Rapid Determination of Meteorolite Composition Based on X-ray Phase Contrast Imaging-Assisted Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
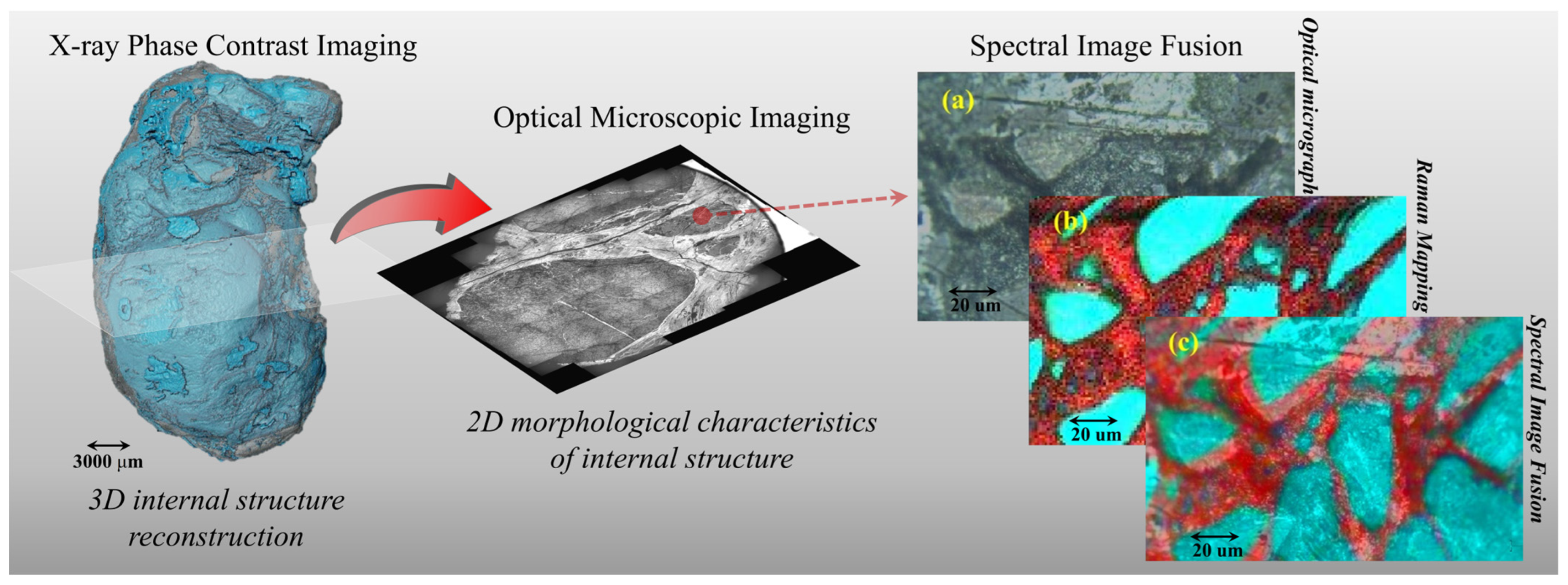
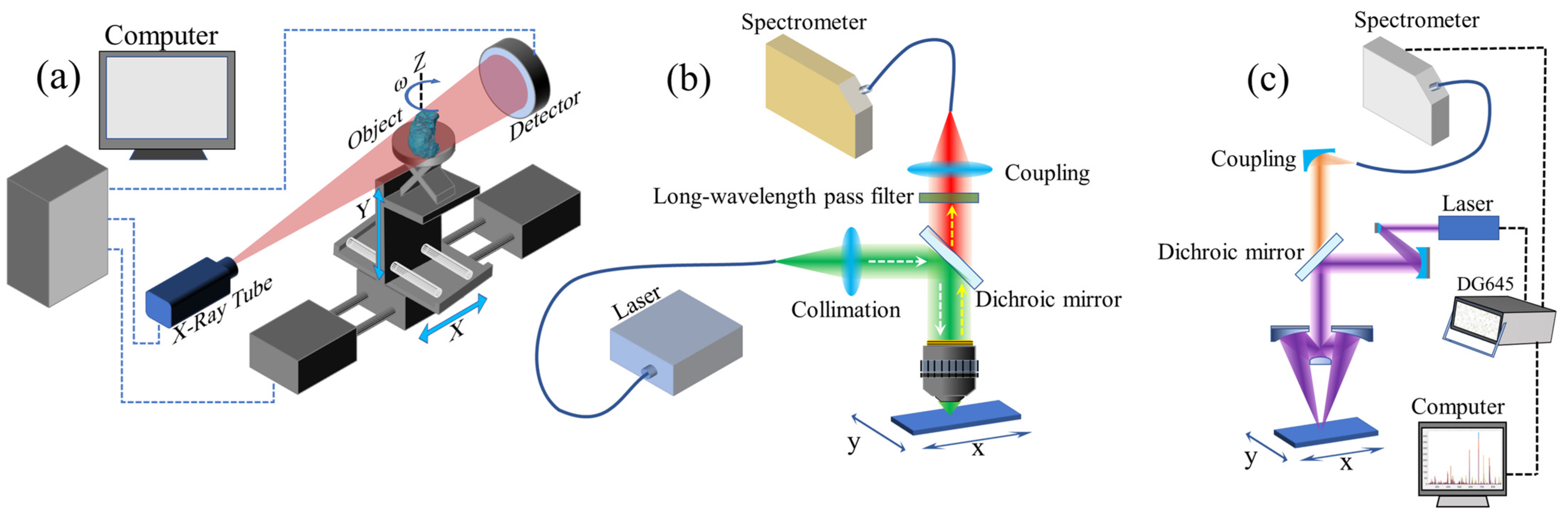
2.1. X-ray Phase Contrast Imaging Technology
2.2. Microphotograph
2.3. Raman Mapping Technology
2.4. Laser-Induced Breakdown Spectroscopy Technology
2.5. Energy Dispersive Spectroscopy (EDS)
3. Results
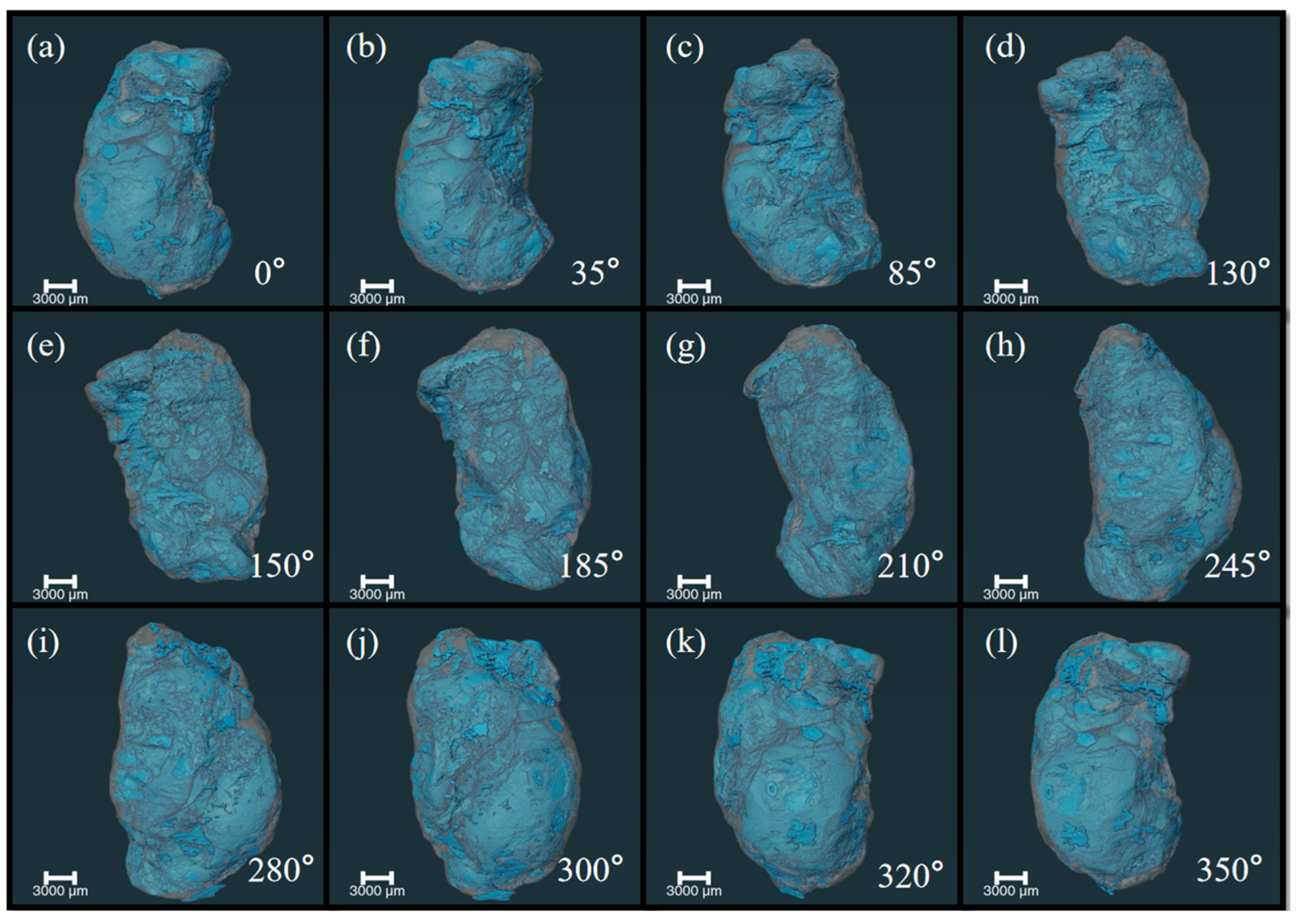
3.1. X-ray Phase Contrast Imaging Technology
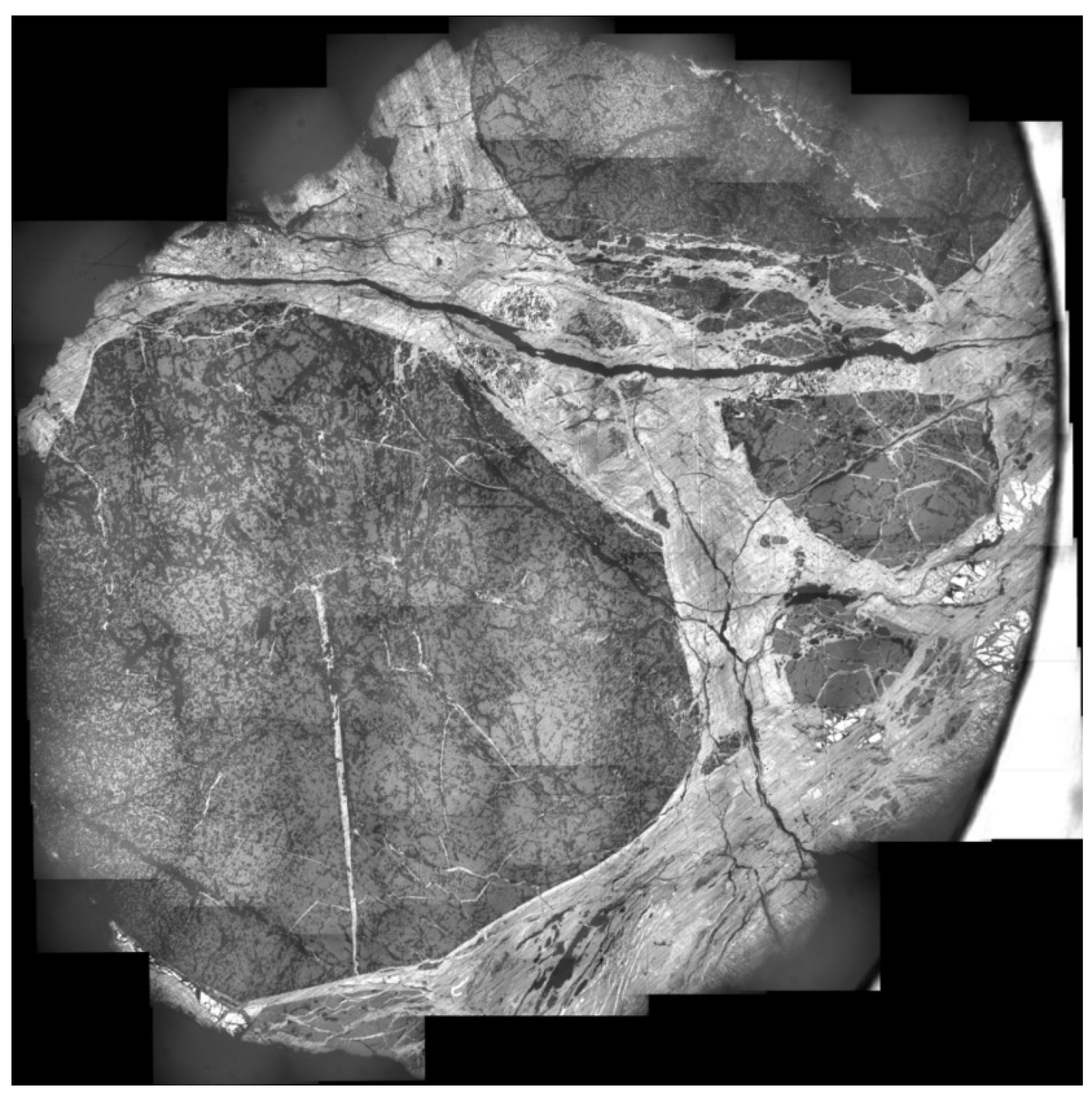
3.2. Microphotograph
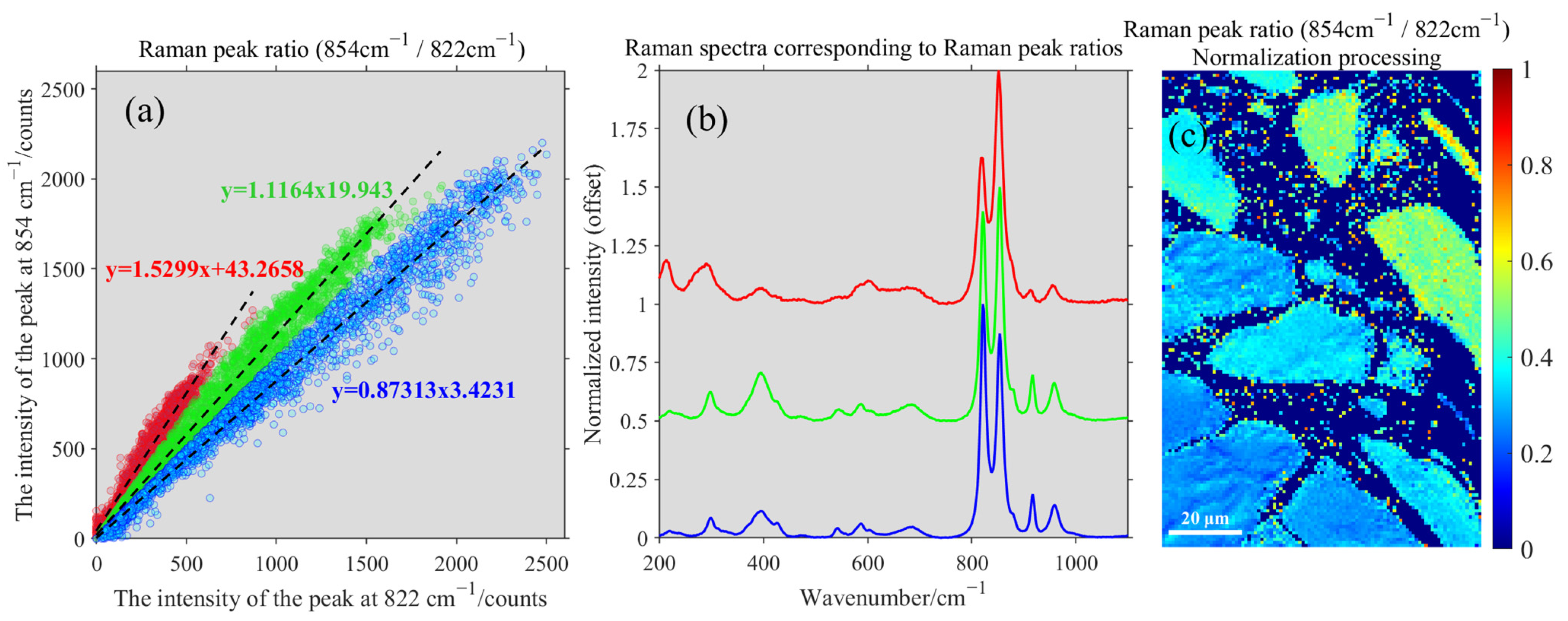
3.3. Spectroscopy Technology
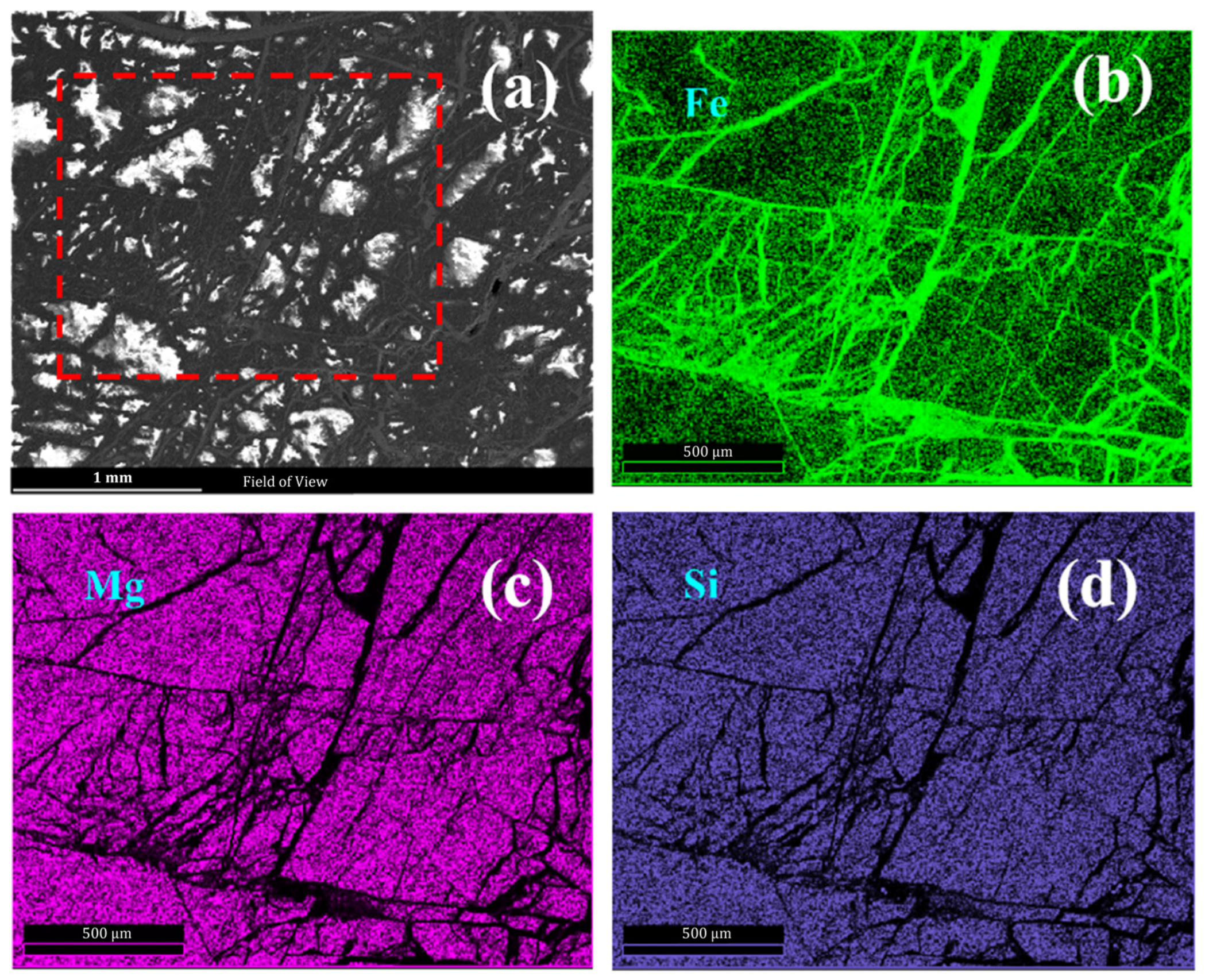
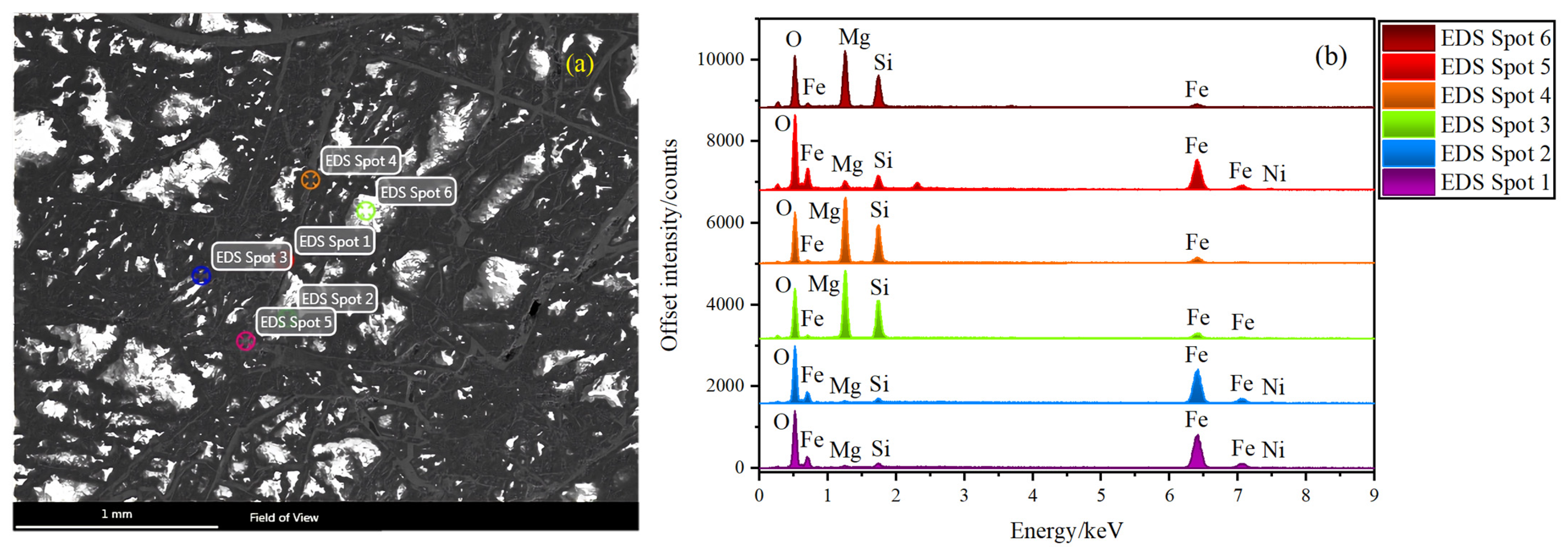
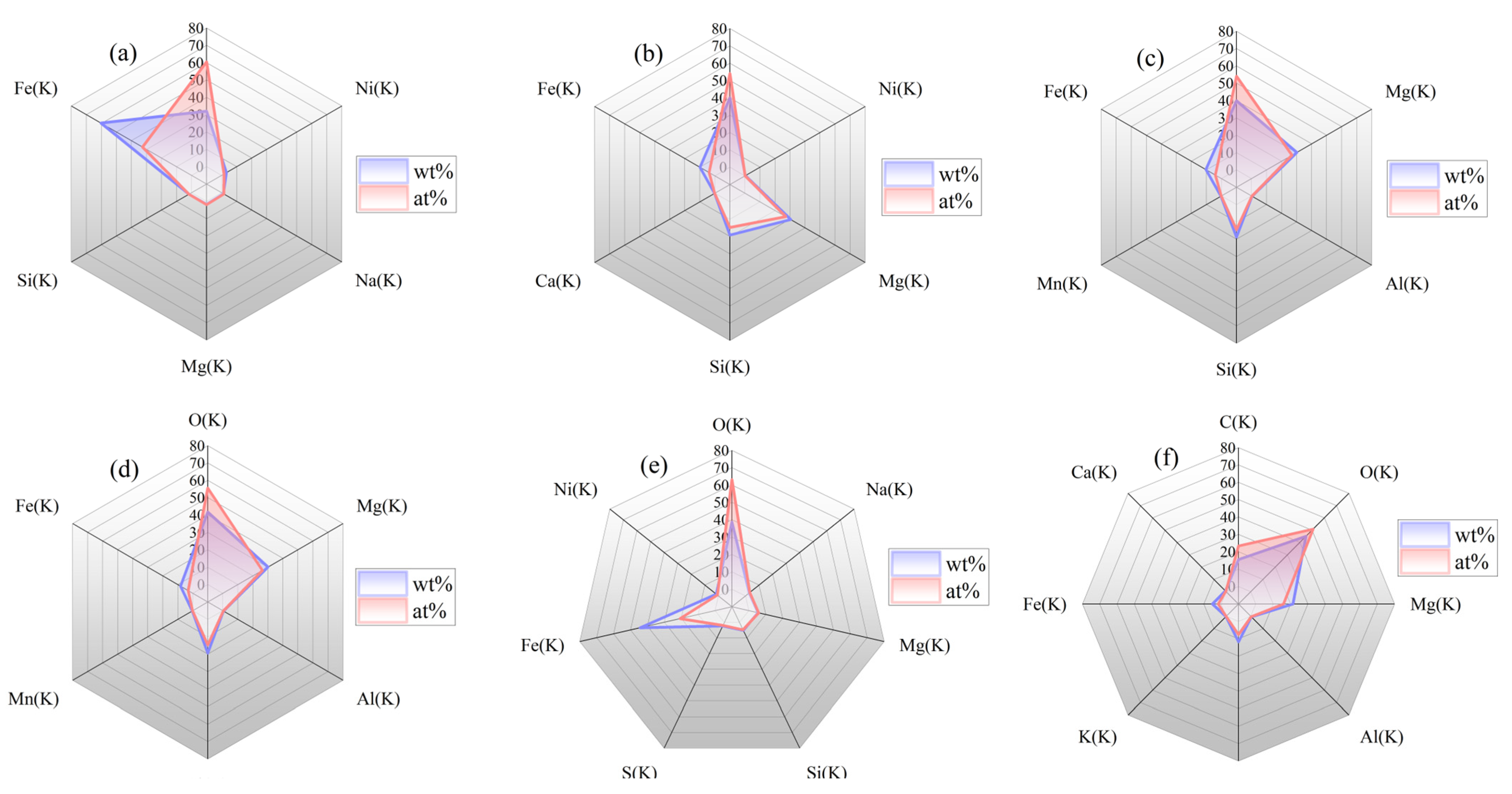
3.4. Energy Dispersive X-ray Spectroscopy Technology
4. Discussion
4.1. Discussion on X-ray Phase Contrast Imaging-Assisted Raman Spectroscopy
4.2. Third Party Test Verification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abderrazak, E.A.; Gabriela, M.M.; Luis, A.B.; Stefan, B.; Armelle, R.; Andrey, B.; Kurt, K.; Timothy, L.; Claire, R.-B.; Olabode, B.; et al. Organism motility in an oxygenated shallow-marine environment 2.1 billion years ago. Proc. Natl. Acad. Sci. USA 2019, 116, 3431–3436. [Google Scholar]
- Xu, M.; Xue, Y.; Chen, R.; Li, K.; Du, G.; Deng, B.; Xie, H.; Xiao, T. A biometrics recognition instrument using X-ray phase contrast imaging for biosafety inspection. Nucl. Tech. 2021, 44, 0802021–08020210. [Google Scholar]
- Bernard, S.; Beyssac, O.; Benzerara, K. Raman Mapping Using Advanced Line-Scanning Systems: Geological Applications. Appl. Spectrosc. 2008, 62, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Clarke, F.C.; Jamieson, M.J.; Clark, D.A.; Hammond, S.V.; Jee, R.D.; Moffat, A.C. Chemical Image Fusion. The Synergy of FT-NIR and Raman Mapping Microscopy to Enable a More Complete Visualization of Pharmaceutical Formulations. Anal. Chem. 2001, 73, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, H.; Su, Y.; Qiu, L.; Ni, H.; Xu, K.-M.; Zhao, W. In-situ high-precision surface topographic and Raman mapping by divided-aperture differential confocal Raman microscopy. Appl. Surf. Sci. 2021, 546, 149061. [Google Scholar] [CrossRef]
- Portillo, H.; Zuluaga, M.C.; Ortega, L.A.; Alonso-Olazabal, A.; Murelaga, X.; Martinez-Salcedo, A. XRD, SEM/EDX and micro-Raman spectroscopy for meteorite ogical and chemical characterization of iron slags from the Roman archaeological site of Forua (Biscay, North Spain). Microchem. J. 2018, 138, 246–254. [Google Scholar] [CrossRef]
- Veneranda, M.; Lopez-Reyes, G.; Manrique-Martinez, J.A.; Sanz-Arranz, A.; Medina, J.; Pérez, C.; Quintana, C.; Moral, A.; Rodríguez, J.A.; Zafra, J.; et al. Raman spectroscopy and planetary exploration: Testing the ExoMars/RLS system at the Tabernas Desert (Spain). Microchem. J. 2021, 165, 106149. [Google Scholar] [CrossRef]
- Cialla-May, D.; Schmitt, M.; Popp, J. Theoretical principles of Raman spectroscopy. Phys. Sci. Rev. 2019, 4, 20170040. [Google Scholar] [CrossRef]
- Prinsloo, L.; Toumié, A.; Colombam, P.; Paris, C.; Bassett, S. In search of the optimum Raman/IR signatures of potential ingredients used in San/Bushman rock art paint. J. Archaeol. Sci. 2013, 40, 2981–2990. [Google Scholar] [CrossRef]
- Iriarte, M.; Hernanz, A.; Gavira-Vallejoa, J.M.; Alcolea-González, J.; de Balbín-Behrmannb, R. μ-Raman spectroscopy of prehistoric paintings from the El Reno cave (Valdesotos, Guadalajara, Spain). J. Archaeol. Sci. Rep. 2017, 14, 454–460. [Google Scholar] [CrossRef]
- Rosina, P.; Collado, H.; Garcês, S.; Gomes, H.; Eftekhari, N.; Nicoli, M.; Vaccaro, C. Benquerencia (La Serena—Spain) rock art: An integrated spectroscopy analysis with FTIR and Raman. Heliyon 2019, 5, e02561. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, P.; Yan, X.; Zhou, Y.; Cheng, Y.; Yao, L.; Jia, J.; He, J.; Wan, X. Study on the Raman spectral characteristics of dynamic and static blood and its application in species identification. J. Photochem. Photobiol. B Biol. 2022, 232, 112478. [Google Scholar] [CrossRef] [PubMed]
- Mistek, E.; Halámková, L.; Doty, K.C.; Muro, C.K.; Lednev, I.K. Race Differentiation by Raman Spectroscopy of a Bloodstain for Forensic Purposes. Anal. Chem. 2016, 88, 7453–7456. [Google Scholar] [CrossRef]
- Pereira, A.F.M.; Rodrigues, B.V.M.; Neto, L.P.; Medeiros de OLopes, L.; da Costa, A.L.F.; Santos, A.S.; Viana, B.C.; Tosato, M.G.; da Silva, G.C.; Gusmão, G.O.M.; et al. On the effect of excessive solar exposure on human skin: Confocal Raman spectroscopy as a tool to assess advanced glycation end products. Vib. Spectrosc. 2021, 114, 103234. [Google Scholar] [CrossRef]
- Voropaev, S.A.; Eliseev, A.A.; Petukhov, D.I. Mineralogy of meteorite chelyabinsk as determined by raman spectroscopy. Lpi Contrib. 2014, 1783. [Google Scholar]
- Ling, Z.C.; Wang, A. Secondary minerals in martian meteorite mil 03346 as detected by raman imaging spectroscopy. Lpi Contrib. 2014, 1783, 5089. [Google Scholar]
- Wang, H.; Wan, X. Effect of chlorophyll fluorescence quenching on quantitative analysis of adulteration in extra virgin olive oil. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119183. [Google Scholar] [CrossRef]
- Barros, I.H.; Paixão, L.S.; Nascimento, M.H.; Lacerda, V., Jr.; Filgueiras, P.R.; Romão, W. Use of portable Raman spectroscopy in the quality control of extra virgin olive oil and adulterated compound oils. Vib. Spectrosc. Int. J. Devoted Appl. Infrared Raman Spectrosc. 2021, 116, 103299. [Google Scholar] [CrossRef]
- Población, I.; Torre-Fdez, I.; Aramendia, J.; López-Reyes, G.; Cabalín, L.M.; Madariaga, J.M.; Rull, F.; Laserna, J.J.; Carrizo, D.; Martínez-Frías, J.; et al. Raman spectroscopy, assisted by X-ray fluorescence and laser-induced breakdown spectroscopy, to characterise original and altered mineral phases in the NWA 2975 Martian shergottite. J. Raman Spectrosc. 2023, 1–15. [Google Scholar] [CrossRef]
- Search RRUFF Sample Data. Available online: https://rruff.info (accessed on 12 July 2023).
- Tu, T.M.; Su, S.C.; Shyu, H.C.; Huang, P.S. A new look at IHS-like image fusion methods. Inf. Fusion 2001, 2, 177–186. [Google Scholar] [CrossRef]
- Rahmani, S.; Strait, M.; Merkurjev, D.; Moeller, M.; Wittman, T. An adaptive IHS pan-sharpening method. IEEE Geosci. Remote Sens. Lett. 2010, 7, 746–750. [Google Scholar] [CrossRef]
- Harris, J.R.; Murray, R.; Hirose, T. IHS transform for the integration of radar imagery with other remotely sensed data. Photogramm. Eng. Remote Sens. 1990, 56, 1631–1641. [Google Scholar]
- Chen, C.M.; Hepner, G.F.; Forster, R.R. Fusion of hyperspectral and radar data using the IHS transformation to enhance urban surface features. ISPRS J. Photogramm. Remote Sens. 2003, 58, 19–30. [Google Scholar] [CrossRef]
- Chopelas, A. Single crystal Raman spectra of forsterite, fayalite, and monticellite. Am. Meteoriteogist 1991, 76, 1101–1109. [Google Scholar] [CrossRef]
- Mouri, T.; Enami, M. Raman spectroscopic study of olivine-group meteorites. J. Meteoriteogical Petrol. Sci. 2008, 103, 100–104. [Google Scholar] [CrossRef]
- Hartigan, J.A.; Wong, M.A. A K-Means Clustering Algorithm. Appl. Stat. 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Wang, A.; Kuebler, K.; Jolliff, B.; Haskin, L.A. Mineralogy of a Martian meteorite as determined by Raman spectroscopy. J. Raman Spectrosc. 2004, 35, 504–514. [Google Scholar] [CrossRef]
- Fortes, F.J.; Moros, J.; Lucena, P.; Cabalín, L.M.; Laserna, J.J. Laser-Induced Breakdown Spectroscopy. Anal. Chem. 2013, 85, 640–669. [Google Scholar] [CrossRef]
- Jung, J.; Yang, J.-H.; Yoh, J.J. An optimal configuration for spark-induced breakdown spectroscopy of bulk meteorites aimed at planetary analysis. J. Anal. Atiomic Spectrom. 2020, 6, 1103–1114. [Google Scholar] [CrossRef]
- Kim, Y.; Fabre, C.; Cauzid, J. Access to quantitative analysis of carbonates using a portable LIBS instrument: First applications to single meteorites and meteorite mixtures. Spectrochim. Acta Part B At. Spectrosc. 2022, 191, 106397. [Google Scholar] [CrossRef]
- Anake, W.U.; Ana, G.R.; Benson, N.U. Study of surface morphology, elemental composition and sources of airborne fine particulate matter in Agbara industrial estate, Nigeria. Int. J. Appl. Environ. Sci. 2016, 11, 881–890. [Google Scholar]
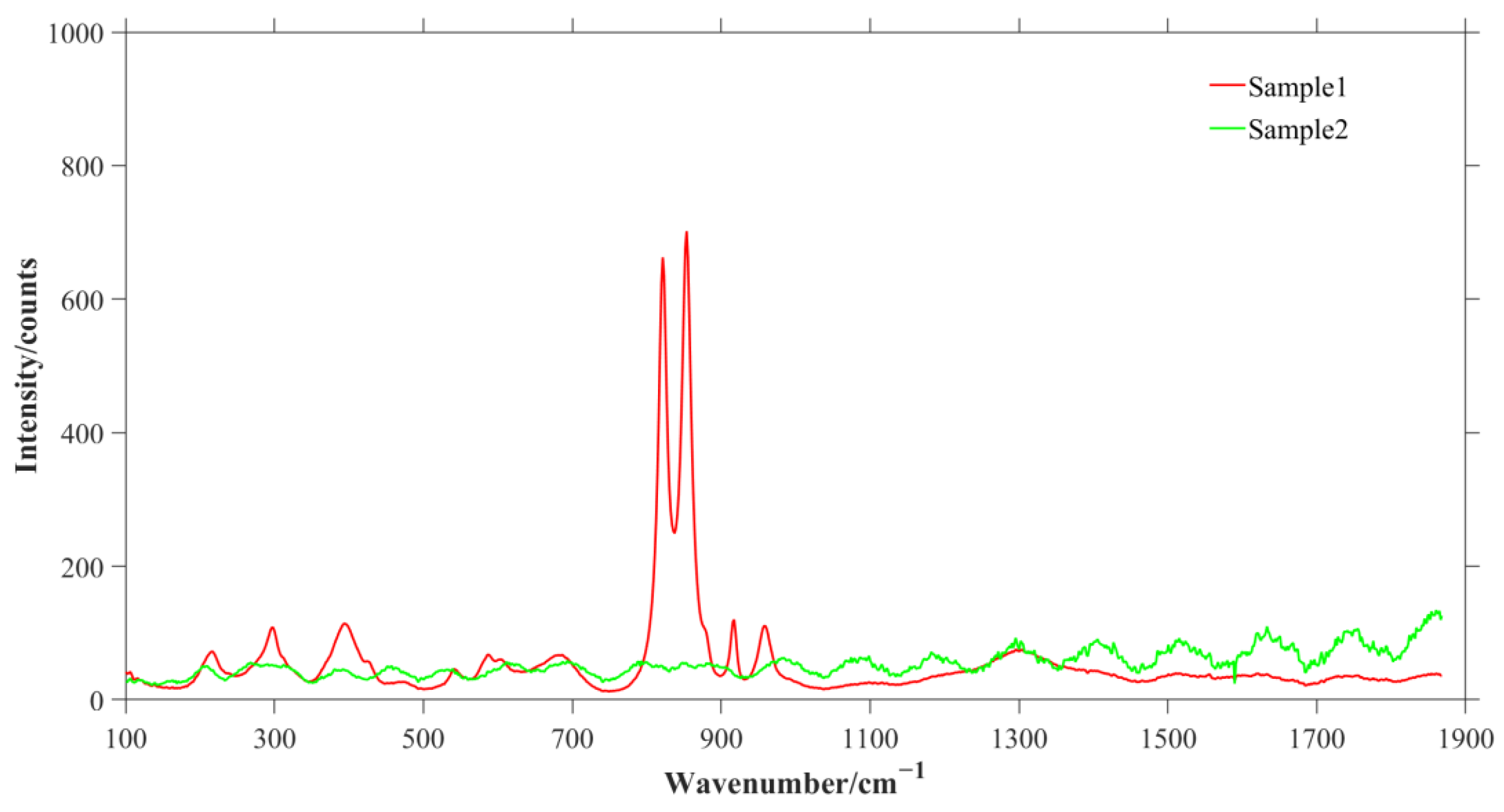
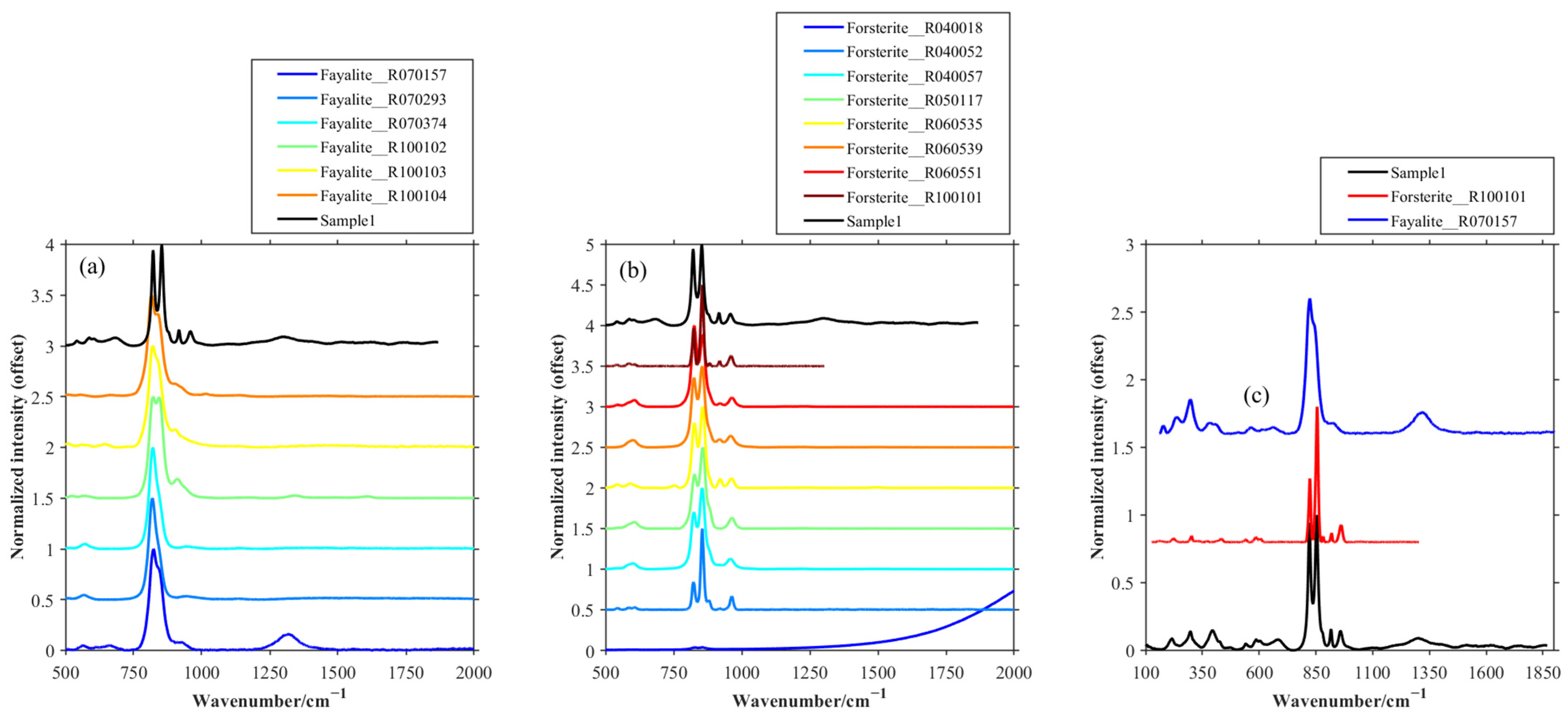
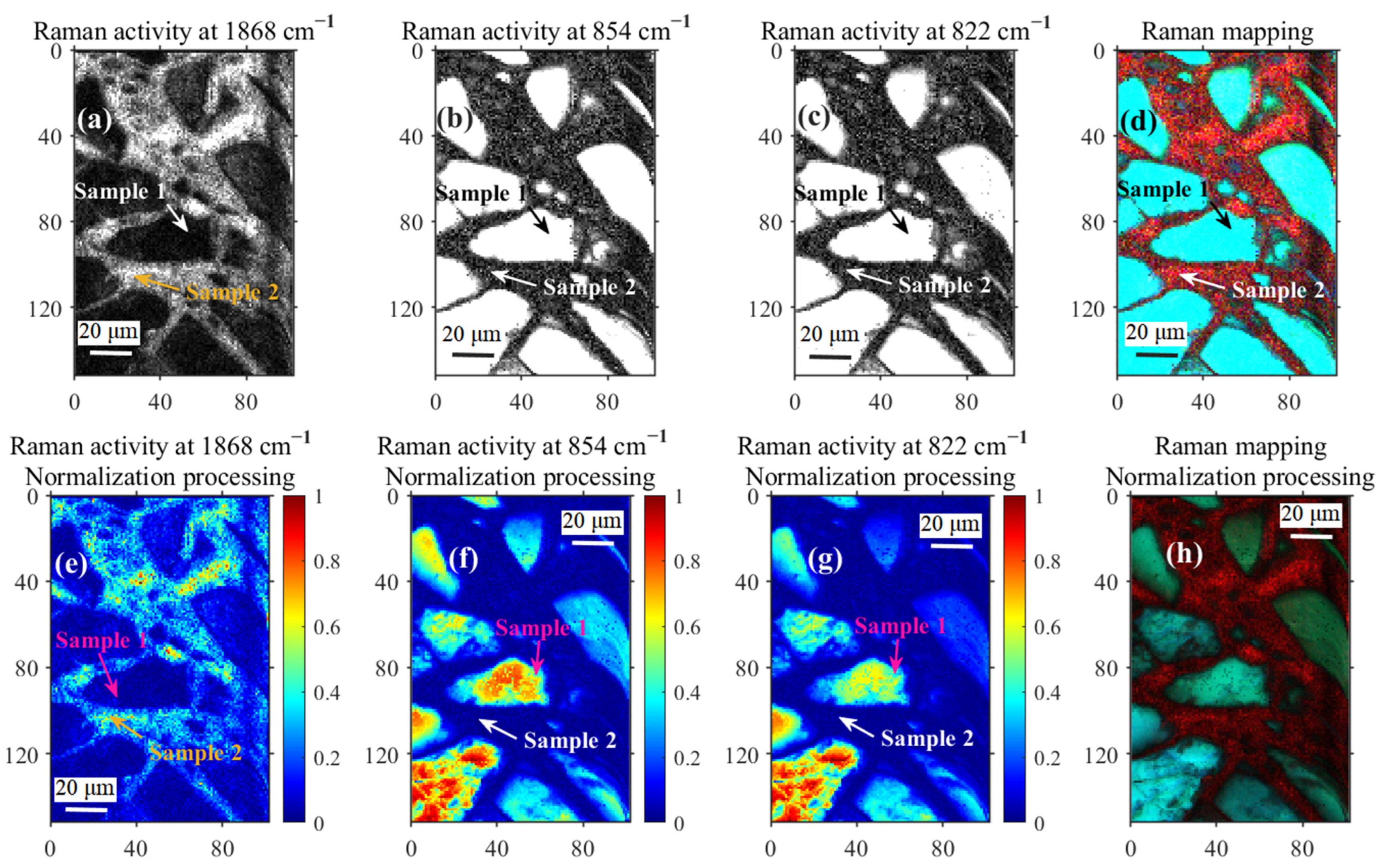
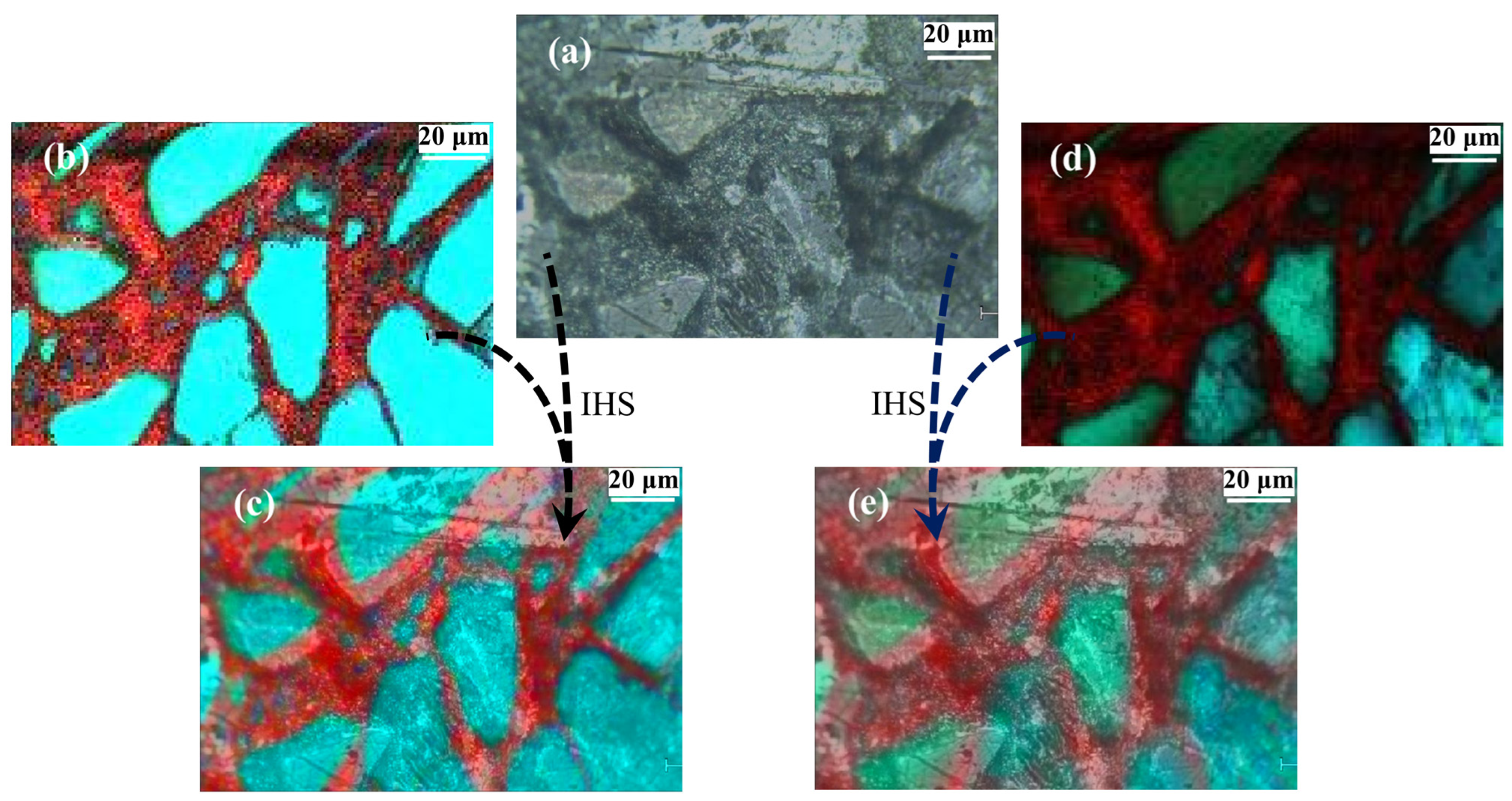


| Sample | Tube Voltage/kV | Tube Current/μA | Exposure Time/s | Distance from Light Source to Sample/mm | Distance from Light Source to Detector/mm | Pixel Size/μm |
|---|---|---|---|---|---|---|
| Pallasite | 140 | 80 | 0.2 | 85 | 367 | 11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Fang, P.; Wang, Y.; Xin, Y.; Xiong, S.; Liu, S.; Xue, Y.; Zhang, L.; Wan, X. Rapid Determination of Meteorolite Composition Based on X-ray Phase Contrast Imaging-Assisted Raman Spectroscopy. Chemosensors 2023, 11, 563. https://doi.org/10.3390/chemosensors11110563
Wang H, Fang P, Wang Y, Xin Y, Xiong S, Liu S, Xue Y, Zhang L, Wan X. Rapid Determination of Meteorolite Composition Based on X-ray Phase Contrast Imaging-Assisted Raman Spectroscopy. Chemosensors. 2023; 11(11):563. https://doi.org/10.3390/chemosensors11110563
Chicago/Turabian StyleWang, Hongpeng, Peipei Fang, Yian Wang, Yingjian Xin, Shengjun Xiong, Sicong Liu, Yanling Xue, Liang Zhang, and Xiong Wan. 2023. "Rapid Determination of Meteorolite Composition Based on X-ray Phase Contrast Imaging-Assisted Raman Spectroscopy" Chemosensors 11, no. 11: 563. https://doi.org/10.3390/chemosensors11110563
APA StyleWang, H., Fang, P., Wang, Y., Xin, Y., Xiong, S., Liu, S., Xue, Y., Zhang, L., & Wan, X. (2023). Rapid Determination of Meteorolite Composition Based on X-ray Phase Contrast Imaging-Assisted Raman Spectroscopy. Chemosensors, 11(11), 563. https://doi.org/10.3390/chemosensors11110563







