Monitoring of Osmotic Swelling Induced Filling Degree Changes in WOW Double Emulsions Using Raman Technologies
Abstract
1. Introduction
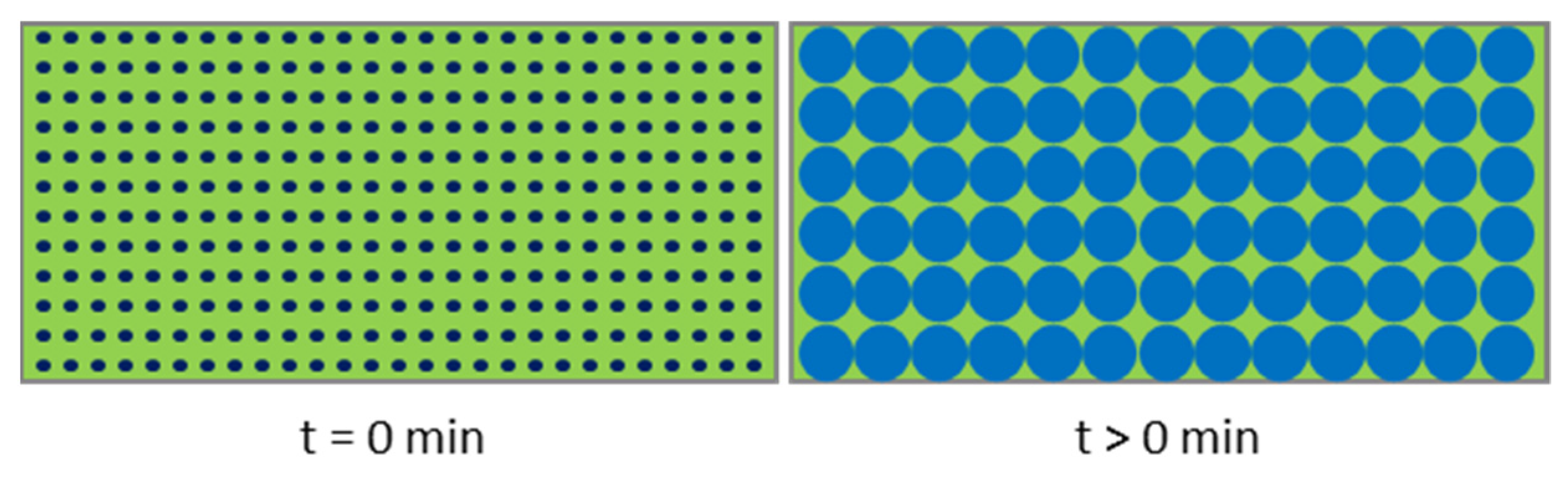
- The lower the osmolality of the inner water phase, the lower the relative filling degree change.
- With increasing osmolality of the outer water phase, the relative filling degree change is also increasing.
2. Materials and Methods
2.1. Emulsion System and Material Properties of the Phases
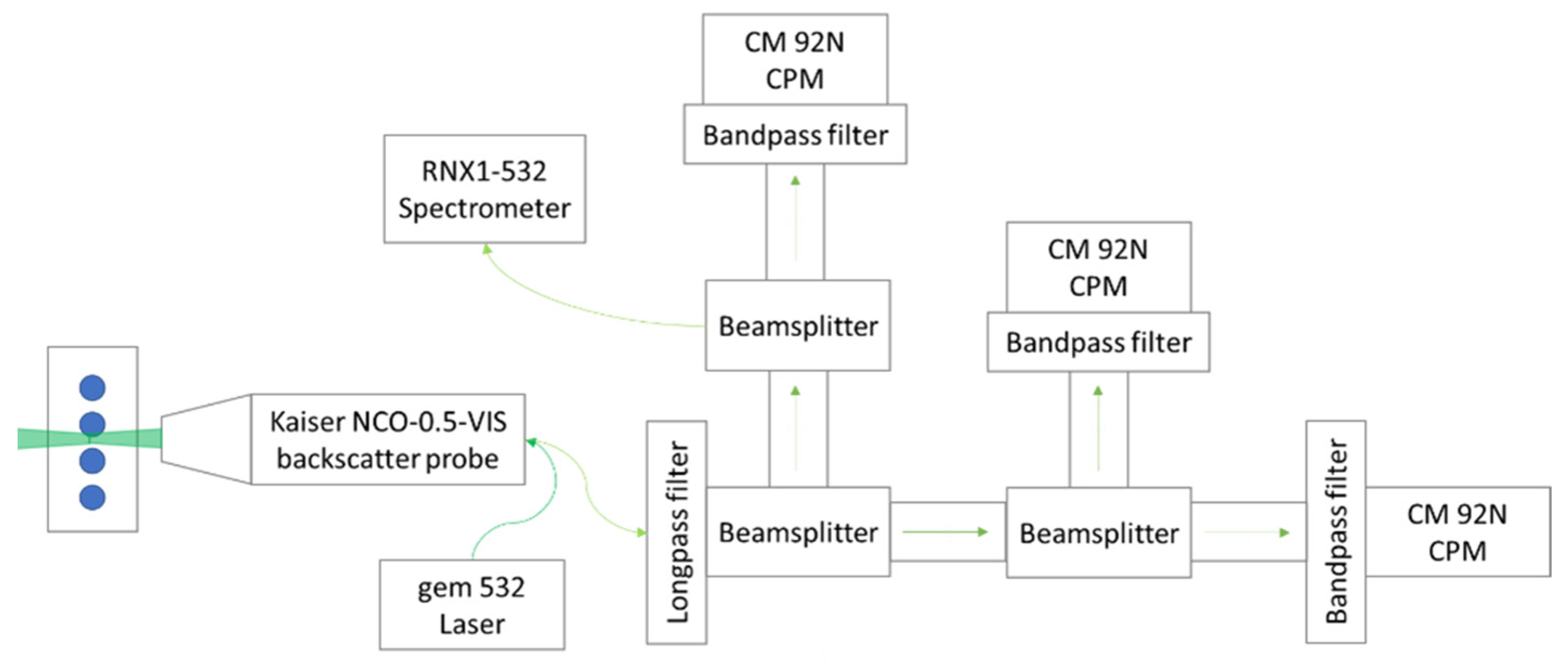
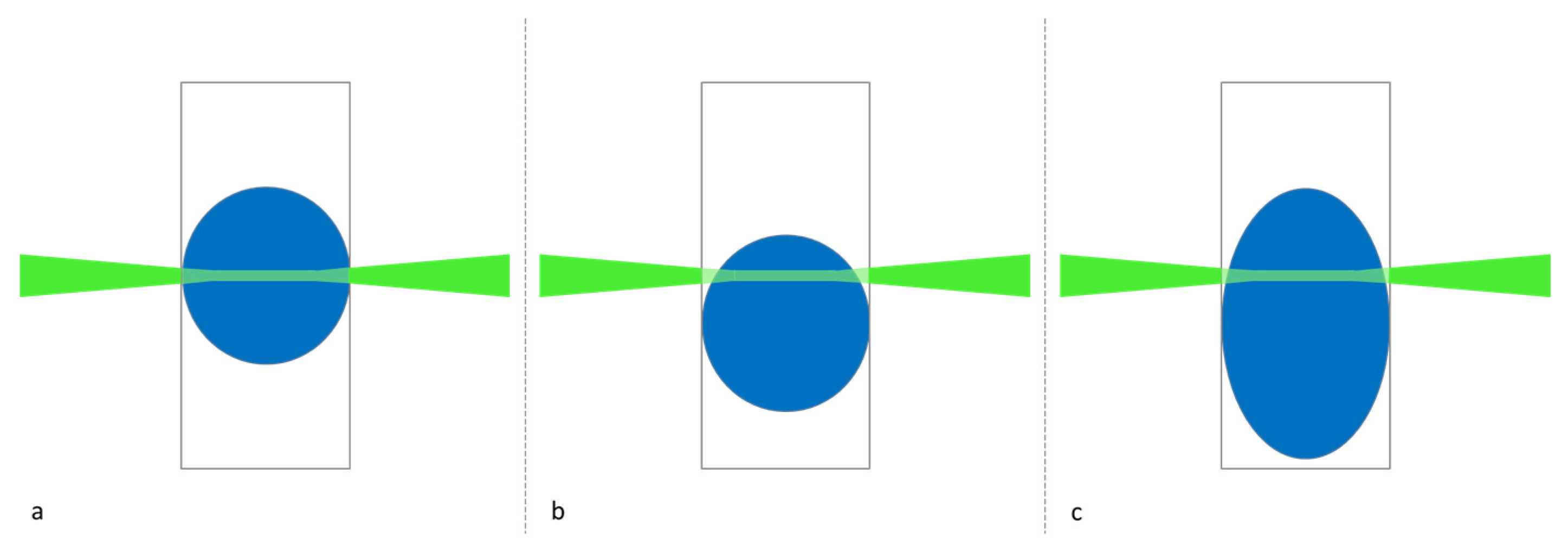
2.2. Optical Setup
2.3. Production of the Double Emulsion
2.4. Experimental Procedure
2.5. Data Analysis
3. Results
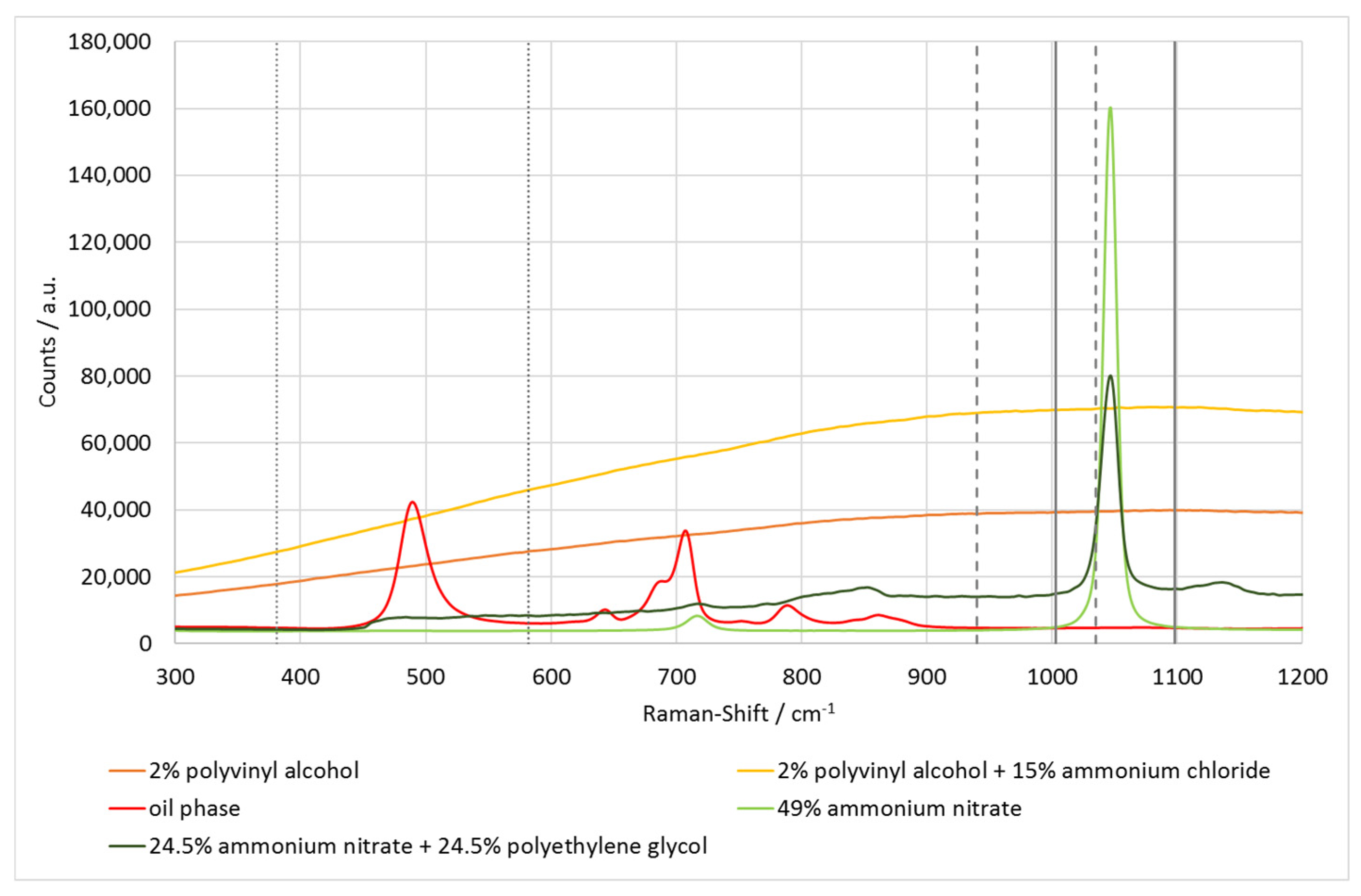
3.1. Raman Spectra of the Emulsion Phases
3.2. Osmolalities of the Aqueous Phases
3.3. Droplet Size Distribution of Inner Emulsions
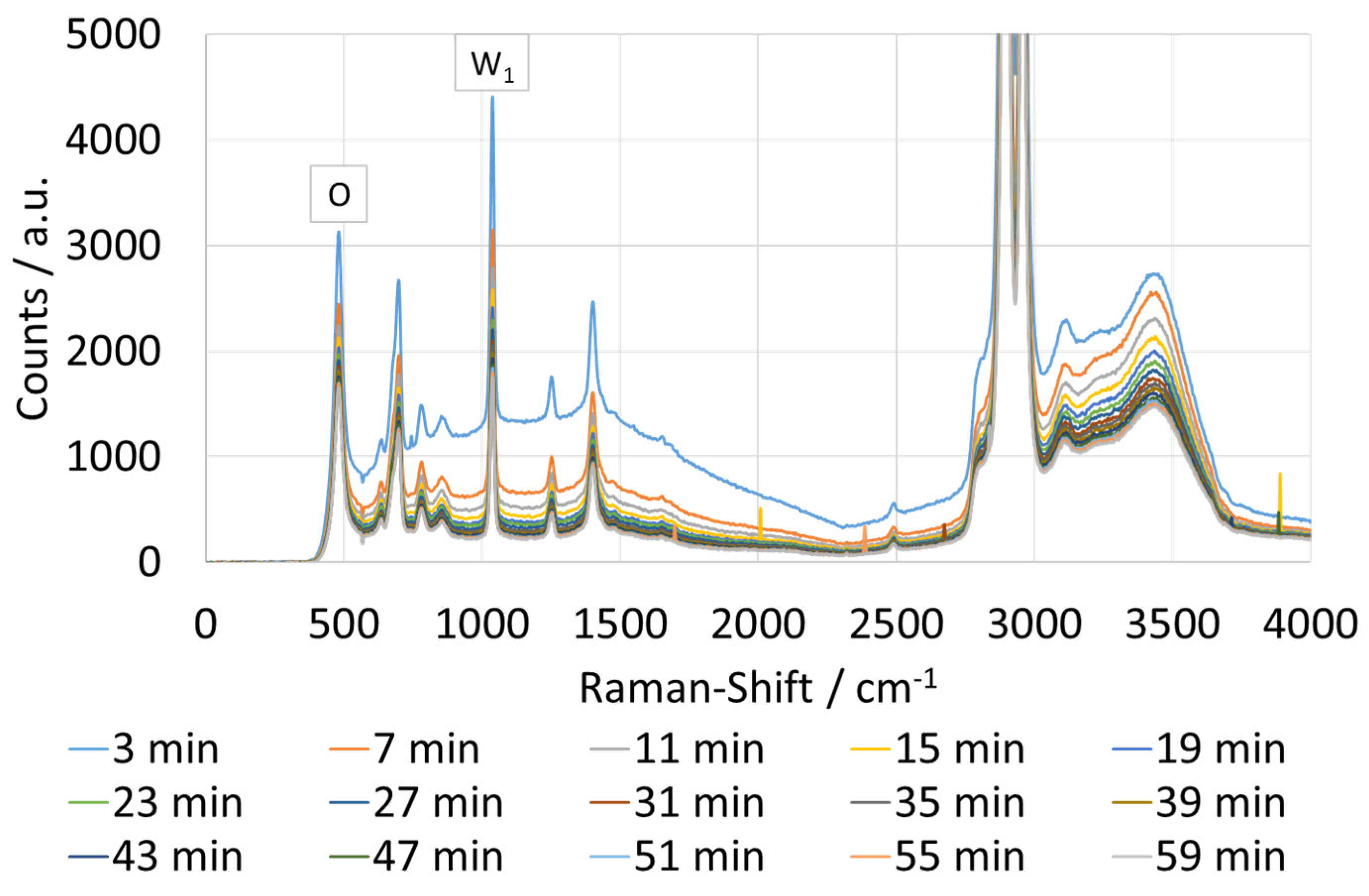
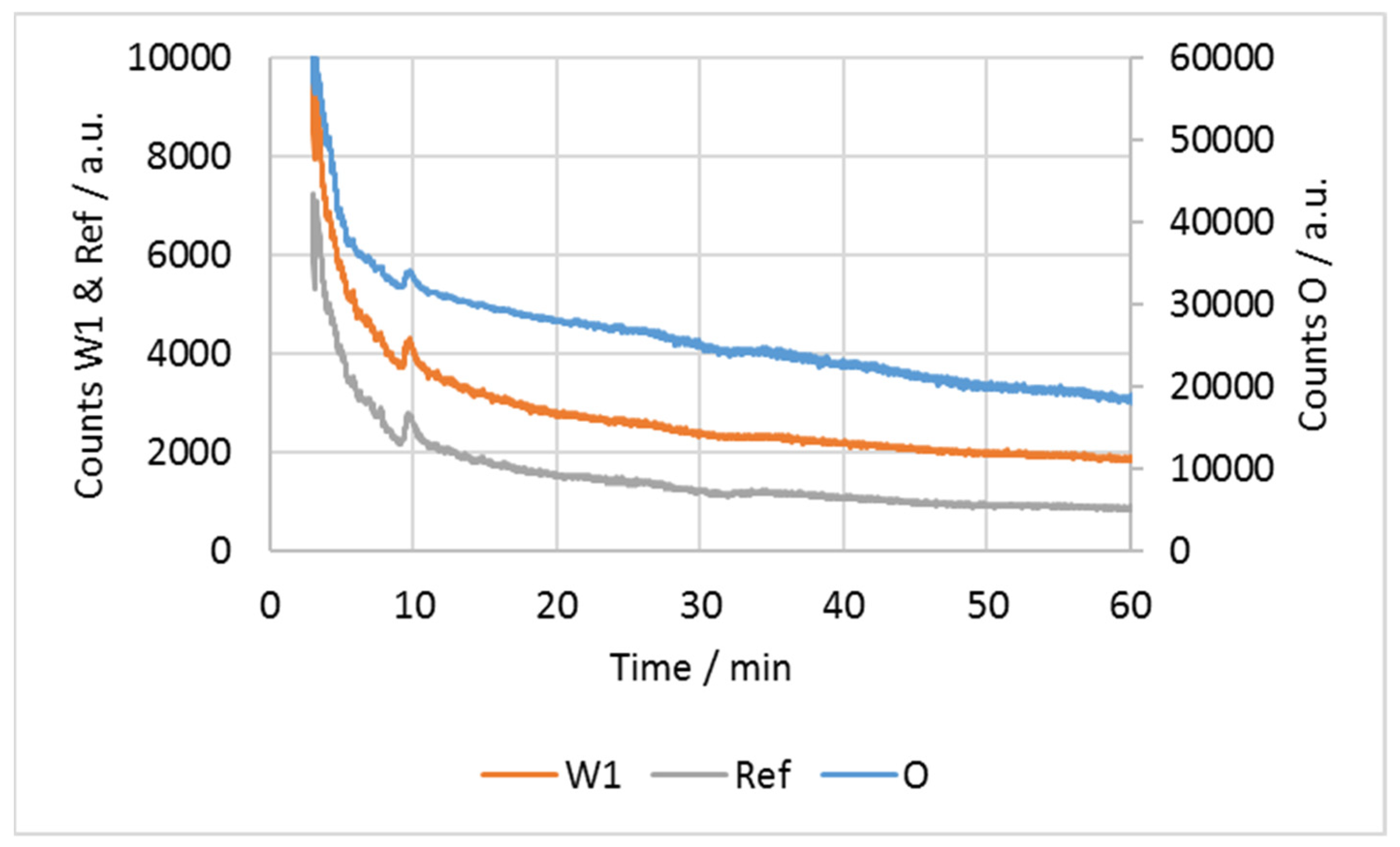
3.4. Raman Spectra and Photometric Data of One Measurement
3.5. Measured Relative Filling Degree Changes
3.6. Changes in Filling Degree of Double Emulsion
4. Discussion
4.1. Interactions of Ammonium Chloride and Polyvinyl Alcohol
4.2. Correlation between Decreasing Signal and Increasing Filling Degree
4.3. Difference between Spectrometric and Photometric Filling Degree Changes
4.4. Decreasing Fluorescence during Measurement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Filter | Center Wavelength | FWHM* | Supplier |
|---|---|---|---|
| Long pass filter | 542 nm ** | - | AHF *** |
| Silicone oil | 546 nm | 12.9 nm | AHF *** |
| Reference | 561 nm | 2 | Laser 2000 **** |
| Ammonium nitrate | 564 nm | 2 | Laser 2000 **** |
References
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.; Tonay, A.N.; Köhler, K.; Schuchmann, H.P. Influence of the second emulsification step during production of W/O/W multiple emulsions: Comparison of different methods to determine encapsulation efficiency in W/O/W emulsions. Can. J. Chem. Eng. 2014, 92, 203–209. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Rojas, O.J.; McClements, D.J. Recent Innovations in Emulsion Science and Technology for Food Applications. J. Agric. Food Chem. 2021, 69, 8944–8963. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H.P. Evaluating the Stability of Double Emulsions—A Review of the Measurement Techniques for the Systematic Investigation of Instability Mechanisms. Colloids Interfaces 2020, 4, 8. [Google Scholar] [CrossRef]
- Rosano, H.; Gandolfo, F.G.; Hidrot, J.-D.P. Stability of W1/O/W2 multiple emulsions influence of ripening and interfacial interactions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 138, 109–121. [Google Scholar] [CrossRef]
- Sela, Y.; Magdassi, S.; Garti, N. Release of markers from the inner water phase of W/O/W emulsions stabilized by silicone based polymeric surfactants. J. Control. Release 1995, 33, 1–12. [Google Scholar] [CrossRef]
- Eisinaite, V.; Duque Estrada, P.; Schroën, K.; Berton-Carabin, C.; Leskauskaite, D. Tayloring W/O/W emulsion composition for effective encapsulation: The role of PGPR in water transfer-induced swelling. Food Res. Int. 2018, 106, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Khadem, B.; Khellaf, M.; Sheibat-Othman, N. Investigating swelling-breakdown in double emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124181. [Google Scholar] [CrossRef]
- Khadem, B.; Sheibat-Othman, N. Modeling droplets swelling and escape in double emulsions using population balance equations. Chem. Eng. J. 2020, 382, 122824. [Google Scholar] [CrossRef]
- Garti, N.; Bisperink, C. Double emulsions: Progress and applications. Curr. Opin. Colloid Interface Sci. 1998, 3, 657–667. [Google Scholar] [CrossRef]
- Neumann, S.M.; van der Schaaf, U.S.; Karbstein, H.P. Investigations on the relationship between interfacial and single droplet experiments to describe instability mechanisms in double emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 464–471. [Google Scholar] [CrossRef]
- Leister, N.; Yan, C.; Karbstein, H.P. Oil Droplet Coalescence in W/O/W Double Emulsions Examined in Models from Micrometer- to Millimeter-Sized Droplets. Colloids Interfaces 2022, 6, 12. [Google Scholar] [CrossRef]
- Lamba, H.; Sathish, K.; Sabikhi, L. Double Emulsions: Emerging Delivery System for Plant Bioactives. Food Bioprocess. Technol. 2015, 8, 709–728. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Saffarionpour, S.; Diosady, L.L. Multiple Emulsions for Enhanced Delivery of Vitamins and Iron Micronutrients and Their Application for Food Fortification. Food Bioprocess. Technol. 2021, 14, 587–625. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Double Emulsions Stabilized by Food Biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H.P. Influence of Hydrophilic Surfactants on the W1–W2 Coalescence in Double Emulsion Systems Investigated by Single Droplet Experiments. Colloids Interfaces 2021, 5, 21. [Google Scholar] [CrossRef]
- Kanouni, M.; Rosano, H.; Naouli, N. Preparation of a stable double emulsion (W1/O/W2): Role of the interfacial films on the stability of the system. Adv. Colloid Interface Sci. 2002, 99, 229–254. [Google Scholar] [CrossRef]
- Yafei, W.; Tao, Z.; Gang, H. Structural evolution of polymer-stabilized double emulsions. Langmuir 2006, 22, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nazir, H.; Zhang, W.; Liu, Y.; Chen, X.; Wang, L.; Naseer, M.M.; Ma, G. Silicone oil emulsions: Strategies to improve their stability and applications in hair care products. Int. J. Cosmet. Sci. 2014, 36, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Garti, N. Progress in Stabilization and Transport Phenomena of Double Emulsions in Food Applications. LWT—Food Sci. Technol. 1997, 30, 222–235. [Google Scholar] [CrossRef]
- Schuch, A.; Wrenger, J.; Schuchmann, H.P. Production of W/O/W double emulsions. Part II: Influence of emulsification device on release of water by coalescence. Colloids Surf. A Physicochem. Eng. Asp. 2014, 461, 344–351. [Google Scholar] [CrossRef]
- Regan, J.O.; Mulvihill, D.M. Water soluble inner aqueous phase markers as indicators of the encapsulation properties of water-in-oil-in-water emulsions stabilized with sodium caseinate. Food Hydrocoll. 2009, 23, 2339–2345. [Google Scholar] [CrossRef]
- Hai, M.; Magdassi, S. Investigation on the release of fluorescent markers from w/o/w emulsions by fluorescence-activated cell sorter. J. Control. Release 2004, 96, 393–402. [Google Scholar] [CrossRef]
- Schuster, S.; Bernewitz, R.; Guthausen, G.; Zapp, J.; Greiner, A.M.; Köhler, K.; Schuchmann, H.P. Analysis of W1/O/W2 double emulsions with CLSM: Statistical image processing for droplet size distribution. Chem. Eng. Sci. 2012, 81, 84–90. [Google Scholar] [CrossRef]
- Guan, X.; Hailu, K.; Guthausen, G.; Wolf, F.; Bernewitz, R. Schuchmann HPPFG-NMR on W 1 /O/W 2 -emulsions: Evidence for molecular exchange between water phases. Eur. J. Lipid Sci. Technol. 2010, 112, 828–837. [Google Scholar] [CrossRef]
- Van Duynhoven, J.P.M.; Goudappel, G.J.W.; van Dalen, G.; van Bruggen, P.C.; Blonk, J.C.G.; Eijkelenboom, A.P.A.M. Scope of droplet size measurements in food emulsions by pulsed field gradient NMR at low field. Magn. Reson. Chem. 2002, 40, S51–S59. [Google Scholar] [CrossRef]
- Bernewitz, R. Charakterisierung von Doppelemulsionen Mittels NMR und CLSM-Struktur und Diffusion; Verlag Dr. Hut: Munich, Germany, 2013. [Google Scholar]
- Schuch, A.; Köhler, K.; Schuchmann, H.P. Differential scanning calorimetry (DSC) in multiple W/O/W emulsions. J. Anal. Calorim. 2013, 111, 1881–1890. [Google Scholar] [CrossRef]
- Neumann, S.M.; van der Schaaf, U.S.; Karbstein, H.P. Structure stability and crystallization behavior of water in oil in water (WOW) double emulsions during their characterization by differential scanning calorimetry (DSC). J. Therm. Anal. Calorim. 2018, 133, 1499–1508. [Google Scholar] [CrossRef]
- Goldrick, S.; Lovett, D.; Montague, G.; Lennox, B. Influence of Incident Wavelength and Detector Material Selection on Fluorescence in the Application of Raman Spectroscopy to a Fungal Fermentation Process. Bioengineering 2018, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Saletnik, A.; Saletnik, B.; Puchalski, C. Overview of Popular Techniques of Raman Spectroscopy and Their Potential in the Study of Plant Tissues. Molecules 2021, 26, 1537. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman Spectroscopic Methods in Food Safety: A Review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Meyer, T.; Akimov, D.; Tarcea, N.; Chatzipapadopoulos, S.; Muschiolik, G.; Kobow, J.; Schmitt, M.; Popp, J. Three-dimensional molecular mapping of a multiple emulsion by means of CARS microscopy. J. Phys. Chem. B 2008, 112, 1420–1426. [Google Scholar] [CrossRef]
- Hufnagel, T.; Rädle, M.; Karbstein, H.P. Influence of Refractive Index Differences on the Signal Strength for Raman-Spectroscopic Measurements of Double Emulsion Droplets. Appl. Sci. 2022, 12, 9056. [Google Scholar] [CrossRef]
- Hufnagel, T.; Stoy, R.; Rädle, M.; Karbstein, H.P. Measurement of the Filling Degree and Droplet Size of Individual Double Emulsion Droplets Using Raman Technologies. Chemosensors 2022, 10, 463. [Google Scholar] [CrossRef]
- Bandulasena, M.V.; Vladisavljević, G.T.; Benyahia, B. Versatile reconfigurable glass capillary microfluidic devices with Lego® inspired blocks for drop generation and micromixing. J. Colloid Interface Sci. 2019, 542, 23–32. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Guo, S.; Bocklitz, T.; Popp, J. Optimization of Raman-spectrum baseline correction in biological application. Analyst 2016, 141, 2396–2404. [Google Scholar] [CrossRef]
- Hufnagel, T.; Leister, N.; Stoy, R.; Rädle, M.; Karbstein, H.P. Research Data to “Monitoring of Osmotic Swelling Induced Filling Degree Changes in WOW Double Emulsions Using Raman Technologies”; Karlsruher Institut für Technologie (KIT): Karlsruhe, Germany, 2023. [Google Scholar]
- Kuchenbecker, P.; Gemeinert, M.; Rabe, T. Inter-laboratory Study of Particle Size Distribution Measurements by Laser Diffraction. Part. Part. Syst. Charact. 2012, 29, 304–310. [Google Scholar] [CrossRef]
- Kurary Poval™. Kuraray Poval™—Basic Physical Properties of PVOH Resin; Kurary Poval™: Tokyo, Japan, 2022. [Google Scholar]




| Phase | Composition | Osmolality [mOsm/kg] |
|---|---|---|
| W1 | 49% ammonium nitrate | 10,255 * |
| 24.5% ammonium nitrate + 24.5% polyethyleneglycol | 5788 * | |
| W2 | 2% poly vinylalcohol 26—88 | 6 ** |
| 2% poly vinylalcohol 26—88 + 5% ammonium chloride | 1877 ** | |
| 2% poly vinylalcohol 26—88 + 10% ammonium chloride | 3717 * | |
| 2% poly vinylalcohol 26—88 + 15% ammonium chloride | 5581 * |
| W1 | W2 | Osmolality Difference W1 – W2/ mOsm/kg | Relative Filling Degree Change/% | |
|---|---|---|---|---|
| Spectrometer | Photometer | |||
| 49% AN | 0% ACL | 10,249 | 56.5 | 89.8 |
| 5% ACL | 8378 | 50.9 | 84.3 | |
| 10% ACL | 6538 | 43.8 | 73.3 | |
| 15% ACL | 4674 | 39.5 | 71.7 | |
| 24.5% AN + 24.5% PEG | 0% ACL | 5782 | 48.8 | 76.2 |
| 5% ACL | 3911 | 39.9 | 61.8 | |
| 10% ACL | 2071 | 25.7 | 47.4 | |
| 15% ACL | 207 | 32.9 | 48.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hufnagel, T.; Leister, N.; Stoy, R.; Rädle, M.; Karbstein, H.P. Monitoring of Osmotic Swelling Induced Filling Degree Changes in WOW Double Emulsions Using Raman Technologies. Chemosensors 2023, 11, 206. https://doi.org/10.3390/chemosensors11040206
Hufnagel T, Leister N, Stoy R, Rädle M, Karbstein HP. Monitoring of Osmotic Swelling Induced Filling Degree Changes in WOW Double Emulsions Using Raman Technologies. Chemosensors. 2023; 11(4):206. https://doi.org/10.3390/chemosensors11040206
Chicago/Turabian StyleHufnagel, Thomas, Nico Leister, Richard Stoy, Matthias Rädle, and Heike P. Karbstein. 2023. "Monitoring of Osmotic Swelling Induced Filling Degree Changes in WOW Double Emulsions Using Raman Technologies" Chemosensors 11, no. 4: 206. https://doi.org/10.3390/chemosensors11040206
APA StyleHufnagel, T., Leister, N., Stoy, R., Rädle, M., & Karbstein, H. P. (2023). Monitoring of Osmotic Swelling Induced Filling Degree Changes in WOW Double Emulsions Using Raman Technologies. Chemosensors, 11(4), 206. https://doi.org/10.3390/chemosensors11040206








