Bioengineered Flagellin–TiO2 Nanoparticle-Based Modified Glassy Carbon Electrodes as a Highly Selective Platform for the Determination of Diclofenac Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization Technique
2.3. Preparation of the Modified Electrode
2.4. Electrode Characterization
2.5. Analysis of the Water Sample
3. Results
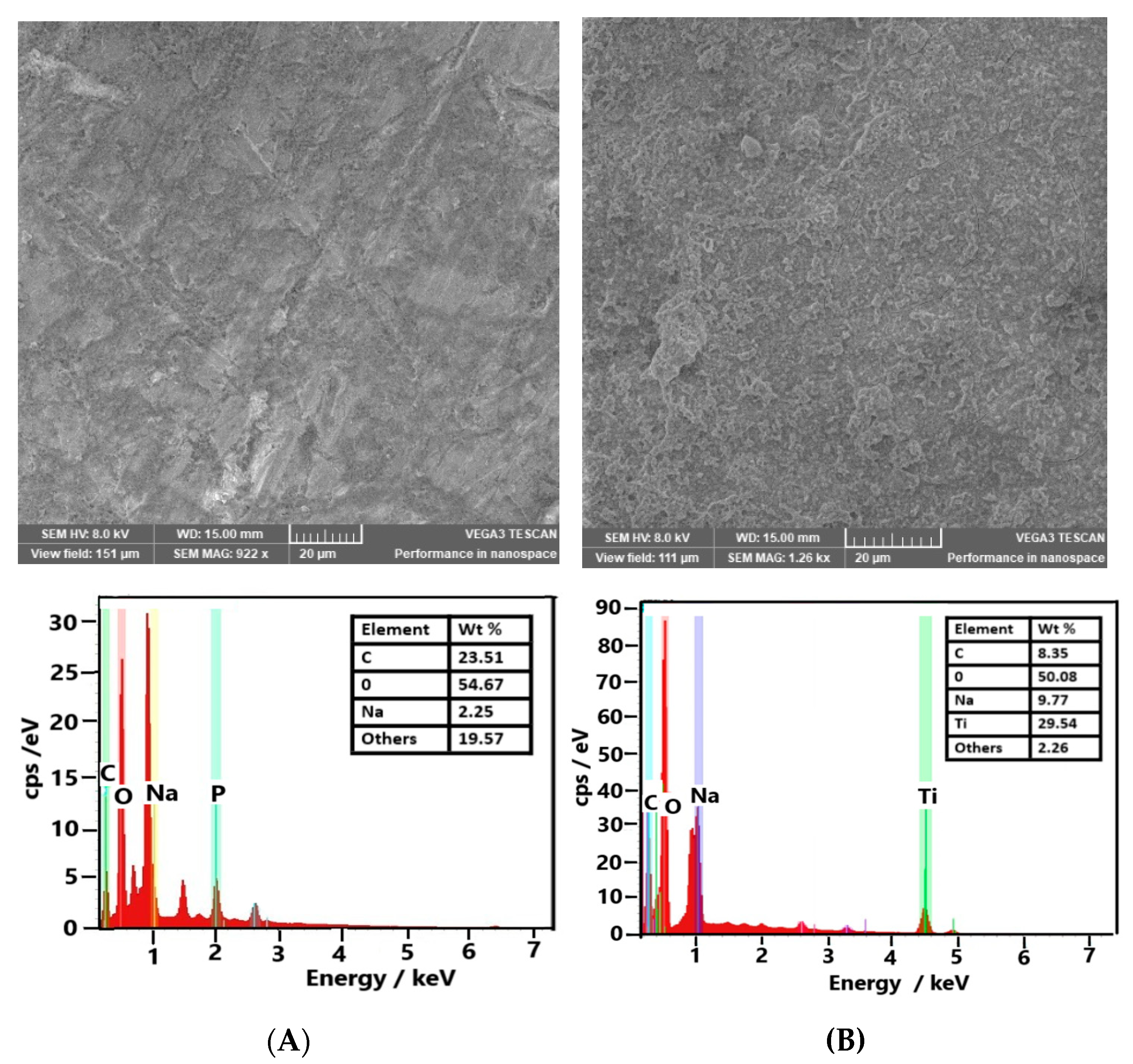
3.1. Morpho Structural Characterization of GCE-Modified Electrodes
3.2. Electrochemical Behavior of GCE-Modified Electrodes for DS Detection
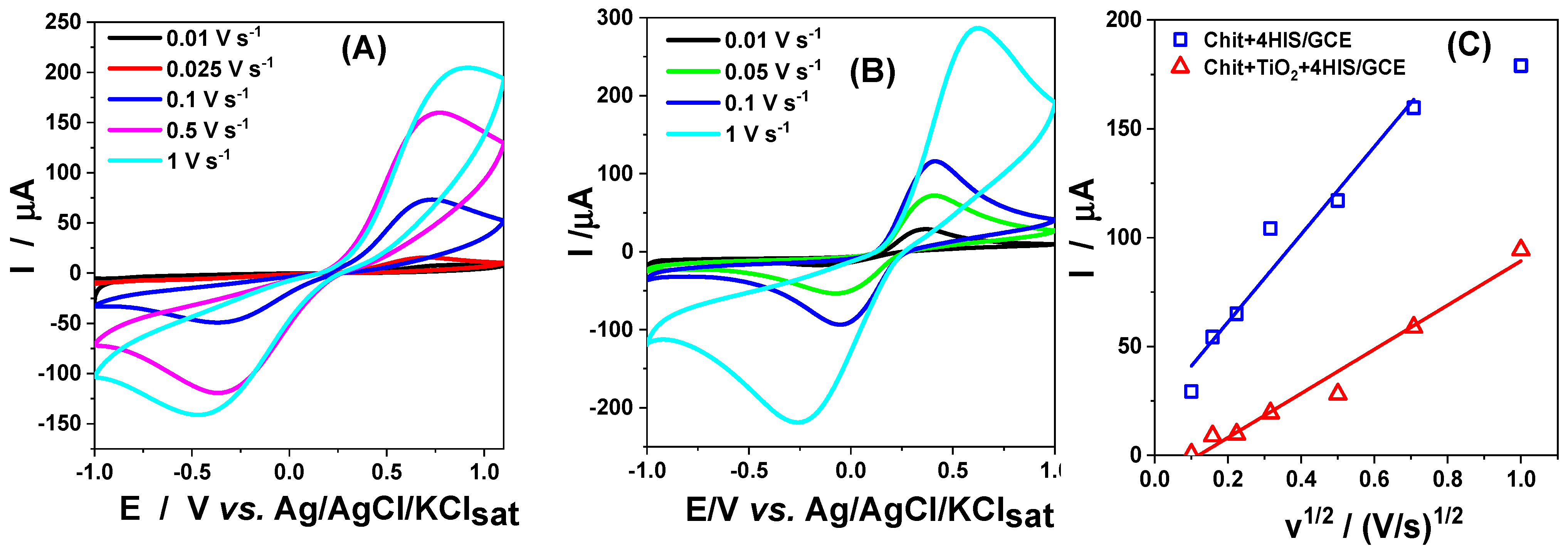
3.2.1. Influence of Scan Rate on DS Oxidation
3.2.2. Influence of the pH on DS Oxidation
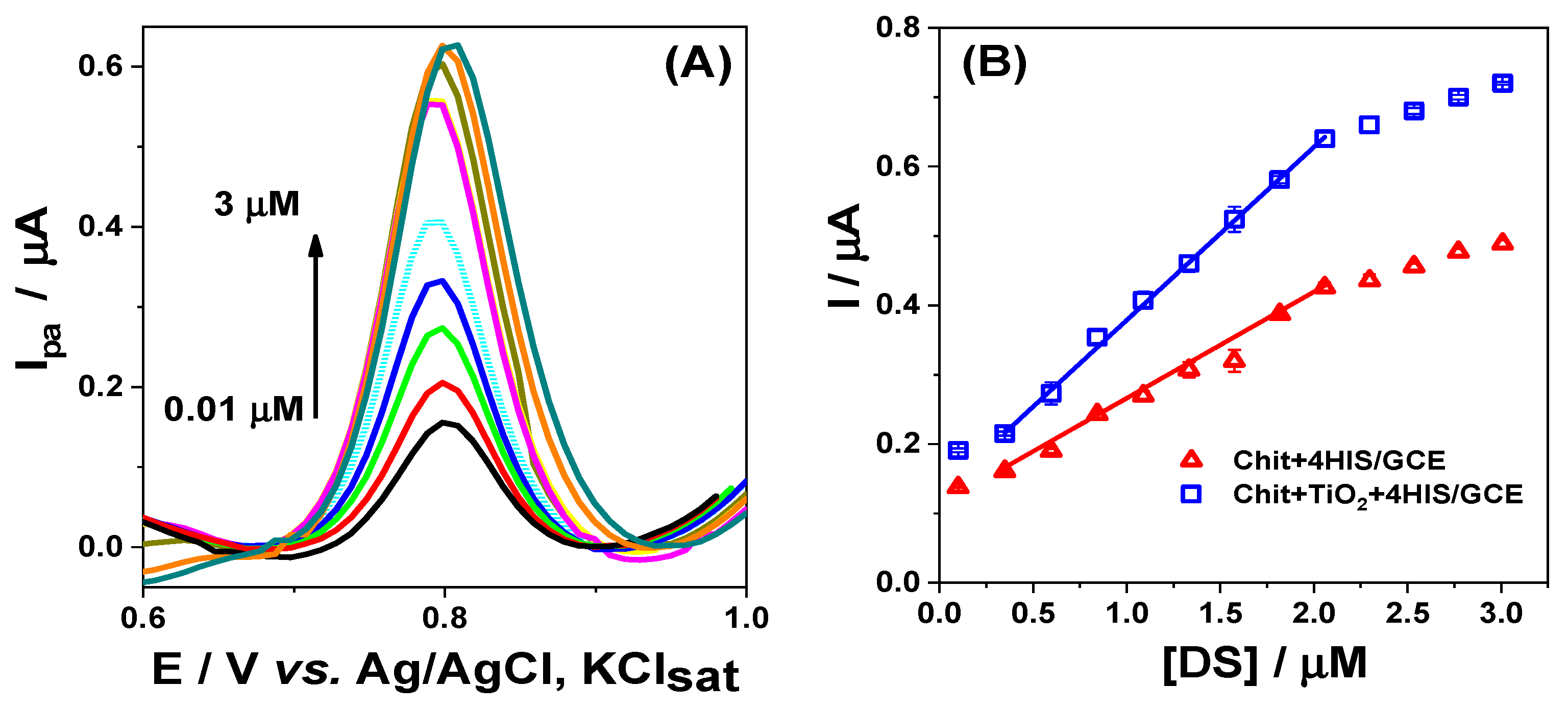
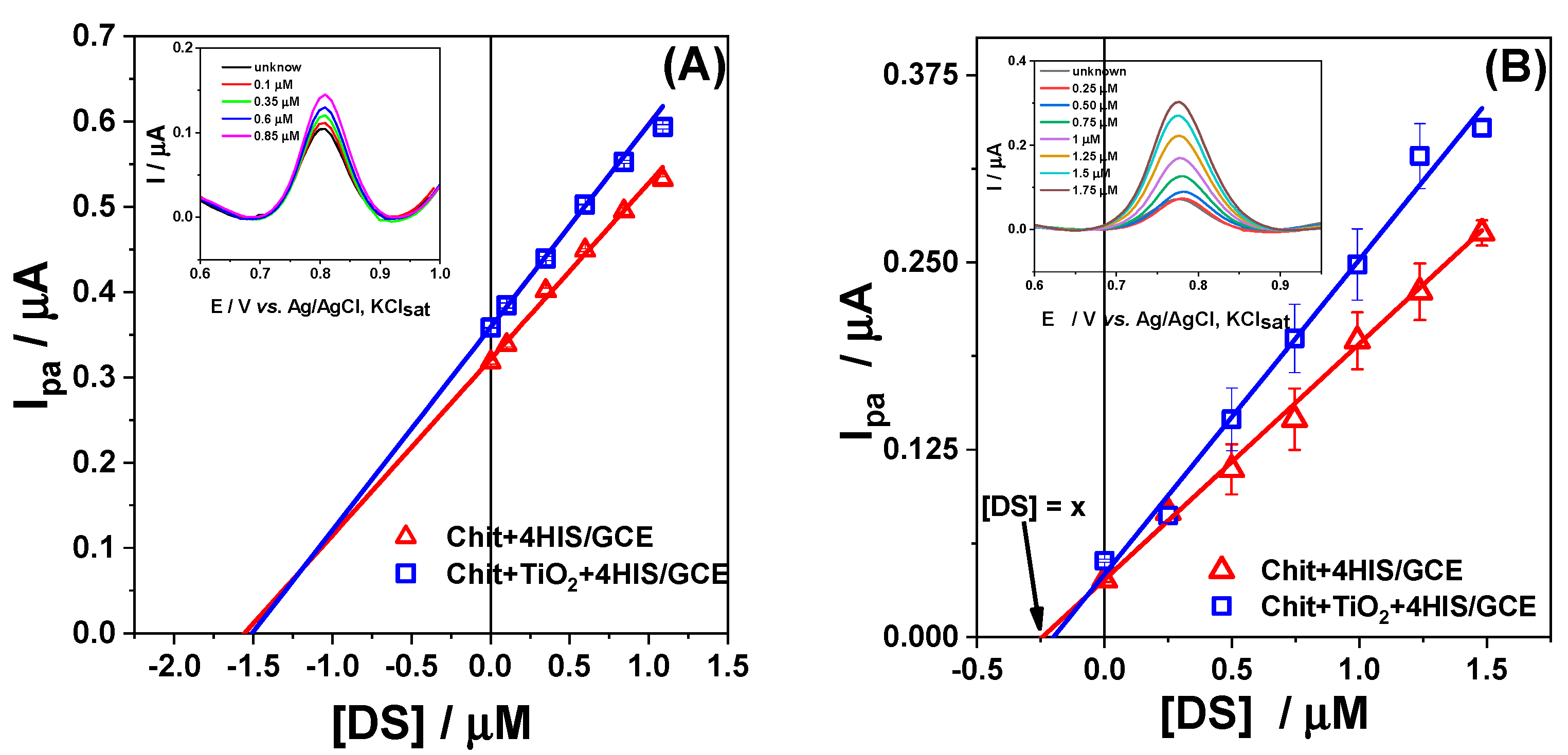
3.3. Analytical Characterization
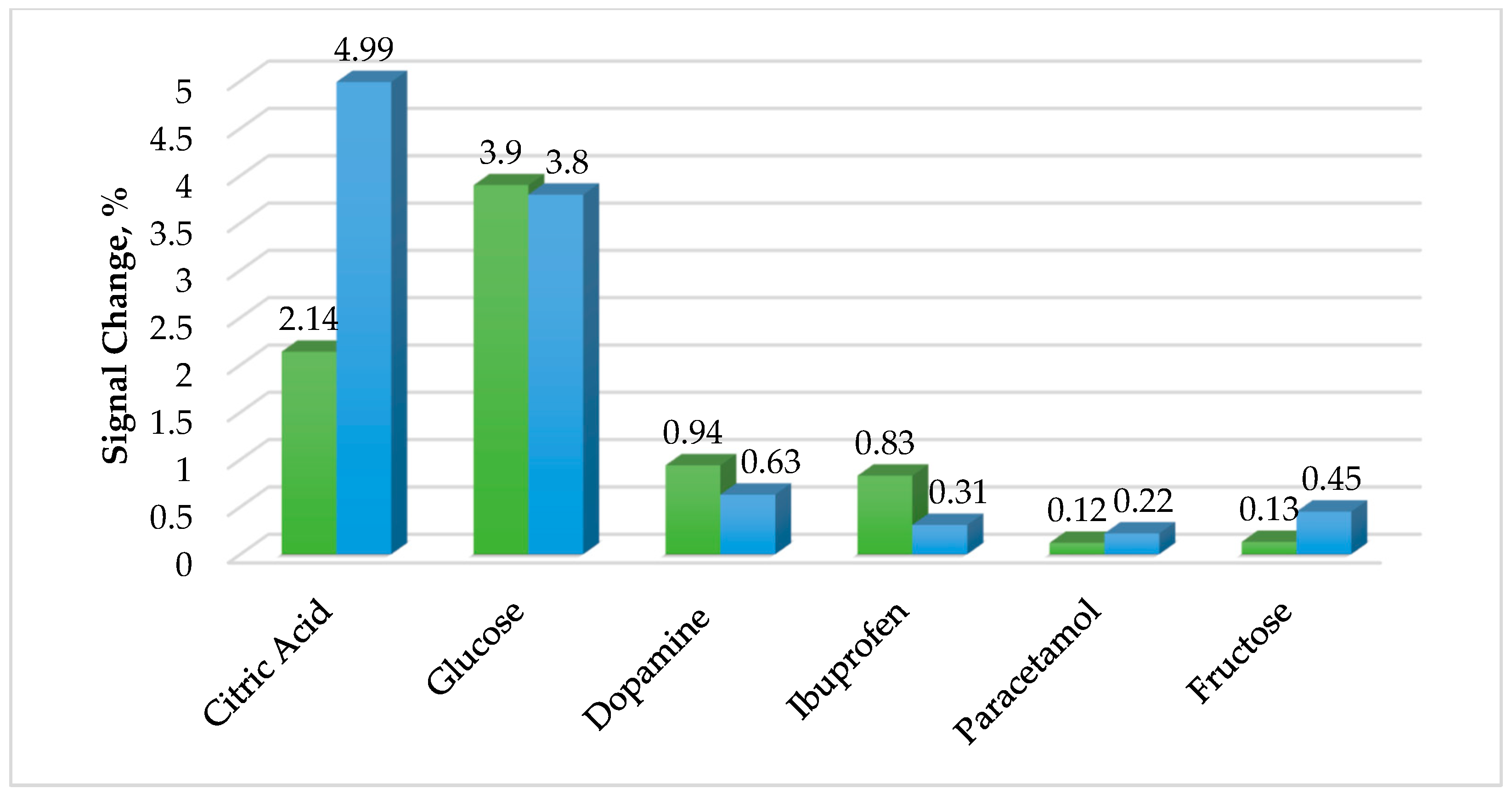
3.4. Interferences Study
3.5. Determination of DS in the Pharmaceutical Sample and in the Wastewater Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.; Shivaprasad, H.L.; Ahmed, S.; Jamshed, M.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2014, 427, 630–633. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document C(2015) 1756); European Commission, Ed.; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Simon, E.; Duffek, A.; Stahl, C.; Frey, M.; Scheurer, M.; Tuerk, J.; Gehrmann, L.; Konemann, S.; Swart, K.; Behnisch, P.; et al. Biological effect and chemical monitoring of Watch List substances in European surface waters: Steroidal estrogens and diclofenac—Effect-based methods for monitoring frameworks. Environ. Int. 2022, 159, 107033. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B. GC–MS determination of diclofenac in human plasma. J. Chromatogr. 2010, 71, 549–551. [Google Scholar] [CrossRef]
- Borenstein, M.; Xue, S.; Cooper, S.; Tzeng, T. Sensitive capillary gas chromatographic-mass spectrometric selected ion monitoring method for the determination of diclofenac concentrations in human plasma. J. Chromatogr. B 1996, 685, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Elkady, E.F. Simultaneous determination of diclofenac potassium and methocarbamol in ternary mixture with guaifenesin by reversed-phase liquid chromatography. Talanta 2010, 82, 1604–1607. [Google Scholar] [CrossRef]
- Bhupendra, L.K.; Kaphalia, S.; Kumar, S.; Kanz, M.; Treinen-Moslen, M. Efficient high-performance liquid chromatography/ultraviolet method for determination of diclofenac and 4′-hydroxy diclofenac in rat serum. J. Chromatogr. B 2006, 830, 231–237. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, C.K.; Choi, S.J.; Kim, S.B.; Lee, M.H.; Ko, G.; Sohn, D.H. Simultaneous determination of aceclofenac and diclofenac in human plasma by narrow bore HPLC using column-switching. J. Pharm. Biomed. Anal. 2020, 23, 775–781. [Google Scholar] [CrossRef]
- Sparidans, R.W.; Lagas, J.S.; Schinkel, A.H.; Schellens, J.H.; Beijnen, J. Liquid chromatography–tandem mass spectrometric assay for diclofenac and three primary metabolites in mouse plasma. J. Chromatogr. B 2008, 872, 77–82. [Google Scholar] [CrossRef]
- Bhushan, R.; Gupta, D.; Mukherjee, A. Liquid chromatographic analysis of certain commercial formulations for non-opioid analgesics. Biomed. Chromatogr. 2007, 21, 1284–1290. [Google Scholar] [CrossRef]
- Botello, J.C.; Caballero, G.P. Spectrophotometric determination of diclofenac sodium with methylene blue. Talanta 1995, 42, 105–108. [Google Scholar] [CrossRef]
- de Souza, R.L.; Tubino, M. Spectrophotometric determination of diclofenac in pharmaceutical preparations. J. Braz. Chem. Soc. 2005, 16, 1068. [Google Scholar] [CrossRef]
- Garcia, Z.M.; Albero, M.I. Flow-injection spectrophotometric determination of diclofenac sodium in pharmaceuticals and urine samples. J. Pharm. Biomed. Anal. 1998, 17, 267–273. [Google Scholar] [CrossRef] [PubMed]
- El-Didamony, A.M.; Amin, A.S. Adaptation of a color reaction for spectrophotometric determination of diclofenac sodium and piroxicam in pure form and in pharmaceutical formulations. Anal. Lett. 2004, 37, 1151–1162. [Google Scholar] [CrossRef]
- de Micalizzi, Y.C.; Pappano, N.B.; Debattista, N.B. First and second-order derivative spectrophotometric determination of benzyl alcohol and diclofenac in pharmaceutical forms. Talanta 1998, 47, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.; Mohana Rao, A.R.; Prasad, N.V. Spectrophotometric analysis of diclofenac sodium and piroxicam and their pharmaceutical preparations. Anal. Lett. 1987, 2, 75–80. [Google Scholar] [CrossRef]
- Arancibia, J.A.; Escandar, G.M. Complexation study of diclofenac with b-cyclodextrin and spectrofluorimetric determination. Analyst 1999, 124, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, J. Determination of diclofenac sodium by capillary zone electrophoresis with electrochemical detection. J. Chromatogr. A 2000, 868, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sarhangzadeh, K.; Khatami, A.A.; Jabbari, M.; Bahari, S. Simultaneous determination of diclofenac and indomethacin using a sensitive electrochemical sensor based on multiwalled carbon nanotube and ionic liquid nanocomposite. J. Appl. Electrochem. 2013, 43, 1217–1224. [Google Scholar] [CrossRef]
- Karuppiah, C.; Cheemalapati, S.M.; Chen, S.M.; Palanisamy, S. Carboxyl-functionalized graphene oxide-modified electrode for the electrochemical determination of nonsteroidal anti-inflammatory drug diclofenac. Ionics 2014, 21, 231–238. [Google Scholar] [CrossRef]
- Baghayeri, M.; Maleki, B.; Zarghani, R. Voltammetric behavior of tiopronin on carbon paste electrode modified with nanocrystalline Fe50Ni50 alloys. Mater. Sci. Eng. C 2014, 44, 175–182. [Google Scholar] [CrossRef]
- Baghayeri, M.; Nazarzadeh Zare, E.; Mansour Lakouraj, M. A simple hydrogen peroxide biosensor based on a novel electro-magnetic poly(p-phenylenediamine) @Fe3O4 nanocomposite. Biosens. Bioelectron. 2014, 55, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, J.; Karimi-Maleh, H.; Baghayeri, M.; Sanati, A.L.; Khalilzadeh, M.A.; Bahrami, M. A fast and sensitive nanosensor based on MgO nanoparticle room-temperature ionic liquid carbon paste electrode for determination of methyldopa in pharmaceutical and patient human urine samples. Ionics 2013, 19, 1907–1914. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Agrawal, B. Electrochemical investigations of diclofenac at edge plane pyrolytic graphite electrode and its determination in human urine. Sens. Actuat. B Chem. 2010, 145, 743–748. [Google Scholar] [CrossRef]
- Eteya, M.; Rounaghi, M.; Deiminiat, B. Fabrication of a new electrochemical sensor based on Au-Pt bimetallic nanoparticles decorated multi-walled carbon nanotubes for determination of diclofenac. Microchem. J. 2019, 144, 254–260. [Google Scholar] [CrossRef]
- de Carvalho, R.C.; Bettsa, A.J.; Cassidya, J.F. Diclofenac determination using CeO2 nanoparticle modified screen-printed electrodes—A study of background correction. Microchem. J. 2020, 158, 105258. [Google Scholar] [CrossRef]
- Salahuddin, M.; Akhter, S.; Basirun, W.J.; Akhtaruzzaman, M.; Mohammed, M.A.; Rahman, N.M.M.A.; Salleh, N.M. Bio-synthesized copper nanoparticle decorated multiwall carbon nanotube-nano cellulose nanocomposite: An electrochemical sensor for the simultaneous detection of acetaminophen and diclofenac sodium. Surf. Interfaces 2022, 34, 102385. [Google Scholar] [CrossRef]
- Naz, S.; Nisar, A.; Qian, L.; Hussain, S.; Karim, S.; Hussain, S.Z.; Liu, Y.; Sun, H.; Ur-Rahman, A.; Ahmad, M. Graphene Oxide Functionalized with Silver Nanoparticles and ZnO Synergic Nanocomposite as an Efficient Electrochemical Sensor for Diclofenac Sodium. Nano Brief Rep. Rev. 2021, 16, 2150139. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Rajkumar, M.; Chen, S. Nano TiO2-PEDOT Film for the Simultaneous Detection of Ascorbic Acid and Diclofenac. Int. J. Electrochem. Sc. 2012, 7, 2109–2122. [Google Scholar] [CrossRef]
- Hajjizadeh, A.; Jabbari, H.; Heli, A.; Movahedi, A.M.; Haghgoo, S. Evaluation of the electrocatalytical properties of NiCo(OH)2 composite modified electrodes. Electrochim. Acta 2007, 53, 1766–1774. [Google Scholar] [CrossRef]
- Jankovics, H.; Szekér, P.; Tóth, E.; Balázs, K.; Lábadi, Z.; Saftics, A.; Kalas, B.; Fried, M.; Petrik, P.; Vonderviszt, F. Flagellin-based electrochemical sensing layer for arsenic detection in water. Sci. Rep. 2021, 11, 3497. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Bessbousse, H.; Elhalil, A.; Barka, N. Electrochemical sensors and biosensors for the determination of diclofenac in pharmaceutical, biological and water samples. Talanta 2021, 3, 100026. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. Trends. Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- John, B. Polymer Nanocomposite-Based Electrochemical Sensors, and Biosensors. In Nanorods and Nanocomposites; InTech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Postolović, K.Ș.; Stanić, Z. Chitosan/TiO2 nanoparticles modified carbon paste electrode as a sensitive volt-ammetric sensor for the determination of diclofenac sodium as an anti-inflammatory drug. Mater. Today Commun. 2023, 37, 107416. [Google Scholar] [CrossRef]
- Dias, G.V.; Joseane, C.; Bernardes, B.N.; Wesling, D.M.; Larissa, S.; Marques, C.R. Preparation, and electrochemical capacitance of high surface area TiO2–RuO2 aerogels. Open Ceram. 2021, 8, 100196. [Google Scholar] [CrossRef]
- Huang, X.J.; Choi, Y.K. Chemical sensors based on nanostructured materials. Sens. Actuat. B 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Seracu, D. Indreptar de Chimie Analitica (Tabele, Diagrame, Programme); Editura Tehnica: Bucuresti, Romania, 1989; p. 116. ISBN 973-3100-935. [Google Scholar]
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A. Design, and construction of a label-free aptasensor for electrochemical detection of sodium diclofenac. Biosens. Bioelectron. 2012, 33, 184–189. [Google Scholar] [CrossRef]
- Deng, A.P.; Himmelsbach, M.; Zhu, Q.S.; Frey, S.; Sengl, M.; Buchberger, W.; Niessner, R.; Knopp, D. Residue analysis of the pharmaceutical diclofenac in different water types using ELISA and GCMS. Environ. Sci. Technol. 2003, 37, 3422–3429. [Google Scholar] [CrossRef]
- Mohamed, M.; El-Wekil, A.; Saad, A.; Alkahtani, H.H.; Ali, R.H.; Mahmoud, A.M. Advanced sensing nanomaterials-based carbon paste electrode for simultaneous electrochemical measurement of esomeprazole and diclofenac sodium in human serum and urine samples. J. Mol. Liq. 2018, 262, 495–503. [Google Scholar] [CrossRef]
- Abbas, A.; Bahiraei, A.; Madrakian, T. Gold nanoparticle/multi-walled carbon nanotube modified glassy carbon electrode as a sensitive voltammetric sensor for the determination of diclofenac sodium. Mat. Sci. Eng. C 2016, 59, 168–176. [Google Scholar] [CrossRef]
- Labadi, Z.; Kalas, B.; Saftics, A.; Illes, L.I.; Jankovics, H.; Bereczk-Tompa, E.; Sebestyén, A.; Tóth, E.; Kakasi, B.; Moldovan, C.; et al. Sensing layer for Ni Detection in Water Created by Immobilization of Bioengineered Flagellar Nanotubes on Gold Surfaces. ACS Biomater. Sci. Eng. 2020, 6, 3811–3820. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2022; pp. 231, 591. [Google Scholar]
- Killedar, L.; Ilager, D.; Shetti, N.P.; Aminabhavi, T.M.; Reddy, K.R. Synthesis of ruthenium doped titanium dioxide nanoparticles for the electrochemical detection of diclofenac sodium. J. Mol. Liq. 2021, 340, 116891. [Google Scholar] [CrossRef]
- Shah, A.; Ullah, A.; Rauf, A.; Rehman, Z.; Shujah, S.; Shah, S.M. Detailed electrochemical probing of a biologically active isoquinoline. J. Electrochem. Soc. 2013, 160, H597. [Google Scholar] [CrossRef]
- Honakeri, N.C.; Malode, S.J.; Kulkarni, R.M.; Shetti, N.P. Electrochemical behavior of diclofenac sodium at core-shell nanostructure modified electrode and its analysis in human urine and pharmaceutical samples. Sens. Int. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Nasraoui, S.; Ameur, S.; Al-Hamry, A.; Mounir, B.; Olfa Kanoun, A. Development of an Efficient Voltammetric Sensor for the Monitoring of 4-Aminophenol Based on Flexible Laser-Induced Graphene Electrodes Modified with MWCNT-PANI. Sensors 2022, 22, 833. [Google Scholar] [CrossRef]
- Harvey, D. Modern Analytical Chemistry, 1st ed.; McGraw-Hill: Boston, MA, USA, 2000; p. 95. [Google Scholar]
- Daizong, J.; Zhouyuanjing, S.; Zixiang, L.; Sze, S.L.; Jingwen, Z.; Tingkai, Z.; Zetao, C.; Xiongjie, Y.; Yanli, L.; Di, L.; et al. Smartphone-based square wave voltammetry system with screen-printed graphene electrodes for norepinephrine detection. Smart Mater. Med. 2020, 1, 1–9. [Google Scholar] [CrossRef]


| Electrode | LOD (μM) | Linear Range (μM) | Real Sample | References |
|---|---|---|---|---|
| GO-COOH/GCE | 0.09 | 1.2–400 | / | [20] |
| Au–Pt NPs/f-MWCNTs/Au | 0.30 | 0.5–1000 | / | [25] |
| CeO2NPs/SPCE | 0.40 | 0.1–26 | Tablets | [26] |
| nanoTiO2/PEDOT/GCE | 0.03 | 4–15 | / | [29] |
| Amino-labeled | 0.27 | 5–1000 | Blood serum | [39] |
| aptamer/Fe3O4/AuNP/CNT/GCE | 20 | 10−5–1.3·× 10−3 | Tap and surface water | [40] |
| NiNPs/erGO/GCE | 0.09 | 0.25–125 | / | [50] |
| Chit + 4HIS/GCE | 0.066 | 0.25–2 | Wastewater | This work |
| Chit + TiO2 + 4HIS/GCE | 0.033 | 0.11–2 | Wastewater | This work |
| Type of Electrode | [DS]/μM Added | [DS]/μM Found | Recovery (%) | R/n |
|---|---|---|---|---|
| Chit + 4HIS/GCE | 1.57 | 1.55 ± 0.63 | 98.72 ± 0.58 | 0.9964/6 |
| Chit + TiO2 + 4HIS/GCE | 1.57 | 1.51 ± 0.77 | 96.18 ± 0.4 | 0.9991/6 |
| Type of Electrode | SWV/ μM | HPLC/ μM | Relative Error/ % | RSD/ % |
|---|---|---|---|---|
| Chit + 4HIS/GCE | 0.24 ± 0.026 | 0.19 ± 0.0018 | 20.83 | 3.53 |
| Chit + TiO2 + 4HIS/GCE | 0.20 ± 0.09 | 5 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, J.S.; Tóth, É.; Jankovics, H.; Fort, C.I.; Turdean, G.L.; Tombacz, E.; Galambos, I. Bioengineered Flagellin–TiO2 Nanoparticle-Based Modified Glassy Carbon Electrodes as a Highly Selective Platform for the Determination of Diclofenac Sodium. Chemosensors 2023, 11, 576. https://doi.org/10.3390/chemosensors11120576
Hidalgo JS, Tóth É, Jankovics H, Fort CI, Turdean GL, Tombacz E, Galambos I. Bioengineered Flagellin–TiO2 Nanoparticle-Based Modified Glassy Carbon Electrodes as a Highly Selective Platform for the Determination of Diclofenac Sodium. Chemosensors. 2023; 11(12):576. https://doi.org/10.3390/chemosensors11120576
Chicago/Turabian StyleHidalgo, Juan Santiago, Éva Tóth, Hajnalka Jankovics, Carmen Ioana Fort, Graziella Liana Turdean, Etelka Tombacz, and Ildiko Galambos. 2023. "Bioengineered Flagellin–TiO2 Nanoparticle-Based Modified Glassy Carbon Electrodes as a Highly Selective Platform for the Determination of Diclofenac Sodium" Chemosensors 11, no. 12: 576. https://doi.org/10.3390/chemosensors11120576
APA StyleHidalgo, J. S., Tóth, É., Jankovics, H., Fort, C. I., Turdean, G. L., Tombacz, E., & Galambos, I. (2023). Bioengineered Flagellin–TiO2 Nanoparticle-Based Modified Glassy Carbon Electrodes as a Highly Selective Platform for the Determination of Diclofenac Sodium. Chemosensors, 11(12), 576. https://doi.org/10.3390/chemosensors11120576







