Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers
Abstract
1. Introduction
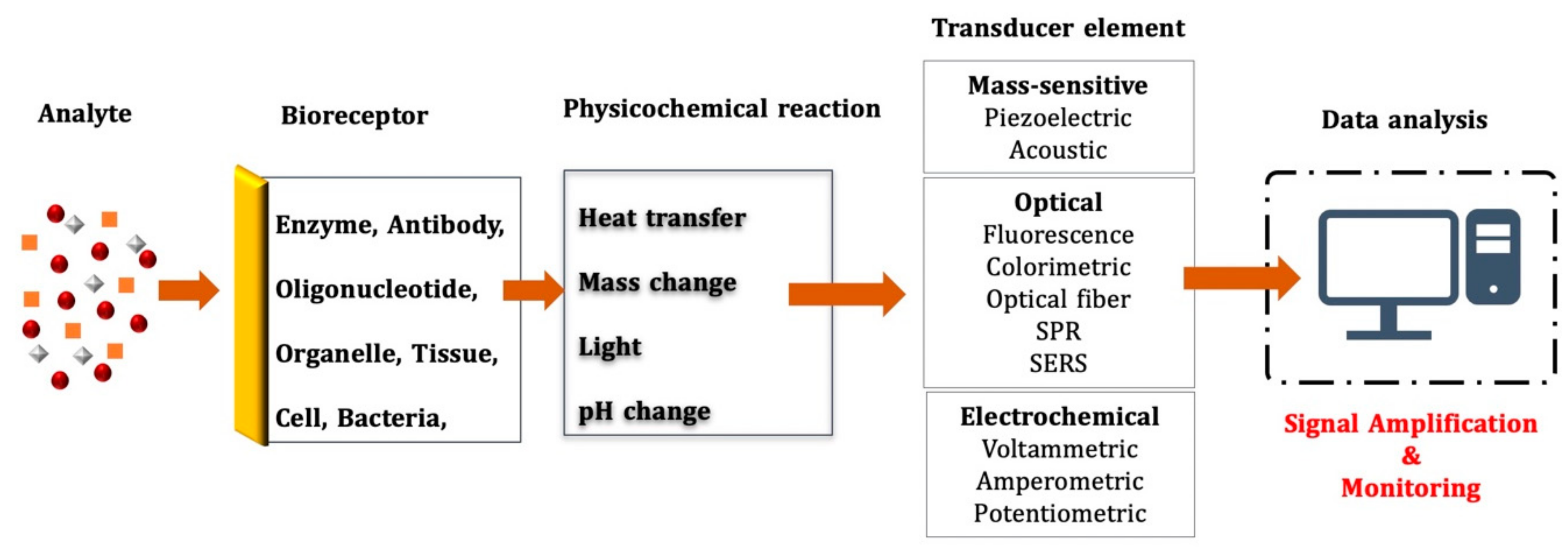
2. Biosensor Devices
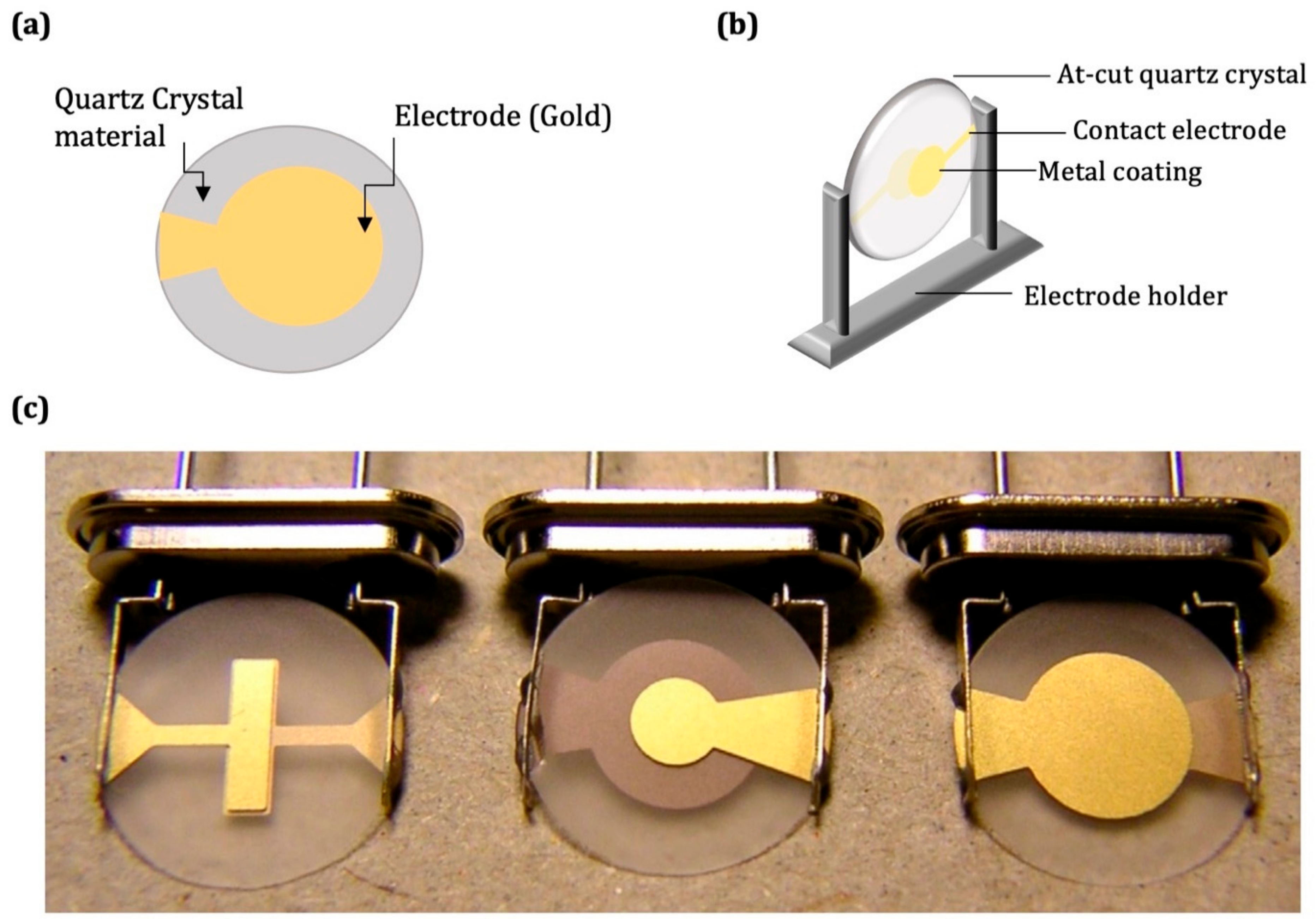
2.1. QCM Technology
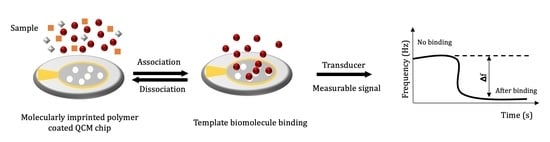
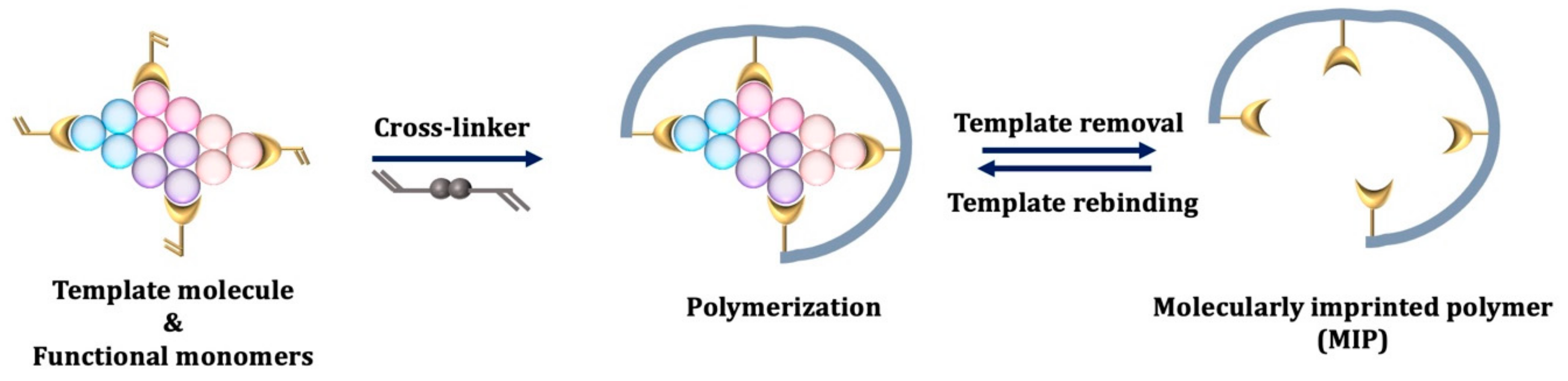
3. Molecular Imprinting Technique
4. QCM Biosensors Based on Molecular Imprinting for Biomarkers
4.1. Protein
4.2. Bacteria
4.3. Virus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skládal, P. Piezoelectric Biosensors. Trends Anal. Chem. 2016, 79, 127–133. [Google Scholar] [CrossRef]
- Ferreira, G.N.M.; Da-Silva, A.-C.; Tome, B. Acoustic Wave Biosensors: Physical Models and Biological Applications of Quartz Crystal Microbalance. Trends Biotechnol. 2009, 27, 689–697. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H. Recent Advances in Acoustic Wave Biosensors for the Detection of Disease-Related Biomarkers: A Review. Anal. Chim. Acta 2021, 1164, 338321. [Google Scholar] [CrossRef] [PubMed]
- Jean, H.; Saha, T.; Tey, T.; Siang, W.; Wei, C. Quartz Crystal Microbalance-Based Biosensors as Rapid Diagnostic Devices for Infectious Diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Kelley, S.O. What Are Clinically Relevant Levels of Cellular and Biomolecular Analytes? ACS Sens. 2017, 2, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, C.; Świtni, M.A.; Halvorsen, T.G.; Cormack, P.A.G.; Sellergren, B.; Reubsaet, L. Automated Protein Biomarker Analysis: On-Line Extraction of Clinical Samples by Molecularly Imprinted Polymers. Sci. Rep. 2017, 7, 44298. [Google Scholar] [CrossRef]
- Kelley, S.O. Advancing Ultrasensitive Molecular and Cellular Analysis Methods to Speed and Simplify the Diagnosis of Disease. Acc. Chem. Res. 2017, 50, 503–507. [Google Scholar] [CrossRef]
- Giamblanco, N.; Conoci, S.; Russo, D.; Marletta, G. Single-Step Label-Free Hepatitis B Virus Detection by a Piezoelectric Biosensor. RSC Adv. 2015, 5, 38152–38158. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Kumar, S.; Swaraj, S.; Neethirajan, S. Advances in Biosensors and Optical Assays for Diagnosis and Detection of Malaria. Biosens. Bioelectron. 2018, 105, 188–210. [Google Scholar] [CrossRef]
- Kosack, C.S.; Page, A.; Klatser, P.R. A Guide to Aid the Selection of Diagnostic Tests. Bull World Health Organ. 2017, 95, 639–645. [Google Scholar] [CrossRef]
- Orazio, P.D. Biosensors in Clinical Chemistry—2011 Update. Clin. Chim. Acta 2011, 412, 1749–1761. [Google Scholar] [CrossRef]
- Tothill, I.E. Biosensors for Cancer Markers Diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef]
- Mascini, M.; Tombelli, S. Biosensors for Biomarkers in Medical Diagnostics. Biomarkers 2008, 13, 637–657. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Horgan, A. The Rational Development of Molecularly Imprinted Polymer-Based Sensors for Protein Detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef]
- Montagut, Y.; Garcia, J.V.; Jimenez, Y.; March, C.; Montoya, A.; Arnau, A. Validation of a Phase-Mass Characterization Concept and Interface for Acoustic Biosensors. Sensors 2011, 11, 4702–4720. [Google Scholar] [CrossRef]
- Özgür, E.; Parlak, O.; Beni, V.; Turner, A.P.F.; Uzun, L. Bioinspired Design of a Polymer-Based Biohybrid Sensor Interface. Sens. Actuators B Chem. 2017, 251, 674–682. [Google Scholar] [CrossRef]
- Marx, K.A. Quartz Crystal Microbalance: A Useful Tool for Studying Thin Polymer Films and Complex Biomolecular Systems at the Solution—Surface Interface. Biomacromolecules 2003, 4, 1009–1120. [Google Scholar] [CrossRef]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly Imprinted Polymers as Receptor Mimics for Selective Cell Recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef]
- Pan, G.; Shinde, S.; Yeung, S.Y.; Jakštaitė, M.; Li, Q.; Wingren, A.G.; Sellergren, B. An Epitope Imprinted Biointerface with Dynamic Bioactivity for Modulating Cell-Biomaterial Interactions. Angew. Chem. Int. Ed. 2017, 56, 15959–15963. [Google Scholar] [CrossRef]
- Mosbach, K. Molecular Imprinting. Techniques 1994, 19, 9–14. [Google Scholar] [CrossRef]
- Saylan, Y.; Denizli, A. Molecularly Imprinted Polymer-Based Microfluidic Systems for Point-of-Care Applications. Micromachines 2019, 10, 766. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly Imprinted Polymer Based Electrochemical Biosensors: Overcoming the Challenges of Detecting Vital Biomarkers and Speeding up Diagnosis. Talanta Open 2020, 2, 100018. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; de Cortalezzi, M.M.F. Molecularly Imprinted Polymers (Mips) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef]
- Murphy, A.C.; Wechsler, M.E.; Peppas, N.A. Recent Advancements in Biosensing Approaches for Screening and Diagnostic Applications. Curr. Opin. Biomed. Eng. 2021, 19, 100318. [Google Scholar] [CrossRef]
- Rong, G.; Corrie, S.R.; Clark, H.A. In Vivo Biosensing: Progress and Perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Bakhshpour, M.; Denizli, A. Molecularly imprinted bionanomaterials and their biomedical applications. In Bionanomaterials Fundamentals and Biomedical Applications; Singh, R.P., Singh, K.R., Eds.; IOP Science: London, UK, 2021; pp. 9–27. ISBN 9780750337670. [Google Scholar]
- Rovira, J.; Domingo, J.L. Human Health Risks Due to Exposure to Inorganic and Organic Chemicals from Textiles: A Review. Environ. Res. 2019, 168, 62–69. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and Their Applications—A Review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Jandas, P.J.; Prabakaran, K.; Luo, J.; Derry Holaday, M.G. Effective Utilization of Quartz Crystal Microbalance as a Tool for Biosensing Applications. Sens. Actuators A. Phys. 2021, 331, 113020. [Google Scholar] [CrossRef]
- Nelson, J.M.; Griffin, E.G. Adsorption of Invertase. J. Am. Chem. Soc. 1916, 38, 1109–1115. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors–Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Mahmoudifard, M. Pivotal Role of Electrospun Nanofibers in Microfluidic Diagnostic Systems-a Review. J. Mater. Chem. B 2019, 7, 4602–4619. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for Cardiac Biomarkers Detection: A Review. Sens. Actuators B Chem. 2012, 171–172, 62–76. [Google Scholar] [CrossRef]
- Tibuzzi, A.; Rea, G.; Pezzotti, G.; Esposito, D.; Johanningmeier, U.; Giardi, M.T. A New Miniaturized Multiarray Biosensor System for Fluorescence Detection. J. Phys. Condens. Matter 2007, 19, 395006. [Google Scholar] [CrossRef]
- Rahtuvanoğlu, A.; Akgönüllü, S.; Karacan, S.; Denizli, A. Biomimetic Nanoparticles Based Surface Plasmon Resonance Biosensors for Histamine Detection in Foods. ChemistrySelect 2020, 5, 5683–5692. [Google Scholar] [CrossRef]
- Heller, D.A.; Baik, S.; Eurell, T.E.; Strano, M.S. Single-Walled Carbon Nanotube Spectroscopy in Live Cells: Towards Long-Term Labels and Optical Sensors. Adv. Mater. 2005, 17, 2793–2799. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Liu, B.; Bai, P.; Wang, C.; Xu, J.; Wang, H. Highly Selective Gas Sensor Based on Hydrophobic Silica Decorated with Trimethoxyoctadecylsilane. ACS Appl. Mater. Interfaces 2021, 13, 1956–1966. [Google Scholar] [CrossRef]
- Kim, K.; Ellis, J.E.; Howard, B.H.; Ohodnicki, P.R. Centimeter-Scale Pillared-Layer Metal—Organic Framework Thin Films Mediated by Hydroxy Double Salt Intermediates for CO 2 Sensor Applications. ACS Appl. Mater. Interfaces 2021, 13, 2062–2071. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, L.; Cao, B.; Xue, X.; Liu, W.; Zhang, C.; Wang, W. Effects of Temperature and Humidity on the Performance of a PECH Polymer Coated SAW Sensor. RSC Adv. 2020, 10, 18099–18106. [Google Scholar] [CrossRef]
- Jeng, M.J.; Sharma, M.; Li, Y.C.; Lu, Y.C.; Yu, C.Y.; Tsai, C.L.; Huang, S.F.; Chang, L.B.; Lai, C.S. Surface Acoustic Wave Sensor for C-Reactive Protein Detection. Sensors 2020, 20, 6640. [Google Scholar] [CrossRef]
- Temel, F. One Novel Calix[4]Arene Based QCM Sensor for Sensitive, Selective and High Performance-Sensing of Formaldehyde at Room Temperature. Talanta 2020, 211, 120725. [Google Scholar] [CrossRef]
- Haghighi, E.; Zeinali, S. Formaldehyde Detection Using Quartz Crystal Microbalance (QCM) Nanosensor Coated by Nanoporous MIL-101(Cr) Film. Microporous Mesoporous Mater. 2020, 300, 110065. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Y.P.; Li, D.M.; Ma, D.; Zheng, S.R.; Wu, C.H.; Li, J.Y.; Zhang, W.G. The Interaction of an Amorphous Metal-Organic Cage-Based Solid (AMOC) with MiRNA/DNA and Its Application on a Quartz Crystal Microbalance (QCM) Sensor. Chem. Commun. 2020, 56, 591–594. [Google Scholar] [CrossRef]
- Ghatak, B.; Banerjee, S.; Ali, S.B.; Das, N.; Tudu, B.; Pramanik, P.; Mukherji, S.; Bandyopadhyay, R. Development of a Low-Cost Portable Aroma Sensing System for Identifying Artificially Ripened Mango. Sens. Actuators A Phys. 2021, 331, 112964. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Glanzing, G.; Leidl, A.; Lieberzeit, P.A.; Dickert, F.L. Imprinted Sol-Gel Materials for Monitoring Degradation Products in Automotive Oils by Shear Transverse Wave. Anal. Chim. Acta 2010, 675, 53–57. [Google Scholar] [CrossRef]
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.Z.; Latif, U.; Feroz, S. Gravimetric Viral Diagnostics: QCM Based Biosensors for Early Detection of Viruses. Chemosensors 2017, 5, 7. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Sadowska, M.; Paulina, Z. Applicability of QCM - D for Quantitative Measurements of Nano- and Microparticle Deposition Kinetics: Theoretical Modeling and Experiments. Anal. Chem. 2020, 92, 15087–15095. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Sadowska, M. Hydrodynamic Solvent Coupling E Ff Ects in Quartz Crystal Microbalance Measurements of Nanoparticle Deposition Kinetics. Anal. Chem. 2020, 92, 3896–3903. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, L.; Liu, Y.; Harms, H.; Wick, L.Y. Effects of Electrokinetic Phenomena on Bacterial Deposition Monitored by Quartz Crystal Microbalance with Dissipation Monitoring. Environ. Sci. Technol. 2020, 54, 14036–14045. [Google Scholar] [CrossRef]
- Son, J.; Ji, S.; Kim, S.; Kim, S.; Kim, S.K.; Song, W.; Lee, S.S.; Lim, J.; An, K.; Myung, S. GC-like Graphene-Coated Quartz Crystal Microbalance Sensor with Microcolumns. ACS Appl. Mater. Interfaces 2021, 13, 4703–4710. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wang, K.; Luo, Z.; Xue, Z.; Gao, H.; Cao, Z.; Cheng, J.; Liu, C.; Zhang, L. Construction of Self-Assembled Polyelectrolyte/Cationic Microgel Multilayers and Their Interaction with Anionic Dyes Using Quartz Crystal. ACS Omega 2021, 6, 5764–5774. [Google Scholar] [CrossRef]
- Chinwangso, P.; Lee, H.J.; Lee, T.R. Self-Assembled Monolayers Generated from Unsymmetrical Partially Fluorinated Spiroalkanedithiols. Langmuir 2015, 31, 13341–13349. [Google Scholar] [CrossRef]
- Sauerbrey, G. Use of Quartz Crystals for Weighing Thin Layers and for Microweighing. Mag. Physic 1959, 155, 206–222. [Google Scholar]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz Crystal Microbalance: Sensing Cell-Substrate Adhesion and Beyond. Biosens. Bioelectron. 2018, 99, 593–602. [Google Scholar] [CrossRef]
- Latif, U.; Can, S.; Hayden, O.; Grillberger, P.; Dickert, F.L. Sauerbrey and Anti-Sauerbrey Behavioral Studies in QCM Sensors—Detection of Bioanalytes. Sens. Actuators B. Chem. 2013, 176, 825–830. [Google Scholar] [CrossRef]
- Anderson, H.; Jönsson, M.; Vestling, L.; Lindberg, U.; Aastrup, T. Quartz Crystal Microbalance Sensor Design. I. Experimental Study of Sensor Response and Performance. Sens. Actuators B Chem. 2007, 123, 27–34. [Google Scholar] [CrossRef]
- Sullivan, C.K.O.; Guilbault, G.G. Commercial Quartz Crystal Microbalances—Theory and Applications. Biosens. Bioelectron. 1999, 14, 663–670. [Google Scholar] [CrossRef]
- Han, D.H.; Kang, L.H. Piezoelectric Properties of Paint Sensor According to Piezoelectric Materials. Funct. Compos. Struct. 2020, 2, 2–13. [Google Scholar] [CrossRef]
- Shindo, Y.; Domon, W.; Narita, F. Dynamic Bending of a Symmetric Piezoelectric Laminated Plate with a through Crack. Theor. Appl. Fract. Mech. 1998, 28, 175–182. [Google Scholar] [CrossRef]
- Fay, B.; Lewin, P.A.; Ludwig, G.; Sessler, G.M.; Yang, G. The influence of spatial polarization distribution on spot poled PVDF membrane hydrophone performance. Ultrasound Med Biol. 1992, 18, 625–635. [Google Scholar] [CrossRef]
- Yoshimura, H.N.; Molisani, A.L.; Narita, N.E.; Manholetti, J.L.A.; Cavenaghi, J.M. Mechanical Properties and Microstructure of Zinc Oxide Varistor Ceramics. Mater. Sci. Forum 2006, 530–531, 408–413. [Google Scholar] [CrossRef]
- Symposium, U. 1983 IEEE Ultrasonics Symposium Abstracts. IEEE Trans. Sonics Ultrason. 2012, 32, 78–122. [Google Scholar] [CrossRef]
- Kamiya, T. Calculation of Crystal Structures, Dielectric Constants and Piezoelectric Properties of Wurtzite-Type Crystals Using Ab-Initio Periodic Hartree-Fock Method. Jpn. J. Appl. Phys. 1996, 35, 4421. [Google Scholar] [CrossRef]
- Narita, F.; Shindo, Y. Piezoelectric Detection and Response Characteristics of Barium Titanate Unimorph Cantilevers Under AC Electric Fields. Int. J. Metall. Mater. Eng. 2015, 1, 10–13. [Google Scholar] [CrossRef][Green Version]
- Hwa, L.G.; Lu, C.L.; Liu, L.C. Elastic Moduli of Calcium Alumino-Silicate Glasses Studied by Brillouin Scattering. Mater. Res. Bull. 2000, 35, 1285–1292. [Google Scholar] [CrossRef]
- Carr, P.H. Measurement of the Piezoelectric Constant of Quartz at Gigacycle Frequencies. J. Acoust. Soc. Am. 1967, 41, 75–83. [Google Scholar] [CrossRef]
- Kuma, S.; Woldemariam, M.M. Structural, Electronic, Lattice Dynamic, and Elastic Properties of SnTiO3 and PbTiO3 Using Density Functional Theory. Adv. Condens. Matter Phys. 2019, 2019, 317614. [Google Scholar] [CrossRef]
- Haun, M.J.; Furman, E.; Jang, S.J.; McKinstry, H.A.; Cross, L.E. Thermodynamic Theory of BiFeO 3 -PbTiO 3. J. Appl. Phys. 1987, 6, 3331. [Google Scholar] [CrossRef]
- Ravina; Dalal, A.; Mohan, H.; Prasad, M.; Pundir, C.S. Detection Methods for Influenza A H1N1 Virus with Special Reference to Biosensors: A Review. Biosci. Rep. 2020, 40, 1–18. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in Biosensors: Principle, Architecture and Applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Pan, M.; Li, R.; Xu, L.; Yang, J.; Cui, X.; Wang, S. Reproducible Molecularly Imprinted Piezoelectric Sensor for Accurate and Sensitive Detection of Ractopamine in Swine and Feed Products. Sensors 2018, 18, 1870. [Google Scholar] [CrossRef]
- Lee, L.T.; Mohamed, M.A.; Yahya, I.; Kulothungan, J.; Muruganathan, M.; Mizuta, H. Comparison of Piezoelectric Energy Harvesting Performance Using Silicon and Graphene Cantilever Beam. Microsyst. Technol. 2018, 24, 3783–3789. [Google Scholar] [CrossRef]
- Rianjanu, A.; Roto, R.; Julian, T.; Hidayat, S.N.; Kusumaatmaja, A.; Suyono, E.A.; Triyana, K. Polyacrylonitrile Nanofiber-Based Quartz Crystal Microbalance for Sensitive Detection of Safrole. Sensors 2018, 18, 1150. [Google Scholar] [CrossRef]
- Battal, D.; Akgönüllü, S.; Yalcin, M.S.; Yavuz, H.; Denizli, A. Molecularly Imprinted Polymer Based Quartz Crystal Microbalance Sensor System for Sensitive and Label-Free Detection of Synthetic Cannabinoids in Urine. Biosens. Bioelectron. 2018. [Google Scholar] [CrossRef]
- Wasilewski, T.; Szulczyński, B.; Kamysz, W.; Gębicki, J.; Namieśnik, J. Evaluation of Three Peptide Immobilization Techniques on a Qcm Surface Related to Acetaldehyde Responses in the Gas Phase. Sensors 2018, 18, 3942. [Google Scholar] [CrossRef]
- Hardoyono, F.; Windhani, K. Identification and Detection of Bioactive Compounds in Turmeric (Curcuma LongaL.) Using a Gas Sensor Array Based on Molecularly Imprinted Polymer Quartz Crystal Microbalance. New J. Chem. 2021, 45, 17930–17940. [Google Scholar] [CrossRef]
- Shim, D.Y.; Chang, S.M.; Kim, J.M. Development of Fast Resettable Gravimetric Aromatic Gas Sensors Using Quartz Crystal Microbalance. Sens. Actuators B Chem. 2021, 329, 129143. [Google Scholar] [CrossRef]
- Meléndrez, D.; Hampitak, P.; Jowitt, T.; Iliut, M.; Vijayaraghavan, A. Development of an Open-Source Thermally Stabilized Quartz Crystal Microbalance Instrument for Biomolecule-Substrate Binding Assays on Gold and Graphene. Anal. Chim. Acta 2021, 1156. [Google Scholar] [CrossRef]
- Komorek, P.; Wałek, M.; Jachimska, B. Mechanism of Lysozyme Adsorption onto Gold Surface Determined by Quartz Crystal Microbalance and Surface Plasmon Resonance. Bioelectrochemistry 2020, 135, 107582. [Google Scholar] [CrossRef]
- Pizzoni, D.; Mascini, M.; Lanzone, V.; Del Carlo, M.; Di Natale, C.; Compagnone, D. Selection of Peptide Ligands for Piezoelectric Peptide Based Gas Sensors Arrays Using a Virtual Screening Approach. Biosens. Bioelectron. 2014, 52, 247–254. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Wang, Y.; Wang, S.; Wang, J. Hollow Molecularly Imprinted Polymer Based Quartz Crystal Microbalance Sensor for Rapid Detection of Methimazole in Food Samples. Food Chem. 2020, 309, 125787. [Google Scholar] [CrossRef]
- Li, B.; Hu, X.; Zhang, Q.; Peng, X.; Xiang, Y. Improved Piezoelectricity of Polylactide Using Vitamin B2 for Poling-Free Mechanical and Acoustic Nanogenerators. J. Mater. Sci. 2021, 56, 902–912. [Google Scholar] [CrossRef]
- Li, D.-M.; Zhang, S.-Y.; Wan, J.-Y.; Zhang, W.-Q.; Yan, Y.-L.; Tang, X.-H.; Zheng, S.-R.; Cai, S.; Zhang, W.-G. A New Hydrazone-Linked Covalent Organic Framework for Fe(Ⅲ) Detection by Fluorescence and QCM Technologies. CrystEngComm 2021, 23, 3594–3601. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; Juan-Borrás, M.; March, C.; Arnau, A.; Escriche, I.; Montoya, Á.; Jiménez, Y. High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCM) Immunosensor for Pesticide Detection in Honey. Food Control 2018, 92, 1–6. [Google Scholar] [CrossRef]
- Su, L.; Zou, L.; Fong, C.C.; Wong, W.L.; Wei, F.; Wong, K.Y.; Wu, R.S.S.; Yang, M. Detection of Cancer Biomarkers by Piezoelectric Biosensor Using PZT Ceramic Resonator as the Transducer. Biosens. Bioelectron. 2013, 46, 155–161. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; Jiménez, Y.; Montoya, Á.; Juan-Borrás, M.; Pascual, N.; Arnau, A.; Escriche, I. High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCMD) Immunosensor for Detection of Sulfathiazole in Honey. Food Control 2020, 115, 107296. [Google Scholar] [CrossRef]
- Buchatip, S.; Ananthanawat, C.; Sithigorngul, P.; Sangvanich, P.; Rengpipat, S.; Hoven, V.P. Detection of the Shrimp Pathogenic Bacteria, Vibrio Harveyi, by a Quartz Crystal Microbalance-Specific Antibody Based Sensor. Sens. Actuators B Chem. 2010, 145, 259–264. [Google Scholar] [CrossRef]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Olfactory Receptor Based Piezoelectric Biosensors for Detection of Alcohols Related to Food Safety Applications. Sens. Actuators B Chem. 2011, 155, 8–18. [Google Scholar] [CrossRef]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. A Highly Selective Biosensor Based on Peptide Directly Derived from the HarmOBP7 Aldehyde Binding Site. Sensors 2019, 19, 4284. [Google Scholar] [CrossRef]
- Lin, W.L.; Lin, C.Y.; Tai, D.F. Preparation of a Molecularly Imprinted Film on Quartz Crystal Microbalance Chip for Determination of Furanic Compounds. Chemosensors 2021, 9, 338. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies—Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular Imprinting Technology for Microorganism Analysis. Trends Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wua, X.; Lia, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Culver, H.R.; Peppas, N.A. Protein-Imprinted Polymers: The Shape of Things to Come? Chem. Mater. 2017, 29, 5753–5761. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, N.; Xu, Y.; Li, Z.; Yan, C.; Mei, K.; Ding, M.; Ding, S.; Guan, P.; Qian, L.; et al. Molecularly Imprinted Materials for Selective Biological Recognition. Macromol. Rapid Commun. 2019, 40, 1900096. [Google Scholar] [CrossRef]
- Regan, B.; Boyle, F.; Kennedy, R.O.; Collins, D. Evaluation of Molecularly Imprinted Polymers for Point-of-Care Testing for Cardiovascular Disease. Sensors 2019, 19, 3485. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Niu, X.; Zhou, Y.; Liu, D.; Falahati, M.; Du, D.; Lin, Y. Integrating Ionic Liquids with Molecular Imprinting Technology for Biorecognition and Biosensing: A Review. Biosens. Bioelectron. 2020, 149, 111830. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Liu, L.; Chen, Y.; Zhou, W.; Pei, J.; Liang, Z.; Zhang, L.; Zhang, Y. Epitope Imprinting Technology: Progress, Applications, and Perspectives toward Artificial Antibodies. Adv. Mater. 2019, 31, 1902048. [Google Scholar] [CrossRef]
- Ge, Y.; Turner, A.P.F. Too Large to Fit ? Recent Developments in Macromolecular Imprinting. Trends Biotechnol. 2008, 26, 218–224. [Google Scholar] [CrossRef]
- Hayden, O. One Binder to Bind Them All. Sensors 2016, 16, 1665. [Google Scholar] [CrossRef]
- Hayden, O.; Lieberzeit, P.A.; Blaas, D.; Dickert, F.L. Artificial Antibodies for Bioanalyte Detection—Sensing Viruses and Proteins. Adv. Funct. Mater. 2006, 16, 1269–1278. [Google Scholar] [CrossRef]
- Dickert, F.L. Molecular Imprinting and Functional Polymers for All Transducers and Applications. Sensors 2018, 18, 327. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-Imprinted Polymer Sensors: Realising Their Potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for Commercial Application in Low-Cost Sensors and Assays—An Overview of the Current Status Quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
- Ye, L.; Haupt, K. Molecularly Imprinted Polymers as Antibody and Receptor Mimics for Assays, Sensors and Drug Discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef]
- Gómez-Arribas, L.N.; Urraca, J.L.; Benito-Penìa, E.; Moreno-Bondi, M.C. Tag-Specific Affinity Purification of Recombinant Proteins by Using Molecularly Imprinted Polymers. Anal. Chem. 2019, 91, 4100–4106. [Google Scholar] [CrossRef]
- Donato, L.; Drioli, E. Imprinted Membranes for Sustainable Separation Processes. Front. Chem. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Mohamed, S.; Balieu, S.; Petit, E.; Galas, L.; Schapman, D.; Hardouin, J.; Baati, R.; Estour, F. A Versatile and Recyclable Molecularly Imprinted Polymer as an Oxidative Catalyst of Sulfur Derivatives: A New Possible Method for Mustard Gas and v Nerve Agent Decontamination. Chem. Commun. 2019, 55, 13243–13246. [Google Scholar] [CrossRef]
- Ceylan Cömert, Ş.; Özgür, E.; Uzun, L.; Odabaşı, M. The Creation of Selective Imprinted Cavities on Quartz Crystal Microbalance Electrode for the Detection of Melamine in Milk Sample. Food Chem. 2022, 372. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly Imprinted Polymer Nanoparticles in Chemical Sensing—Synthesis, Characterisation and Application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Wang, X.; Fu, L.; Luo, L.; Chen, L. Rotational Paper-Based Microfluidic-Chip Device for Multiplexed and Simultaneous Fluorescence Detection of Phenolic Pollutants Based on a Molecular-Imprinting Technique. Anal. Chem. 2018, 90, 11827–11834. [Google Scholar] [CrossRef]
- Muhammad, P.; Tu, X.; Liu, J.; Wang, Y.; Liu, Z. Molecularly Imprinted Plasmonic Substrates for Specific and Ultrasensitive Immunoassay of Trace Glycoproteins in Biological Samples. ACS Appl. Mater. Interfaces 2017, 9, 12082–12091. [Google Scholar] [CrossRef]
- Parisi, O.I.; Morelli, C.; Puoci, F.; Saturnino, C.; Caruso, A.; Sisci, D.; Trombino, G.E.; Picci, N.; Sinicropi, M.S. Magnetic Molecularly Imprinted Polymers (MMIPs) for Carbazole Derivative Release in Targeted Cancer Therapy. J. Mater. Chem. B 2014, 2, 6619–6625. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Molecularly Imprinted Polymer as Drug Delivery Carrier in Alginate Dressing. Mater. Lett. 2017, 201, 46–49. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, Z.; Ding, Q.; Zhao, C.; Yu, H.; Zhu, X.; Fu, M.; Liu, Q. Magnetic-Graphene Oxide Based Molecular Imprinted Polymers for Selective Extraction of Glycoprotein at Physiological PH. Polymer 2021, 215, 123384. [Google Scholar] [CrossRef]
- Cao, J.; Wu, X.; Wang, L.; Shao, G.; Qin, B.; Wang, Z.; Wang, T.; Fu, Y. A Cellulose-Based Temperature Sensitivity Molecular Imprinted Hydrogel for Specific Recognition and Enrichment of Paclitaxel. Int. J. Biol. Macromol. 2021, 181, 1231–1242. [Google Scholar] [CrossRef]
- Lu, Z.; Du, X.; Sun, M.; Zhang, Y.; Li, Y.; Wang, X.; Wang, Y.; Du, H.; Yin, H.; Rao, H. Novel Dual-Template Molecular Imprinted Electrochemical Sensor for Simultaneous Detection of CA and TPH Based on Peanut Twin-like NiFe2O4/CoFe2O4/NCDs Nanospheres: Fabrication, Application and DFT Theoretical Study. Biosens. Bioelectron. 2021, 190, 113408. [Google Scholar] [CrossRef]
- Gao, R.; Hao, Y.; Zhang, L.; Cui, X.; Liu, D.; Zhang, M.; Tang, Y.; Zheng, Y. A Facile Method for Protein Imprinting on Directly Carboxyl-Functionalized Magnetic Nanoparticles Using Non-Covalent Template Immobilization Strategy. Chem. Eng. J. 2016, 284, 139–148. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Zhao, H.T.; Yang, X.; Zhang, H.; Dong, A.J.; Wang, J.; Li, B. Selective Recognition and Fast Enrichment of Anthocyanins by Dummy Molecularly Imprinted Magnetic Nanoparticles. J. Chromatogr. A 2018, 1572, 9–19. [Google Scholar] [CrossRef]
- Ndunda, E.N. Molecularly Imprinted Polymers—A Closer Look at the Control Polymer Used in Determining the Imprinting Effect: A Mini Review. J. Mol. Recognit. 2020, 33, e2855. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Tsalbouris, A.; Kabir, A.; Furton, K.G.; Samanidou, V.F. Synthesis and Application of Molecularly Imprinted Polymers Using Sol–Gel Matrix Imprinting Technology for the Efficient Solid-Phase Extraction of BPA from Water. Microchem. J. 2020, 157, 104965. [Google Scholar] [CrossRef]
- Yao, T.; Gu, X.; Li, T.; Li, J.; Li, J.; Zhao, Z.; Wang, J.; Qin, Y.; She, Y. Enhancement of Surface Plasmon Resonance Signals Using a MIP/GNPs/RGO Nano-Hybrid Film for the Rapid Detection of Ractopamine. Biosens. Bioelectron. 2016. [Google Scholar] [CrossRef]
- Özgür, E.; Saylan, Y.; Bereli, N.; Türkmen, D.; Denizli, A. Molecularly Imprinted Polymer Integrated Plasmonic Nanosensor for Cocaine Detection. J. Biomater. Sci. Polym. Ed. 2020, 31, 1211–1222. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5008–5945. [Google Scholar] [CrossRef]
- Nezhadali, A.; Mojarrab, M. Computational Design and Multivariate Optimization of an Electrochemical Metoprolol Sensor Based on Molecular Imprinting in Combination with Carbon Nanotubes. Anal. Chim. Acta 2016, 924, 86–98. [Google Scholar] [CrossRef]
- Garcia-Cruz, A.; Ahmad, O.S.; Alanazi, K.; Piletska, E.; Piletsky, S.A. Generic Sensor Platform Based on Electro-Responsive Molecularly Imprinted Polymer Nanoparticles (e-NanoMIPs). Microsyst. Nanoeng. 2020, 6, 83. [Google Scholar] [CrossRef]
- Alanazi, K.; Garcia Cruz, A.; Di Masi, S.; Voorhaar, A.; Ahmad, O.S.; Cowen, T.; Piletska, E.; Langford, N.; Coats, T.J.; Sims, M.R.; et al. Disposable Paracetamol Sensor Based on Electroactive Molecularly Imprinted Polymer Nanoparticles for Plasma Monitoring. Sens. Actuators B Chem. 2021, 329, 129128. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Battal, D.; Yalcin, M.S.; Yavuz, H.; Denizli, A. Rapid and Sensitive Detection of Synthetic Cannabinoids JWH-018, JWH-073 and Their Metabolites Using Molecularly Imprinted Polymer-Coated QCM Nanosensor in Artificial Saliva. Microchem. J. 2020, 153, 104454. [Google Scholar] [CrossRef]
- Bartold, K.; Pietrzyk-le, A.; Huynh, T.; Sosnowska, M.; Noworyta, K.; Lisowski, W.; Sannicolo, F.; Cauteruccio, S.; Licandro, E.; Souza, F.D.; et al. Programmed Transfer of Sequence Information into a Molecularly Imprinted Polymer for Hexakis (2,2′-Bithien-5-Yl) DNA Analogue Formation toward Single-Nucleotide-Polymorphism Detection. ACS Appl. Mater. Interfaces 2017, 9, 3948–3958. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Xiong, C.; Zheng, L.; Ding, Y.; Lu, H. Selective Recognition of Histidine Enantiomers Using Novel Molecularly Imprinted Organic Transistor Sensor. Org. Electron. 2018, 61, 254–260. [Google Scholar] [CrossRef]
- Fang, M.; Zhuo, K.; Chen, Y.; Zhao, Y.; Bai, G.; Wang, J. Fluorescent Probe Based on Carbon Dots / Silica / Molecularly Imprinted Polymer for Lysozyme Detection and Cell Imaging. Anal. Bioanal. Chem. 2019, 411, 5799–5807. [Google Scholar] [CrossRef]
- Esentürk, M.K.; Akgönüllü, S.; Yılmaz, F.; Denizli, A. Molecularly Imprinted Based Surface Plasmon Resonance Nanosensors for Microalbumin Detection. J. Biomater. Sci. Polym. Ed. 2019, 30, 646–661. [Google Scholar] [CrossRef]
- Guo, J.; Fang, G.; Wang, S.; Wang, J. Quartz Crystal Microbalance Sensor Based on 11-Mercaptoundecanoic Acid Self-Assembly and Amidated Nano-Titanium Film for Selective and Ultrafast Detection of Phosphoproteins in Food. Food Chem. 2021, 344, 128656. [Google Scholar] [CrossRef]
- Malik, A.A.; Nantasenamat, C.; Piacham, T. Molecularly Imprinted Polymer for Human Viral Pathogen Detection. Mater. Sci. Eng. C 2017, 77, 1341–1348. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Yan, J.; Luan, D.; Wu, J.; Bian, X. Rapid, Sensitive and Label-Free Detection of Pathogenic Bacteria Using a Bacteria-Imprinted Conducting Polymer Film-Based Electrochemical Sensor. Talanta 2021, 226, 122135. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Molecularly Imprinted Polymer-Based Sensors for Priority Pollutants. Sensors 2021, 21, 2406. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xing, H.; Li, G.; Wu, M. Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater. Sensors 2020, 20, 5506. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, Y.; Wang, Y.; Guo, H.; Nie, L. A Novel Molecularly Imprinted Nanosensor Based on Quartz Crystal Microbalance for Specific Recognition of α-Amanitin. Microchem. J. 2020, 159, 105383. [Google Scholar] [CrossRef]
- Yang, J.C.; Lee, J.; Hong, S.W.; Park, J. Molecularly Imprinted Quartz Crystal Microbalance Sensors with Lithographically Patterned Frisbee-like Pillar Arrays for Sensitive and Selective Detection of Iprodione. Sens. Actuators B Chem. 2020, 320, 128366. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR Nanosensor Based on Molecularly Imprinted Polymer Film with Gold Nanoparticles for Sensitive Detection of Aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. Development of Gold Nanoparticles Decorated Molecularly Imprinted–Based Plasmonic Sensor for the Detection of Aflatoxin M1 in Milk Samples. Chemosensors 2021, 9, 363. [Google Scholar] [CrossRef]
- Burnham-marusich, A.R.; Berninsone, P.M. Multiple Proteins with Essential Mitochondrial Functions Have Glycosylated Isoforms. Mitochondrion 2012, 12, 423–427. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Pan, J.; Jia, H.; Chen, Z.; Guo, T. Multifunctional Oligomer Immobilized on Quartz Crystal Microbalance: A Facile and Stabilized Molecular Imprinting Strategy for Glycoprotein Detection. Anal. Bioanal. Chem. 2019, 411, 3941–3949. [Google Scholar] [CrossRef]
- Diltemiz, S.E.; Hür, D.; Keçili, R.; Ersöz, A.; Say, R. New Synthesis Method for 4-MAPBA Monomer and Using for the Recognition of IgM and Mannose with MIP-Based QCM Sensors. Analyst 2013, 138, 1558–1563. [Google Scholar] [CrossRef]
- Ma, X.; He, X.; Li, W.; Zhang, Y. Epitope Molecularly Imprinted Polymer Coated Quartz Crystal Microbalance Sensor for the Determination of Human Serum Albumin. Sens. Actuators B. Chem. 2017, 246, 879–886. [Google Scholar] [CrossRef]
- Lee, M.H.; Thomas, J.L.; Tseng, H.Y.; Lin, W.C.; Liu, B.D.; Lin, H.Y. Sensing of Digestive Proteins in Saliva with a Molecularly Imprinted Poly(Ethylene-Co-Vinyl Alcohol) Thin Film Coated Quartz Crystal Microbalance Sensor. ACS Appl. Mater. Interfaces 2011, 3, 3064–3071. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, R.S.; Shah, K.; Prasad, R.; Singh, M. Epitope Imprinting of Iron Binding Protein of Neisseria Meningitidis Bacteria through Multiple Monomers Imprinting Approach. J. Mol. Recognit. 2018, 31, e2709. [Google Scholar] [CrossRef]
- Nasrullah, A.; Afzal, A.; Mujahid, A.; Lieberzeit, P.; Bajwa, S.Z.; Mustafa, G.; Latif, U. Imprinted Polymer and Cu2O-Graphene Oxide Nanocomposite for the Detection of Disease Biomarkers. Meas. Sci. Technol. 2021, 32, 105111. [Google Scholar] [CrossRef]
- Danstism, S.; Röhlen, D.; Wagner, T.; Wagner, P.; Schöning, M.J. A LAPS-Based Differential Sensor for Parallelized Metabolism Monitoring of Various Bacteria. Sensors 2019, 19, 4692. [Google Scholar] [CrossRef]
- Latif, U.; Can, S.; Sussitz, H.F.; Dickert, F.L. Molecular Imprinted Based Quartz Crystal Microbalance Sensors for Bacteria and Spores. Chemosensors 2020, 8, 64. [Google Scholar] [CrossRef]
- Yilmaz, E.; Majidi, D.; Ozgur, E.; Denizli, A. Whole Cell Imprinting Based Escherichia Coli Sensors: A Study for SPR and QCM. Sens. Actuators B. Chem. 2015, 209, 714–721. [Google Scholar] [CrossRef]
- Guha, A.; Sheej, O.; Guerreiro, A.; Karim, K.; Sandstr, N.; Ostanin, V.P.; Van Der Wijngaart, W.; Piletsky, S.A.; Ghosh, S.K. Direct Detection of Small Molecules Using a Nano-Molecular Imprinted Polymer Receptor and a Quartz Crystal Resonator Driven at a Fixed Frequency and Amplitude. Biosens. Bioelectron. 2020, 158, 112176. [Google Scholar] [CrossRef]
- Kushwaha, A.; Srivastava, J.; Singh, A.K.; Anand, R.; Raghuwanshi, R.; Rai, T.; Singh, M. Epitope Imprinting of Mycobacterium Leprae Bacteria via Molecularly Imprinted Nanoparticles Using Multiple Monomers Approach. Biosens. Bioelectron. 2019, 145, 111698. [Google Scholar] [CrossRef]
- Cornelis, P.; Givanoudi, S.; Yongabi, D.; Iken, H.; Duwé, S.; Deschaume, O.; Robbens, J.; Dedecker, P.; Bartic, C.; Wübbenhorst, M.; et al. Sensitive and Specific Detection of E. Coli Using Biomimetic Receptors in Combination with a Modified Heat-Transfer Method. Biosens. Bioelectron. 2019, 136, 97–105. [Google Scholar] [CrossRef]
- Tokonami, S.; Nakadoi, Y.; Takahashi, M.; Ikemizu, M.; Kadoma, T.; Saimatsu, K.; Dung, L.Q.; Shiigi, H.; Nagaoka, T. Label-Free and Selective Bacteria Detection Using a Film with Transferred Bacterial Configuration. Anal. Chem. 2013, 85, 4925–4929. [Google Scholar] [CrossRef]
- Wangchareansak, T.; Thitithanyanont, A.; Chuakheaw, D.; Gleeson, M.P.; Lieberzeit, P.A.; Sangma, C. A Novel Approach to Identify Molecular Binding to the Influenza Virus H5N1: Screening Using Molecularly Imprinted Polymers (MIPs). Medchemcomm 2014, 5, 617–621. [Google Scholar] [CrossRef]
- Lu, C.H.; Zhang, Y.; Tang, S.F.; Fang, Z.B.; Yang, H.H.; Chen, X.; Chen, G.N. Sensing HIV Related Protein Using Epitope Imprinted Hydrophilic Polymer Coated Quartz Crystal Microbalance. Biosens. Bioelectron. 2012, 31, 439–444. [Google Scholar] [CrossRef]
- Tai, D.; Lin, C.; Wu, T.; Chen, L. Recognition of Dengue Virus Protein Using Epitope-Mediated Molecularly Imprinted Film of a Pentadecapeptide onto a Quartz Crystal Microbalance. Anal. Chem. 2005, 77, 5140–5143. [Google Scholar] [CrossRef]
- Klangprapan, S.; Choke-arpornchai, B.; Lieberzeit, P.A.; Choowongkomon, K. Sensing the Classical Swine Fever Virus with Molecularly Imprinted Polymer on Quartz Crystal Microbalance. Heliyon 2020, 6, e04137. [Google Scholar] [CrossRef]
- Jenik, M.; Schirhagl, R.; Schirk, C.; Hayden, O.; Lieberzeit, P.; Blaas, D.; Paul, G.; Dickert, F.L. Sensing Picornaviruses Using Molecular Imprinting Techniques on a Quartz Crystal Microbalance. Anal. Chem. 2009, 81, 5320–5326. [Google Scholar] [CrossRef]
- Selvolini, G.; Marrazza, G. MIP-Based Sensors: Promising New Tools for Cancer Biomarker Determination. Sensors 2017, 17, 718. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. Applications of Commercial Biosensors in Clinical, Food, Environmental, and Biothreat/Biowarfare Analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
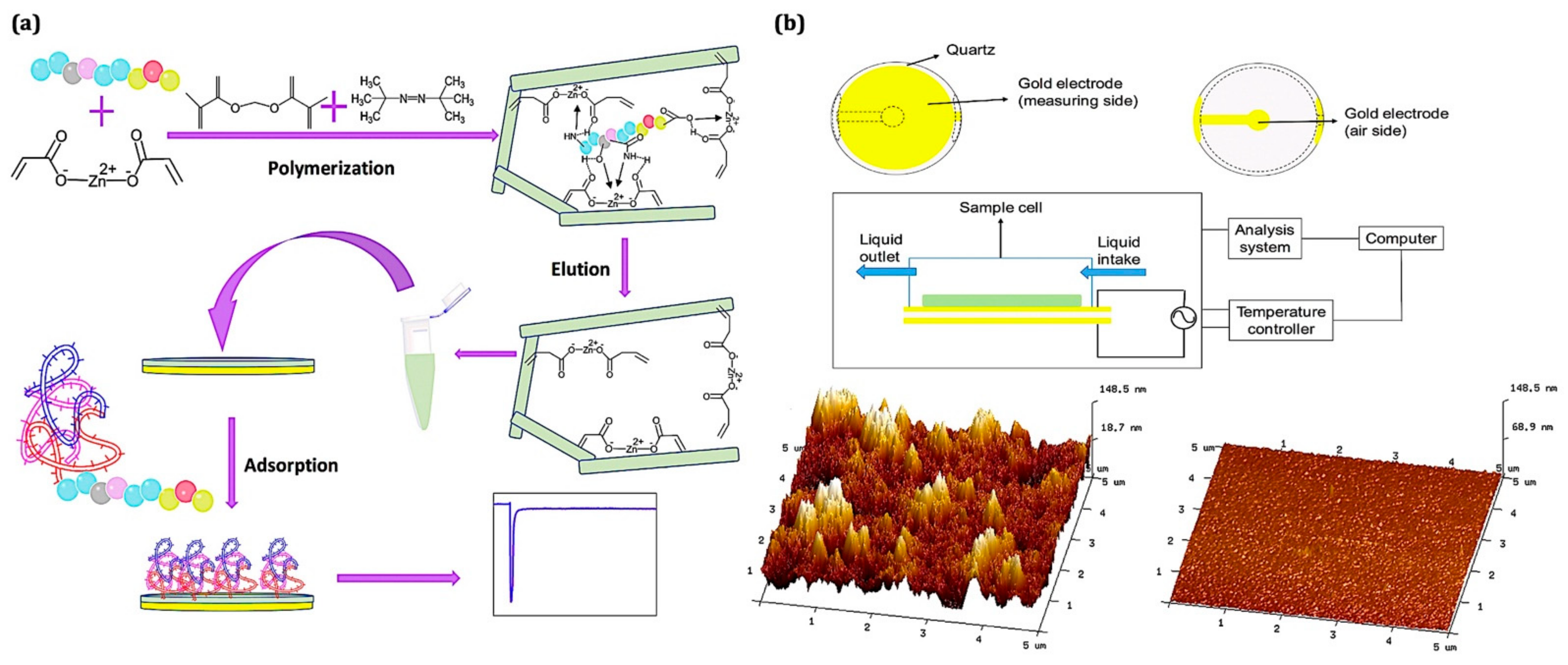
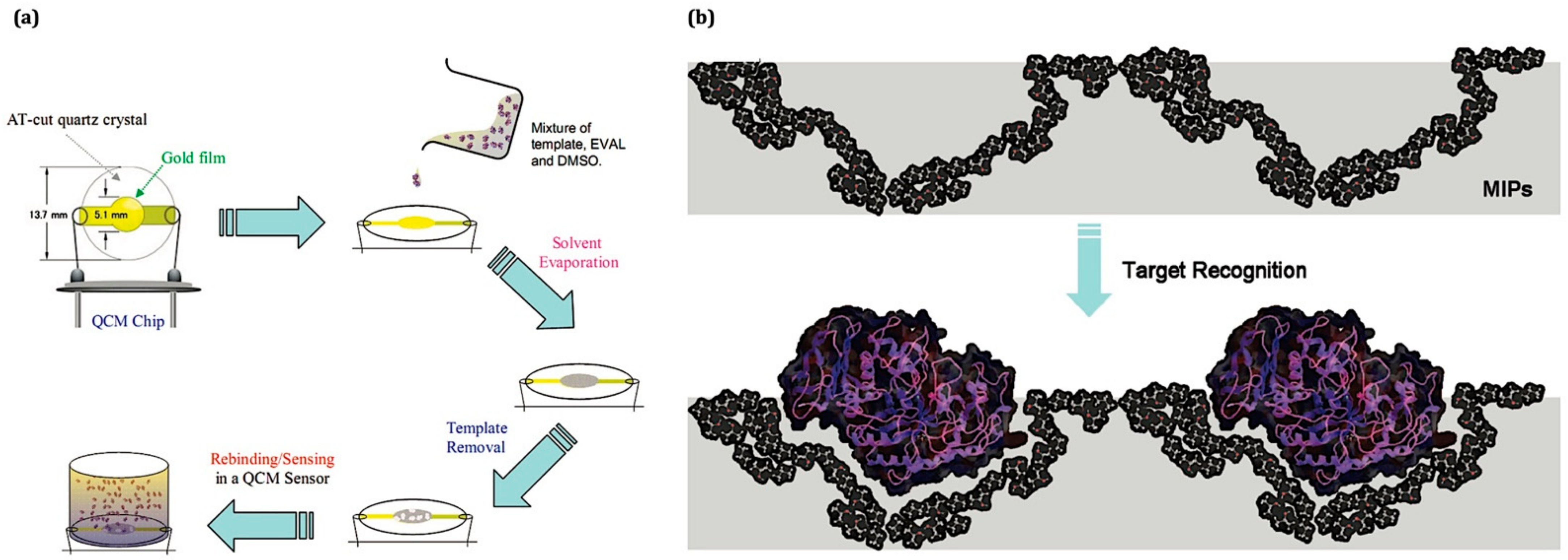


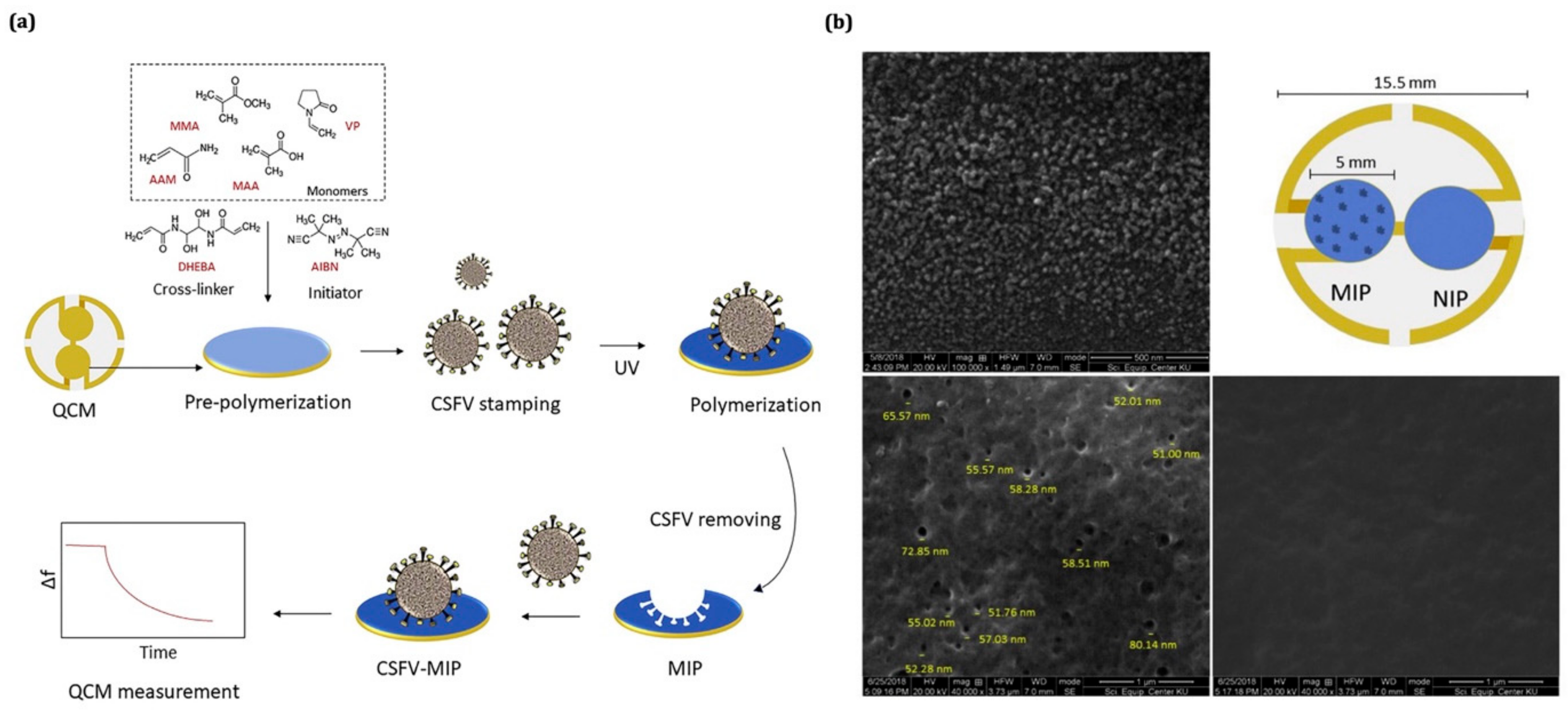
| Analyte | Functional Monomer | Linear Range | LOD | IF or k′ | Ref. |
|---|---|---|---|---|---|
| Protein | |||||
| Glycoprotein | 4-Vinylphenylboronic acid (VPBA) | 1 × 10−7–1 × 10−4 g/mL | - | ∼3.5 | [149] |
| IgM | Methacryloylamidophenylboronic acid (MAPBA) | 0.01–0.1 mM | - | 6.1 k′ | [150] |
| Albumin | N,N-dimethylformamide | 0.050–0.500 μg/mL. | 0.026 μg/mL | 6.9 | [151] |
| Amylase | Poly-(ethylene-co-vinyl alcohol) | 0–1.0 μg/mL | ∼pM | 2.13–2.47 | [152] |
| Iron requisition protein (fbpA) | 3-sulfopropyl methacrylate potassium-salt and benzyl methacrylate, N,N-methylene-bis-acrylamide | 5–30 ng/mL | 1.39 ng/mL | 12.27 | [153] |
| α1-Acid glycoprotein (α1-AGp) | Boronate-affinity | 150–200 ng/mL | 0.25 ng/mL | ∼7 | [154] |
| Bacteria | |||||
| E. coli and B. subtilis | Bulk imprinting | 0–25 × 107 cells/mL | - | - | [156] |
| E. coli | N-methacryloyl-l-histidine methylester | 0.5–3.0 McFarland | 3.72 × 105 CFU/mL | 3 k′ and 43.44 k′ | [157] |
| Mycobacterium leprae | 3-sulphopropyl methacrylate potassium salt, benzyl methacrylate and 4-aminothiophenol | 10–140 nM | 0.161 nM | 8.28 | [159] |
| E. Coli | Polyurethane | 102–106 CFU/mL | 100 CFU/mL | - | [160] |
| P. aeruginosa | Polypyrrole (OPPy) | 1011–103 CFU/mL | 103 CFU/mL | 3.92 k′ | [161] |
| Virus | |||||
| H5N1 | Methacrylic acid and methyl-methacrylate | 1–8 HAU | 1 HA | ∼4 | [162] |
| HIV type-I | Dopamine | 2–200 ng/mL | 2 ng/mL | 6 | [163] |
| Dengue virus | Pentadecapeptide | 0.5–50,000 ng/mL | - | - | [164] |
| Swine fever virus | Multi-functional monomer/surface imprinting technique | 4–21 μg/mL | 1.7 μg/mL | 2 and 62 | [165] |
| Picornavirus | Polyurethane MIPs | 100–300 μg/mL | 100 μg/mL | ∼9 | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgönüllü, S.; Özgür, E.; Denizli, A. Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors 2022, 10, 106. https://doi.org/10.3390/chemosensors10030106
Akgönüllü S, Özgür E, Denizli A. Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors. 2022; 10(3):106. https://doi.org/10.3390/chemosensors10030106
Chicago/Turabian StyleAkgönüllü, Semra, Erdoğan Özgür, and Adil Denizli. 2022. "Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers" Chemosensors 10, no. 3: 106. https://doi.org/10.3390/chemosensors10030106
APA StyleAkgönüllü, S., Özgür, E., & Denizli, A. (2022). Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors, 10(3), 106. https://doi.org/10.3390/chemosensors10030106