A Dinitrophenol-Based Colorimetric Chemosensor for Sequential Cu2+ and S2− Detection
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Instrumentations
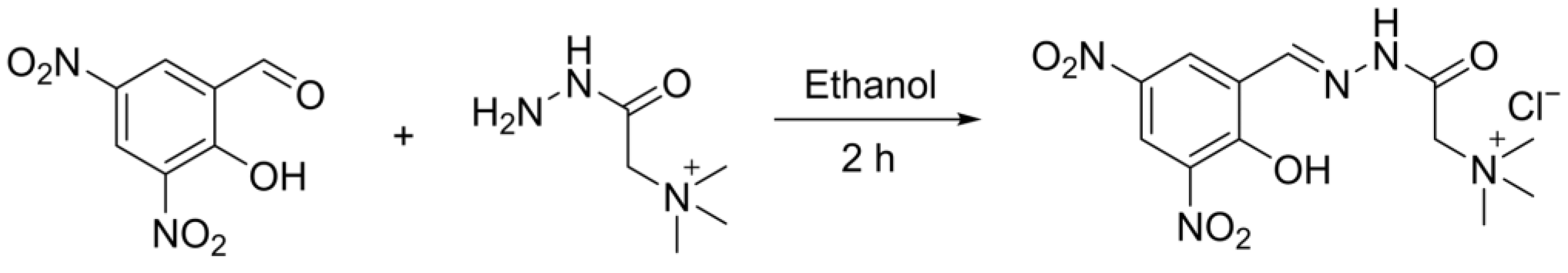
2.2. Synthesis of Chemosensor HDHT ((E)-2-(2-(2-Hydroxy-3,5-dinitrobenzylidene)hydrazineyl)-N,N,N-trimethyl-2-oxoethan-1-aminium) Chloride)
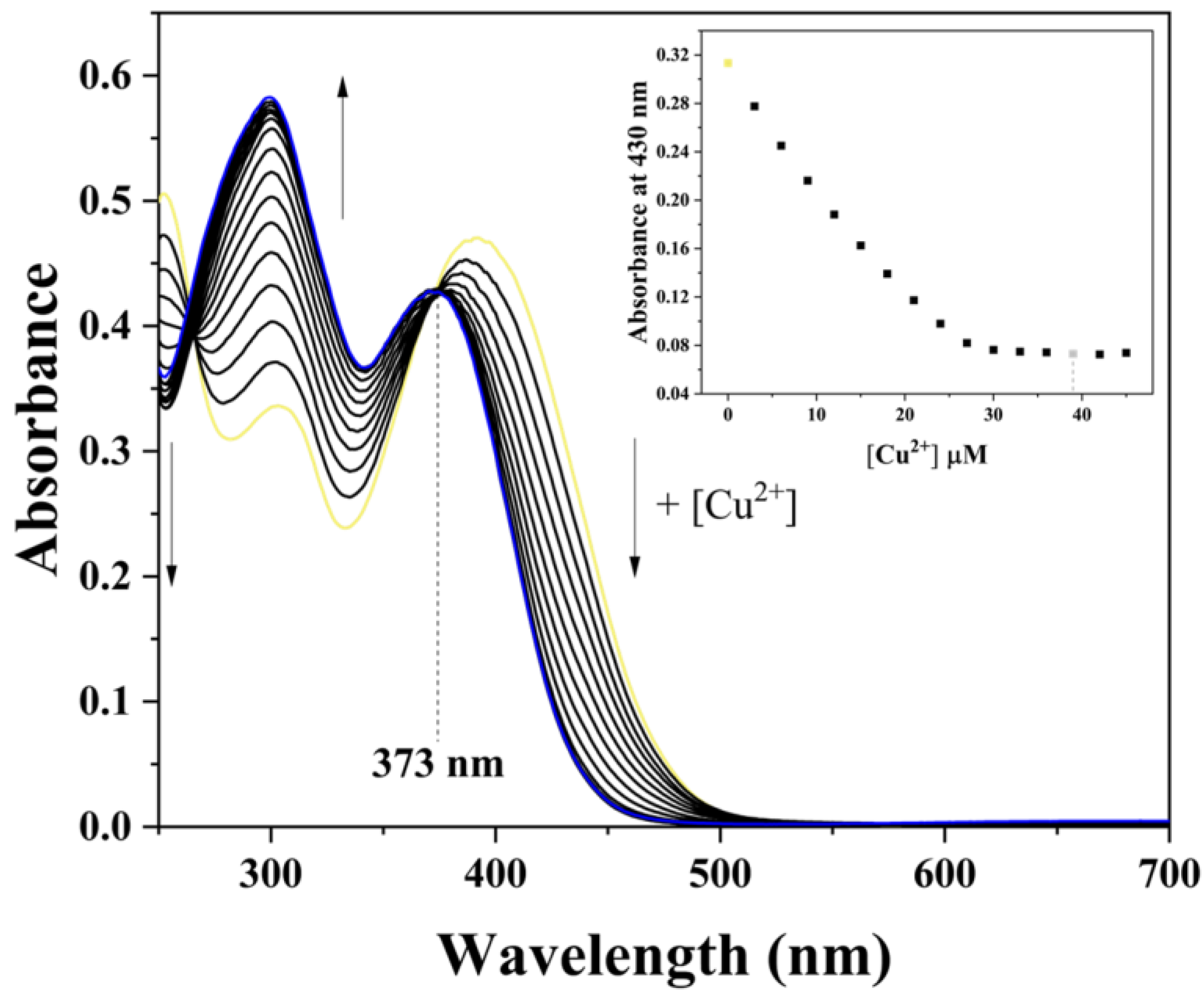
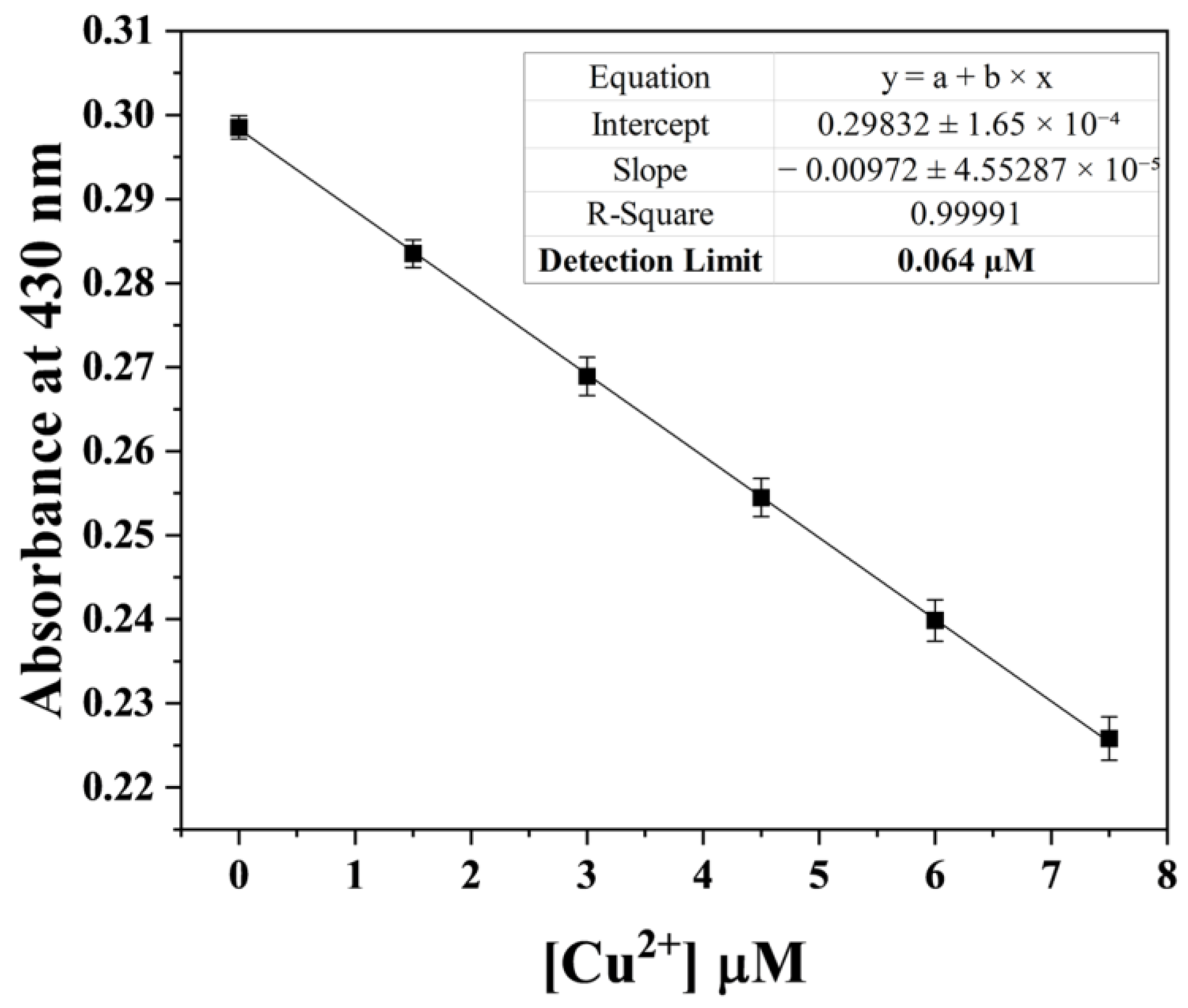
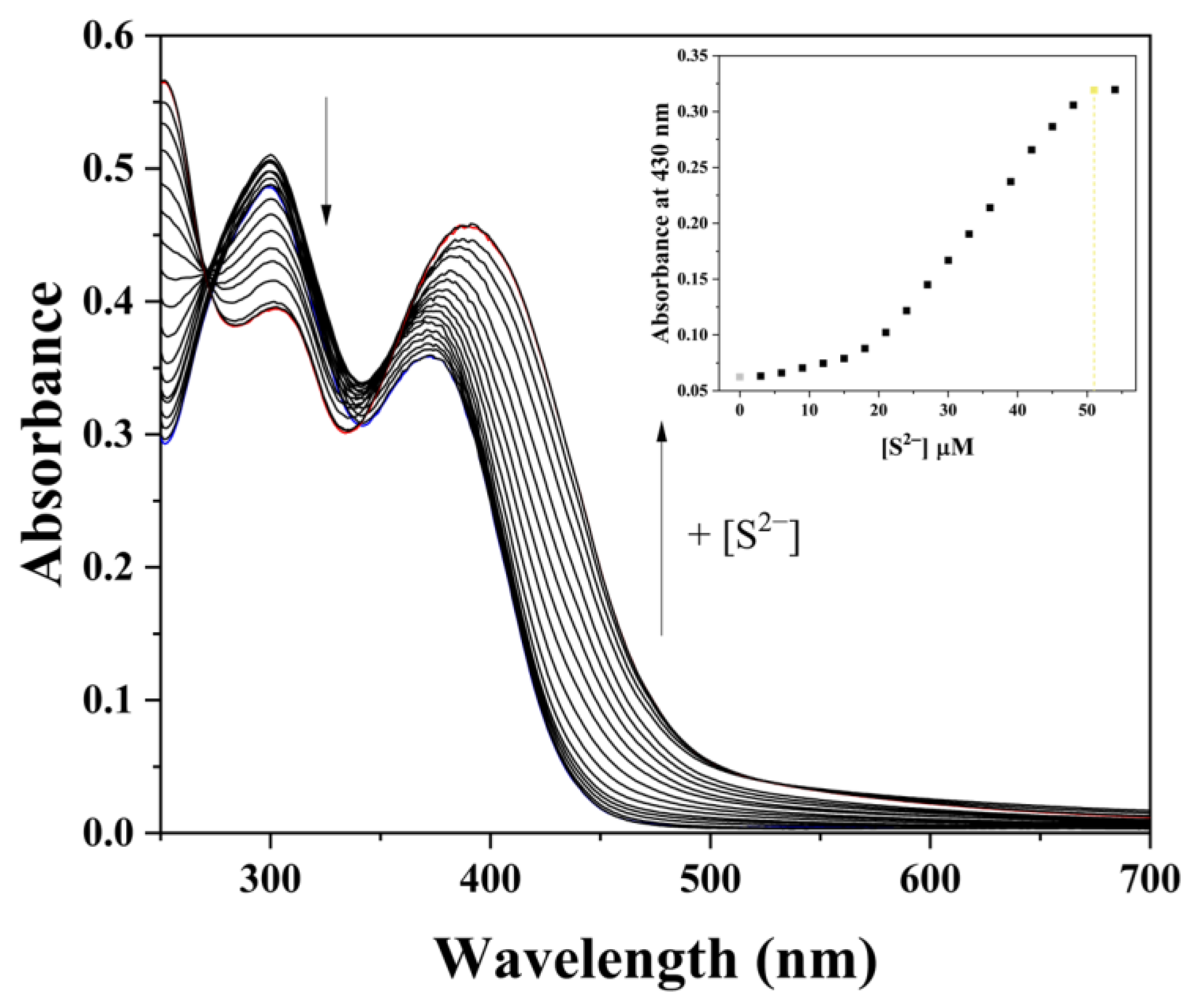
2.3. UV–Vis Titration
2.4. Job-Plot Analysis
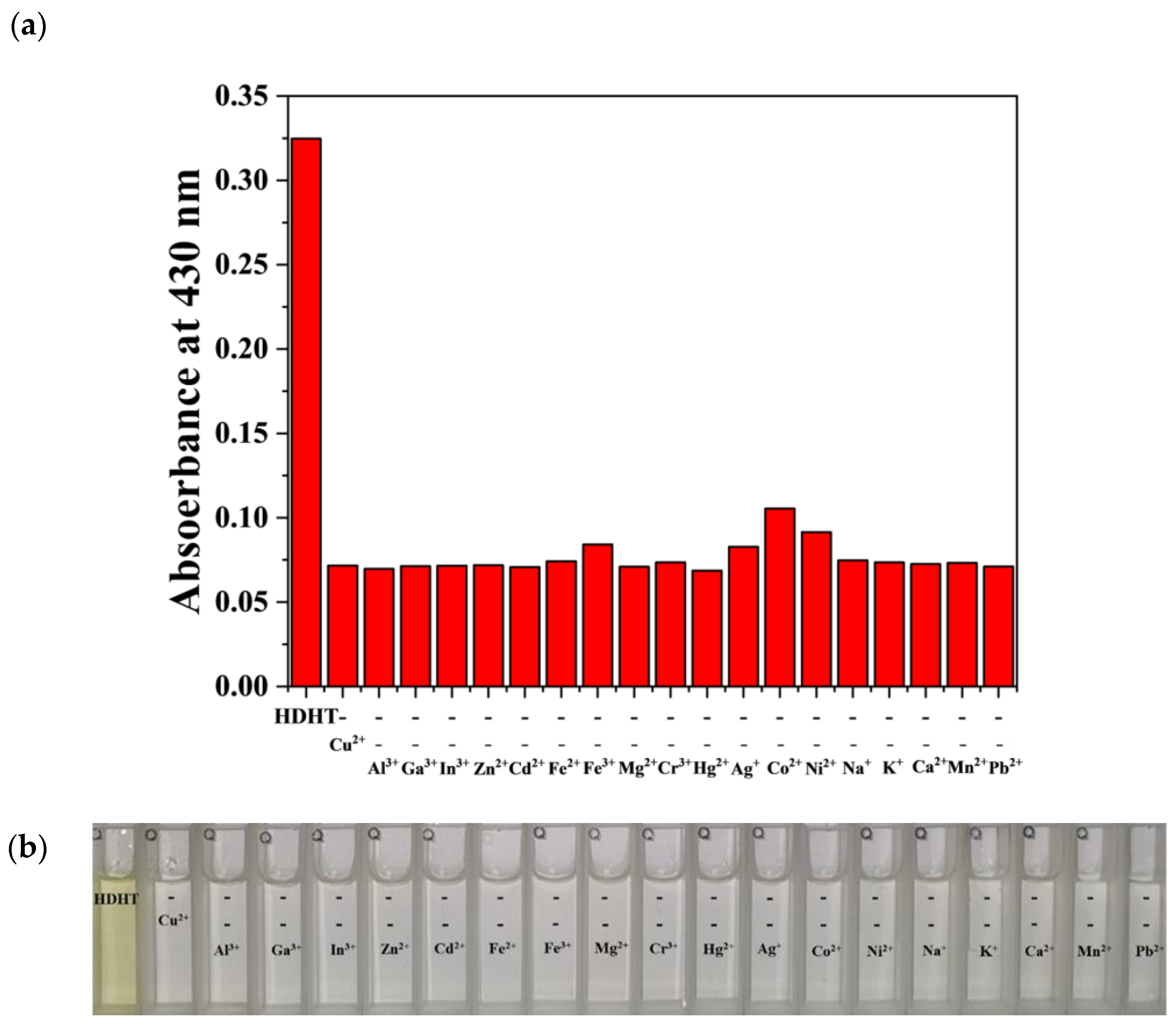
2.5. UV–Vis Inhibition Tests
2.6. pH Test
2.7. Real-Water-Sample Detection
2.8. Theoretical Calculations
3. Results and Discussion
3.1. Synthesis and Structural Characteristics of HDHT
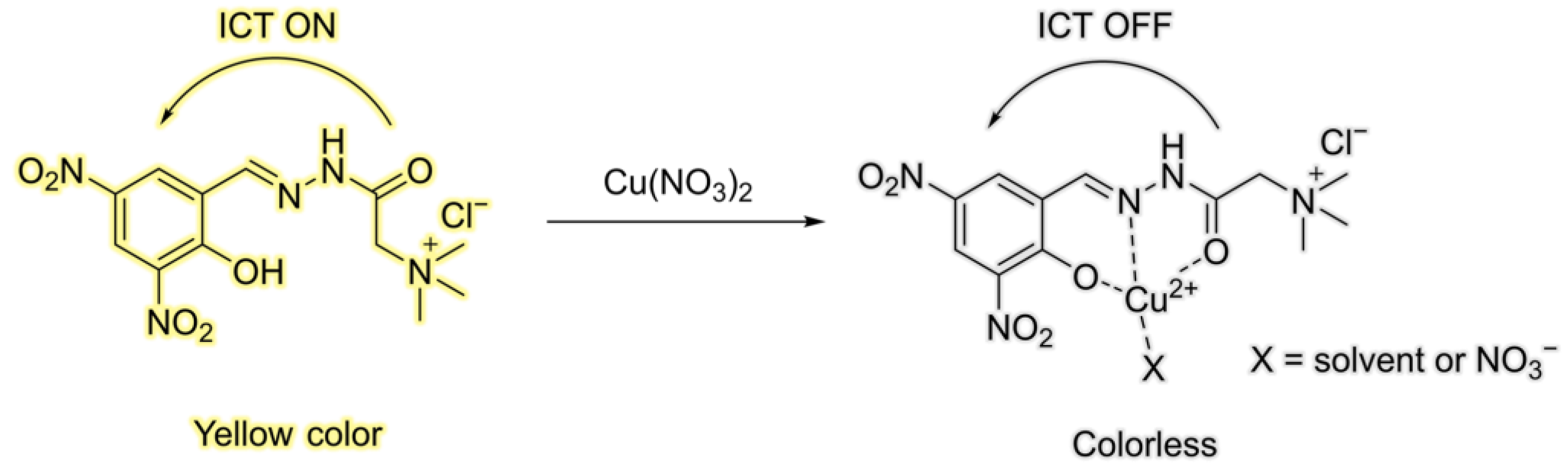
3.2. Application of HDHT with Cu2+
3.3. Theoretical Study
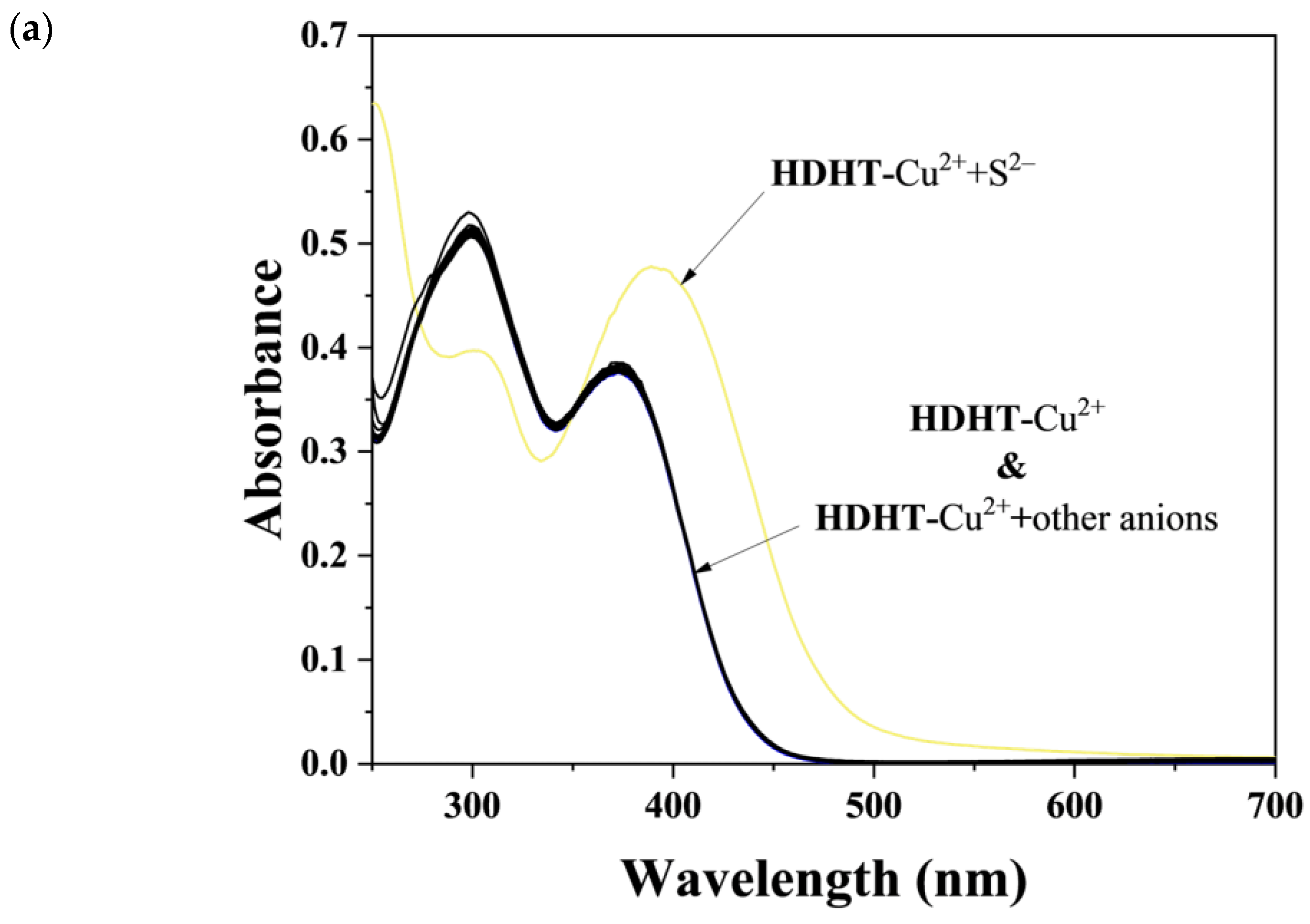
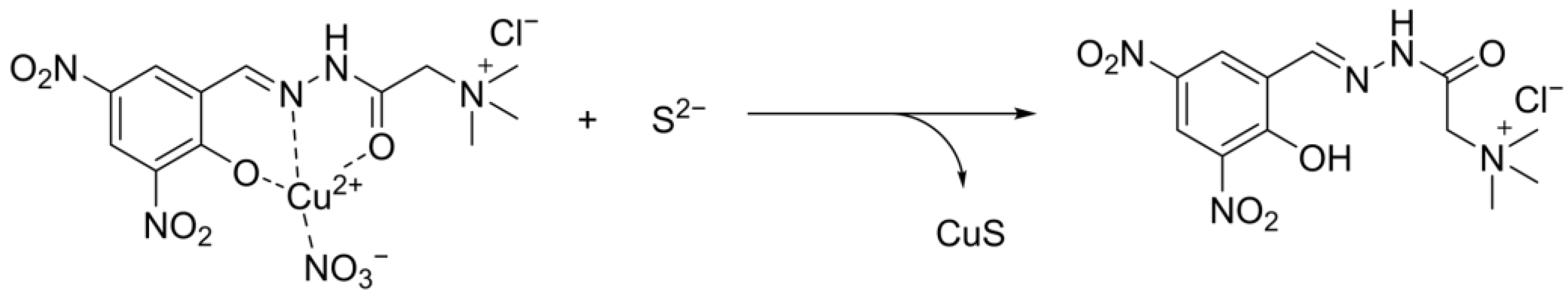
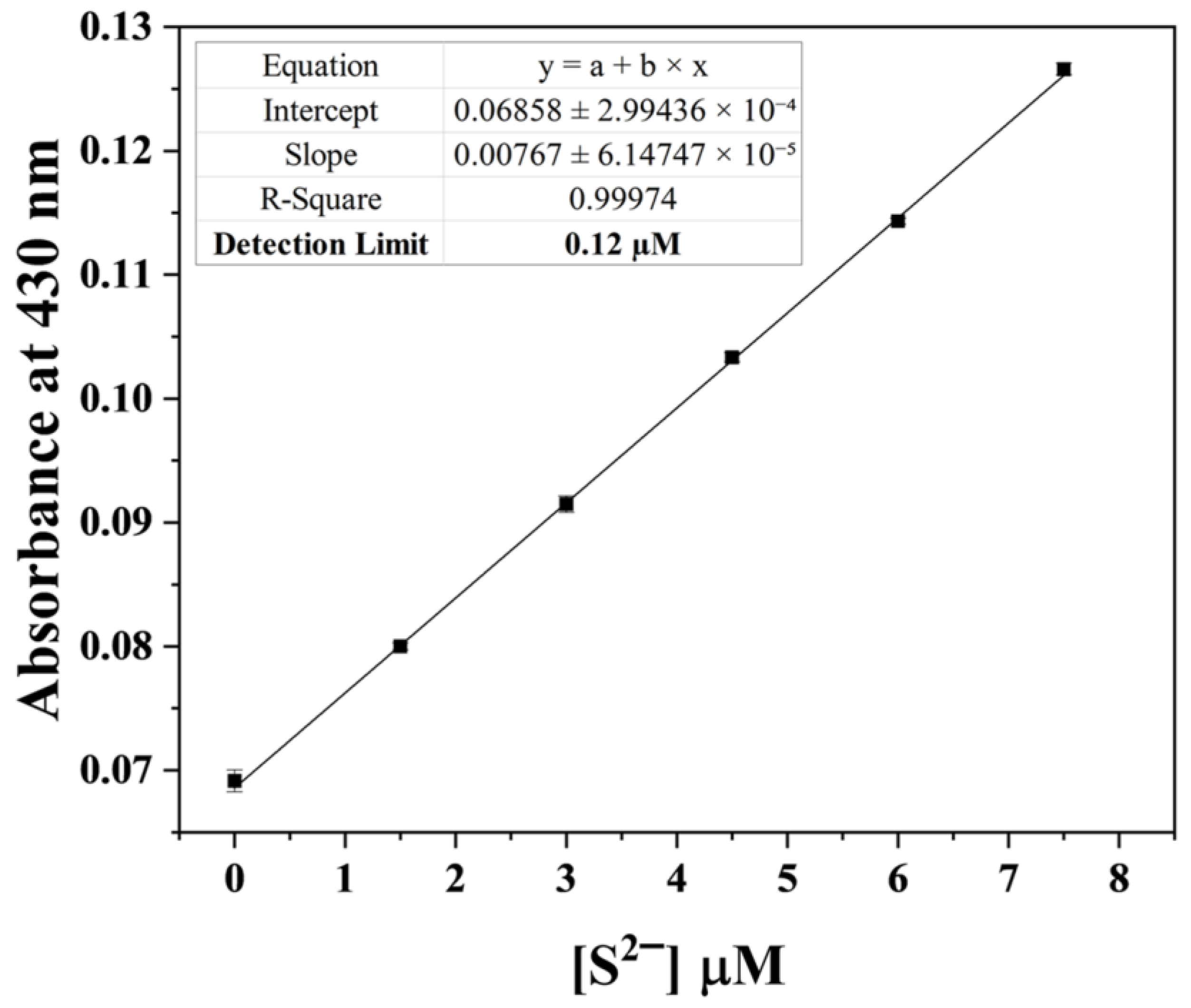
3.4. Application of HDH–Cu2+ Complex for S2− Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choe, D.; Kim, C. A Benzothiadiazole-Based Colorimetric Chemosensor for Detecting Cu2+ and Sequential H2S in Practical Samples. Inorg. Chim. Acta 2022, 543, 121180. [Google Scholar] [CrossRef]
- He, L.; Tao, H.; Koo, S.; Chen, G.; Sharma, A.; Chen, Y.; Lim, I.T.; Cao, Q.Y.; Kim, J.S. Multifunctional Fluorescent Nanoprobe for Sequential Detections of Hg2+ Ions and Biothiols in Live Cells. ACS Appl. Bio Mater. 2018, 1, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Manna, A.K.; Patra, G.K. A Fluorescent Colorimetric Vanillin Di-Schiff Base Chemosensor for Detection of Cu(II) and Isolation of Trinuclear Cu(II)-Dihydrazide. Mater. Adv. 2022, 3, 2495–2504. [Google Scholar] [CrossRef]
- Asadpour Chounechenan, S.; Mohammadi, A.; Ghafouri, H. A New and Efficient Diaminopyrimidine-Based Colorimetric and Fluorescence Chemosensor for the Highly Selective and Sensitive Detection of Cu2+ in Aqueous Media and Living Cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120507. [Google Scholar] [CrossRef]
- Kavitha, B.S.; Sridevi, S.; Makam, P.; Ghosh, D.; Govindaraju, T.; Asokan, S.; Sood, A.K. Highly Sensitive and Rapid Detection of Mercury in Water Using Functionalized Etched Fiber Bragg Grating Sensors. Sens. Actuators B Chem. 2021, 333, 129550. [Google Scholar]
- Guan, W.L.; Zhang, Y.F.; Zhang, Q.P.; Zhang, Y.M.; Wei, T.B.; Yao, H.; Lin, Q. A Novel Fluorescent Chemosensor Based on Naphthofuran Functionalized Naphthalimide for Highly Selective and Sensitive Detecting Hg2+ and CN−. J. Lumin. 2022, 244, 118722. [Google Scholar] [CrossRef]
- Abebe, F.; Gonzalez, J.; Makins-Dennis, K.; Shaw, R. A New Bis(Rhodamine)-Based Colorimetric Chemosensor for Cu2+. Inorg. Chem. Commun. 2020, 120, 108154. [Google Scholar] [CrossRef]
- Aysha, T.S.; Mohamed, M.B.I.; El-Sedik, M.S.; Youssef, Y.A. Multi-Functional Colorimetric Chemosensor for Naked Eye Recognition of Cu2+, Zn2+ and Co2+ Using New Hybrid Azo-Pyrazole/Pyrrolinone Ester Hydrazone Dye. Dyes Pigm. 2021, 196, 109795. [Google Scholar] [CrossRef]
- Tavallali, H.; Deilamy-Rad, G.; Karimi, M.A.; Rahimy, E. A Novel Dye-Based Colorimetric Chemosensors for Sequential Detection of Cu2+ and Cysteine in Aqueous Solution. Anal. Biochem. 2019, 583, 113376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Y.; Zheng, X.; Li, Z.; Xu, S. High Selective and Sensitive Optical Probe with Effective Recognition for Cu2+ Based on a Novel Aniline Squarylium Dye. Inorg. Chem. Commun. 2020, 121, 108198. [Google Scholar] [CrossRef]
- Hu, Z.F.; Dou, L.; Zhang, J.; Zhang, Y.; Sun, Y.X.; Dong, W.K. A Novel “on-off-on” Halogen-Substituted Bis(Salamo)-like Fluorogenic Chemosensor for Sequentially Identifying Cu2+ Ions and Cysteine. Inorg. Chim. Acta 2022, 541, 121090. [Google Scholar] [CrossRef]
- Ko, Y.G.; Mayank; Singh, N.; Jang, D.O. Single Chemosensor for Sensing Multiple Analytes: Selective Fluorogenic Detection of Cu2+ and Br−. Tetrahedron Lett. 2018, 59, 3839–3844. [Google Scholar] [CrossRef]
- Pungut, N.A.S.; Heng, M.P.; Saad, H.M.; Sim, K.S.; Lee, V.S.; Tan, K.W. From One to Three, Modifications of Sensing Behavior with Solvent System: DFT Calculations and Real-Life Application in Detection of Multianalytes (Cu2+, Ni2+ and Co2+) Based on a Colorimetric Schiff Base Probe. J. Mol. Struct. 2021, 1238, 130453. [Google Scholar] [CrossRef]
- Lee, J.C.; Gray, H.B.; Winkler, J.R. Copper (II) Binding to Alpha-Synuclein, the Parkinson’ s Protein. J. Am. Chem. Soc. 2008, 130, 6898–6899. [Google Scholar] [CrossRef]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for Copper Acquisition, Distribution and Regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef]
- Kshtriya, V.; Koshti, B.; Pandey, D.K.; Kharbanda, S.; Chandra Kanth, P.; Singh, D.K.; Bhatia, D.; Gour, N. Sequential and Cellular Detection of Copper and Lactic Acid by Disaggregation and Reaggregation of the Fluorescent Panchromatic Fibres of an Acylthiourea Based Sensor. Soft Matter 2021, 17, 4304–4316. [Google Scholar] [CrossRef]
- Kim, A.; Kang, J.H.; Jang, H.J.; Kim, C. Fluorescent Detection of Zn(II) and In(III) and Colorimetric Detection of Cu(II) and Co(II) by a Versatile Chemosensor. J. Ind. Eng. Chem. 2018, 65, 290–299. [Google Scholar] [CrossRef]
- Cheah, P.W.; Heng, M.P.; Saad, H.M.; Sim, K.S.; Tan, K.W. Specific Detection of Cu2+ by a PH-Independent Colorimetric Rhodamine Based Chemosensor. Opt. Mater. 2021, 114, 110990. [Google Scholar] [CrossRef]
- Mohanasundaram, D.; Bhaskar, R.; Sankarganesh, M.; Nehru, K.; Gangatharan Vinoth Kumar, G.; Rajesh, J. A Simple Pyridine Based Fluorescent Chemosensor for Selective Detection of Copper Ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 120395. [Google Scholar] [CrossRef]
- Ozmen, P.; Demir, Z.; Karagoz, B. An Easy Way to Prepare Reusable Rhodamine-Based Chemosensor for Selective Detection of Cu2+ and Hg2+ Ions. Eur. Polym. J. 2022, 162, 110922. [Google Scholar] [CrossRef]
- Rajasekaran, D.; Venkatachalam, K.; Periasamy, V. “On–off–on” Pyrene-Based Fluorescent Chemosensor for the Selective Recognition of Cu2+ and S2− Ions and Its Utilization in Live Cell Imaging. Appl. Organomet. Chem. 2020, 34, e5342. [Google Scholar] [CrossRef]
- Feng, S.S.; Wei, Y.X.; Li, M.; Dong, W.K. A Highly Selective Naphthalene-Fluorophore Salamo-Based Chemosensor for Sequential Identification of Cu2+ and S2− Ions in Water Applications. J. Mol. Struct. 2022, 1261, 132923. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Xu, X.; Zhang, Y.; Zhang, Y.; Dong, W.K. A Highly Sensitive and Selective Bis(Salamo)-Type Fluorescent Chemosensor for Identification of Cu2+ and the Continuous Recognition of S2−, Arginine and Lysine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117927. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.R.; Yu, M.; Wang, L.; Zhang, Y.; Ding, Y.J. Catching S2− and Cu2+ by a Highly Sensitive and Efficient Salamo-like Fluorescence-Ultraviolet Dual Channel Chemosensor. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 730–739. [Google Scholar] [CrossRef]
- Kargar, M.; Darabi, H.R.; Sharifi, A.; Mostashari, A. A New Chromogenic and Fluorescent Chemosensor Based on a Naphthol-Bisthiazolopyridine Hybrid: A Fast Response and Selective Detection of Multiple Targets, Silver, Cyanide, Sulfide, and Hydrogen Sulfide Ions and Gaseous H2S. Analyst 2020, 145, 2319–2330. [Google Scholar] [CrossRef]
- Jung, J.M.; Kang, J.H.; Han, J.; Lee, H.; Lim, M.H.; Kim, K.T.; Kim, C. A Novel “off-on” Type Fluorescent Chemosensor for Detection of Zn2+ and Its Zinc Complex for “on-off” Fluorescent Sensing of Sulfide in Aqueous Solution, in Vitro and in Vivo. Sens. Actuators B Chem. 2018, 267, 58–69. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, D.; Xue, S.; Chen, B.; Wen, S.; Yang, X.; Wu, J. Rational Design of Dual-Functional Peptide-Based Chemosensor for Sequential Detection of Ag+ (AgNPs) and S2− Ions by Fluorescent and Colorimetric Changes and Its Application in Live Cells, Real Water Samples and Test Strips. Microchem. J. 2022, 177, 107326. [Google Scholar] [CrossRef]
- Chen, F.; Han, D.; Liu, H.; Wang, S.; Li, K.B.; Zhang, S.; Shi, W. A Tri-Site Fluorescent Probe for Simultaneous Sensing of Hydrogen Sulfide and Glutathione and Its Bioimaging Applications. Analyst 2018, 143, 440–448. [Google Scholar] [CrossRef]
- Thai, D.A.; Lee, N.Y. A Paper-Based Colorimetric Chemosensor for Rapid and Highly Sensitive Detection of Sulfide for Environmental Monitoring. Anal. Methods 2021, 13, 1332–1339. [Google Scholar] [CrossRef]
- Kim, M.S.; Jung, J.M.; Ahn, H.M.; Kim, C. A Simple Colorimetric Chemosensor for Relay Detection of Cu2+ and S2− in Aqueous Solution. J. Coord. Chem. 2018, 71, 355–370. [Google Scholar] [CrossRef]
- Singh, N.; Chandra, R. A Naked-Eye Colorimetric Sensor Based on Chalcone for the Sequential Recognition of Copper(II) and Sulfide Ions in Semi-Aqueous Solution: Spectroscopic and Theoretical Approaches. New J. Chem. 2021, 45, 10340–10348. [Google Scholar] [CrossRef]
- Wang, P.; Sun, L.; Wu, J.; Yang, X.; Lin, P.; Wang, M. A Dual-Functional Colorimetric and Fluorescent Peptide-Based Probe for Sequential Detection of Cu2+ and S2− in 100% Aqueous Buffered Solutions and Living Cells. J. Hazard. Mater. 2021, 407, 124388. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Niu, Q.; Li, T.; Guo, Z.; Liu, H. A Simple, Reversible, Colorimetric and Water-Soluble Fluorescent Chemosensor for the Naked-Eye Detection of Cu2 + in ~ 100% Aqueous Media and Application to Real Samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choe, D.; Lee, J.J.; Kim, C. A Benzyl Carbazate-Based Colorimetric Chemosensor for Relay Detection of Cu2+ and S2− in near-Perfect Aqueous Media. J. Mol. Struct. 2021, 1240, 130576. [Google Scholar] [CrossRef]
- So, H.; Chae, J.B.; Kim, C. A Thiol-Containing Colorimetric Chemosensor for Relay Recognition of Cu2+ and S2− in Aqueous Media with a Low Detection Limit. Inorg. Chim. Acta 2019, 492, 83–90. [Google Scholar] [CrossRef]
- Parua, S.P.; Sinha, D.; Rajak, K.K. A Highly Selective “on-off-on” Optical Switch for Sequential Detection of Cu2+ and S2– Ions Based on 2, 6-Diformyl-4-Methyl Phenol and Catecholase Activity by Its Copper Complex. ChemistrySelect 2018, 3, 1120–1128. [Google Scholar] [CrossRef]
- Jin, X.; Chen, H.; Zhang, W.; Wang, B.; Shen, W.; Lu, H. A Novel Purine Derivative-Based Colorimetric Chemosensor for Sequential Detection of Copper Ion and Sulfide Anion. Appl. Organomet. Chem. 2018, 32, e4577. [Google Scholar] [CrossRef]
- Kwon, N.; Chen, Y.; Chen, X.; Kim, M.H.; Yoon, J. Recent Progress on Small Molecule-Based Fluorescent Imaging Probes for Hypochlorous Acid (HOCl)/Hypochlorite (OCl−). Dyes Pigm. 2022, 200, 110132. [Google Scholar] [CrossRef]
- Lee, S.; Jen, M.; Jang, T.; Lee, G.; Pang, Y. Twisted Intramolecular Charge Transfer of Nitroaromatic Push–Pull Chromophores. Sci. Rep. 2022, 12, 6557. [Google Scholar] [CrossRef]
- Kajetanowicz, A.; Grela, K. Nitro and Other Electron Withdrawing Group Activated Ruthenium Catalysts for Olefin Metathesis Reactions. Angew. Chemie Int. Ed. 2021, 60, 13738–13756. [Google Scholar] [CrossRef]
- Vojinović-Ješić, L.S.; Češljević, V.I.; Bogdanović, G.A.; Leovac, V.M.; Szécsényi, K.M.; Divjaković, V.; Joksović, M.D. Transition Metal Complexes with Girard Reagent-Based Ligands. Part V. Synthesis, Characterization and Crystal Structure of Pentagonal-Bipyramidal Manganese(II) Complex with 2,6-Diacetylpyridine Bis(Girard-T Hydrazone). Inorg. Chem. Commun. 2010, 13, 1085–1088. [Google Scholar] [CrossRef]
- Moussa, M.N.H.; El-Far, A.A.; El-Shafei, A.A. The Use of Water-Soluble Hydrazones as Inhibitors for the Corrosion of C-Steel in Acidic Medium. Mater. Chem. Phys. 2007, 105, 105–113. [Google Scholar] [CrossRef]
- Bogdanov, A.V.; Zaripova, I.F.; Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Voronina, J.K.; Mironov, V.F. Synthesis and Antimicrobial Study of Novel 1-Benzylated Water-Soluble Isatin-3-Hydrazones. Chem. Biodivers. 2018, 15, 1800088. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian, Inc.: Walingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent Molecular Orbital Methods. XXIII. A Polarization-type Basis Set for Second-row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for Main Group Elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef]
- Ershov, A.Y.; Lagoda, I.V.; Yakimovich, S.I.; Pakal’Nis, V.V.; Zerova, I.V.; Dobrodumov, A.V.; Shamanin, V.V. Tautomerism and Conformational Isomerism of Mercaptoacetylhydrazones of Aliphatic and Aromatic Aldehydes. Russ. J. Org. Chem. 2009, 45, 660–666. [Google Scholar] [CrossRef]
- Gil, D.; Lee, J.J.; Lee, H.; Kim, K.-T.; Kim, C. Detection of Environmentally Hazardous Hypochlorite in Pure Water with a Novel Fluorescent Chemosensor: Application to Water Samples, Commercial Disinfectants, Test Strips, and Zebrafish. Dyes Pigm. 2022, 207, 110714. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Mahmoud, A.M.; Alkahtani, S.A.; Ali, R.; El-Wekil, M.M. A Novel Imidazole Derived Colorimetric and Fluorometric Chemosensor for Bifunctional Detection of Copper (II) and Sulphide Ions in Environmental Water Samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117846. [Google Scholar] [CrossRef]
- Rajendra Prasad, Y.; Kumar, P.P.; Kumar, P.R.; Rao, A.S. Synthesis and Antimicrobial Activity of Some New Chalcones of 2-Acetyl Pyridine. J. Chem. 2008, 5, 144–148. [Google Scholar]
- Rout, K.C.; Mondal, B. Copper(II) Complex as Selective Turn-on Fluorescent Probe for Nitrite Ion. Inorg. Chim. Acta 2015, 437, 54–58. [Google Scholar] [CrossRef]
- Nouri Moghadam, F.; Amirnasr, M.; Meghdadi, S.; Eskandari, K.; Buchholz, A.; Plass, W. A New Fluorene Derived Schiff-Base as a Dual Selective Fluorescent Probe for Cu2+ and CN−. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 207, 6–15. [Google Scholar] [CrossRef]
- Rha, C.J.; Lee, H.; Kim, C. An Effective Phthalazine-Imidazole-Based Chemosensor for Detecting Cu2+, Co2+ and S2− via the Color Change. Inorg. Chim. Acta 2020, 511, 119788. [Google Scholar] [CrossRef]




| Sample | Cu2+ Added (μM) | Cu2+ Added (μM) | Recovery (%) | R.S.D. (n = 3) (%) |
|---|---|---|---|---|
| Drinking water | 0 | 0 | - | - |
| 9.00 | 9.17 | 101.89 | 3.71 | |
| Tap water | 0 | 0 | - | - |
| 9.00 | 8.81 | 97.89 | 2.29 |
| Sample | Na2S Added (μM) | Na2S Added (μM) | Recovery (%) | R.S.D. (n = 3) (%) |
|---|---|---|---|---|
| Drinking water | 0 | 0 | - | - |
| 4.5 | 4.38 | 97.33 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.; Moon, S.; Gil, D.; Kim, C. A Dinitrophenol-Based Colorimetric Chemosensor for Sequential Cu2+ and S2− Detection. Chemosensors 2023, 11, 143. https://doi.org/10.3390/chemosensors11020143
Nam H, Moon S, Gil D, Kim C. A Dinitrophenol-Based Colorimetric Chemosensor for Sequential Cu2+ and S2− Detection. Chemosensors. 2023; 11(2):143. https://doi.org/10.3390/chemosensors11020143
Chicago/Turabian StyleNam, Hyejin, Sungjin Moon, Dongkyun Gil, and Cheal Kim. 2023. "A Dinitrophenol-Based Colorimetric Chemosensor for Sequential Cu2+ and S2− Detection" Chemosensors 11, no. 2: 143. https://doi.org/10.3390/chemosensors11020143
APA StyleNam, H., Moon, S., Gil, D., & Kim, C. (2023). A Dinitrophenol-Based Colorimetric Chemosensor for Sequential Cu2+ and S2− Detection. Chemosensors, 11(2), 143. https://doi.org/10.3390/chemosensors11020143







