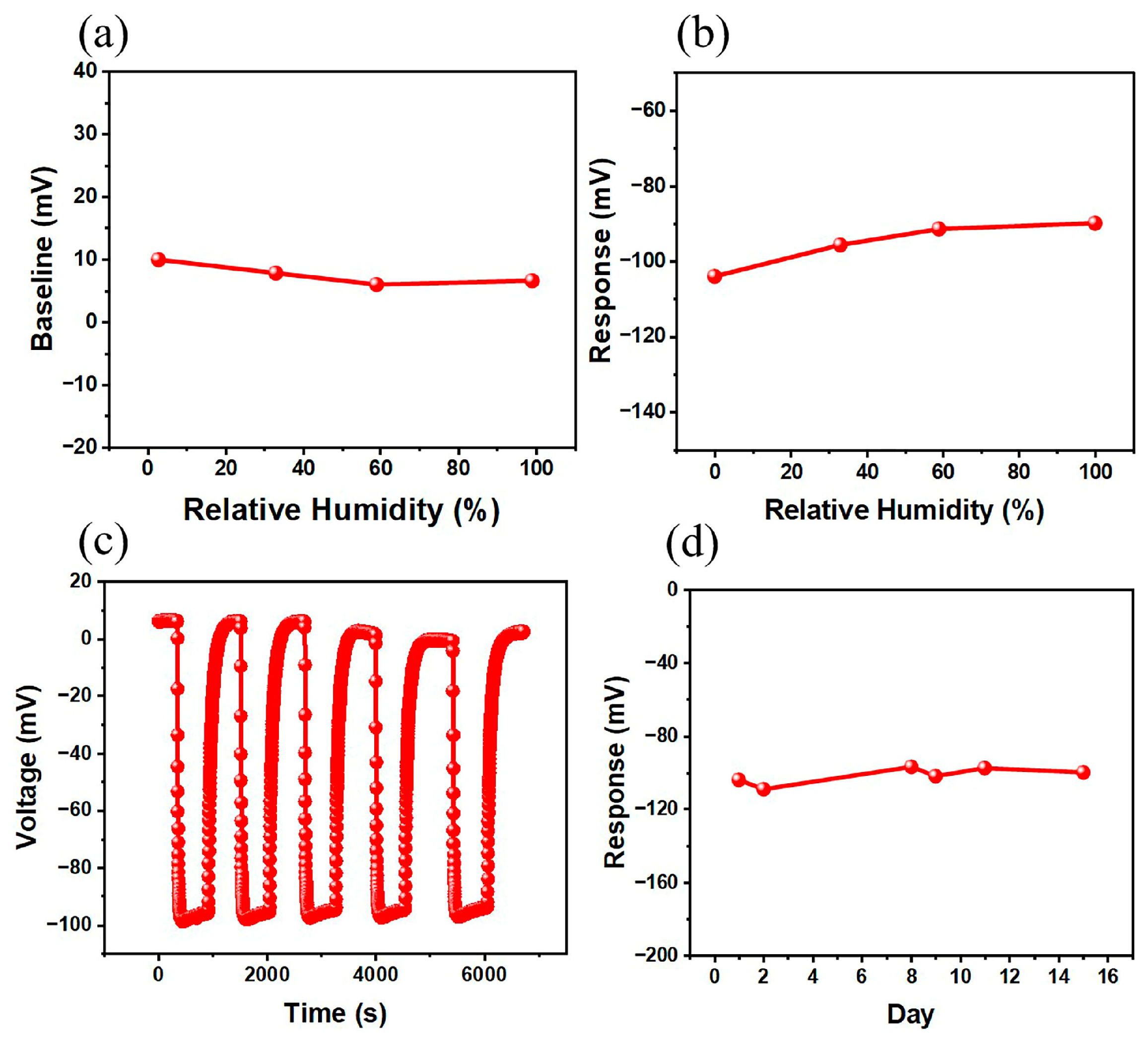
3.2. Sensing Performance
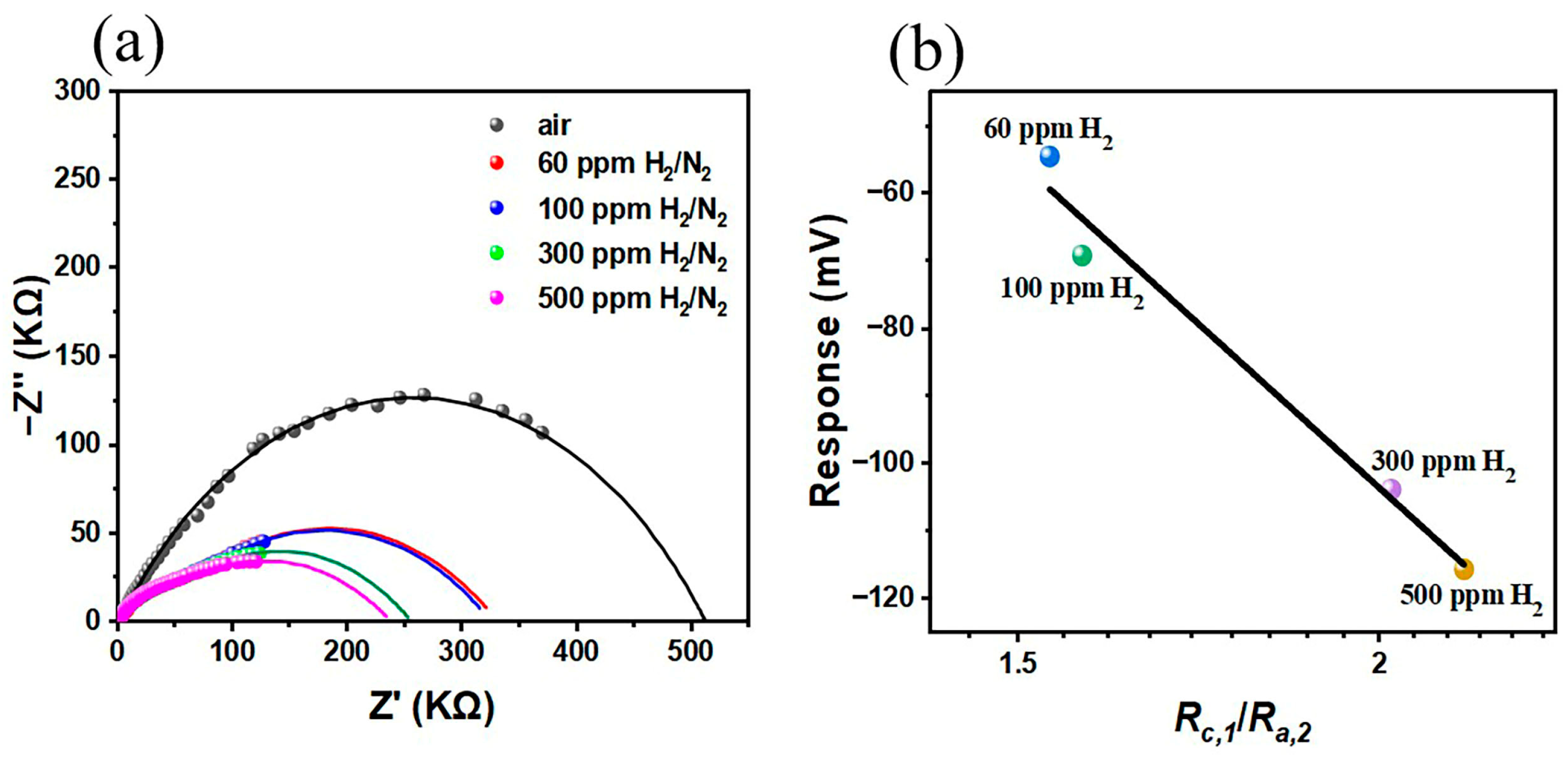
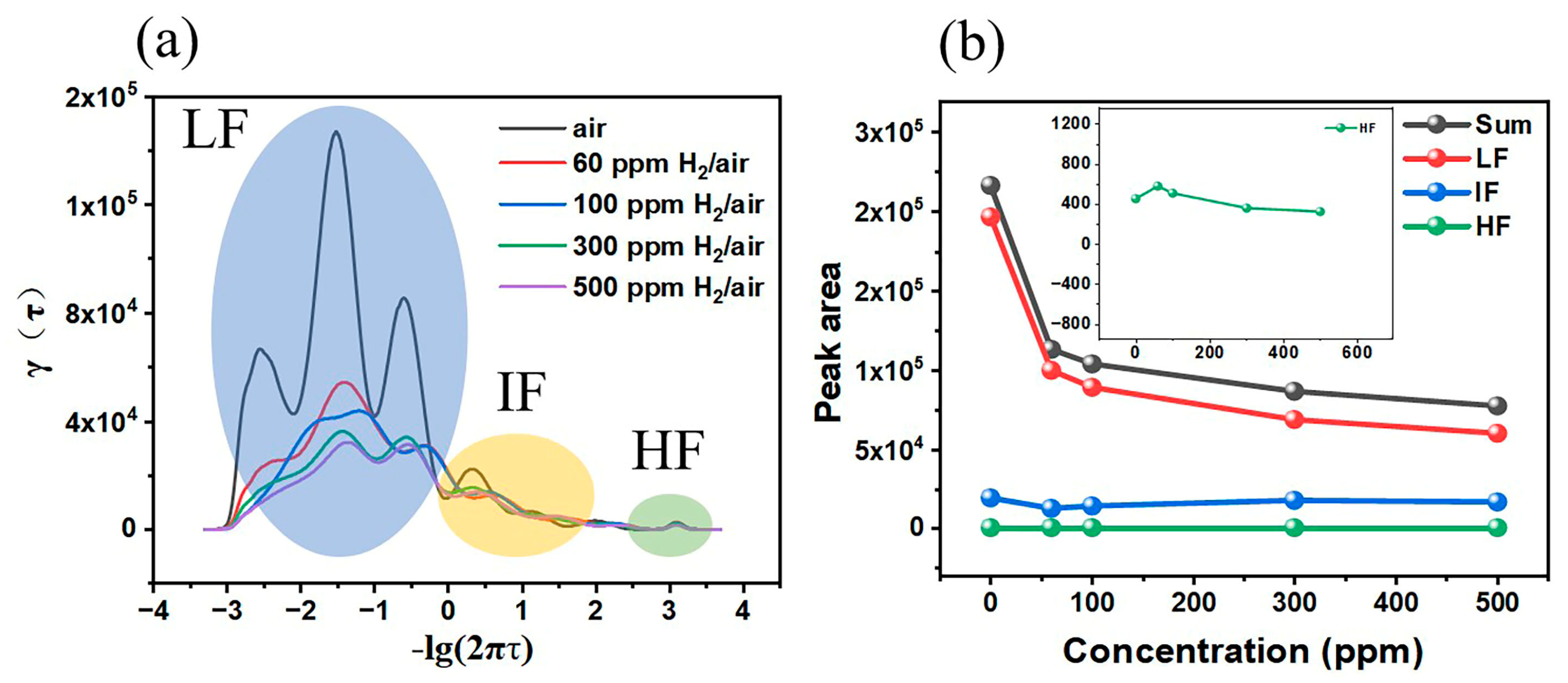
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, K.; Tung, T.T.; Yap, P.L.; Nine, M.J.; Kim, H.C.; Losic, D. Fast response hydrogen gas sensor based on Pd/Cr nanogaps fabricated by a single-step bending deformation. Anal. Chim. Acta 2020, 1138, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrog. Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Pak, Y.; Lim, N.; Kumaresan, Y.; Lee, R.; Kim, K.; Kim, T.H.; Kim, S.-M.; Kim, J.T.; Lee, H.; Ham, M.-H.; et al. Palladium Nanoribbon Array for Fast Hydrogen Gas Sensing with Ultrahigh Sensitivity. Adv. Mater. 2015, 27, 6945–6952. [Google Scholar] [CrossRef] [PubMed]
- Gutmacher, D.; Hoefer, U.; Wöllenstein, J. Gas sensor technologies for fire detection. Sens. Actuators B Chem. 2012, 175, 40–45. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Toribio, J.; Lorenzo, M.; Aguado, L. Innovative Design of Residual Stress and Strain Distributions for Analyzing the Hydrogen Embrittlement Phenomenon in Metallic Materials. Materials 2022, 15, 9063. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Bhattacharya, S. Hydrogen gas sensing methods, materials, and approach to achieve parts per billion level detection: A review. Int. J. Hydrogen Energy 2019, 44, 26076–26099. [Google Scholar] [CrossRef]
- Shen, B.; Luo, J.; Xie, Y.; Zhang, D.; Fan, P.; Zhong, A. Hydrogen gas ppb-level detection based on AlGaN/GaN high electron mobility transistor with 2.0 nm thick Pt gate layer. Appl. Phys. Lett. 2019, 115, 254104. [Google Scholar] [CrossRef]
- Podlepetsky, B.; Samotaev, N.; Kovalenko, A. Responses’ parameters of hydrogen sensors based on MISFET with Pd(Ag)-Ta2O5-SiO2-Si structure. Sens. Actuators B Chem. 2019, 290, 698–705. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Baranov, A.M.; Talipov, V.A.; Mironov, S.M.; Akbari, S.; Kolesnik, I.V.; Orlova, E.D.; Napolskii, K.S. Investigation of catalytic hydrogen sensors with platinum group catalysts. Sens. Actuators B Chem. 2021, 346, 130515. [Google Scholar] [CrossRef]
- Wu, R.-J.; Tian, X.-M.; Hua, Z.-Q.; Lu, N.; Wang, P. A low temperature catalytic combustible gas sensor based on Ru supported zeolite catalyst films. Chin. J. Anal. Chem. 2021, 49, 63–68. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, A.; Bae, H.; Park, C.J.; Song, S.J. Surface decorated spinel-oxide electrodes for mixed-potential ammonia sensor: Performance and DRT analysis. J Hazard Mater. 2020, 396, 122601. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, F.; Yang, X.; Guan, Y.; Ma, C.; Hao, X.; Liang, X.; Liu, F.; Sun, P.; Zhang, T.; et al. Fabrication of Well-Ordered Three-Phase Boundary with Nanostructure Pore Array for Mixed Potential-Type Zirconia-Based NO2 Sensor. ACS Appl. Mater. Interfaces 2016, 8, 16752–16760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yi, J.; Jiang, X. Fast Response, Highly Sensitive and Selective Mixed-Potential H2 Sensor Based on (La, Sr)(Cr, Fe)O3-δ Perovskite Sensing Electrode. ACS Appl. Mater. Interfaces 2017, 9, 17218–17225. [Google Scholar] [CrossRef] [PubMed]
- Anggraini, S.A.; Ikeda, H.; Miura, N. Tuning H2 Sensing Performance of Zirconia-based Sensor using ZrSiO4(+Au) Sensing-electrode. Electrochim. Acta 2015, 171, 7–12. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, J.; Zhang, Z.; Zhang, H. The relation between mixed-potential hydrogen response and electrochemical activities for perovskite oxides. Sens. Actuators B Chem. 2022, 352, 130988. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, J.; Han, H.; Meng, Y.; Zhang, H.; Jiang, Y. Electrochemical Response of Mixed Conducting Perovskite Enables Low-Cost High-Efficiency Hydrogen Sensing. ACS Appl. Mater. Interfaces 2022, 14, 33580–33588. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, L.; Hao, X.; Li, W.; Wang, B.; Wang, T.; Liang, X.; Liu, F.; Wang, C.; Lu, G. Mixed potential type NH3 sensor based on YSZ solid electrolyte and metal oxides (NiO, SnO2, WO3) modified FeVO4 sensing electrodes. Sens. Actuators B Chem. 2021, 343, 130043. [Google Scholar] [CrossRef]
- Lu, G.; Miura, N.; Yamazoe, N. High-temperature hydrogen sensor based on stabilized zirconia and a metal oxide electrode. Sens. Actuators B Chem. 1996, 35, 130–135. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Yuan, L.; Yu, J. A review of high-temperature electrochemical sensors based on stabilized zirconia. Solid State Ion. 2015, 283, 91–102. [Google Scholar] [CrossRef]
- Nicollet, C.; Toparli, C.; Harrington, G.F.; Defferriere, T.; Yildiz, B.; Tuller, H.L. Acidity of surface-infiltrated binary oxides as a sensitive descriptor of oxygen exchange kinetics in mixed conducting oxides. Nat. Catal. 2020, 3, 913–920. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon Nanotube Sensors for Gas and Organic Vapor Detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Balamurugan, S.; Naresh, N.; Prakash, I.; Satyanarayana, N. Capacity fading mechanism of Li2O loaded NiFe2O4/SiO2 aerogel anode for lithium-ion battery: Ex-situ XPS analysis. Appl. Surf. Sci. 2021, 535, 147677. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, W.; Zhang, J.; Lu, Y.; Liu, C.; Adimi, S.; Zhou, J.; Ruan, S.; Chen, Y. Construction of p-n heterojunctions by modifying MOF-derived α-Fe2O3 with partially covered cobalt tungstate for high-performance ethyl acetate detection. Sens. Actuators B Chem. 2021, 344, 130129. [Google Scholar] [CrossRef]
- Yan, W.; Fan, H.; Zhai, Y.; Yang, C.; Ren, P.; Huang, L. Low temperature solution-based synthesis of porous flower-like α-Fe2O3 superstructures and their excellent gas-sensing properties. Sens. Actuators B Chem. 2011, 160, 1372–1379. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Teng, Y.; Yi, J. Simulation of dynamic response of mixed-potential hydrogen sensors. Int. J. Hydrog. Energy 2022, 47, 33891–33902. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, H.; Zhang, Z.; Chen, D. Hierarchical porous hollow SnO2 nanofiber sensing electrode for high performance potentiometric H2 sensor. Sens. Actuators B Chem. 2018, 268, 456–464. [Google Scholar] [CrossRef]
- Yi, J.; Han, H. Analysis of factors affecting response for mixed potential gas sensors. Electrochim. Acta 2021, 379, 138129. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Xiao, G.; Zhao, F.; Chen, F. A Novel Electrode Material for Symmetrical SOFCs. Adv. Mater. 2010, 22, 5478–5482. [Google Scholar] [CrossRef]
- Lu, M.Y.; Scipioni, R.; Park, B.-K.; Yang, T.; Chart, Y.A.; Barnett, S.A. Mechanisms of PrOx performance enhancement of oxygen electrodes for low and intermediate temperature solid oxide fuel cells. Mater. Today Energy 2019, 14, 100362. [Google Scholar] [CrossRef]
- Adler, S.B. Factors Governing Oxygen Reduction in Solid Oxide Fuel Cell Cathodes. Chem. Rev. 2004, 104, 4791–4844. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, P.K.; Brosha, E.L.; Mukundan, R.; Nelson, M.A.; Williamson, T.L.; Garzon, F.H. Development and testing of a miniaturized hydrogen safety sensor prototype. Sens. Actuators B Chem. 2010, 148, 469–477. [Google Scholar] [CrossRef]
- Anggraini, S.A.; Breedon, M.; Miura, N. Sensing characteristics of aged zirconia-based hydrogen sensor utilizing Zn–Ta-based oxide sensing-electrode. Electrochem. Commun. 2013, 31, 133–136. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Yang, J.; Yu, J.; Wang, J.; Tang, Z. Mixed potential hydrogen sensor using ZnWO4 sensing electrode. Sens. Actuators B Chem. 2014, 195, 520–525. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Tang, Z.; Tang, Z.; Yu, J.; Wang, J. Hydrogen sensing of the mixed-potential-type MnWO4/YSZ/Pt sensor. Sens. Actuators B Chem. 2015, 206, 176–180. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Tang, Z.; Wang, J.; Yu, J.; Tang, Z. Potentiometric hydrogen sensors based on yttria-stabilized zirconia electrolyte (YSZ) and CdWO4 interface. Sens. Actuators B Chem. 2016, 223, 365–371. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, J.; Jun, Y.; Tang, Z. A cobalt tungstate compound sensing electrode for hydrogen detection based upon mixed-potential type sensors. RSC Adv. 2017, 7, 2919–2925. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-Performance Nanostructured Palladium-Based Hydrogen Sensors—Current Limitations and Strategies for Their Mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yi, J. Enhancing the Potentiometric H2 Sensing of Pr0.1Ce0.9O2−δ Using Fe2O3 Surface Modification. Chemosensors 2023, 11, 250. https://doi.org/10.3390/chemosensors11040250
Wang L, Yi J. Enhancing the Potentiometric H2 Sensing of Pr0.1Ce0.9O2−δ Using Fe2O3 Surface Modification. Chemosensors. 2023; 11(4):250. https://doi.org/10.3390/chemosensors11040250
Chicago/Turabian StyleWang, Liang, and Jianxin Yi. 2023. "Enhancing the Potentiometric H2 Sensing of Pr0.1Ce0.9O2−δ Using Fe2O3 Surface Modification" Chemosensors 11, no. 4: 250. https://doi.org/10.3390/chemosensors11040250
APA StyleWang, L., & Yi, J. (2023). Enhancing the Potentiometric H2 Sensing of Pr0.1Ce0.9O2−δ Using Fe2O3 Surface Modification. Chemosensors, 11(4), 250. https://doi.org/10.3390/chemosensors11040250





